Abstract
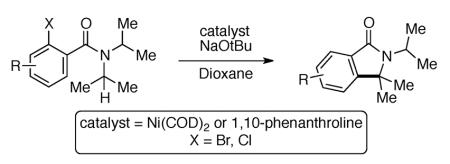
The development of an intramolecular arylation of sp3 C–H bonds adjacent to nitrogen using aryl halides is described. Arylation was accomplished using either Ni(COD)2 or 1,10-phenanthroline in sub-stoichiometric amounts and the reaction conditions were applied to a variety of electronically differentiated benzamide substrates. Preliminary studies suggest a mechanism involving aryl and alkyl radical intermediates.
The direct functionalization of C–H bonds is a powerful method for the introduction of aryl groups into organic molecules. The majority of known methods for the arylation of sp3 C–H bonds utilize precious metal (e.g. Pd, Ru, Rh) catalysts and/or expensive phosphine ligands.1,2 In recent years a number of reports on Cu-3 and Fe-4 catalyzed sp3 C–H bond arylation using heteroarenes5 or transmetallating reagents6 have been described. Additionally, an example of Ni-catalyzed intermolecular oxidative arylation of C–H bonds adjacent to ether oxygen or amine nitrogen atoms has been published.7 Although these reports represent important advances toward general systems for alkyl C–H arylations using inexpensive catalysts, most of these protocols require the use of potentially hazardous oxidants (e.g., TBHP, DDQ) at high temperatures. This drawback could be addressed by replacing transmetallating reagents with aryl halides, which can serve as both the aryl source and the oxidant. To the best of our knowledge, no examples of alkyl C–H arylations using aryl halides and sub-stoichiometric amounts of first row transition metals have been disclosed.1
Herein, we describe a method for the intramolecular coupling of aryl halides (halide = Br, Cl) with alkyl C–H bonds adjacent to nitrogen in benzamide substrates using sub-stoichiometric Ni(COD)2. During the course of these investigations we discovered that the same transformation could often be effected using sub-stoichiometric 1,10-phenanthroline in place of Ni(COD)2. These latter results are the first demonstration of the use of transition metal-free catalysts for the arylation of alkyl C–H bonds adjacent to heteroatoms using aryl halides.8,9,10,11
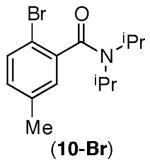
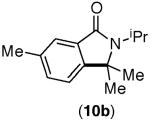
Our studies commenced with the investigation of reaction parameters for the intramolecular arylation of amide 1-Br using the Ni(COD)2/PCy3 catalyst system (Table 1). Although the use of carbonate and phosphate bases provided only trace cyclization product (entries 1–4), higher conversion was obtained with NaOtBu in xylene or dioxane (entries 5 and 6). Strikingly, in contrast to the previously reported Pd-catalyzed reactions (which afford 1a),12 isoindolinone 1b was formed as the major product via arylation of the cyclohexyl C–H bond.
Table 1.
Optimization of the Intramolecular Arylation of 1-Br[a]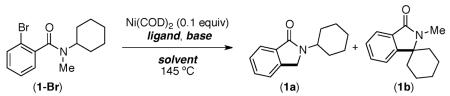
| entry | ligand | base | solvent | 1a (% yield)[b] | 1b (% yield)[b] | conversion(%)[c] |
|---|---|---|---|---|---|---|
| 1 | PCy3HBF4 | Cs2CO3 | xylene | 0 | 0 | 33 |
| 2 | PCy3HBF4 | Rb2CO3 | xylene | 0 | 0 | 31 |
| 3 | PCy3HBF4 | K2CO3 | xylene | 0 | 2 | 5 |
| 4 | PCy3HBF4 | K3PO4 | xylene | 0 | 2 | 34 |
| 5 | PCy3HBF4 | NaOtBu | xylene | 8 | 30 | 88 |
| 6 | PCy3HBF4 | NaOtBu | dioxane | 8 | 30 | 83 |
| 7 | none | NaOtBu | dioxane | 9 | 45 | 100 |
| 8[d] | none | NaOtBu | dioxane | 2 | 11 | 52 |
| 9[e] | none | NaOtBu | dioxane | 0 | 24 | 69 |
| 10[f] | none | NaOtBu | dioxane | 0 | 11 | 56 |
General conditions: Ni(COD)2 (0.1 equiv), PCy3HBF4 (0 or 0.2 equiv), base (1.5 equiv), solvent, 145 °C, 15 h.
