Abstract
Previous studies have demonstrated that increased gastric pH from the use of acid-reducing agents, such as proton-pump inhibitors or H2-receptor antagonists, can significantly impact the absorption of weakly basic drugs that exhibit pH-dependent solubility. Clinically practical strategies to mitigate this interaction have not been developed. This pilot study evaluated the extent and time course of gastric re-acidification after a solid oral dosage form of anhydrous betaine HCl in healthy volunteers with pharmacologically-induced hypochlorhydria. Six healthy volunteers with baseline normochlorhydria (fasting gastric pH < 4) were enrolled in this single period study. Hypochlorhydria was induced via 20 mg oral rabeprazole twice daily for four days. On the fifth day, an additional 20 mg dose of oral rabeprazole was given and gastric pH was monitored continuously using the Heidelberg pH capsule. After gastric pH > 4 was confirmed for 15 minutes, 1500 mg of betaine HCl was given orally with 90 mL of water and gastric pH was continuously monitored for 2 hours. Betaine HCl significantly lowered gastric pH by 4.5 (±0.5) units from 5.2 (±0.5) to 0.6 (±0.2) (P <0.001) during the 30 minute interval after administration. The onset of effect of betaine HCl was rapid, with a mean time to pH < 3 of 6.3 (±4.3) minutes. The re-acidification period was temporary with a gastric pH < 3 and < 4 lasting 73 (±33) and 77 (±30) minutes, respectively. Betaine HCl was well tolerated by all subjects. In healthy volunteers with pharmacologically-induced hypochlorhydria, betaine HCl was effective at temporarily lowering gastric pH. The rapid onset and relatively short duration of gastric pH reduction gives betaine HCl the potential to aid the absorption of orally administered weakly basic drugs that exhibit pH-dependent solubility when administered under hypochlorhydric conditions.
Keywords: betaine hydrochloride, gastric re-acidification, hypochlorhydria, pH-dependent solubility, proton pump inhibitor (PPI), drug-drug interaction (DDI)
Introduction
Previous studies have investigated the effects of gastric pH on the absorption of orally administered drugs,1–3 emphasizing the importance of gastric acid to the dissolution and solubilization processes. These pH effects on absorption are also dependent on the physicochemical properties of each individual compound, such as native ionic state, acid dissociation constants (pKa), and partition coefficient (logP). Moreover, the magnitude of these effects can be amplified for compounds that exhibit pH-dependent solubility, where exponential changes in solubility are observed as the pH rises or falls.
This gastric pH interaction is highlighted for weakly-basic drugs that exhibit decreasing solubility over the pH range 1 to 4, as elevations in gastric pH reduce the solubility of these drugs and can lead to reduced drug absorption and systemic exposure. An example of such a compound is atazanavir, which is a protease inhibitor that is weakly basic with a pKa of 4.25.4 When administered in the presence of a low dose of omeprazole (20 mg orally), atazanavir area under the plasma concentration-time curve (AUC0–24) and trough levels (Cmin) were reduced by 42% (90% CI of geometric mean ratio: 0.44–0.75) and 46% (90% CI of geometric mean ratio: 0.41–0.71), respectively.5 Many synthetic triazole antifungal agents are also weak bases with pH-dependent solubility and are affected by elevations in gastric pH. Itraconazole for example, which has a pKa of 3.7,6 showed significant reductions in plasma AUC0–24 and maximum plasma concentration (Cmax) of 64% and 66% (P <0.05), respectively, when administered to healthy volunteers pre-treated with oral omeprazole (40 mg daily) for 14 days.7 In a similar fashion, ketoconazole, when co-administered with cimetidine, suffered reductions in AUC and Cmax of greater than 90% in healthy volunteers.8
In an attempt to mitigate the impact of acid-reducing agents on drug absorption for weakly-basic drugs with pH-dependent solubility, Chin et al.1 conducted a prospective clinical study investigating the effects of an acidic beverage (Coca-Cola®) on the pharmacokinetics of ketoconazole. When ketoconazole was dosed with Coca-Cola® (pH = 2.5) under the effects of omeprazole, ketoconazole AUC0–∞ and Cmax were both increased by greater than 10-fold when compared to the omeprazole treatment without Coca-Cola®,1 though Coca-Cola® did not completely restore the omeprazole decrease in ketoconazole exposure. Nonetheless, re-acidification strategies to increase drug absorption in hypochlorhydric conditions seem to be a viable strategy. However, in a study with atazanavir, Coca-Cola® did not markedly reverse the decreased absorption due to omeprazole dosing.9 In order to sufficiently influence gastric pH in such a way as to mitigate reduced drug absorption, a large quantity of an acidic beverage would need to be given over a short period of time to facilitate dissolution of an oral drug. Though it may seem viable, this method is flawed by the lack of consistency in the ability to provide ample gastric re-acidification under hypochlorhydric conditions. Additionally, the impact on the absorption of these weakly basic drugs may be limited as the pH of Coca-Cola® is only 2.5, which, for some drugs, may not contain enough acid to effectively mitigate this interaction. Moreover, repeated oral ingestion of a large volume of an acidic beverage is potentially harmful to oral and dental health (i.e. dental caries), and is likely not clinically acceptable in patients with symptoms of gastroesophageal reflux disease (GERD) due to the associated esophageal pain and discomfort of an acidic beverage. Therefore, we sought to develop a more reproducible and reliable strategy of gastric re-acidification under hypochlorhydric conditions that also has the potential to be more clinically acceptable.
