Abstract
Mutations in the connexin 26 gene (GJB2) are the most common genetic cause of deafness, leading to congenital bilateral non-syndromic sensorineural hearing loss. Here we report the generation of a mouse model for a connexin 26 (C×26) mutation, in which cre-Sox10 drives excision of the C×26 gene from non-sensory cells flanking the auditory epithelium. We determined that these conditional knockout mice, designated Gjb2-CKO, have a severe hearing loss. Immunocytochemistry of the auditory epithelium confirmed absence of C×26 in the non-sensory cells. Histology of the organ of Corti and the spiral ganglion neurons (SGNs) performed at ages 1, 3, or 6 months revealed that in Gjb2-CKO mice, the organ of Corti began to degenerate in the basal cochlear turn at an early stage, and the degeneration rapidly spread to the apex. In addition, the density of SGNs in Rosenthal’s canal decreased rapidly along a gradient from the base of the cochlea to the apex, where some SGNs survived until at least 6 months of age. Surviving neurons often clustered together and formed clumps of cells in the canal. We then assessed the influence of brain derived neurotrophic factor (BDNF) gene therapy on the SGNs of Gjb2-CKO mice by inoculating Adenovirus BDNF (Ad.BDNF) into the base of the cochlea via the scala tympani or scala media. We determined that over-expression of BDNF beginning around 1 month of age resulted in a significant rescue of neurons in Rosenthal’s canal of the cochlear basal turn but not in the middle or apical portions. This data may be used to design therapies for enhancing the SGN physiological status in all GJB2 patients and to a sub-group of GJB2 patients where the hearing loss progresses due to ongoing degeneration of the auditory nerve, thereby improving the outcome of cochlear implant therapy in these ears.
Keywords: Deafness, Connexin 26, Spiral ganglion, BDNF, Gene therapy, GJB2
1. Introduction
Hearing loss is a frequent disorder, affecting one in every 1,000 children, and highly heterogeneous, with many known genetic and environmental causes. The genetic causes account for 50% of childhood deafness. Mutations in the GJB2 gene are the most common cause of hereditary impairment, affecting approximately 1 in 2,000 newborn children in some geographical regions (Hochman et al., 2010; Liu et al., 2001). GJB2 encodes C×26, a member of the connexin family of gap junction-forming proteins, which are involved in direct cell-to-cell transfer of small molecules and ions such as potassium (Bruzzone et al., 1996; Kikuchi et al., 2000; Kumar et al., 1996). While not completely clear, the molecular mechanisms underlying the deafness caused by connexin mutations are likely related to defects in recycling potassium ions due to dysfunction of gap junctions in non-sensory cells that flank the organ of Corti medially and to some extent also laterally. In the most severe cases of sensorineural hearing loss, which includes individuals with GJB2 mutations, the only treatment currently available are cochlear implants, which electrically stimulate the SGNs via an electrode array located in the scala tympani (Cullen et al., 2004). In many human patients, the hearing deficit due to GJB2 mutations is stable, as is the function of the cochlear implant (Kong et al., 2013; Tarkan et al., 2013; Yoshida et al., 2013). However, in some cases, the hearing loss progresses and the benefits derived from the implanted prosthesis gradually decline as well. The decline in hearing may be associated with degenerative changes in the auditory nerve. Preservation of the neural components in inner ears with dysfunctional or non-functional C×26 is essential for deriving optimal benefits from cochlear implants. Work on animal models can aid our understanding of the pathology of mutant inner ears and our development of therapies to enhance the function of cochlear implants.
The first mutant mouse to model GJB2 exhibited cochlear hair cell loss as well as degeneration of SGNs (Cohen-Salmon et al., 2002). It is unclear why hair cells degenerate due to a mutation in non-sensory cells, and whether the neuronal degeneration is a direct outcome of the mutation or an indirect response to the loss of cells in the auditory epithelium. To more closely investigate the pathology in cochlear tissues associated with a C×26 deficiency, and to begin to design therapeutic interventions for GJB2 patients, we generated a mouse. The mice, C×26Sox10Cre, were obtained by crossing C×26loxp/loxp mice, carrying the floxed GJB2 gene, and Sox10Cre mice, which express a Cre recombinase under the Sox10 promoter (Anselmi et al., 2008; Cohen-Salmon et al., 2002; Crispino et al., 2011; Matsuoka et al., 2005).
