Abstract
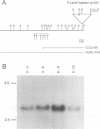
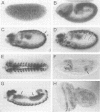
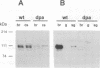
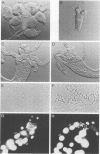
We have isolated the Drosophila disc proliferation abnormal (dpa) gene, a member of the MCM family of DNA replication factors. Members of this family of proteins are required for DNA replication in yeast. A dpa null mutant dies during pupal stages because imaginal tissues necessary for the formation of the adult fly fail to proliferate normally. Beginning in late embryogenesis BrdU labeling reveals DNA replication defects in mitotically proliferating cells. In contrast, dpa is dispensable for endoreplication, a specialized cell cycle consisting of consecutive rounds of S phases without intervening mitosis. Our studies suggest an essential role for dpa in mitotic DNA replication but not in endoreplication. Thus, dpa is not a general replication factor but may play a specialized regulatory role in DNA replication.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axton J. M., Shamanski F. L., Young L. M., Henderson D. S., Boyd J. B., Orr-Weaver T. L. The inhibitor of DNA replication encoded by the Drosophila gene plutonium is a small, ankyrin repeat protein. EMBO J. 1994 Jan 15;13(2):462–470. doi: 10.1002/j.1460-2075.1994.tb06281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell S. P., Stillman B. ATP-dependent recognition of eukaryotic origins of DNA replication by a multiprotein complex. Nature. 1992 May 14;357(6374):128–134. doi: 10.1038/357128a0. [DOI] [PubMed] [Google Scholar]
- Bier E., Vaessin H., Shepherd S., Lee K., McCall K., Barbel S., Ackerman L., Carretto R., Uemura T., Grell E. Searching for pattern and mutation in the Drosophila genome with a P-lacZ vector. Genes Dev. 1989 Sep;3(9):1273–1287. doi: 10.1101/gad.3.9.1273. [DOI] [PubMed] [Google Scholar]
- Blow J. J., Laskey R. A. A role for the nuclear envelope in controlling DNA replication within the cell cycle. Nature. 1988 Apr 7;332(6164):546–548. doi: 10.1038/332546a0. [DOI] [PubMed] [Google Scholar]
- Bodmer R., Carretto R., Jan Y. N. Neurogenesis of the peripheral nervous system in Drosophila embryos: DNA replication patterns and cell lineages. Neuron. 1989 Jul;3(1):21–32. doi: 10.1016/0896-6273(89)90112-8. [DOI] [PubMed] [Google Scholar]
- Chong J. P., Mahbubani H. M., Khoo C. Y., Blow J. J. Purification of an MCM-containing complex as a component of the DNA replication licensing system. Nature. 1995 Jun 1;375(6530):418–421. doi: 10.1038/375418a0. [DOI] [PubMed] [Google Scholar]
- Coxon A., Maundrell K., Kearsey S. E. Fission yeast cdc21+ belongs to a family of proteins involved in an early step of chromosome replication. Nucleic Acids Res. 1992 Nov 11;20(21):5571–5577. doi: 10.1093/nar/20.21.5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crevel G., Cotterill S. DNA replication in cell-free extracts from Drosophila melanogaster. EMBO J. 1991 Dec;10(13):4361–4369. doi: 10.1002/j.1460-2075.1991.tb05014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffley J. F., Cocker J. H., Dowell S. J., Rowley A. Two steps in the assembly of complexes at yeast replication origins in vivo. Cell. 1994 Jul 29;78(2):303–316. doi: 10.1016/0092-8674(94)90299-2. [DOI] [PubMed] [Google Scholar]
- Duronio R. J., O'Farrell P. H. Developmental control of a G1-S transcriptional program in Drosophila. Development. 1994 Jun;120(6):1503–1515. doi: 10.1242/dev.120.6.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard J. P., Poupon M. F., Press P., Favez G. Effet du sérum de patients tuberculeux sur la transformation blastique de lymphocytes de sarcoïdose. Schweiz Med Wochenschr. 1970 Nov 14;100(46):1986–1988. [PubMed] [Google Scholar]