Calibrated GC yields against hexadecane as the internal standard.
Calibrated yield of remaining 1-Br determined by gas chromatographic analysis of the crude reaction mixtures.
General conditions, but with no Ni(COD)2.
In the presence of TEMPO (0.5 equiv).
In the presence of galvinoxyl (0.5 equiv).
Control experiments revealed that the yield of 1b could be improved to 45% in the absence of PCy3 (entry 7). However, diminished product yields were obtained when Ni(COD)2 was excluded (entry 8). The preferential arylation of the more substituted C–H bond (cyclohexyl versus methyl) using the Ni(COD)2/NaOtBu system suggested the possible involvement of alkyl radicals in these reactions. As would be expected, in this scenario the use of radical scavengers such as TEMPO (2,2,6,6-tetramethylpiperidin-1-yl)oxidanyl) and galvinoxyl led to lower yields of 1b (entries 9 and 10).
The potential involvement of radicals and the requirement for strong bases is reminiscent of the recently reported 1,10-phenanthroline catalyzed arylation of sp2 C–H bonds using aryl halides.8,9 As shown in Scheme 1, 1,10-phenanthroline could be used in place of Ni(COD)2 to afford 1b in comparable yield (38%) albeit with higher catalyst loadings (20 mol %). Although complete conversion of 1-Br is observed using either Ni(COD)2 or 1,10-phenanthroline, the modest yield of 1b is in part due to significant demethylation of the protodebrominated substrate (producing a 2° amide). The observation of this product suggested that 1° C–H bonds are not amenable to the desired arylation.
Scheme 1.
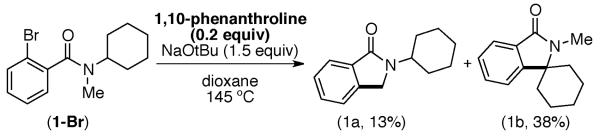
Arylation Using 1,10-Phenanthroline
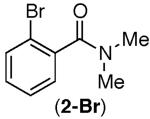
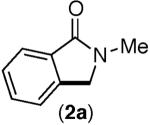
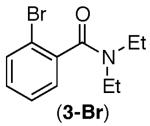
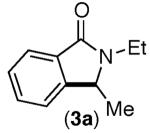
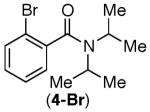
Consistent with this proposal, the dimethyl substrate 2-Br provided the desired product only in trace amounts under both sets of conditions (Table 2, entry 1). Gratifyingly, substrates bearing only 3° C–H bonds adjacent to nitrogen led to higher yields of products than those bearing 1° or 2° C–H bonds (entries 1–4).13
Table 2.
Scope of Arylation Using Bromide Substrates
| entry | substrate | product | Conditions A yield[a],[b] | Conditions B yield[c],[d] |
|---|---|---|---|---|
| 1 |
|
|
<10% | <10% |
| 2 |
|
|
53% | 47% |
| 3 |
|

|
76% | 89% |
| 4 |
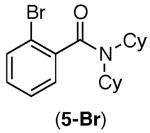
|
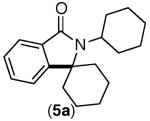
|
92% | 95% |
| 5 |
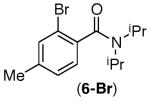
|
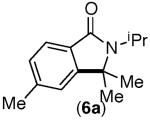
|
83% | 90% |
| 6 |

|
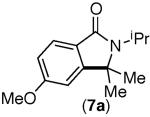
|
80%[d] | trace |
| 7 |
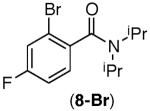
|
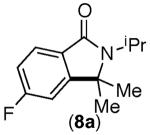
|
60%[d] | 13%[b] |
Conditions A: Ni(COD)2 (0.1 equiv), NaOtBu (1.5 equiv), dioxane, 145 °C.