Using a hydrochloric acid (HCl) supplement given in a solid oral dosage form such as a tablet or capsule would be more beneficial for this purpose, as the HCl would not directly come into contact with the mouth or esophagus. Additionally, the acidifying effect of the supplement would only activate once it reaches the stomach, where the salt can dissociate and produce free HCl. A paper published in 1991 characterized the effects of a solid oral dose of glutamic acid hydrochloride on gastric pH in healthy male volunteers receiving ranitidine. Glutamic acid hydrochloride lowered gastric pH to less than pH 2 within 15 minutes and remained under pH 3 for 45 minutes.10 While glutamic acid hydrochloride is no longer commercially available, a comparable salt, betaine hydrochloride (BHCl), has been more recently used as a gastric acid supplement and is available over the counter as a nutraceutical. Betaine is a naturally occurring substance that can be found in a variety of foods including beets, spinach, and whole wheat foods. Although BHCl has been used to supplement gastric acid, the time course and potency of this effect has not been thoroughly investigated in humans. Therefore, this pilot study aimed to characterize the ability of BHCl to reduce gastric pH under the influence of a proton-pump inhibitor, rabeprazole (Aciphex®) in healthy volunteers.
Experimental Section
Subject Enrollment
Six healthy volunteers (4 male, 2 female) with ages between 25 and 57 years were enrolled in this single period, single treatment study (National Clinical Trials ID: #NCT01237353). Volunteers were non-smokers and had a mean (±SD) body mass index (BMI) of 25.2 (±3.8) kg/m2. After providing written informed consent, all subjects underwent a screening visit to determine eligibility to participate in the study. The screening visit consisted of a physical examination, medical history review, and measurement of baseline gastric pH using the Heidelberg pH Diagnostic System (Heidelberg Medical, Inc., Mineral Bluff, GA). This radio-telemetry system utilizes a small Heidelberg capsule that is tethered to a thin, pre-measured surgical string and given to subjects to swallow. Gastric pH measurements are then wirelessly transmitted from the capsule to a designated computer, which records pH data at 1-second intervals. Enrolled subjects did not have hypo- or achlorhydria (i.e. fasting pH was < 2), nor did they possess any history of gastrointestinal diseases. This study was approved by the Committee on Human Research of the University of California, San Francisco.
Study Design
This study was conducted at the Clinical & Translational Science Institute’s Clinical Research Center (CCRC) at the University of California, San Francisco. Healthy subjects were first pre-treated with 20 mg oral rabeprazole sodium (Aciphex®; Eisai Inc., Woodcliff Lake, NJ) twice daily, with food, for four days to induce hypochlorhydria. All subjects were also responsible for maintaining a medication diary, in which the time of each rabeprazole dose was recorded, and whether or not it was taken with food. On the fifth day, the Heidelberg capsule was placed and gastric pH was observed for 30 minutes to achieve baseline measurements and an additional 20 mg of rabeprazole sodium was administered. When gastric pH had remained above 4 for at least 15 minutes, 1500 mg of BHCl (2–750 mg tablets (Lot #01010); Designs for Health, Windsor, CT) were given with 250 mL of water (Designs for Health is a company specializing in the production of nutritional products, is certified by the National Sanitation Foundation, and is compliant with Good Manufacturing Practices11). Gastric pH was monitored an additional 2 hours, or until the pH rose above 4 for 15 minutes. All subjects were fasted from midnight the night prior and remained fasted until the end of the study day.