The C×26 conditional knockout mutant mice (Gjb2-CKO) described in this work represent complete loss of C×26 in the cells driven by the Sox10 promoter, representing the most extreme form of hearing impairment in humans with GJB2 mutations.
Preliminary examination of these mice revealed that in addition to hearing loss due to the lack of C×26, hair cells and auditory neurons degenerate rapidly over the first few months of their life. We tested the outcome of neurotrophin gene therapy on the fate of hair cells and neurons in these mice. Neurotrophins, in particular BDNF and neurotrophin-3 (NT-3), have a role in the development of afferent neurons in the organ of Corti (Fritzsch et al., 1999; Ylikoski et al., 1998) and in protecting SGNs in ears where hair cells are lost (Aarnisalo et al., 2000; Duan et al., 2000; Nakaizumi et al., 2004; Staecker et al., 1996; Van De Water et al., 1996). Reports on the protective effects of neurotrophins in ears exposed to environmental lesions were recently extended to include deaf ears in Pou4f3 mutant mice with no hair cells, in which BDNF gene therapy enhanced survival of the auditory nerve (Fukui et al., 2012). In contrast, the Gjb2-CKO mice used in our current study are born with hair cells. We characterized ABR hearing thresholds as well as the histology of the auditory epithelium and the auditory nerve in these mutants at different time points from 1 to 6 months of age, to determine the influence of BDNF gene therapy on these tissues.
2. Materials and methods
2.1. Animals and groups
Animal care and handling and all procedures described in this work were approved by the University Committee on the Use and Care of Animals of the University of Michigan and the Animal Care and Use Committee of Tel Aviv University (M-10-087) and performed using accepted veterinary standards.
We generated C×26Sox10Cre mice using C×26loxP mice, provided by Prof. Klaus Willecke (Cohen-Salmon et al., 2002). These mice are on a C57BL/6 background with two lox sites around exon 2 of the Gjb2 gene. Mice were considered to be wild type as they presented no abnormalities. The Sox10-Cre mouse line was provided by Prof. William D. Richardson (Matsuoka et al., 2005). These mice express Cre recombinase under the Sox10 promoter. In the cochlear epithelium, Sox10 is expressed in the non-sensory cells around the organ of Corti (Anselmi et al., 2008). The two mouse lines were outcrossed several times to wild type mice. When crossing the two transgenic lines in order to achieve a double transgenic mouse, only the transference of Cre via the maternal germline resulted in viable mutant mice. Both male and female mice were used.
Animals were divided into 3 age groups: 1, 3 or 6 months old. We examined 5 mutant and 5 wild type mice in each age group. In addition, another 14 mice in the 1 month age group received Ad.BDNF inoculation into the scala media (N=9) or into the scala tympani (N=5) and were sacrificed 1 month later.
2.2. Immunocytochemistry
Animals at 1 month of age were sacrificed and their temporal bones removed. Samples were fixed in 4% paraformaldehyde in phosphate buffer for 2 hours, rinsed, and the area of the auditory epithelium dissected as whole-mount preparations. Whole-mounts were permeabilized in 0.3% Triton X-100 for 20 minutes and then blocked against non-specific binding of secondary antibody with 5% normal goat serum for 30 minutes. Tissues were reacted with primary antibody, rinsed, and incubated with the secondary antibody. As primary antibody we used a rabbit antibody specific for C×26 (Invitrogen, Cat. No. 51-2800) diluted 1:200 in blocking buffer, overnight at 4°C. Secondary antibody was a goat-an ti rabbit Alexa Fluor® 594, diluted 1:200 in PBS, for 60 minutes. To double stain for F-actin, we used Alexa Fluor® 488 phalloidin, diluted 1:500, for 30 minutes. Specimens were mounted on glass slides using Gel/Mount (Biomeda, Foster City, CA USA). Whole-mounts were examined and recorded using a Leica DMRB epi-fluorescence microscope (Leica, Eaton, PA, USA) using 20× oil objective lenses and a CCD Cooled SPOT-RT digital camera (Diagnostic Instruments, Sterling Heights, MI).