- Hayashi S., Hirose S., Metcalfe T., Shirras A. D. Control of imaginal cell development by the escargot gene of Drosophila. Development. 1993 May;118(1):105–115. doi: 10.1242/dev.118.1.105. [DOI] [PubMed] [Google Scholar]
- Heichman K. A., Roberts J. M. Rules to replicate by. Cell. 1994 Nov 18;79(4):557–562. doi: 10.1016/0092-8674(94)90541-x. [DOI] [PubMed] [Google Scholar]
- Heitzler P., Coulson D., Saenz-Robles M. T., Ashburner M., Roote J., Simpson P., Gubb D. Genetic and cytogenetic analysis of the 43A-E region containing the segment polarity gene costa and the cellular polarity genes prickle and spiny-legs in Drosophila melanogaster. Genetics. 1993 Sep;135(1):105–115. doi: 10.1093/genetics/135.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson D. S., Banga S. S., Grigliatti T. A., Boyd J. B. Mutagen sensitivity and suppression of position-effect variegation result from mutations in mus209, the Drosophila gene encoding PCNA. EMBO J. 1994 Mar 15;13(6):1450–1459. doi: 10.1002/j.1460-2075.1994.tb06399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy K. M., Clark C. D., Botstein D. Subcellular localization of yeast CDC46 varies with the cell cycle. Genes Dev. 1990 Dec;4(12B):2252–2263. doi: 10.1101/gad.4.12b.2252. [DOI] [PubMed] [Google Scholar]
- Hilliker A. J., Trusis-Coulter S. N. Analysis of the functional significance of linkage group conservation in Drosophila. Genetics. 1987 Oct;117(2):233–244. doi: 10.1093/genetics/117.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H., Nozaki N., Sugimoto K. DNA polymerase alpha associated protein P1, a murine homolog of yeast MCM3, changes its intranuclear distribution during the DNA synthetic period. EMBO J. 1994 Sep 15;13(18):4311–4320. doi: 10.1002/j.1460-2075.1994.tb06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y., Mimura S., Nishimoto S., Takisawa H., Nojima H. Identification of the yeast MCM3-related protein as a component of Xenopus DNA replication licensing factor. Cell. 1995 May 19;81(4):601–609. doi: 10.1016/0092-8674(95)90081-0. [DOI] [PubMed] [Google Scholar]
- Li J. J., Herskowitz I. Isolation of ORC6, a component of the yeast origin recognition complex by a one-hybrid system. Science. 1993 Dec 17;262(5141):1870–1874. doi: 10.1126/science.8266075. [DOI] [PubMed] [Google Scholar]
- Madine M. A., Khoo C. Y., Mills A. D., Laskey R. A. MCM3 complex required for cell cycle regulation of DNA replication in vertebrate cells. Nature. 1995 Jun 1;375(6530):421–424. doi: 10.1038/375421a0. [DOI] [PubMed] [Google Scholar]
- Mauhin V., Lutz Y., Dennefeld C., Alberga A. Definition of the DNA-binding site repertoire for the Drosophila transcription factor SNAIL. Nucleic Acids Res. 1993 Aug 25;21(17):3951–3957. doi: 10.1093/nar/21.17.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake S., Okishio N., Samejima I., Hiraoka Y., Toda T., Saitoh I., Yanagida M. Fission yeast genes nda1+ and nda4+, mutations of which lead to S-phase block, chromatin alteration and Ca2+ suppression, are members of the CDC46/MCM2 family. Mol Biol Cell. 1993 Oct;4(10):1003–1015. doi: 10.1091/mbc.4.10.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A. The substrates of the cdc2 kinase. Semin Cell Biol. 1991 Aug;2(4):261–270. [PubMed] [Google Scholar]
- Poole S. J., Kauvar L. M., Drees B., Kornberg T. The engrailed locus of Drosophila: structural analysis of an embryonic transcript. Cell. 1985 Jan;40(1):37–43. doi: 10.1016/0092-8674(85)90306-x. [DOI] [PubMed] [Google Scholar]
- Prokop A., Technau G. M. Normal function of the mushroom body defect gene of Drosophila is required for the regulation of the number and proliferation of neuroblasts. Dev Biol. 1994 Feb;161(2):321–337. doi: 10.1006/dbio.1994.1034. [DOI] [PubMed] [Google Scholar]