Venous blood samples for serum gastrin measurements (Quest Diagnostics, San Jose, CA) were taken at the following times, with respect to administration of the Heidelberg capsule: 0.5 h, 2.5 h, and at the end of the study.
Data Analysis
Gastric pH data was recorded by the Heidelberg pH Diagnostic System over 1-second intervals. From this, pH data at 1-minute intervals were then reported and used for subsequent analyses, as it had been previously shown that pH data sampled at 1-minute intervals can accurately depict the pH-time profile.10 To evaluate the potency of BHCl, the lowest pH during a 15- and 30-minute interval after BHCl administration was subtracted from the median pH value over a 15- and 30-minute interval before BHCl administration, respectively.
The onset of effect of BHCl was determined by the time necessary for gastric pH to fall to less than pH 3, while the duration of effect of BHCl was measured by calculating the rebound time necessary for gastric pH to rise above pH 3 and pH 4, relative to BHCl administration. As a secondary method to evaluate BHCl efficacy, area under the pH versus time curves (AUCpH) were calculated using a modification of the Trapezoidal Rule, beginning with BHCl administration until the end of the study. To calculate the AUCpH below pH 3 or pH 4, the difference between the observed pH and pH 3 or pH 4 was multiplied by the time interval (1 minute) for each time point. At any given time, if the observed pH exceeded the pH 3 or pH 4, those AUCs were reported as zero.
Statistical Analyses
The sample size was calculated based on a previously reported change in gastric pH of −4.6 ± 1.0 pH units after 1360 mg (7.6 mmol H+) of glutamic acid HCl was administered to healthy volunteers treated with ranitidine.10 With a two-sided α=0.05 and 90% power, 5 subjects would be necessary to detect a 2 unit difference in gastric pH before and after the administration of 1500 mg (9.7 mmol H+) of betaine HCl. Thus, a sample size of 6 was chosen to account for patient compliance and improve the estimations of our study endpoints.
All statistical tests were performed using GraphPad Prism 5 for Windows Version 5.02 (La Jolla, CA). Differences in gastric pH values before and after BHCl administration were tested for statistical significance using a Student’s Paired, Two-tailed t-test, whereas AUCpH correlations were tested for statistical significance using a non-parametric Spearman Rank Correlation test. Statistical significance was achieved with P ≤0.05.
Results
Gastric pH Monitoring
Representative gastric pH plots from two subjects during the fifth study day can be found in Figure 1. Though all subjects were pre-treated with multiple doses of rabeprazole, fasting gastric pH at the beginning of the fifth study day was not always above 4. This may most likely be due to the nocturnal gastric acid breakthrough that occurs overnight, even after twice-daily dosing of a PPI.12 It is interesting to note that those subjects who had a fasting gastric pH > 4 at the beginning of the study day took their doses with a meal, which supports the hypothesis that PPIs only inhibit active proton pumps in the stomach.12
Figure 1. Sample gastric pH data of two subjects captured by the Heidelberg pH Capsule.
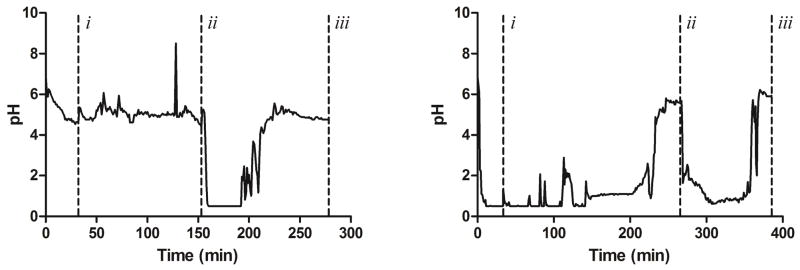
Gastric pH measurements at 1-minute intervals were plotted versus time to produce the following curves. Vertical dashed lines (- - -) mark the following study events: (i) administration of 20 mg oral rabeprazole with 90 mL of water, (ii) administration of 1500 mg oral BHCl with 250 mL of water, and (iii) study end. The left curve represents a “best-case scenario,” where a subject began the study day with a gastric pH > 4 after pre-treatment with rabeprazole for 4 days. In contrast, the right curve represents a “worst-case scenario,” where a subject began the study with pH < 4. In these instances, BHCl was not administered until gastric pH remained above 4 for at least 15 minutes.