2.3 Auditory brainstem response measurement (ABR)
Auditory brainstem responses (ABRs) were assessed in wild type and Gjb2-CKO mice at 1 month of age. ABR recordings were collected at frequencies of 12, 16 and 24 kHz on the left ear of each animal. Prior to ABR testing, mice were anesthetized intraperitoneally with ketamine (65 mg/kg), xylazine (7 mg/kg) and acepromazine (2 mg/kg) and placed on a water-circulating heating pad to maintain body temperature. The anticholinergic drug, glycopyrrolate was administered subcutaneously at 0.2 mg/kg. Additional ketamine was administered if needed to maintain anesthesia depth sufficient to insure immobilization and relaxation. ABRs were recorded in an electrically and acoustically shielded chamber (C A Tegner AB, Bromma, Sweden). Tucker Davis Technologies (TDT) System III hardware and SigGen/BioSig software (TDT, Alachua, FL USA) were used to present the stimulus and record responses. Neural activity in response to brief tone bursts were measured using sterile needle electrodes inserted subcutaneously posterior to each pinna and at the vertex of the skull. Each tone burst was 15 milliseconds in duration, with 1 ms rise/fall times, presented 10 per second through an EC1 driver (TDT, aluminum enclosure made in-house), with the speculum placed just inside the tragus. Up to 1024 responses were averaged for each stimulus level. Responses were collected for stimulus levels in 10 dB steps at higher stimulus levels, with additional 5 dB steps near threshold. Thresholds were interpolated between the lowest stimulus level where a response was observed, and 5 dB lower, where no response was observed.
2.4. Adenovirus
Animals in the experimental group received an adenoviral vector with a mouse BDNF gene insert, driven by the cytomegalovirus (CMV) promoter (Di Polo et al., 1998). The effectiveness of the vector and its specific BDNF activity were previously established (Di Polo et al., 1998). In groups that received inoculation into the scala media, we injected 1 μl of Ad.BDNF and for scala tympani inoculations, we injected 2 μl (to partially offset the larger volume of that space). In both cases the viral vector titer of the injected solution was 4×1012 adenoviral particles per ml. The viral suspension was stored at −80 °C and thawed on ice before use.
2.5.Surgery
Mice were inoculated with 1μl of Ad.BDNF into the scala media endolymph via a cochleostomy and 2μl of Ad.BDNF into the scala tympani perilymph via the round window. Prior to the initial incision, the following devices were prepared. A polyethylene tube was connected to a fine polyimide tube and the end of the polyethylene tube was connected by a 30G needle to a 5 μl Hamilton syringe filled with sterile water. Fast green FCF (Sigma-Aldrich) was added to the Ad.BDNF vector solution to trace it during the injection process. The mice were anesthetized with ketamine (120 mg/kg, i.p.) and xylazine (7 mg/kg, i.p.).
For injection into the scala media via a cochleostomy, the left post-auricular region was shaved and an incision was made behind the pinna of the left ear. The muscles overlying the bulla were divided with forceps and scissors, and the tympanic bulla was exposed without damaging the facial nerve. The bulla was perforated with a fine gauge needle (26G) and the hole was expanded with forceps. After the stapedial artery was identified as it coursed over the basal cochlear turn, the cochlear lateral wall was thinned just beneath the stapedial artery with a surgical drill. The tip of the polyimide tube was inserted into the cochlea and advanced toward the scala media. The infusion speed was controlled by a syringe pump. Successful injections into the cochlea were confirmed by observation of Fast green. After the inoculation was complete, the tube was left in place for 2 minutes; after it was removed, the hole in the cochlear wall was covered with muscle tissue and fat. The skin was closed with a 6-0 prolene suture.
For injection into the scala tympani, the tympanic bulla was exposed and opened as above, then the tip of the polyimide tube was inserted into the round window and advanced toward the scala tympani. The vector solution was injected in the same manner as in the scala media approach (described above). Both procedures (scala media or scala tympani inoculations) took approximately 30 minutes to complete. Afterwards, the mice were allowed to awaken from anesthesia, and their pain was controlled with ketoprofen (5 mg/kg, s.c.).
