Abstract
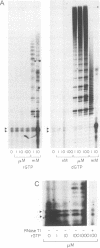
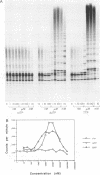
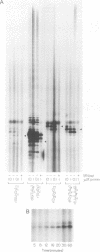
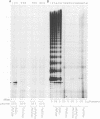
Telomerase is a ribonucleoprotein (RNP) DNA polymerase involved in telomere synthesis. A short sequence within the telomerase RNA component provides a template for de novo addition of the G-rich strand of a telomeric simple sequence repeat onto chromosome termini. In vitro, telomerase can elongate single-stranded DNA primers processively: one primer can be extended by multiple rounds of template copying before product dissociation. Telomerase will incorporate dNTPs or ddNTPs and will elongate any G-rich, single-stranded primer DNA. In this report, we show that Tetrahymena telomerase was able to incorporate a ribonucleotide, rGTP, into product polynucleotide. Synthesis of the product [d(TT)r(GGGG)]n was processive, suggesting that the chimeric product remained associated with the enzyme both at the active site and at a second, previously characterized, template-independent product binding site. As predicted by this finding, RNA-containing oligonucleotides served as primers for elongation. More than 3 nt of RNA at a primer 3' end decreased the quantity of product synthesis but increased the affinity of the primer for telomerase. Thus, RNA-containing primers were effective as competitive inhibitors of DNA primer elongation by telomerase. These results support the possible evolutionary origin of telomerase as an RNA-dependent RNA polymerase.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borukhov S., Sagitov V., Goldfarb A. Transcript cleavage factors from E. coli. Cell. 1993 Feb 12;72(3):459–466. doi: 10.1016/0092-8674(93)90121-6. [DOI] [PubMed] [Google Scholar]
- Collins K., Greider C. W. Tetrahymena telomerase catalyzes nucleolytic cleavage and nonprocessive elongation. Genes Dev. 1993 Jul;7(7B):1364–1376. doi: 10.1101/gad.7.7b.1364. [DOI] [PubMed] [Google Scholar]
- Collins K., Kobayashi R., Greider C. W. Purification of Tetrahymena telomerase and cloning of genes encoding the two protein components of the enzyme. Cell. 1995 Jun 2;81(5):677–686. doi: 10.1016/0092-8674(95)90529-4. [DOI] [PubMed] [Google Scholar]
- Counter C. M., Gupta J., Harley C. B., Leber B., Bacchetti S. Telomerase activity in normal leukocytes and in hematologic malignancies. Blood. 1995 May 1;85(9):2315–2320. [PubMed] [Google Scholar]
- Counter C. M., Hirte H. W., Bacchetti S., Harley C. B. Telomerase activity in human ovarian carcinoma. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):2900–2904. doi: 10.1073/pnas.91.8.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989 Jan 26;337(6205):331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985 Dec;43(2 Pt 1):405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- Greider C. W. Mammalian telomere dynamics: healing, fragmentation shortening and stabilization. Curr Opin Genet Dev. 1994 Apr;4(2):203–211. doi: 10.1016/s0959-437x(05)80046-2. [DOI] [PubMed] [Google Scholar]
- Greider C. W. Telomerase is processive. Mol Cell Biol. 1991 Sep;11(9):4572–4580. doi: 10.1128/mcb.11.9.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley C. B., Kim N. W., Prowse K. R., Weinrich S. L., Hirsch K. S., West M. D., Bacchetti S., Hirte H. W., Counter C. M., Greider C. W. Telomerase, cell immortality, and cancer. Cold Spring Harb Symp Quant Biol. 1994;59:307–315. doi: 10.1101/sqb.1994.059.01.035. [DOI] [PubMed] [Google Scholar]
- Ishihama A., Mizumoto K., Kawakami K., Kato A., Honda A. Proofreading function associated with the RNA-dependent RNA polymerase from influenza virus. J Biol Chem. 1986 Aug 5;261(22):10417–10421. [PubMed] [Google Scholar]
- Johnson T. L., Chamberlin M. J. Complexes of yeast RNA polymerase II and RNA are substrates for TFIIS-induced RNA cleavage. Cell. 1994 Apr 22;77(2):217–224. doi: 10.1016/0092-8674(94)90314-x. [DOI] [PubMed] [Google Scholar]
- Kassavetis G. A., Geiduschek E. P. RNA polymerase marching backward. Science. 1993 Feb 12;259(5097):944–945. doi: 10.1126/science.7679800. [DOI] [PubMed] [Google Scholar]