1H NMR yields against 1,4-dinitrobenzene as the internal standard.
Conditions B: 1,10-phenanthroline (0.2 equiv), NaOtBu (1.5 equiv), dioxane, 145 °C.
Isolated yields (isolated yields were generally within 5% of the crude 1H NMR yields).
Under the Ni-mediated conditions (Conditions A), the isoindolinone products are formed in good yields from electronically varied substrates. Although good yield of 8a is obtained from 8-Br, competitive substitution of the F and Br groups by tBuO− ion was observed with this substrate. In contrast to the Ni-mediated transformations, the efficiency of the corresponding phenanthroline-mediated reactions (Conditions B) is significantly influenced by the substrate electronics. While substrates bearing electron neutral aryl rings were transformed to the desired products in excellent yields (entries 3–5), those bearing substituents that are electron withdrawing with respect to the ipso C–Br position afforded only trace cyclization products (7-Br and 8-Br, entries 6 and 7). The observed poor reaction efficiencies of these substrates is partly due to a faster rate of nucleophilic aromatic substitution by tBuO− than the desired C–C bond formation, as this SNAr product is detected at the end of the reaction.
We next explored the efficiency of the analogous transformations of aryl chlorides. As shown in Table 3, the use of Ni(COD)2 generally led to significantly higher yields than the phenanthroline conditions for aryl chlorides. Overall the functional group tolerance and electronic effects were similar to those observed with aryl bromides.
Table 3.
Scope of Arylation Using Chloride Substrates
| entry | substrate | product | Conditions A yield[a],[b] | Conditions B yield[c],[d] |
|---|---|---|---|---|
| 1 |
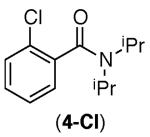
|

|
86% | 45% |
| 2 |
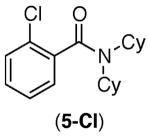
|
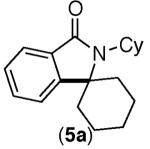
|
81% | 68% |
| 3 |
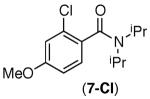
|
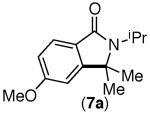
|
66% | trace |
| 4 |
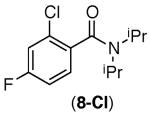
|
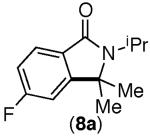
|
65% | trace |
| 5 |
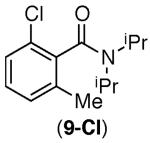
|
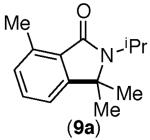
|
67% | 46% |
Conditions A: Ni(COD)2 (0.1 equiv), NaOtBu (1.5 equiv), dioxane, 145 °C.
Isolated yields, (isolated yields were generally within 5% of the crude NMR yields).
Conditions B: 1,10-phenanthroline (0.2 equiv), NaOtBu (1.5 equiv), dioxane, 145 °C.
1H NMR yields against 1,4-dinitrobenzene as the internal standard.
The reaction of substrates bearing a substituent meta to the amide moiety was next examined (Table 4). The reaction of meta-Me, -OMe and -F substituted benzamides revealed that C–C bond formation does not take place exclusively at the ipso (CAr–X) position. Instead a mixture of isomeric products was formed, exhibiting a slight preference for C–C bond formation at the more sterically hindered position on the aryl ring (i.e., ortho to the preexisting aryl substituent, entries 1–3, 5, and 6).
Table 4.
Site-Selectivity of C-C Bond Formation
| entry | substrate | major product | minor product | yield (selectivity) |
|
|---|---|---|---|---|---|
| Conditions | |||||
| A[a] | B[b],[c] | ||||
| 1 |
|

|
|
84% (1.1:1)[d],[e] | 93% (1.3:1)[e] |
| 2 |
|
|
|
71% (2.0:1)[d],[e] | 94% (1.7:1)[e] |
| 3 |
|
|
|
83% (1.3:1)[d],[e] | 94% (1.3:1)[e] |
| 4 |
|
|
|
79% (2.2:1)[c],[e] | trace |
| 5 |
|

|
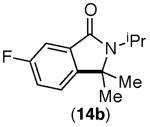
|
80% (2.7:1)[c],[e] | trace |
| 6 |

|
|
|
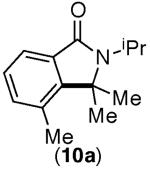
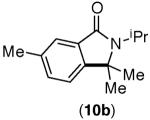
67% (1.2:1)[d],[e] | 82% (1.4:1)[c],[e] |
Reaction conditions: Ni(COD)2 (0.1 equiv), NaOtBu (1.5 equiv), dioxane, 145 °C.