2.6. Transcardiac perfusion fixation
Prior to the initial incision, a perfusion pump was attached to a 26G perfusion needle. Two syringes were used, one filled with 2% glutaraldehyde and the other with PBS. The mice were anesthetized with ketamine (120 mg/kg, i.p.) and xylazine (7 mg/kg, i.p.), placed supine on the operating table, with extremities firmly taped to the table. A transverse incision was made through the abdomen along the length of the diaphragm, then the diaphragm was cut from the lateral and ventral thoracic walls. A longitudinal cut was made through the ribs on each side to open the thoracic cavity. The inferior vena cava was clamped by a Kocher clamp. The needle was inserted directly into the left ventricle, while holding the heart steady with forceps, and a drain cut was made in the right atrium with scissors. PBS was infused until the blood cleared from the body, followed by infusion of a 2% glutaraldehyde solution (5ml) at the rate of 3 ml/min with a perfusion pump.
2.7. Plastic sections
Two hours after cardiac perfusion with 2% glutaraldehyde, cochleae were placed in 5% EDTA with 0.25% glutaraldehyde for two days and then embedded in JB-4 (Electron Microscopy Sciences). The tissues were sectioned with a glass knife (2 μm) at a near-mid-modiolar plane and stained with 1% toluidine blue. Cross-sections were examined and recorded using a Leica DMRB microscope using 10x, 40x and 100x oil objective lenses and a Cooled CCD SPOT-RT3 digital camera.
2.8. Quantitative analyses of SGN density
To evaluate SGN survival, images of 5 cross-sections were randomly selected from a total of 44 sections obtained for each cochlea. SGNs that exhibited a clear nucleus and cytoplasm in Rosenthal’s canal of the apex, middle and base were counted. In each plastic section that was analyzed, we collected data from 3 profiles of Rosenthal’s canal: basal, middle and apical. The area of Rosenthal’s canal was measured using tpsDig2 software (F. James Rohlf, Ecology & Evolution, SUNY at Stony Brook). The number of SGNs per 10,000 μm2 was calculated for each profile.
For 1, 3 and 6 month samples (untreated), a two-factor ANOVA was used to simultaneously test for effects of age, genotype and their interaction; the counts of SGN in each profile of Rosenthal’s canal (apex, middle and base) were treated as separate measurement variables. Age was treated as a categorical variable to avoid assuming that the counts are linear or progressive functions of time. The interaction term tests whether the two main factors (age and genotype) influence each other – for example, does genotype affect aging, or does age affect the expression of the genotype? The ANOVA was performed first on the complete data set, evaluating all 3 Rosenthal’s canal profiles simultaneously. After determining that there were significant effects, ad hoc tests were performed on each profile separately. The sequential Bonferroni criterion was used to determine whether p-values were significant. Analyses were performed using utilities in R (http://cran.r-project.org/).
Differences between treated ears and contralateral controls were tested using Student’s t-test for paired samples, with Bonferroni adjustment for multiple comparisons.
3. Results
3.1. C×26 immunocytochemistry
We compared C×26 immunocytochemistry using whole mount preparations of the auditory epithelium in wild type mice (Fig. 1 A-B) to mutant mice (Fig. 1C) at 1 month of age. Immunoreactivity of C×26 (red) appears as a distinctive dotted line associated with the perimeter of the cells. Staining is most pronounced in the inner sulcus cell region. Hair cells are negative. Figure A inset area is shown in Figure B at higher magnification, clearly depicting the distribution of C×26. In contrast, the auditory epithelium of Gjb2-CKO ears lacks staining for C×26 in the auditory epithelium, as shown in the epithelial cells of the inner sulcus cell region (Fig. 1C). In mutant ears, supporting cells in the auditory epithelium are seen in some areas (left side of Fig. 1C) but missing in others, as the tissue becomes a flat epithelium (right side of Fig. 1C). Adherens junctions in areas of cell-cell contacts reveal the poor organization of the tissue.
Figure 1.

