Abstract
Triple negative breast cancer (TNBC) is currently the only major breast tumor subtype without effective targeted therapy and as a consequence in general has poor outcome. To identify new therapeutic targets in TNBC we performed an shRNA screen for protein kinases commonly amplified and overexpressed in breast cancer. Using this approach, we identified AKT3 as a gene preferentially required for the growth of TNBCs. Downregulation of Akt3 significantly inhibits the growth of TNBC lines in 3D spheroid cultures and in mouse xenograft models whereas loss of Akt1 or Akt2 have more modest effects. Akt3 silencing markedly upregulates the p27 cell cycle inhibitor and this is critical for the ability of Akt3 to inhibit spheroid growth. In contrast to Akt1, Akt3 silencing results in only a minor enhancement of migration and does not promote invasion. Depletion of Akt3 in TNBC sensitizes cells to the pan-Akt inhibitor GSK690693. These results imply that Akt3 has a specific function in TNBCs, thus, its therapeutic targeting may provide a new treatment option for this tumor subtype.
Introduction
Breast cancer is the most common cancer amongst women worldwide. Based on gene expression profiling, this disease is categorized into three major subtypes: luminal, HER2+/ER−, and basal-like (1). Recent developments in endocrine therapy for the treatment of luminal breast cancer and Her2 targeted therapy, such as trastuzumab for HER2+/ER− tumors, have led to improved survival for a subset of breast cancer patients (2–4). However, the basal-like subtype, which comprises ~15% of invasive breast cancers and is generally triple-negative (TN; ER−, PR−, HER2−), lacks targeted therapy (5, 6). Currently, chemotherapy is the only option for the treatment of triple negative breast cancers (TNBCs), but its clinical benefit is limited to a subset of patients. Due to poor prognosis and a more aggressive phenotype, there is an urgent clinical need to identify novel therapeutic targets for TNBCs.
Akt is a key regulator of numerous cellular phenotypes associated with cancer, including cell survival, proliferation and metastasis (7). Hyperactivation of Akt due to mutations in the PIK3CA, the catalytic subunit of the p110α subunit of phosphoinositide 3-kinase, PTEN loss, INPP4B loss or HER2 amplification are common features of many tumors (8, 9). The three mammalian Akt isoforms (Akt1, Akt2 and Akt3) are encoded by distinct genes, have high sequence similarity and are activated by near-identical mechanisms (10, 11). The critical role of Akt in modulating cancer cell survival and growth has been well-characterized (12). However, the role played by individual Akt isoforms in different molecular subtypes of breast cancer has not been extensively evaluated. In particular, it is not known whether a specific Akt isoform plays a predominant role in TNBC. In the context of breast cancer invasion and metastasis, Akt isoforms have non-redundant roles whereby Akt1 inhibits invasion and metastasis, yet Akt2 promotes these phenotypes both in vitro and in mouse models of breast cancer progression (11–14). Akt3 is arguably the least studied isoform, and its function in breast cancer cell proliferation, survival and migration is not known. Nevertheless, isoform-specific functions of Akt3 have been evaluated, especially in knockout mice where the brain size of Akt3 null mice is reduced (15, 16). Akt3 has a putative oncogenic function is supported by the observation that it is overexpressed with high enzymatic activity in ER− breast cancer cells (17). This agrees with the analysis by TCGA that has reported upregulation of AKT3 expression in 28% of TNBCs (5). The recent identification of somatic mutations of AKT3 including MAGI3-Akt3 and Akt3E17K in different cancers also points to an important role of this isoform in tumorigenesis (18, 19). However, a causal role for Akt3 in breast cancer initiation and growth has not been examined.
Here, we report that Akt3 is a critical regulator of the growth of TNBCs. Downregulation of Akt3 using shRNA inhibits tumor spheroid growth in 3D as well as in xenografts. Akt3 depletion is accompanied by robust upregulation of the cell cycle inhibitor p27. Silencing p27 rescues spheroid growth inhibition mediated by Akt3 depletion, indicating that Akt3 modulates tumor growth, at least in part, via p27. These findings point to a previously underappreciated isoform-selective role for Akt3 in the tumorigenesis of TNBC, and demonstrate that inhibition of Akt3-specific signaling might be exploited for therapeutic purposes.
Materials and Methods
Cell Culture
MCF7, MDA-MB-231, MDA-MB-468, T47D, Hs578T and HEK293T cells were obtained from ATCC and maintained in Dulbecco’s modified Eagle medium (DMEM; Cellgro) supplemented with 10% Fetal Bovine Serum (FBS; HyClone). SKBR3 and MDA-MB-453 cells obtained from ATCC were cultured in McCoy’s 5A medium (Cambrex) supplemented with 10% FBS. BT-549 cells obtained from ATCC were grown in RPMI 1640 medium supplemented with 10% FBS. SUM-159-PT cells were cultured in Ham’s F12 medium (Cellgro) supplemented with 5% FBS, 1 μg ml−1 hydrocortisone (Sigma-Aldrich) and 5 μg ml−1 insulin (Sigma-Aldrich). ZR-75-30 and BT474 cells obtained from ATCC were cultured in RPMI 1640 medium (Cambrex) supplemented with 10% FBS and 10 μg ml−1 insulin. MCF10ADCIS.com (20) were grown in DMEM/Ham’s F12 medium supplemented with 5% equine serum (Gibco-brl), 10 μg ml−1 insulin, 500 ng ml−1 hydrocortisone (Sigma-Aldrich), 20 ng ml−1 EGF (R&D Systems) and 100 ng ml−1 cholera toxin (List Biological Labs). All cell lines obtained from the cell banks listed above are tested for authentication using STR (short tandem repeat) profiling and passaged for fewer than 6 months, and routinely assayed for mycoplasma contamination.
3D cultures
3D cultures were prepared as previously described (21). Briefly, chamber slides were coated with growth factor-reduced Matrigel (BD Biosciences) and allowed to solidify for 30 min. 4,000 MCF10ADCIS or MDA-MB-231 cells in assay medium were seeded on coated chamber slides. Assay medium for MCF10ADCIS contains DMEM/Ham’s F12 supplemented with 2% equine serum, 10 μg ml−1 insulin, 500 ng ml−1 hydrocortisone, 5 ng ml−1 EGF, 100 ng ml−1 cholera toxin, 2 μg ml−1 puromycin and 2% Matrigel. MDA-MB-231 assay medium contains DMEM supplemented with 2% tet system approved FBS (Clontech) and 5% Matrigel. The assay medium was replaced every 4 days. 100 ng ml−1 doxycycline (dox) was added every 2 or 3 days.