Reaction conditions: 1,10-phenanthroline (0.2 equiv), NaOtBu (1.5 equiv), dioxane, 145 °C.
Isolated yields (all isolated yields were within 5% of the crude NMR yields).
1H NMR yields against 1,4-dinitrobenzene as the internal standard.
Selectivities determined by 1H NMR spectroscopic analysis of the crude reaction mixtures.
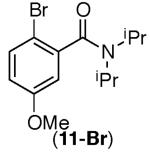
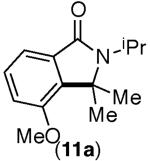
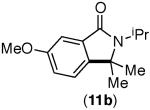
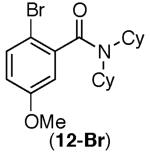
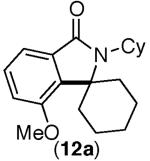
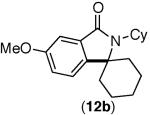
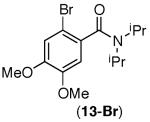
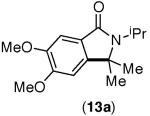
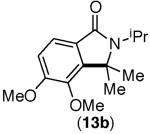
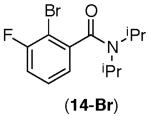
This selectivity trend was upheld for both substrates 11-Br and 12-Br, which differ in the nature of the alkyl groups on the amide. Interestingly however, the reaction of dimethoxy substrate 13-Br displayed modest selectivity for alkylation at the less sterically hindered ortho position, para to one of the OMe groups (entry 4). The site-selectivity of the phenanthroline-mediated transformations was comparable to that of the Ni-mediated process described above (entries 1–3, 6). Consistent with the poor reactivity of substrates 7-Br and 8-Br under phenanthroline conditions (Table 2), substrates 13-Br and 14-Br led to trace product with phenanthroline, while displaying good reactivity with Ni(COD)2 (Table 4, entries 4–5).
A plausible mechanism for the Ni-mediated intramolecular arylation involves (i) an initiation step via single electron transfer from Ni0 to the substrate to generate radical anion B, (ii) loss of Br− to form the aryl radical C, (iii) intramolecular H-atom abstraction to provide the stabilized alkyl radical D, (iv) radical addition into the arene to generate isomeric aryl radical intermediates E and F, and finally (v) re-aromatization to afford the cyclized product with concomitant regeneration of the radical anion B (Scheme 2). Alternatively, under phenanthroline conditions, the 1 e− transfer steps (i and v) are mediated by a phenanthroline/tBuO− adduct analogous to that previously documented in sp2 C–H arylations.8,9,14
Scheme 2.
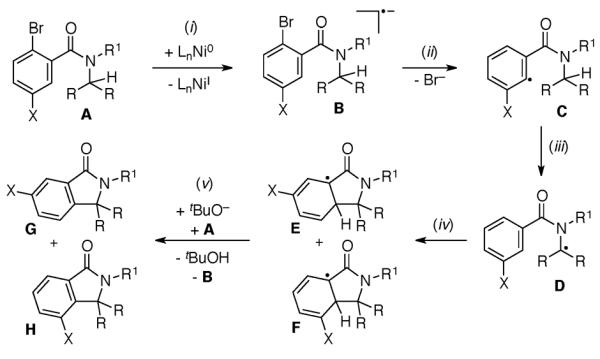
Plausible Mechanism
This mechanism is consistent with several observations described above. First, radical scavengers such as TEMPO and galvinoxyl inhibit the Ni-mediated transformation (Table 1, entries 9 and 10). Second, the efficiency of the arylation increases with more substituted alkyl C–H bonds, which is in accord with a mechanism involving alkyl radical intermediates like D. Third, meta-substituted amides lead to mixtures of isomeric products. This latter result is expected for a mechanism involving the proposed step iv (Scheme 2). Finally, the diminished efficiencies of the reactions of aryl chloride substrates (versus the analogous aryl bromides) under phenanthroline conditions parallels the trend in reduction potentials of aryl halides (Ar–Br>Ar–Cl).14,15
In summary, this paper describes the development of intramolecular arylation of sp3 C–H bonds adjacent to an amide nitrogen using Ar–X (X = Br, Cl) using sub-stoichiometric Ni(COD)2 or 1,10-phenanthroline. Preliminary studies suggest the involvement of aryl and alkyl radical intermediates.