Potency of Betaine Hydrochloride
The average (±SD) gastric pH during the 15- and 30-minute interval just before BHCl administration was found to be 5.1 (±0.5) and 5.2 (±0.5), respectively, confirming that the rabeprazole dosing regimen chosen for the study was sufficient to induce hypochlorhydria in each subject. As seen in Figure 2, gastric pH was significantly reduced (P <0.001) after the administration of BHCl, with the lowest pH values over a 15- and 30-minute interval of 0.9 (±0.4) and 0.6 (±0.2), respectively. This corresponds to a mean change in pH (ΔpH) of 4.2 (±0.3) and 4.6 (±0.5) units for the 15- and 30-minute intervals, respectively.
Figure 2. Potency of BHCl administration in Healthy Volunteers with Rabeprazole-Induced Hypochlorhydria.
Gastric pH was compared at equal time intervals (15 and 30 minutes) before and after BHCl administration. In both time intervals, gastric pH ranged from 4.5–5.9 before BHCl, with a mean pH of 5.1 (±0.5) and 5.2 (±0.5) for the 15- and 30-minute intervals, respectively. After BHCl administration, the mean gastric pH was dropped to 0. 9 (±0.4) and 0.6 (±0.2), ranging between 0.5–1.6 and 0.5–1.1, for the 15- and 30-minute intervals, respectively. Means of 6 subjects are represented by horizontal bars. (*** : P<0.001)
Time Course of Betaine Hydrochloride
Table 1 summarizes the time course of BHCl for each subject. The onset of effect of BHCl was rapid, taking only 6.27 (±4.29) minutes to reach a pH < 3 when 1500 mg BHCl was given orally with 250 mL of water. The duration of BHCl re-acidification was also temporary, with rebound times to pH 3 and pH 4 averaging 73 (±33) and 77 (±30) minutes, respectively.
Table 1.
Onset and duration measures of gastric re-acidification using BHCl after rabeprazole-induced hypochlorhydria. Onset of effect is characterized by the time to pH < 3, while the duration of effect is characterized by the rebound times to pH > 3 and pH > 4.
| Subject | Time to pH < 3 (Post-BHCl, min) | Rebound Time to pH > 3 (min) | Rebound Time to pH > 4 (min) |
|---|---|---|---|
| P-001 | 2.13 | 29 | 42 |
| P-002 | 12.6 | 83 | 83 |
| P-003 | 4.05 | 46 | 53 |
| P-004 | 1.83 | 90 | 90 |
| P-005 | 8.83 | 121 | 127 |
| P-006 | 8.23 | 66 | 66 |
|
| |||
| Mean (±SD) | 6.27 (±4.29) | 73 (±33) | 77 (±30) |
AUCpH Below pH 3 and pH 4
The individual AUCpH values for each patient can be found in Table 2. When comparing AUCpH below pH 3 or AUCpH below pH 4 with the ΔpH after BHCl administration, no correlations were found (P=0.66 and P=0.42 respectively). However, as expected, correlations were found between the AUCpH below pH 3 and rebound time to pH > 3 (r=0.9; P=0.03) and AUCpH below pH 4 and rebound time to pH > 4 (r=1.0; P=0.003).
Table 2.
Individual AUCpH values calculated below thresholds of pH 3 and pH 4.
| Subject | AUC Below pH < 3 (ΔpH*min) | AUC Below pH < 4 (ΔpH*min) |
|---|---|---|
| P-001 | 70.1 | 108 |
| P-002 | 178 | 259 |
| P-003 | 104 | 156 |
| P-004 | 169 | 261 |
| P-005 | 281 | 409 |
| P-006 | 91.0 | 157 |
|
| ||
| Mean (±SD) | 149 (±77.9) | 225 (±109) |
|
| ||
| Spearman-r Coefficienta | 0.89 | 1.0 |
Spearman-r coefficients are reported for correlations between AUCpH measures and their respective rebound times to pH > 3 and pH > 4.