Antibodies
Anti-Akt1 monoclonal antibody, anti-Akt2 monoclonal antibody, anti-Akt3 monoclonal antibody, anti-phospho-Akt S473 (pAkt) monoclonal antibody, anti-p27 monoclonal antibody were obtained from Cell Signaling Technology. Anti-β-actin monoclonal antibody was purchased from Sigma-Aldrich. Anti-bromodeoxyuridine (BrdU) monoclonal antibody was obtained from Roche. Anti-p85 polyclonal antibody was generated in house and has been described (22). Horseradish peroxidase-conjugated anti-mouse and anti-rabbit immunoglobulin G (IgG) antibody were purchased from Chemicon.
RNA interference
For dox-inducible shRNA-mediated knockdown of Akt isoforms, a set of single-stranded oligonucleotides encoding the Akt1, Akt2 and Akt3 target shRNA and its complement were synthesized. The hairpin sequences have been validated previously (11). Akt1, sense, 5′-CCGGGAGTTTGAGTACCTGAAGCTGCTCGAGCAGCTTCAGGTACTCAAACTCTTTTTG–3′; Akt2, sense, 5′–CCGGGCGTGGTGAATACATCAAGACCTCGAGGTCTTGATGTATTCACCACGCTTTTTG–3′; Akt3, sense, 5′–CCGGCTGCCTTGGACTATCTACATTCTCGAGAATGTAGATAGTCCAAGGCAGTTTTTG–3′. The oligonucleotide sense and antisense pair was annealed and inserted into tet-on pLKO. To produce lentiviral supernatants, 293T cells were co-transfected with control or shRNA-containing tet-on pLKO vectors, VSVG and psPAX2 for 48 h. p27 shRNA sequence (sense, 5′–CCGGAAGTACGAGTGGCAAGAGGTGCTCGAGCACCTCTTGCCACTCGTACTTTTTTTG–3′) previously validated (23) was cloned into the tet-on pLKO lentiviral expression system as described above. Cells stably expressing dox-inducible shRNA were cultured in medium containing puromycin (0.5–2 μg ml−1) Gene knockdown was induced by incubating cells with 100 ng ml−1 dox for 48 to 72 h.
Plasmids
For dox-inducible over-expression of Akt3, HA-Akt3/pTRIPZ was constructed. HA-Akt3 cDNA was amplified by PCR from HA-Akt3/pcDNA3. The resulting PCR product was digested with restriction enzymes AgeI and ClaI, followed by insertion into pTRIPZ lentiviral vector (Thermo Scientific).
shRNA screen
Screening was performed as described previously (24). Briefly, cells were infected with a library of lentiviral shRNAs directed against 26 human kinases commonly gained and overexpressed in breast cancer. Infected cells were selected for puromycin resistance, and cultured for 6 days. Cell viability/proliferation was assessed using CellTiter-Glo (Promega). Suppression of growth was defined as the capacity for at least two clones of shRNA targeting the same gene to decreased proliferation by ≥ 30% compared to control.
Xenograft studies
Female nude mice (6–8 weeks old) were purchased from Taconic and maintained and treated under specific pathogen-free conditions. All procedures were approved by the Institutional Animal Care and Use Committee at Dana-Faber Cancer Institute and conform to the federal guidelines for the care and maintenance of laboratory animals. The mice were injected subcutaneously with 1 × 105 MCF10ADCIS or 2 × 106 MDA-MB-468 cells in media with 50% Matrigel. The mice were randomly divided into control and treatment groups and treated with water supplemented with 1 mg ml−1 dox and 2% sucrose, or standard water supply. Tumor formation was examined every 2 to 3 days for the whole duration of the experiment. One hour prior to tumor harvesting, BrdU was injected intraperitoneally to assess cell proliferation. Tumors were harvested and weighed at the experimental endpoint.
Immunohistochemistry of FFPE xenograft tumors
Xenograft tumors were deparaffinized in xylene and hydrated in a series of ethanol. After heat-induced antigen retrieval in citrate buffer (pH 6), the samples were blocked with donkey serum. Slides were then incubated with primary antibodies for overnight at 4°C. Samples were washed three times with TBS followed by incubation with biotinylated secondary antibodies for one hour. The slides were washed and developed using the Diaminobenzidine (DAB) metal enhanced kit (Vector lab) and counter-stained with hematoxylin.
Transwell migration assays
1 × 105 cells in serum-free medium containing 0.1% BSA were added to the upper chambers in triplicate. NIH 3T3-cell-conditioned medium was added to the lower chambers. After 2–16 h incubation at 37°C, non-migrated cells on Transwell filters (8 μm pore size; Corning) were removed. Cells that had migrated to the bottom of the filters were fixed and stained using the Hema-3 stain set (Protocol).
Cell viability assays
MDA-MB-231 and SKBR3 cells were seeded 24 h before inhibitor treatment into 96-well plates at density of 2000–4000 cells per well in 100 μl medium. Cell viability was measured 72 h after inhibitor treatment using the WST-1 assay (Clontech) according to manufacturer’s protocol.
Quantitative real-time-PCR
Total RNA was isolated with Trizol (Invitrogen, Camarillo, CA) according to manufacturer’s protocol. Reverse transcription was performed using random hexamers and multiscribe reverse transcriptase (Applied Biosystems, Foster City, CA). Quantitative real-time PCR was performed using an ABI Prism 7700 sequence detector (Foster City, CA). Akt isoform-specific primers have been validated previously (25). Akt1 primer: sense, 5′–CAAGCCCAAGCACCGC–3′; Akt2 primer: sense, 5′–GCAAGGCACGGGCTAAAG–3′; Akt3 primer: sense, 5′–GAAGAGGAGAGAATGAATTGTAGTCCA–3′. PCR reactions were carried out in triplicate. Quantification of mRNA expression was calculated by the dCT method with GAPDH as the reference gene.