Supplementary Material
Acknowledgements
This work was supported by St. Olaf College, the American Chemical Society Petroleum Research Fund (PRF#52224-UNI3), and the NIH NIGMS (R15GM107892). L. C. W. is thankful for the SURF fellowship from ACS, Division of Organic Chemistry.
Supporting Information Available: Experimental details and spectroscopic and analytical data for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For representative reviews on C–H arylation, see: Alberico D, Scott ME, Lautens M. Chem. Rev. 2007;107:174. doi: 10.1021/cr0509760. Kakiuchi F, Kochi T. Synthesis. 2008:3013. McGlacken GP, Bateman LM. Chem. Soc. Rev. 2009;38:2447. doi: 10.1039/b805701j. Chen X, Engle KM, Wang D-H, Yu J-Q. Angew. Chem., Int. Ed. 2009;48:5094. doi: 10.1002/anie.200806273. Ackermann L, Vincente R, Kapdi AR. Angew. Chem., Int. Ed. 2009;48:9792. doi: 10.1002/anie.200902996. Bellina F, Rossi R. Tetrahedron. 2009;65:10269. Lyons TW, Sanford MS. Chem. Rev. 2010;110:1147. doi: 10.1021/cr900184e. Daugulis O. Top. Curr. Chem. 2010;292:57. doi: 10.1007/128_2009_10. Yamaguchi J, Kei M, Itami K. Eur. J. Org. Chem. 2013:19.
- 2.For representative reviews on sp3 C–H functionalization, see: Giri R, Shi B-F, Engle K-M, Maguel N, Yu J-Q. Chem. Soc. Rev. 2009;38:3242. doi: 10.1039/b816707a. Jazzar R, Hitse J, Renaudat A, Sofack-Kreutzer J, Baudoin O. Chem. Eur. J. 2010;16:2654. doi: 10.1002/chem.200902374. Wasa M, Engle KM, Yu J-Q. Isr. J. Chem. 2010;50:605. doi: 10.1002/ijch.201000038. Bellina F, Rossi R. Chem. Rev. 2010;110:1082. doi: 10.1021/cr9000836. Baudoin O. Chem. Soc. Rev. 2011;40:4902. doi: 10.1039/c1cs15058h.