Serum Gastrin Measurements
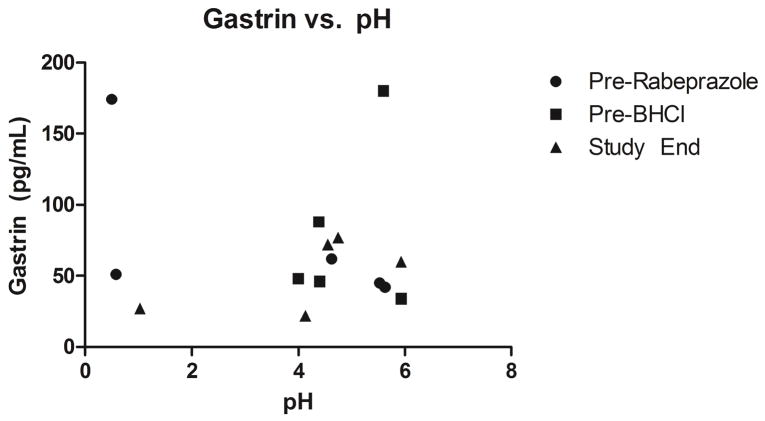
Serum gastrin levels measured during the study day are depicted in Figure 3. No significant relationships were found between gastrin levels and gastric pH measures.
Figure 3. Serum Gastrin Levels Measured at Major Study Events and the Corresponding Observed Gastric pH.
Serum gastrin levels (pg/mL) were measured three-times on each study day: before rabeprazole dosing, before BHCl dosing, and at the end of the study day. No significant relationships were observed between serum gastrin and gastric pH with respect to the artificial changes in pH due to rabeprazole and BHCl.
Safety
All 6 subjects who were enrolled completed the study. No significant adverse events were observed. Moreover, no gastrointestinal side effects as a result of BHCl administration were observed.
Discussion
In this pilot study, we demonstrated that 1500 mg of BHCl significantly (P <0.001) and safely reduced gastric pH in healthy subjects with pharmacologically-induced hypochlorhydria by over 4 pH units (Figure 2), lowering gastric pH from above pH 5 to below pH 1 for more than an hour. These findings support the further investigation of BHCl as a solid oral dosage form of HCl that can potentially aid in the absorption of orally administered weakly basic drugs with pH-dependent solubility given under hypochlorhydric conditions.
Hypochlorhydria (or achlorhydria) is defined as a lack (or absence) of acid in the gastric fluid, and can be caused by medical conditions such as Helicobacter pylori infection13 or autoimmune metaplastic atrophic gastritis.14 Hypochlorhydria can also be induced through the repeated administration of acid-reducing agents, such as proton-pump inhibitors (PPIs) or H2-receptor antagonists (H2-RAs), commonly used to manage symptoms of gastroesophageal reflux disesase (GERD) or peptic ulcer disease (PUD). Individuals on daily drug regimens of acid-reducing agents therefore will have high gastric pH. From a pharmacologic perspective, gastric acid is important for the dissolution and solubilization of many solid oral dosage form medications and therefore is critical in oral drug absorption. Thus, conditions in which gastric acid levels are lowered or suppressed can lead to drug-drug interactions (DDIs) resulting in altered systemic concentrations and drug exposures. For example, for weakly basic drugs that exhibit pH-dependent solubility over a pH range of 1 to 4, the fraction of the dose available to the body for absorption will be severely reduced when gastric pH is > 4, limiting drug absorption and systemic exposure during acid-reducing agent therapy. In this scenario, temporary gastric re-acidification during drug administration may be helpful.