Immunoblots
Cells were washed with PBS at 4°C and lysed in RIPA buffer (1% NP-40, 0.5% deoxycholic acid (SDC), 0.1% SDS, 150 mM NaCl, 50 mM Tris-HCl (pH 7.5), proteinase inhibitor cocktail, 50 nM calyculin, 1 mM sodium pyrophosphate, 20 mM sodium fluoride) for 15 min at 4°C. Cell extracts were pre-cleared by centrifugation at 13,000 rpm for 10 min at 4°C and protein concentration was measured with Bio-Rad protein assay reagent using a Beckman Coulter DU-800 machine. Lysates were then resolved on 10% acrylamide gels by SDS-PAGE and transferred electrophoretically to nitrocellulose membrane (BioRad) at 100 V for 60 min. The blots were blocked in TBST buffer (10 mM Tris-HCl, pH 8, 150 mM NaCl, 0.2% Tween 20) containing 5% (w/v) nonfat dry milk for 30 min, and then incubated with the specific primary antibody diluted in blocking buffer at 4°C overnight. Membranes were washed three times in TBST, and incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. Membranes were washed 3 times and developed using enhanced chemiluminescence substrate (Pierce).
Survival Analyses in Clinical Cohorts of TNBC
To determine the association of AKT3 mRNA expression with patient survival, we used two publicly available clinically annotated breast cancer gene expression datasets. The first is a published gene expression microarray (GEM) meta-dataset that integrates gene expression and survival data from a total of 19 published studies, including a total of 2,731 patients, as described in (26). 527 of these patients were molecularly subtyped using the three-gene model of (27) as ER−/HER2−. Given that true PR expression is not observed in ER− breast cancer (28), this group is representative of TNBC. To assess the association of AKT3 expression with survival among these ER−/HER2− breast cancer patients, we performed a Cox Proportional Hazards analysis. To assess the association of a binary AKT3 expression score with survival, we thresholded the AKT3 expression at the TNBC population median and plotted Kaplan-Meier curves in AKT3-high expressing vs. ATK3-low expressing tumors. We assessed the significance in difference between survival curves by performing a Logrank test. We performed a similar set of analyses on the METABRIC dataset, which is comprised of a total of approximately 2,000 breast cancer cases with clinical follow-up, as described in (29). Pathological data for ER, PR, and HER2 expression were available for this cohort, and based on these data we identified 319 TNBC patients. The METABRIC dataset contained 4 probes for the AKT3 mRNA. For each probe, we performed Cox Proportional Hazards analysis to overall survival. To assess the association of a binary AKT3 expression score with survival, we computed the mean AKT3 expression for each patient and thresholded the mean AKT3 expression score at the TNBC population median. We plotted Kaplan-Meier curves in AKT3-high expressing vs. AKT3-low expressing tumors and performed a Logrank test to assess differences in the curves.
Results
Overexpression of Akt3 in TN breast cancer
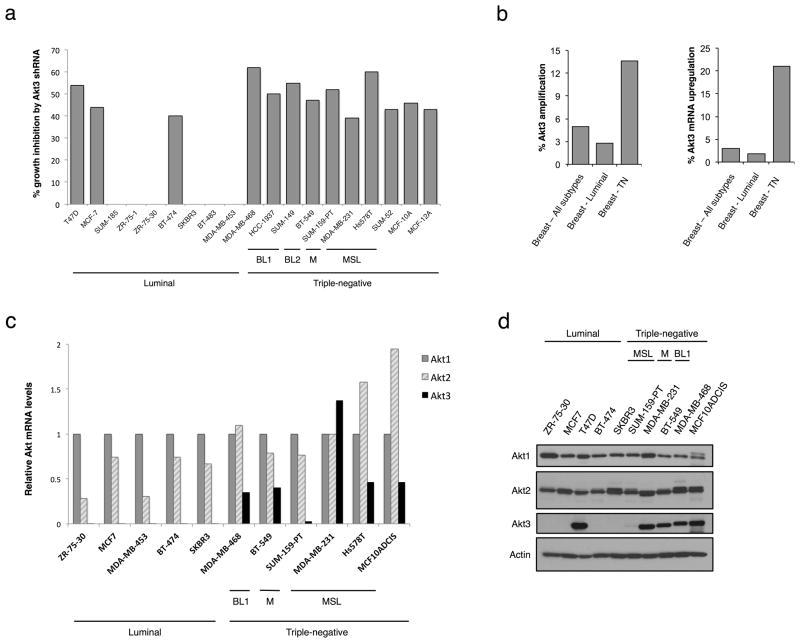
To identify genes that are necessary for growth of TNBC, we screened a panel of 17 commonly-studied breast cancer lines as well as two TN non-tumorigenic, immortalized mammary epithelial lines (MCF-10A and MCF-12A) with a library of lentiviral shRNAs targeting 26 protein kinases amplified in breast tumors. Akt3 was found to be one candidate gene that was preferentially required for TNBC growth. In 10 out of 10 TNBC lines tested (including both mesenchymal and basal-like subtypes), Akt3 depletion leads to growth inhibition (Fig. 1A). In contrast, proliferation is inhibited in only 3 out of 9 luminal cancer lines transduced with Akt3 shRNA (P for difference in proportions = 0.009). To investigate if there is a correlation between Akt3 expression and breast tumor subtypes, we analyzed a dataset of breast cancer from The Cancer Genome Atlas Project (TCGA) (n = 825). Samples with Akt3 amplification have an over-representation in the TNBC subtype (14%) and an underrepresentation in luminal tumors (3%) (Fig. 1B). In addition, whereas Akt3 mRNA is upregulated in 2% luminal breast tumors, its expression is significantly higher in 21% of TNBCs. To determine whether this is also observed in breast cancer cell lines, we quantified mRNA levels for individual Akt isoforms. Whereas all cell lines tested express Akt1 and Akt2, Akt3 is exclusively expressed in TNBC lines (Fig. 1C). Correspondingly, 5 out of 5 TN lines express Akt3 at the protein level as observed by immunoblot analysis, whereas only one of the tested luminal lines shows detectable Akt3 expression (T47D, Fig. 1D).
Figure 1. Preferential amplification and overexpression of Akt3 in TNBCs.
A. Plot showing growth inhibition by Akt3 shRNAs in a panel of breast cell lines using a loss-of-function screen. Inhibition was scored when growth was inhibited by ≥ 30% with at least 2 clones of Akt3 shRNA. B. Bar graph depicting the percentage of Akt3 amplification and mRNA upregulation in the indicated tumors, using a dataset from The Cancer Genome Atlas. C. Analysis of Akt isoform mRNA expression in various breast cancer cell lines by real-time RT-PCR. The levels of Akt isoform mRNAs are expressed as a ratio relative to the Akt1 mRNA in each cell line. D. Immunoblotting showing expression of Akt isoforms in a panel of breast cancer lines. Actin was used as loading control. BL1, basal-like 1; BL2, basal-like 2; M, mesenchymal-like; MSL, mesenchymal stem-like.