- 3.(a) Wu J-C, Song R-J, Wang Z-Q, Huang X-C, Xie Y-X, Li J-H. Angew. Chem. Int. Ed. 2012;51:3453. doi: 10.1002/anie.201109027. [DOI] [PubMed] [Google Scholar]; (b) Boess E, Schmitz C, Klussmann M. J. Am. Chem. Soc. 2012;134:5317. doi: 10.1021/ja211697s. [DOI] [PubMed] [Google Scholar]; (c) Zhang G, Miao J, Zhao Y, Ge HB. Angew. Chem. Int. Ed. 2012;51:8318. doi: 10.1002/anie.201204339. [DOI] [PubMed] [Google Scholar]; (d) Park SJ, Price JR, Todd MH. J. Org. Chem. 2012;77:949. doi: 10.1021/jo2021373. [DOI] [PubMed] [Google Scholar]; (e) Huang L, Niu T, Wu J, Zhang Y. J. Org. Chem. 2011;76:1759. doi: 10.1021/jo1023975. [DOI] [PubMed] [Google Scholar]; (f) Su W, Yu J, Li Z, Jiang Z. J. Org. Chem. 2011;76:9144. doi: 10.1021/jo2015533. [DOI] [PubMed] [Google Scholar]; (g) Li Z, Li C-J. J. Am. Chem. Soc. 2005;127:6968. doi: 10.1021/ja0516054. [DOI] [PubMed] [Google Scholar]
- 4.(a) Shirakawa E, Yoneda T, Moriya K, Ota K, Uchiyama N, Nishikawa R, Hayashi T. Chem. Lett. 2011;40:1041. [Google Scholar]; (b) Ohta M, Quick MP, Yamaguchi J, Wunsch B, Itami K. Chem. Asian. J. 2009;4:1416. doi: 10.1002/asia.200900157. [DOI] [PubMed] [Google Scholar]; (c) Li Y-Z, Li B-J, Lu X-Y, Lin S, Shi Z-J. Angew. Chem. Int. Ed. 2009;48:3817. doi: 10.1002/anie.200900341. [DOI] [PubMed] [Google Scholar]
- 5.(a) Mitchell EA, Peschiulli A, Lefevre N, Meerpoel L, Maes BUW. Chem. Eur. J. 2012;18:10092. doi: 10.1002/chem.201201539. [DOI] [PubMed] [Google Scholar]; (b) Zhang C, Tang C, Jiao N. Chem. Soc. Rev. 2012;41:3464. doi: 10.1039/c2cs15323h. [DOI] [PubMed] [Google Scholar]; (c) Wendlandt AE, Suess AM, Stahl SS. Angew. Chem. Int. Ed. 2011;50:11062. doi: 10.1002/anie.201103945. [DOI] [PubMed] [Google Scholar]; (d) Shi W, Liu C, Lei A. Chem. Soc. Rev. 2011;40:2761. doi: 10.1039/c0cs00125b. [DOI] [PubMed] [Google Scholar]
- 6.(a) Sekine M, Ilies L, Nakamura E. Org. Lett. 2013;15:714. doi: 10.1021/ol400056z. [DOI] [PubMed] [Google Scholar]; (b) Shang R, Ilies L, Matsumoto A, Nakamura E. J. Am. Chem. Soc. 2013;135:6030. doi: 10.1021/ja402806f. [DOI] [PubMed] [Google Scholar]; (c) Singh PP, Gudup S, Aruri H, Singh U, Ambala S, Yadav M, Sawant SD, Vishwakarma RA. Org. Biomol. Chem. 2012;10:1587. doi: 10.1039/c1ob06660a. [DOI] [PubMed] [Google Scholar]; (d) Singh PP, Gudup S, Ambala S, Singh U, Dadhwal S, Singh B, Sawant SD, Vishwakarma RA. Chem. Commun. 2011;47:5852. doi: 10.1039/c1cc11094b. [DOI] [PubMed] [Google Scholar]; (e) Yoshikai N, Mieczkowski A, Matsumoto A, Ilies L, Nakamura E. J. Am. Chem. Soc. 2010;132:5568. doi: 10.1021/ja100651t. [DOI] [PubMed] [Google Scholar]; (f) Basle O, Li C-J. Org.. Lett. 2008;10:3661. doi: 10.1021/ol8012588. [DOI] [PubMed] [Google Scholar]
- 7.Liu D, Liu C, Li H, Lei A. Angew. Chem. Int. Ed. 2013;52:4453. doi: 10.1002/anie.201300459. [DOI] [PubMed] [Google Scholar]
- 8.For recent examples on biaryl formation in the absence of transition metal catalysts see: Buden ME, Guastavino JF, Rossi RA. Org. Lett. 2013;15:1174. doi: 10.1021/ol3034687. Liu W, Tian F, Wang X, Yu H, Bi Y. Chem. Commun. 2013;49:2983. doi: 10.1039/c3cc40695d. Cheng Y, Gu X, Li P. Org. Lett. 2013;15:2664. doi: 10.1021/ol400946k. Mehta VP, Punji B. RSC Adv. 2013:11957. Wu Y, Wong, Mao F, Chan TL, Kwong FY. Org. Lett. 2012;14:5306. doi: 10.1021/ol302489n. Bhakuni BS, Kumar A, Balkrishna SJ, Sheikh JA, Konar S, Kumar S. Org. Lett. 2012;14:2838. doi: 10.1021/ol301077y. De S, Ghosh S, Bhunia S, Sheikh JA, Bisai A. Org. Lett. 2012;14:4466. doi: 10.1021/ol3019677. Shirakawa E, Itoh K-I, Higashino T, Hayashi T. J. Am Chem. Soc. 2010;132:15537. doi: 10.1021/ja1080822. Liu W, Cao H, Zhang H, Chung KH, He C, Wang H, Kwong FY, Lei A. J. Am Chem. Soc. 2010;132:16737. doi: 10.1021/ja103050x. Sun C-L, Li H, Yu D-G, Yu M, Zhou X, Lu X-Y, Huang K, Zheng S-F, Li B-J, Shi J-Z. Nature Chem. 2010;2:1044. doi: 10.1038/nchem.862.