A gastric pH ≤ 2 was thought to be sufficiently acidic to improve the solubility of weakly basic drugs with pH-dependent solubility. Therefore, a 1500 mg dose of BHCl (2–750 mg tablets) was chosen as it provides the equivalent of 9.7 mmol of hydrogen ions (H+), which equates to pH 1.41 in 250 mL of water. Though 1–750 mg tablet will produce over 4.8 mmol of H+ and a pH of 1.71, two tablets were used to guarantee a more consistent gastric re-acidification across patients. An acidic beverage, such as Coca-Cola® for example, was reported to have a pH of 2.5.1 If a drug was administered with 250 mL of the beverage, this would only provide 0.79 mmol of H+, which is much less than a single 750 mg tablet of BHCl that could provide over 4.8 mmol of H+. BHCl provides an additional benefit over acidic beverages as the solid oral dosage form supplies acid directly to the stomach with no contact to the esophageal walls, minimizing potential patient discomfort and poor oral hygiene associated with repeated dosing. Furthermore, betaine is a safe and important human nutrient obtained from the diet from a variety of foods. Intake of betaine from foods is estimated to be 200–400 mg/day,15 and betaine doses of 6 to 20 grams per day are used safely to therapeutically treat homocystinuria.16
Drug absorption can only be improved for weakly basic compounds if there is adequate exposure to a more acidic environment. If a drug was administered following BHCl dosing and the duration of re-acidification was not adequate, gastric pH would recover too quickly and there would be a minimal effect on the drug’s dissolution and solubility. However, BHCl should provide ample time for gastric re-acidification (time of gastric pH < 4 ranged from 42 to 127 minutes), considering that for “rapidly dissolving” drugs, 85% of the labeled amount of drug is dissolved within 30 minutes.17
The onset of the re-acidification was also favorable, with a mean onset of 6.3 minutes, ranging between 1.8 and 12 minutes (Table 1). This rapid onset of gastric re-acidification is important from a practical standpoint, as this minimizes the time between the dosing of BHCl and a theoretical victim drug. Having a short activation time also allows this strategy to be more applicable in a real-life patient scenario, where co-administration of BHCl and a victim drug would be more commonplace. Nonetheless, while this may help improve overall drug absorption, we do recognize the potential risks of having exposure to high gastric acidity in actual patients who are on an acid-reducing agent for gastrointestinal disorders. As a proof-of-concept study, only one dose of BHCl was chosen and only healthy volunteers were enrolled. Thus, further studies are warranted to investigate the dose-response of BHCl on the potency and duration of gastric re-acidification and the tolerability of BHCl in patients on acid-reducing agents for GERD or other gastrointestinal disorders.
While ΔpH and rebound times to pH > 3 and > 4 were used as the primary markers of BHCl activity, the AUCpH’s below pH 3 and pH 4 have also shown to have some value. Significant correlations were found between the AUCpH below pH 3 and pH 4 and their respective rebound times, making it a secondary measure of the duration of gastric re-acidification as opposed to the potency. As each subject received the same dose of BHCl, the observed ΔpHs did not vary significantly, with coefficients of variation less than 10%. Although the pH-time data can directly provide an estimate of the length of the re-acidification period, the method can be flawed if the data are significantly affected by fluctuations in the pH above and below a given threshold. Using the AUCpH can account for these fluctuations because only the parts of pH-time curves that are actually spent below pH 3 and pH 4 are integrated into the AUCpH calculations, thereby limiting the effect that the variability in pH measurements may have.
In addition to the potency and duration parameters, serum gastrin was examined for its ability to be a predictor of gastric re-acidification following BHCl administration. Gastrin is a hormone produced by antral G cells that is a potent inducer of gastric acid secretion18 and can be affected via negative feedback in the presence of gastric acid, where its release is inhibited.19 We found no significant relationships between serum gastrin levels and gastric pH (Figure 3), indicating that while the hormone is actively involved in the regulation of gastric acid, it is not predictive of the presence of gastric acid from BHCl administration.
The results of this pilot study have shown that BHCl can be used as an effective method to rapidly and temporarily lower gastric pH in healthy volunteers with drug-induced hypochlorhydria. This strategy has several advantages over previous methods of gastric re-acidification. It utilizes a commercially available, oral, natural supplement that can be purchased over the counter, making it easy for potential patients to obtain and use, thereby avoiding the harm and discomfort that can arise as a result of the ingestion of a large quantity of acidic fluid. BHCl has a rapid onset of effect, which allows convenient co-administration with a potential victim drug a possible outcome. Additionally, the duration of gastric re-acidification is temporary, yet sufficient, giving ample time for the dissolution of victim drugs that may be formulated as an immediate release dosage form. Lastly, it is possible that the degree of gastric re-acidification may be tailored to specific patients by altering the administered dose of BHCl, though the dose-dependencies of these effects still require further evaluation.
For weakly basic drugs that are administered orally under drug-induced hypochlorhydria, co-administration of BHCl may have a positive impact on the pharmacokinetics of the compound. Small-molecule, molecularly targeted anti-cancer agents are a class of drugs that have pH-dependent solubility and can be negatively affected by the effects of acid-reducing agents.20 Moreover, many cancer patients on a drug regimen consisting of these anti-cancer medications are also on some type of acid-reducing agent to mitigate adverse gastrointestinal side effects as a result of their disease or treatment.21 Furthermore, the potential for DDIs is heightened given the high availability of acid-reducing agents over the counter, combined with the frequency of the practice of poly-pharmacy in any given patient population. Therefore, we hypothesize that the use of BHCl can be a viable strategy to improve the reduced drug absorption of these anti-cancer agents when given under drug-induced hypochlorhydria.