To evaluate the association of Akt3 mRNA expression with patient survival in cancer gene expression data. The first is a published gene expression microarray (GEM) meta-dataset that integrates gene expression and survival data from a total of 19 published studies, including a total of 2,731 patients, as described (26). The second analysis was performed on the full METABRIC dataset, which is comprised of a total of approximately 2,000 breast cancer cases with clinical follow-up, as described (30). Analysis of both data sets provides no evidence to suggest that AKT3 mRNA expression levels are an important predictor of survival in TNBC (Supplemental Fig. S1). However, mRNA expression levels may show poor correlation with levels of activated Akt3, and thus may be poor markers of pathway activation. Thus, to definitely determine the association of Akt3 signaling with patient survival, it will be important to perform analyses assessing the relationship between phosphorylated/activated Akt3 and patient survival on large clinical cohorts. These data are currently unavailable from public databases of clinically annotated molecular profiling data in breast cancer.
Akt3 depletion potently inhibits breast tumor spheroid growth and sensitizes TNBC to an Akt inhibitor
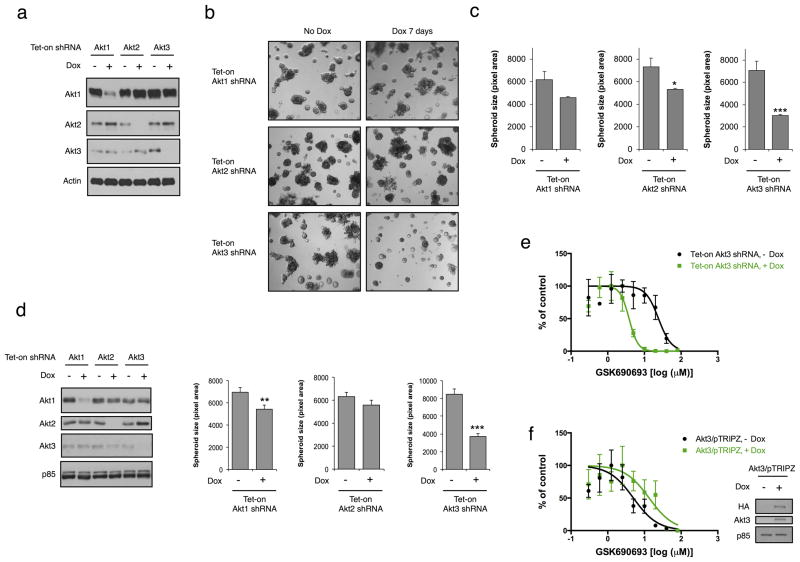
To explore the function of Akt3 in TNBC, we generated a panel of breast cancer lines with tet-on doxycycline (Dox)-inducible Akt1, Akt2 or Akt3 shRNA. Upon dox administration, Akt isoforms are depleted specifically and quantitatively (Fig. 2A). We investigated the consequence of Akt3 silencing on tumor spheroid growth using a three- dimensional (3D) culture system in vitro that more accurately recapitulates phenotypes that govern tumor growth in vivo. Depletion of Akt3 in MDA-MB-231 cells potently inhibits spheroid growth (Fig. 2B), resulting in a 57% reduction of spheroid size (Fig. 2C). In contrast, the effect of Akt1 or Akt2 depletion on spheroid growth is much more modest. A 26% reduction in spheroid size is observed in Akt2-depleted cells, whereas there is no significant change in spheroid size when Akt1 is silenced. Knockdown of Akt3 in another TN line, MCF10ADCIS, cloned from a xenograft lesion formed by premalignant MCF-10AT cells and has the oncogenic mutation H1047R in the p110α catalytic subunit (PIK3CA) (31), has similar effect on the inhibition of spheroid growth (56% reduction; Fig. 2D). Conversely, depletion of Akt1 or Akt2 has minimal effect on the growth of spheroids. These data suggest that Akt3 has a selective role in promoting tumor spheroid growth of TNBCs.
Figure 2. An essential role of Akt3 for the growth of TN breast tumor spheroids.
A. MDA-MB-231 cells expressing tet-on Akt isoform shRNA were treated with dox (100 ng ml−1) for 3 days. Whole cell lysates were subjected to immunoblotting. B. MDA-MB-231 cells were grown in 3D cultures for 7 days in the presence or absence of dox (100 ng ml−1). Representative phase-contrast images are shown. C. The size of spheroids (n ≥ 50) grown in B was quantified and depicted in bar graph. Error bars represent mean ± standard error of the mean (SEM). D. Immunoblotting showing knockdown of Akt isoforms in tet-on MCF10ADCIS cells when treated with dox (100 ng ml−1) for 3 days. p85 was used as a loading control. Bar graph depicts spheroid size of MCF10ADCIS cells grown in 3D culture for 7 days in the presence or absence of dox (100 ng ml−1). *P < 0.05; **P < 0.01; ***P < 0.001. E. MDA-MB-231 cells expressing tet-on Akt3 shRNA were treated with or without dox (100 ng ml−1) for 2 days. Cells were then seeded to 96 well plates, followed by GSK690693 treatment for 3 days. Cell viability was assessed by WST assays. Error bars represent SEM between replicates (n = 3). F. SKBR3 cells expressing tet-on Akt3/pTRIPZ were treated with or without dox (100 ng ml−1) for 3 days. Cells were treated with inhibitor and assessed for viability as described in E. Whole cell lysates were subjected to immunoblotting.
Data from a recent study have demonstrated that the majority of TNBC are resistant to the pan-Akt inhibitor GDC-0068 (32). Since most of the TNBC lines tested express Akt3, we next determined whether Akt3 loss in TNBC might result in increased sensitivity to Akt inhibition. Whereas MDA-MB-231 cells are resistant to the ATP-competitive inhibitor GSK690693 (IC50: 24.2 μM), loss of Akt3 results in a 6-fold decrease in IC50 (3.8 μM, p < 0.0001; Fig. 2E). Similarly, Akt3 depletion in MCF10ADCIS cells leads to a 28-fold decrease in IC50 (0.54 μM vs. 15.4 μM in control cells; P < 0.0001; data not shown). To further determine the role of Akt3 in drug sensitivity, we overexpressed Akt3 in a luminal breast cancer line, SKBR3, which does not express this Akt isoform. Overexpression of Akt3 results in a 2.7-fold increase in IC50 for GSK690693 compared to control cells (13.4 μM vs. 5 μM in control cells; P < 0.05; Fig. 2F). Together, these data indicate that Akt3 plays an important role in conferring resistance to pan-Akt inhibitors in TNBCs.