- 9.For recent reviews on biaryl formation in the absence of transition metal catalysts see: Shirakawa E, Hayashi T. Chem. Lett. 2012;41:130. Yanagisawa S, Itami K. Chem. Cat. Chem. 2011;3:827. Studer A, Curran DP. Angew. Chem. Int. Ed. 2011;50:5018. doi: 10.1002/anie.201101597. Lei A, Lei W, Liu C, Chen M. Dalton Trans. 2010;39:10352. doi: 10.1039/c0dt00486c.
- 10.Metal free intramolecular alpha-arylation adjacent to a carbonyl using aryl halides has been reported. See: Khan TA, Tripoli R, Crawford JJ, Martin CG, Murphy JA. Org. Lett. 2003;5:2971. doi: 10.1021/ol035173i. Arylations adjacent to heteroatoms using aryl halides in the presence of radical initiators such as Bu3SnH/AIBN have been reported (See ref 11d).
- 11.For other representative reports on sp3 C–H arylation adjacent to nitrogen atoms see: Dastbaravardeh N, Kirchner K, Schnürch M, Mihovilovic MD. J. Org. Chem. 2013;78:658. doi: 10.1021/jo302547q. (b) McNally A, Prier CK, MacMillan DWC. Science. 2011;334:1114. doi: 10.1126/science.1213920. Prokopkova H, Bergman SD, Aelvoet K, Smout V, Herrebout W, Van der Veken B, Meerpoel L, Maes BUW. Chem. Eur. J. 2010;16:13063. doi: 10.1002/chem.201001887. Campos KR. Chem. Soc. Rev. 2007;36:1069. doi: 10.1039/b607547a. Pastine SJ, Gribkov DV, Sames D. J. Am. Chem. Soc. 2006;128:14220. doi: 10.1021/ja064481j.. Campos KR, Klapars A, Waldman JH, Dormer PG, Chen CY. J. Am. Chem. Soc. 2006;128:3538. doi: 10.1021/ja0605265.
- 12.(a) Rousseaux S, Gorelsky SI, Chung BKW, Fagnou K. J. Am. Chem. Soc. 2010;132:10692. doi: 10.1021/ja103081n. [DOI] [PubMed] [Google Scholar]; (b) Rousseaux S, Davi M, Sofack-Kreutzer J, Pierre C, Kefalidis CE, Clot E, Fagnou K, Baudoin O. J. Am. Chem. Soc. 2010;132:10706. doi: 10.1021/ja1048847. [DOI] [PubMed] [Google Scholar]
- 13.Product 3a has been isolated previously from the reaction of 3-Br in the absence of any catalyst. In this report, 3a was isolated as a side product in the context of a Pd-catalyzed reaction. MacNeil SL, Gray M, Gusev DG, Brigs LE, Snieckus V. J. Org. Chem. 2008;73:9710. doi: 10.1021/jo801856n.
- 14.Tsou TT, Kochi JK. J. Am. Chem. Soc. 1979;101:7547. [Google Scholar]
- 15.The mechanism in Scheme 2 is just one proposal. The regeneration of Ni0 in step (v) cannot be excluded amongst other possibilities.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