A clinical study in healthy volunteers is currently ongoing in our laboratory, investigating the use of BHCl and its ability to improve dasatinib (Sprycel®) absorption under drug-induced hypochlorhydria (National Clinical Trials ID: #NCT01398046).
Acknowledgments
The authors would like to acknowledge the staff and nurses of the UCSF-CCRC for their assistance in this study. The UCSF-CCRC is supported by an NIH/NCRR Grant (Number UL1 RR024131). This study was supported by a grant from Genentech, Inc., and M. R. Yago was supported in part by NIH Training Grant T32 GM007175.
List of Abbreviations
- pKa
Acid Disassociation Constant
- logP
Partition Coefficient
- AUC0–24
Area Under the Concentration-Time Curve from 0–24 hours
- Cmin
Trough Levels; Minimum Plasma Concentration
- CI
Confidence Interval
- Cmax
Maximum Plasma Concentration
- GERD
Gastroesophageal Reflux Disease
- HCl
Hydrochloric Acid
- BHCl
Betaine Hydrochloride
- BMI
Body Mass Index
- AUCpH
Area Under the pH-Time Curve
- ΔpH
Change in pH
- PPI
Proton Pump Inhibitor
- H2-RA
H2-Receptor Antagonist
- PUD
Peptic Ulcer Disease
- DDI
Drug-Drug Interaction
References
- 1.Chin TW, Loeb M, Fong IW. Effects of an Acidic Beverage (Coca-Cola) on Absorption of Ketoconazole. Antimicrob Agents Chemother. 1995;39:1671–1675. doi: 10.1128/aac.39.8.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yin OQ, Gallagher N, Fischer D, Demirhan E, Zhou W, Golor G, Schran H. Effect of the Proton Pump Inhibitor Esomeprazole on the Oral Absorption and Pharmacokinetics of Nilotinib. J Clin Pharmacol. 2010;50:960–967. doi: 10.1177/0091270009346061. [DOI] [PubMed] [Google Scholar]
- 3.Eley T, Luo FR, Agrawal S, Sanil A, Manning J, Li T, Blackwood-Chirchir A, Bertz R. Phase I Study of the Effect of Gastric Acid Ph Modulators on the Bioavailability of Oral Dasatinib in Healthy Subjects. J Clin Pharmacol. 2009;49:700–709. doi: 10.1177/0091270009333854. [DOI] [PubMed] [Google Scholar]
- 4.Kis O, Zastre JA, Ramaswamy M, Bendayan R. pH Dependence of Organic Anion-Transporting Polypeptide 2b1 in Caco-2 Cells: Potential Role in Antiretroviral Drug Oral Bioavailability and Drug-Drug Interactions. J Pharmacol Exp Ther. 2010;334:1009–1022. doi: 10.1124/jpet.110.166314. [DOI] [PubMed] [Google Scholar]
- 5.Zhu L, Persson A, Mahnke L, Eley T, Li T, Xu X, Agarwala S, Dragone J, Bertz R. Effect of Low-Dose Omeprazole (20 Mg Daily) on the Pharmacokinetics of Multiple-Dose Atazanavir with Ritonavir in Healthy Subjects. J Clin Pharmacol. 2011;51:368–377. doi: 10.1177/0091270010367651. [DOI] [PubMed] [Google Scholar]
- 6.Itraconazole (Package Insert) Eon Labs, Inc; Laurelton, New York: 2012. [Google Scholar]
- 7.Jaruratanasirikul S, Sriwiriyajan S. Effect of Omeprazole on the Pharmacokinetics of Itraconazole. Eur J Clin Pharmacol. 1998;54:159–161. doi: 10.1007/s002280050438. [DOI] [PubMed] [Google Scholar]
- 8.Blum RA, D’Andrea DT, Florentino BM, Wilton JH, Hilligoss DM, Gardner MJ, Henry EB, Goldstein H, Schentag JJ. Increased Gastric pH and the Bioavailability of Fluconazole and Ketoconazole. Ann Intern Med. 1991;114:755–757. doi: 10.7326/0003-4819-114-9-755. [DOI] [PubMed] [Google Scholar]
- 9.Agarwala SGK, Eley T, Wang Y, Hughes E, Grasela D. Pharmacokinetic Interaction between Atazanavir and Omeprazole in Healthy Subjects. Presented at the 3rd IAS Conference on HIV Pathogenesis and Treatment; Rio de Janiero, Brazil. July 24–27: 2005; Poster #163. [Google Scholar]
- 10.Knapp MJ, Berardi RR, Dressman JB, Rider JM, Carver PL. Modification of Gastric pH with Oral Glutamic Acid Hydrochloride. Clin Pharm. 1991;10:866–869. [PubMed] [Google Scholar]
- 11.Designs for Health. [accessed August 19: 2013];Quality and Manufacturing - Nsf Certification. http://www.designsforhealth.com/QualityManufacturing_QualityandEfficacy_NSFCertification.html.