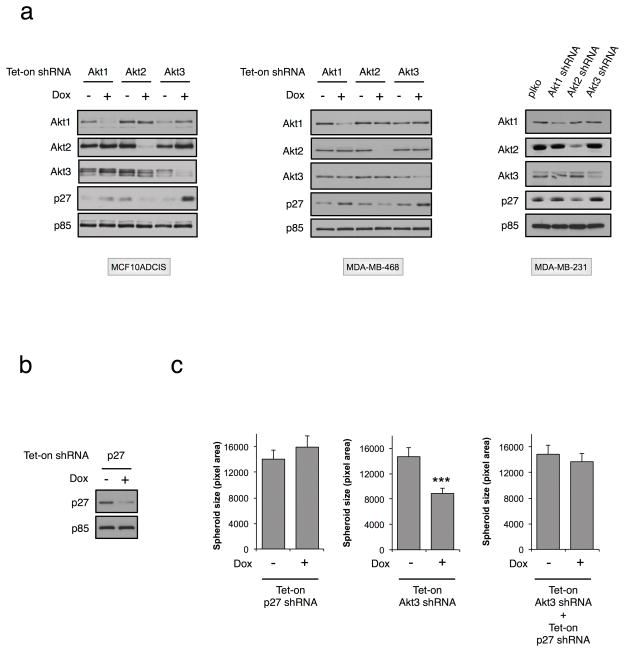
p27 is a key regulator of cell cycle progression. Studies have shown that Akt inhibits p27 expression via the transcription factor Forkhead Box O (FoxO) (33). Moreover, phosphorylation by Akt has been shown to promote the proteasomal degradation of p27 (34), thereby regulating its expression at both the transcriptional and post-translational level. However, it is not known whether p27 is regulated redundantly by all Akt isoforms, or whether specificity exists. In this context, in MCF10ADCIS cells Akt1 silencing results in increased p27 protein expression (Fig. 3A), whereas p27 levels are diminished upon Akt2 silencing. Interestingly, p27 is significantly elevated in Akt3-depleted cells. Similar results are observed in two other TNBC lines, MDA-MB-231 and MDA-MB-468 (Fig. 3A). To determine if Akt3 promotes tumor cell proliferation by modulating p27 expression levels, we performed a rescue experiment by silencing p27. As shown in Fig. 3B, ~50% of p27 is depleted by specific shRNA. Downregulation of p27 alone has minimal effect on 3D spheroid growth (Fig. 3C). In contrast, Akt3 shRNA-mediated spheroid growth inhibition is completely rescued by p27 silencing, indicating that the effect of Akt3 on tumor spheroid growth is mediated, at least in part, by p27.
Figure 3. p27 is a downstream effector of Akt3 in regulating tumor spheroid growth.
A. MCF10ADCIS and MDA-MB-468 cells expressing tet-on Akt isoform shRNA were treated with dox (100 ng ml−1) for 3 days, followed by immunoblot analysis. MDA-MB-231 cells were infected with Akt isoform shRNA/pLKO lentiviral vectors for 3 days, followed by immunoblot analysis. B. MCF10ADCIS cells were infected with tet-on p27 shRNA lentiviral vector. Three days after dox (100 ng ml−1) treatment, cell lysates were immunoblotted with anti-p27 and anti-p85. C. MCF10ADCIS cells expressing tet-on p27 shRNA and/or Akt3 shRNA were seeded to 3D culture for 24 h, followed by treatment with dox (100 ng ml−1) for an additional 7 days. Spheroid size (n = 100) was quantified. *** indicates a P-value of <0.001.
Role of Akt isoforms in modulating invasiveness of tumor spheroids
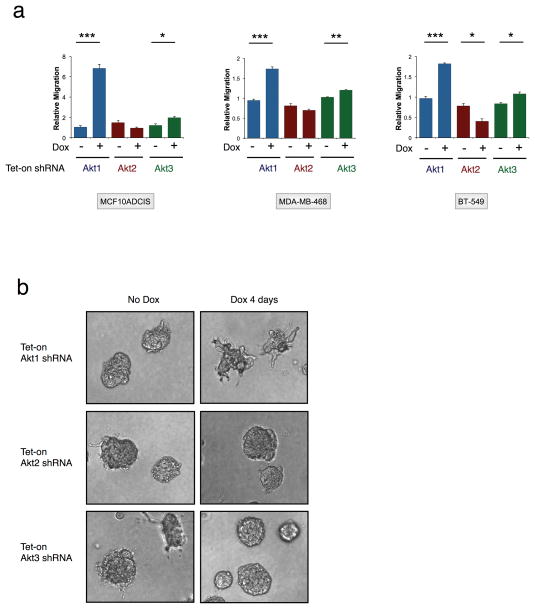
We and others have shown that Akt1 and Akt2 have opposing functions in regulating breast cancer cell invasion and metastasis (12). However, a functional role for Akt3 in modulating cancer cell migration has not been evaluated. To assess chemotactic cell migration, Transwell migration assays were performed. In three TNBC lines (MCF10ADCIS, MDA-MB-468 and BT-549), Akt1 and Akt2 silencing leads to increased and decreased cell migration, respectively (Fig. 4A), in agreement with published studies. A statistically-significant but modest enhancement of migration is observed upon Akt3 silencing in all three lines. Furthermore, Akt1 silencing promotes an abnormal branching morphology with cells protruding into Matrigel (Fig. 4B). In contrast, spheroids remain round and uniform when Akt2 or Akt3 is depleted. These results indicate that Akt3 plays a critical role in modulating tumor spheroid growth in the absence of significant effects on breast cancer cell invasive migration.
Figure 4. Isoform-specific functions of Akt isoforms on breast cancer cell migration and invasion.