- Kim N. W., Piatyszek M. A., Prowse K. R., Harley C. B., West M. D., Ho P. L., Coviello G. M., Wright W. E., Weinrich S. L., Shay J. W. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994 Dec 23;266(5193):2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- Lazcano A., Fastag J., Gariglio P., Ramírez C., Oró J. On the early evolution of RNA polymerase. J Mol Evol. 1988;27(4):365–376. doi: 10.1007/BF02101199. [DOI] [PubMed] [Google Scholar]
- Lee M. S., Blackburn E. H. Sequence-specific DNA primer effects on telomerase polymerization activity. Mol Cell Biol. 1993 Oct;13(10):6586–6599. doi: 10.1128/mcb.13.10.6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingner J., Hendrick L. L., Cech T. R. Telomerase RNAs of different ciliates have a common secondary structure and a permuted template. Genes Dev. 1994 Aug 15;8(16):1984–1998. doi: 10.1101/gad.8.16.1984. [DOI] [PubMed] [Google Scholar]
- Mantell L. L., Greider C. W. Telomerase activity in germline and embryonic cells of Xenopus. EMBO J. 1994 Jul 1;13(13):3211–3217. doi: 10.1002/j.1460-2075.1994.tb06620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick-Graham M., Romero D. P. Ciliate telomerase RNA structural features. Nucleic Acids Res. 1995 Apr 11;23(7):1091–1097. doi: 10.1093/nar/23.7.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melek M., Davis B. T., Shippen D. E. Oligonucleotides complementary to the Oxytricha nova telomerase RNA delineate the template domain and uncover a novel mode of primer utilization. Mol Cell Biol. 1994 Dec;14(12):7827–7838. doi: 10.1128/mcb.14.12.7827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin G. B. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989 Nov 3;59(3):521–529. doi: 10.1016/0092-8674(89)90035-4. [DOI] [PubMed] [Google Scholar]
- Poch O., Sauvaget I., Delarue M., Tordo N. Identification of four conserved motifs among the RNA-dependent polymerase encoding elements. EMBO J. 1989 Dec 1;8(12):3867–3874. doi: 10.1002/j.1460-2075.1989.tb08565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prowse K. R., Avilion A. A., Greider C. W. Identification of a nonprocessive telomerase activity from mouse cells. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1493–1497. doi: 10.1073/pnas.90.4.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero D. P., Blackburn E. H. A conserved secondary structure for telomerase RNA. Cell. 1991 Oct 18;67(2):343–353. doi: 10.1016/0092-8674(91)90186-3. [DOI] [PubMed] [Google Scholar]
- Shampay J., Szostak J. W., Blackburn E. H. DNA sequences of telomeres maintained in yeast. Nature. 1984 Jul 12;310(5973):154–157. doi: 10.1038/310154a0. [DOI] [PubMed] [Google Scholar]
- Shippen-Lentz D., Blackburn E. H. Functional evidence for an RNA template in telomerase. Science. 1990 Feb 2;247(4942):546–552. doi: 10.1126/science.1689074. [DOI] [PubMed] [Google Scholar]
- Shippen-Lentz D., Blackburn E. H. Telomere terminal transferase activity from Euplotes crassus adds large numbers of TTTTGGGG repeats onto telomeric primers. Mol Cell Biol. 1989 Jun;9(6):2761–2764. doi: 10.1128/mcb.9.6.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M. S., Gottschling D. E. TLC1: template RNA component of Saccharomyces cerevisiae telomerase. Science. 1994 Oct 21;266(5184):404–409. doi: 10.1126/science.7545955. [DOI] [PubMed] [Google Scholar]
- Watson J. D. Origin of concatemeric T7 DNA. Nat New Biol. 1972 Oct 18;239(94):197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- Yu G. L., Blackburn E. H. Developmentally programmed healing of chromosomes by telomerase in Tetrahymena. Cell. 1991 Nov 15;67(4):823–832. doi: 10.1016/0092-8674(91)90077-c. [DOI] [PubMed] [Google Scholar]
- Yu G. L., Bradley J. D., Attardi L. D., Blackburn E. H. In vivo alteration of telomere sequences and senescence caused by mutated Tetrahymena telomerase RNAs. Nature. 1990 Mar 8;344(6262):126–132. doi: 10.1038/344126a0. [DOI] [PubMed] [Google Scholar]
- Zahler A. M., Prescott D. M. Telomere terminal transferase activity in the hypotrichous ciliate Oxytricha nova and a model for replication of the ends of linear DNA molecules. Nucleic Acids Res. 1988 Jul 25;16(14B):6953–6972. doi: 10.1093/nar/16.14.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]