- 12.Peghini PL, Katz PO, Bracy NA, Castell DO. Nocturnal Recovery of Gastric Acid Secretion with Twice-Daily Dosing of Proton Pump Inhibitors. Am J Gastroenterol. 1998;93:763–767. doi: 10.1111/j.1572-0241.1998.221_a.x. [DOI] [PubMed] [Google Scholar]
- 13.El-Omar EM, Oien K, El-Nujumi A, Gillen D, Wirz A, Dahill S, Williams C, Ardill JE, McColl KE. Helicobacter Pylori Infection and Chronic Gastric Acid Hyposecretion. Gastroenterology. 1997;113:15–24. doi: 10.1016/s0016-5085(97)70075-1. [DOI] [PubMed] [Google Scholar]
- 14.Cohen S. Gastritis and Peptic Ulcer Disease. In: Albert RK, Cohen S, editors. The Merck Manual for Health Care Professionals. 19. Merck Sharp & Dohme Corp; Whitehouse Station, NJ: 2012. [accessed May 1: 2013]. [Online] Gastrointestinal Disorders. http://www.merckmanuals.com/professional/gastrointestinal_disorders/gastritis_and_peptic_ulcer_disease/overview_of_acid_secretion.html. [Google Scholar]
- 15.Fischer LM, Scearce JA, Mar MH, Patel JR, Blanchard RT, Macintosh BA, Busby MG, Zeisel SH. Ad Libitum Choline Intake in Healthy Individuals Meets or Exceeds the Proposed Adequate Intake Level. J Nutr. 2005;135:826–829. doi: 10.1093/jn/135.4.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Betaine Anhydrous for Oral Solution (Cystadane®, Package Insert) Rare Disease Therapeutics, Inc; Franklin, TN: [Google Scholar]
- 17.The US Food and Drug Administration. Guidance for Industry - Waiver of In Vitro Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmacetics Classification System. 2000 [Google Scholar]
- 18.Hoogerwerf WA, Pasricha PJ. Pharmacotherapy of Gastric Acidity, Peptic Ulcers, and Gastroesophageal Reflux Disease. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 11. McGraw-Hill; New York: 2006. pp. 967–981. [Google Scholar]
- 19.Walsh JH, Richardson CT, Fordtran JS. pH Dependence of Acid Secretion and Gastrin Release in Normal and Ulcer Subjects. J Clin Invest. 1975;55:462–468. doi: 10.1172/JCI107952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Budha NR, Frymoyer A, Smelick GS, Jin JY, Yago MR, Dresser MJ, Holden SN, Benet LZ, Ware JA. Drug Absorption Interactions between Oral Targeted Anticancer Agents and PPIs: Is pH-Dependent Solubility the Achilles Heel of Targeted Therapy? Clin Pharmacol Ther. 2012;92:203–213. doi: 10.1038/clpt.2012.73. [DOI] [PubMed] [Google Scholar]
- 21.Smelick GS, Heffron T, Chu L, West DA, DuVall SL, Lum B, Budha NR, Holden SN, Benet LZ, Frymoyer AR, Dresser MJ, Ware JA. Prevalence of Acid-Reducing Agents (ARA) in Cancer Populations: ARA Drug-Drug Interaction Potential for Molecularly Targeted Agents in Clinical Development. Submitted to Molecular Pharmaceutics. 2011 doi: 10.1021/mp400403s. [DOI] [PubMed] [Google Scholar]