A. MCF10ADCIS, MDA-MB-468 and BT-549 cells expressing tet-on Akt isoform shRNA were treated with dox (100 ng ml−1). Three days after treatment, cells were subjected to Transwell migration assays. Relative migration (y axis) = ratio of the number of migrated cells in test versus control. Error bars represent mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001. B. MCF10ADCIS cells expressing tet-on Akt isoform shRNA were seeded to 3D culture for 2 days, followed by treatment with dox (100 ng ml−1) for an additional 4 days.
Akt3 downregulation attenuates breast tumor growth in vivo
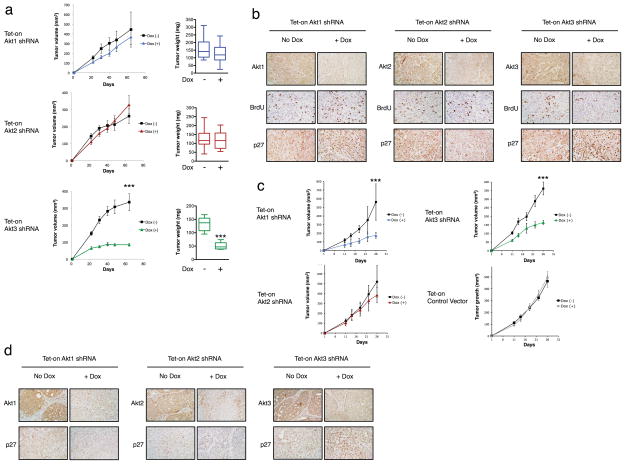
Next we evaluated the contribution of Akt3 to tumorigenesis in vivo. Because Akt3 selectively inhibits tumor spheroid growth of TNBC lines, MDA-MB-468 cells stably expressing tet-on Akt1, Akt2 and Akt3 shRNA were injected subcutaneously into nude mice. When Akt3 is depleted by inducing shRNA expression via dox in drinking water, there is a significant reduction in tumor growth (Fig. 5A). In striking contrast, silencing Akt1 or Akt2 has no significant effect on tumor growth. To confirm the knockdown of Akt isoforms and to assess their effect on cell proliferation as well as p27 expression, immunohistochemistry was performed on xenografts harvested at the end of the study. Akt protein levels are dramatically reduced in dox-treated tumors when compared to their vehicle-treated counterparts (Fig. 5B). Depletion of Akt3, but not Akt1 or Akt2, is concomitant with a significant reduction in BrdU staining, consistent with the notion that Akt3 plays a critical role in tumor cell proliferation in vivo. Similar to what is observed with the in vitro analysis, depletion of Akt3, but not Akt1 or Akt2, leads to a marked elevation of p27 expression. Interestingly, silencing of either Akt1 or Akt3 markedly reduces tumor growth in a distinct TN line, MCF10ADCIS (Fig. 5C). The more marked effect of Akt1 on MCF10ADCIS tumor growth in vivo compared to in vitro could be due to paracrine function of Akt1 in the tumor-associated stroma and microenvironment (12). Dox itself has no effect on tumor growth since the growth of tumors containing empty vector in the absence of dox is indistinguishable from dox-treated tumors (Fig 5C). Similar to what is observed in MDA-MB-468 cells, silencing Akt3, but not Akt1 or Akt2, leads to robust upregulation of p27 expression (Fig. 5D). Taken together, these data demonstrate that TNBC growth can be inhibited by silencing Akt3 alone.
Figure 5. Akt3 depletion attenuates tumor growth in vivo.
A. Dox (1 mg ml−1) was administered to mice in drinking water started 1 day after subcutaneous injection of MDA-MB-468 cells expressing Akt isoform shRNA (n = 10). Tumor volume was measured on day 22, 31, 40, 48 and 64 after injection. Bar graphs showing tumor xenograft weights 64 days after injection. ***P < 0.001. B. Akt isoforms, BrdU and p27 immunohistochemistry staining for xenografts in A. C. MCF10ADCIS cells expressing Akt isoform shRNA or empty vector were injected subcutaneously into nude mice (n = 10). Dox (1 mg ml−1) was administered to mice in drinking water started 1 day after injection. Plots depict tumor volumes measured on day 11, 14, 18, 22 and 26 after injection. D. Akt isoforms and p27 immunohistochemistry staining for xenografts in C.
Discussion
TNBCs are especially aggressive and have a relatively poor prognosis compared to other breast tumor subtypes. In the present study we set out to identify molecular targets of TNBC with therapeutic potential using an RNAi functional genetic screen. We find that Akt3 silencing preferentially inhibits the proliferation of TNBC cell lines both in vitro and in xenografts. Consistent with this, Akt3 is overexpressed in 21% and 2% of TN and luminal breast tumors, respectively. To determine the contribution of individual Akt isoforms in TN tumor growth, we used an inducible shRNA strategy. Silencing Akt3, but not Akt1 or Akt2, dramatically reduces tumor spheroid growth in 3D. The selective effect of Akt3 in TN tumor is noteworthy since it has been largely assumed that Akt isoforms play redundant roles in regulating tumor proliferation and growth. Furthermore, an isoform-specific function of Akt3 in any cancer context has not yet demonstrated. In breast cancer, published studies have focused on the opposing functions of Akt1 and Akt2 on cell migration and invasion. Several downstream Akt targets have been shown to contribute to the differential functions of these two isoforms in motility, including palladin, Nuclear Factor of Activated T cells (NFAT), Tuberous Sclerosis Complex 2, and β1 integrins (35–38). In this study, we find that while Akt3 is required for TNBC proliferation and tumor growth, unlike Akt1 it does not promote an invasive phenotype. These findings have significant implications for targeted therapeutics, since currently all Akt small molecule inhibitors in phase I and II clinical trials are not isoform selective. One prediction is that pan Akt inhibitors could have undesired clinical effects by actually promoting invasion and even metastasis, since Akt1 has been shown to have both and anti-invasive and anti-metastatic activity in vivo. In contrast, targeting Akt3 exclusively in TNBCs may have the advantage of inhibiting tumor growth without any significant impact on metastatic dissemination. Furthermore, due to the critical roles of Akt isoforms in tissue homeostasis, pan-Akt inhibitors are expected to be dose-limiting. Instead, we would argue that isoform-specific inhibitors, including Akt3-specific drugs would likely have less associated toxicity and provide a better therapeutic window.