- Prokop A., Technau G. M. The origin of postembryonic neuroblasts in the ventral nerve cord of Drosophila melanogaster. Development. 1991 Jan;111(1):79–88. doi: 10.1242/dev.111.1.79. [DOI] [PubMed] [Google Scholar]
- Raff J. W., Glover D. M. Nuclear and cytoplasmic mitotic cycles continue in Drosophila embryos in which DNA synthesis is inhibited with aphidicolin. J Cell Biol. 1988 Dec;107(6 Pt 1):2009–2019. doi: 10.1083/jcb.107.6.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. V., King J. A., Orr-Weaver T. L. Identification of genomic regions required for DNA replication during Drosophila embryogenesis. Genetics. 1993 Nov;135(3):817–829. doi: 10.1093/genetics/135.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. V., Orr-Weaver T. L. The regulation of the cell cycle during Drosophila embryogenesis: the transition to polyteny. Development. 1991 Aug;112(4):997–1008. doi: 10.1242/dev.112.4.997. [DOI] [PubMed] [Google Scholar]
- Stern B., Ried G., Clegg N. J., Grigliatti T. A., Lehner C. F. Genetic analysis of the Drosophila cdc2 homolog. Development. 1993 Jan;117(1):219–232. doi: 10.1242/dev.117.1.219. [DOI] [PubMed] [Google Scholar]
- Tautz D., Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989 Aug;98(2):81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Todorov I. T., Pepperkok R., Philipova R. N., Kearsey S. E., Ansorge W., Werner D. A human nuclear protein with sequence homology to a family of early S phase proteins is required for entry into S phase and for cell division. J Cell Sci. 1994 Jan;107(Pt 1):253–265. doi: 10.1242/jcs.107.1.253. [DOI] [PubMed] [Google Scholar]
- Treisman J. E., Follette P. J., O'Farrell P. H., Rubin G. M. Cell proliferation and DNA replication defects in a Drosophila MCM2 mutant. Genes Dev. 1995 Jul 15;9(14):1709–1715. doi: 10.1101/gad.9.14.1709. [DOI] [PubMed] [Google Scholar]
- Truman J. W., Bate M. Spatial and temporal patterns of neurogenesis in the central nervous system of Drosophila melanogaster. Dev Biol. 1988 Jan;125(1):145–157. doi: 10.1016/0012-1606(88)90067-x. [DOI] [PubMed] [Google Scholar]
- Vässin H., Bremer K. A., Knust E., Campos-Ortega J. A. The neurogenic gene Delta of Drosophila melanogaster is expressed in neurogenic territories and encodes a putative transmembrane protein with EGF-like repeats. EMBO J. 1987 Nov;6(11):3431–3440. doi: 10.1002/j.1460-2075.1987.tb02666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitebread L. A., Dalton S. Cdc54 belongs to the Cdc46/Mcm3 family of proteins which are essential for initiation of eukaryotic DNA replication. Gene. 1995 Mar 21;155(1):113–117. doi: 10.1016/0378-1119(94)00925-i. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Date T., Matsukage A. Distribution of PCNA in Drosophila embryo during nuclear division cycles. J Cell Sci. 1991 Dec;100(Pt 4):729–733. doi: 10.1242/jcs.100.4.729. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Kuroda K., Hirose F., Matsukage A. Distribution of DNA polymerase alpha during nuclear division cycles in Drosophila melanogaster embryo. Cell Struct Funct. 1992 Apr;17(2):105–112. doi: 10.1247/csf.17.105. [DOI] [PubMed] [Google Scholar]
- Yan H., Gibson S., Tye B. K. Mcm2 and Mcm3, two proteins important for ARS activity, are related in structure and function. Genes Dev. 1991 Jun;5(6):944–957. doi: 10.1101/gad.5.6.944. [DOI] [PubMed] [Google Scholar]
- Yan H., Merchant A. M., Tye B. K. Cell cycle-regulated nuclear localization of MCM2 and MCM3, which are required for the initiation of DNA synthesis at chromosomal replication origins in yeast. Genes Dev. 1993 Nov;7(11):2149–2160. doi: 10.1101/gad.7.11.2149. [DOI] [PubMed] [Google Scholar]