Further support for the need to develop Akt3-specific inhibitors for the therapeutic targeting of TNBC tumors comes from a recent study on the sensitivities of different breast cancer lines to the pan-Akt inhibitor GDC-0068 (32). Of all luminal lines tested in this study, 5 out of 6 lines are sensitive to GDC-0068 with an IC50 < 2 μM. In agreement with our expression analysis, most of these lines have undetectable levels of Akt3. In contrast, 5 out of 5 TNBC lines tested express Akt3, and 3 of them are insensitive to GDC-0068 (MDA-MB-468, MDA-MB-231, MCF10A: IC50 >10 μM; BT-549: IC50 5.6 μM; Hs578T IC50 2.2 μM). Consistent with this, we show that MDA-MB-231 and MCF10ADCIS cells are resistant to the pan-Akt inhibitor GSK690693. Importantly, Akt3 depletion in both lines resulted in robust sensitization to the drug, suggesting a critical role of Akt3 in conferring resistance to pan-Akt inhibitor in TNBCs. It will be interesting to determine if there is a correlation between Akt3 expression and sensitivity to pan-Akt inhibitors in clinical samples.
Based on gene expression signatures, seven distinct subtypes of TNBC have been proposed, with a classification that comprises two basal-like, immunomodulatory, mesenchymal, mesenchymal stem-like, and luminal androgen receptor subtypes (39). Recent reports indicated that different subtypes of TNBCs may respond differently to targeted and chemotherapies (40). Our data point to a distinct function of Akt3 in MDA-MB-468 and MDA-MB-231 breast cancer lines, which belong to the basal-like 1 and mesenchymal stem-like subtype, respectively. Future studies warrant evaluating the impact of Akt isoform-specific signaling in the various proposed TNBC subtypes.
Our study has also uncovered distinct roles of Akt isoforms in the regulation of p27. It has been shown that Akt is a negative regulator of p27. Here we demonstrate that Akt2, in contrast to Akt1, positively regulates p27 expression. Surprisingly, silencing Akt3 results in a robust elevation of p27 expression concomitant with inhibition of the growth of TNBC lines in vitro as well as in xenografts. Using an shRNA rescue strategy, we show that p27 plays a critical function in the regulation of tumor spheroid growth mediated by Akt3. These findings not only lend further support to the notion of non-redundant functions of Akt isoforms at the molecular level, they also suggest the potential use of p27 as a biomarker of Akt3 activity.
Depletion of Akt3 alone inhibits MCF10ADCIS and MDA-MB-468 xenograft tumor growth. Interestingly, although Akt1 has minimal effect in modulating spheroid growth, silencing Akt1 in MCF10ADCIS in xenografts leads to a reduction of tumor growth comparable to that observed with Akt3 depletion. This is likely due to the functional importance of Akt1 signaling in the tumor-associated stroma, since this isoform plays an important role in promoting VEGF-mediated angiogenesis (41). Whether angiogenesis contributes to the effect of Akt1 on MCF10ADCIS growth in our model remains to be determined.
In conclusion, our studies demonstrate that targeting Akt3 and downstream signaling may be an effective approach to inhibit growth of TNBC. Since Akt3-specific inhibitors have yet to be developed, we propose that the development of such inhibitors could constitute the basis for Akt3 as a novel target for single agent or combination therapy.
Supplementary Material
Acknowledgments
We thank the confocal imaging core and histology core at BIDMC for their technical support with immunohistochemistry; Casey Stottrup for technical assistance with 3D cultures, migration assays and immunoblotting; Evan Lien for providing pTRIPZ-Akt3 plasmid; and members of the Toker laboratory for discussions. This study was supported in part by grants from the National Institutes of Health (R.C., K99CA157945; A.T., CA092644; T32 CA081156-09), the Susan G. Komen Foundation (K.P.), from a sponsored research grant from ImClone/Eli Lilly (A.T and R.C), and from an award from the Klarman Family Foundation (A.B.).
Footnotes
The authors disclose no potential conflicts of interest.
References
- 1.Risbridger GP, Davis ID, Birrell SN, Tilley WD. Breast and prostate cancer: more similar than different. Nat Rev Cancer. 2010;10:205–12. doi: 10.1038/nrc2795. [DOI] [PubMed] [Google Scholar]
- 2.Ariazi EA, Ariazi JL, Cordera F, Jordan VC. Estrogen receptors as therapeutic targets in breast cancer. Current topics in medicinal chemistry. 2006;6:181–202. [PubMed] [Google Scholar]
- 3.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–26. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 4.Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2010;28:92–8. doi: 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwase H, Kurebayashi J, Tsuda H, Ohta T, Kurosumi M, Miyamoto K, et al. Clinicopathological analyses of triple negative breast cancer using surveillance data from the Registration Committee of the Japanese Breast Cancer Society. Breast cancer. 2010;17:118–24. doi: 10.1007/s12282-009-0113-0. [DOI] [PubMed] [Google Scholar]
- 7.Bellacosa A, Testa JR, Staal SP, Tsichlis PN. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991;254:274–7. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- 8.Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–64. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 9.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nature reviews. 2006;7:606–19. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 10.Woodgett JR. Recent advances in the protein kinase B signaling pathway. Curr Opin Cell Biol. 2005;17:150–7. doi: 10.1016/j.ceb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Irie HY, Pearline RV, Grueneberg D, Hsia M, Ravichandran P, Kothari N, et al. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. The Journal of cell biology. 2005;171:1023–34. doi: 10.1083/jcb.200505087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chin YR, Toker A. Function of Akt/PKB signaling to cell motility, invasion and the tumor stroma in cancer. Cellular signalling. 2009;21:470–6. doi: 10.1016/j.cellsig.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hutchinson JN, Jin J, Cardiff RD, Woodgett JR, Muller WJ. Activation of Akt-1 (PKB-alpha) can accelerate ErbB-2-mediated mammary tumorigenesis but suppresses tumor invasion. Cancer research. 2004;64:3171–8. doi: 10.1158/0008-5472.can-03-3465. [DOI] [PubMed] [Google Scholar]
- 14.Maroulakou IG, Oemler W, Naber SP, Tsichlis PN. Akt1 ablation inhibits, whereas Akt2 ablation accelerates, the development of mammary adenocarcinomas in mouse mammary tumor virus (MMTV)-ErbB2/neu and MMTV-polyoma middle T transgenic mice. Cancer research. 2007;67:167–77. doi: 10.1158/0008-5472.CAN-06-3782. [DOI] [PubMed] [Google Scholar]
- 15.Chen EP, Smyth EM. COX-2 and PGE2-dependent immunomodulation in breast cancer. Prostaglandins & other lipid mediators. 2011;96:14–20. doi: 10.1016/j.prostaglandins.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tschopp O, Yang ZZ, Brodbeck D, Dummler BA, Hemmings-Mieszczak M, Watanabe T, et al. Essential role of protein kinase B gamma (PKB gamma/Akt3) in postnatal brain development but not in glucose homeostasis. Development. 2005;132:2943–54. doi: 10.1242/dev.01864. [DOI] [PubMed] [Google Scholar]
- 17.Nakatani K, Thompson DA, Barthel A, Sakaue H, Liu W, Weigel RJ, et al. Up-regulation of Akt3 in estrogen receptor-deficient breast cancers and androgen-independent prostate cancer lines. The Journal of biological chemistry. 1999;274:21528–32. doi: 10.1074/jbc.274.31.21528. [DOI] [PubMed] [Google Scholar]
- 18.Banerji S, Cibulskis K, Rangel-Escareno C, Brown KK, Carter SL, Frederick AM, et al. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature. 2012;486:405–9. doi: 10.1038/nature11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies MA, Stemke-Hale K, Tellez C, Calderone TL, Deng W, Prieto VG, et al. A novel AKT3 mutation in melanoma tumours and cell lines. Br J Cancer. 2008;99:1265–8. doi: 10.1038/sj.bjc.6604637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller FR, Santner SJ, Tait L, Dawson PJ. MCF10DCIS.com xenograft model of human comedo ductal carcinoma in situ. Journal of the National Cancer Institute. 2000;92:1185–6. doi: 10.1093/jnci/92.14.1185a. [DOI] [PubMed] [Google Scholar]
- 21.Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918–22. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kapeller R, Toker A, Cantley LC, Carpenter CL. Phosphoinositide 3-kinase binds constitutively to alpha/beta-tubulin and binds to gamma-tubulin in response to insulin. The Journal of biological chemistry. 1995;270:25985–91. doi: 10.1074/jbc.270.43.25985. [DOI] [PubMed] [Google Scholar]
- 23.Ronty M, Taivainen A, Heiska L, Otey C, Ehler E, Song WK, et al. Palladin interacts with SH3 domains of SPIN90 and Src and is required for Src-induced cytoskeletal remodeling. Experimental cell research. 2007;313:2575–85. doi: 10.1016/j.yexcr.2007.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marotta LL, Almendro V, Marusyk A, Shipitsin M, Schemme J, Walker SR, et al. The JAK2/STAT3 signaling pathway is required for growth of CD44(+)CD24(−) stem cell- like breast cancer cells in human tumors. J Clin Invest. 2011;121:2723–35. doi: 10.1172/JCI44745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ringel MD, Hayre N, Saito J, Saunier B, Schuppert F, Burch H, et al. Overexpression and overactivation of Akt in thyroid carcinoma. Cancer research. 2001;61:6105–11. [PubMed] [Google Scholar]
- 26.Beck AH, Knoblauch NW, Hefti MM, Kaplan J, Schnitt SJ, Culhane AC, et al. Significance analysis of prognostic signatures. PLoS computational biology. 2013;9:e1002875. doi: 10.1371/journal.pcbi.1002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haibe-Kains B, Desmedt C, Loi S, Culhane AC, Bontempi G, Quackenbush J, et al. A three-gene model to robustly identify breast cancer molecular subtypes. J Natl Cancer Inst. 2012;104:311–25. doi: 10.1093/jnci/djr545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hefti MM, Hu R, Knoblauch NW, Collins LC, Haibe-Kains B, Tamimi RM, et al. Estrogen receptor negative/progesterone receptor positive breast cancer is not a reproducible subtype. Breast Cancer Res. 2013;15:R68. doi: 10.1186/bcr3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–52. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–52. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnabas N, Cohen D. Phenotypic and Molecular Characterization of MCF10DCIS and SUM Breast Cancer Cell Lines. International journal of breast cancer. 2013;2013:872743. doi: 10.1155/2013/872743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin J, Sampath D, Nannini MA, Lee BB, Degtyarev M, Oeh J, et al. Targeting activated Akt with GDC-0068, a novel selective Akt inhibitor that is efficacious in multiple tumor models. Clin Cancer Res. 2013;19:1760–72. doi: 10.1158/1078-0432.CCR-12-3072. [DOI] [PubMed] [Google Scholar]
- 33.Stahl M, Dijkers PF, Kops GJ, Lens SM, Coffer PJ, Burgering BM, et al. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J Immunol. 2002;168:5024–31. doi: 10.4049/jimmunol.168.10.5024. [DOI] [PubMed] [Google Scholar]
- 34.Vervoorts J, Luscher B. Post-translational regulation of the tumor suppressor p27(KIP1) Cell Mol Life Sci. 2008;65:3255–64. doi: 10.1007/s00018-008-8296-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chin YR, Toker A. The Actin-Bundling Protein Palladin Is an Akt1-Specific Substrate that Regulates Breast Cancer Cell Migration. Molecular cell. 2010;38:333–44. doi: 10.1016/j.molcel.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoeli-Lerner M, Yiu GK, Rabinovitz I, Erhardt P, Jauliac S, Toker A. Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Molecular cell. 2005;20:539–50. doi: 10.1016/j.molcel.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 37.Liu H, Radisky DC, Nelson CM, Zhang H, Fata JE, Roth RA, et al. Mechanism of Akt1 inhibition of breast cancer cell invasion reveals a protumorigenic role for TSC2. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:4134–9. doi: 10.1073/pnas.0511342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arboleda MJ, Lyons JF, Kabbinavar FF, Bray MR, Snow BE, Ayala R, et al. Overexpression of AKT2/protein kinase Bbeta leads to up-regulation of beta1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells. Cancer research. 2003;63:196–206. [PubMed] [Google Scholar]
- 39.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–67. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masuda H, Baggerly KA, Wang Y, Zhang Y, Gonzalez-Angulo AM, Meric-Bernstam F, et al. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin Cancer Res. 2013;19:5533–40. doi: 10.1158/1078-0432.CCR-13-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shoji K, Oda K, Nakagawa S, Hosokawa S, Nagae G, Uehara Y, et al. The oncogenic mutation in the pleckstrin homology domain of AKT1 in endometrial carcinomas. Br J Cancer. 2009;101:145–8. doi: 10.1038/sj.bjc.6605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.