Epi-fluorescence of whole mounts (middle turn) showing C×26 immunoreactivity (red) and phalloidin (green) (A-C) and ABR thresholds recorded at 1 month of age in wild type and mutant mice (D). (A-B) In wild type auditory epithelium C×26 immunoreactivity appears as a conspicuous dotted line surrounding apical surfaces of non-sensory cells. The brightest staining was found in the inner sulcus cell region. Panel B is a high-magnification images of the inset in A. (C) In a mutant cochlea, C×26 immunoreactivity is absent in the inner sulcus and other regions. Hair cells are missing and supporting cells are present in one area (on left) but no in other areas, where they are replaced by a flat epithelium. Scale bars indicate 50 μm. (D) ABR thresholds at 12, 16, and 24 kHz in wild type and mutant mice measured at 1 month of age. Auditory thresholds were significantly higher in Gjb2-CKO mice (p<0.001) than in wild type mice at 12 and 16 and 24 kHz. Sample size for Gjb2-CKO mice: n=8 at 12 & 16 kHz, n=5 at 24 kHz (thresholds for 3 mice could not be detected at the maximum stimulation level of 110 dB). Sample size for wild type mice: n=4 at all three frequencies. Error bars indicate the standard deviation of the mean.
3.2.ABR data
ABR thresholds were measured at 12, 16, and 24 kHz in wild type and mutant mice at 1 month of age (Fig. 1D). The wild type mice exhibited normal hearing. However, Gjb2-CKO mice exhibited significant threshold shifts at 12, 16 and 24 kHz, indicating substantial hearing loss at those frequencies. In some cases, thresholds at 24 kHz could not be detected even at the maximum stimulation level of 110 dB SPL.
3.3. Organ of Corti degeneration
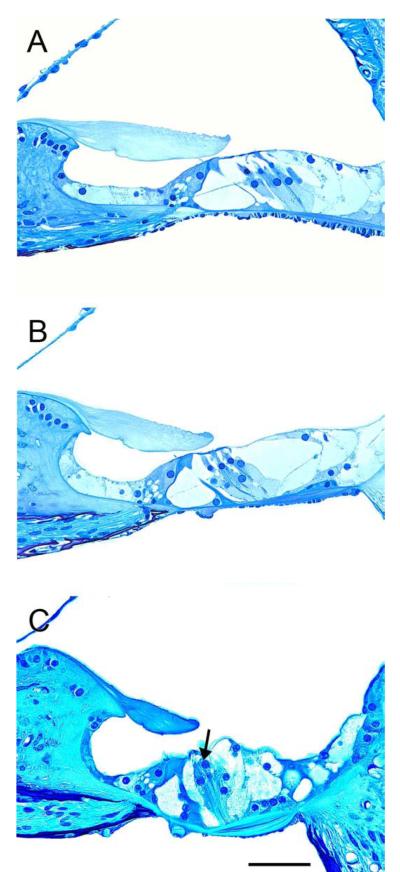
Survival of the organ of Corti was assessed at ages 1, 3 or 6 months. In cochleae of wild type mice (Fig. 2) the organ of Corti appears normal in all cochlear turns of 1 and 3 month old animals (data not shown). At 6 months, the apex (Fig. 2A) and the middle (Fig. 2B) still appear normal but the base begins to display pathology (Fig. 2C). At this stage, the inner hair cells appear normal and the tunnel of Corti is preserved but partial outer hair cell degeneration is observed (Fig. 2C, arrow). These age-related changes in the organ of Corti are typical to the C57BL/6 mouse (Idrizbegovic et al., 2003; Idrizbegovic et al., 2004; Someya et al., 2008).
Figure 2.
A panel of photomicrographs of cross sections through the organ of Corti of wild type mice at 6 months of age. (A-B) The organ of Corti region in the apex (A) and middle (B) contain the normal cellular components including hair cells and supporting cells and appears normally organized. (C) In the base area the inner hair cell appears normal but partial outer hair cells degeneration is observed (arrow). Scale bar indicates 50 μm.
In contrast, cochleae of Gjb2-CKO mice (Fig. 3) display a severe and rapid degeneration of the organ of Corti. At 1 month of age, hair cells are partly degenerated and although pillar cells are visible, the tunnel of Corti is not well formed in the apex and middle (Fig. 3A-B). In the base, the organ of Corti is completely replaced by a flat epithelium (Fig. 3C). At 3 months, the organ of Corti in the apex appears similar to the apical region of 1 month old ears (Fig. 3D, compare to 3A), but now both the middle and base regions of the auditory epithelium are replaced by a flat epithelium (Fig. 3E-F). At 6 months, the organ of Corti in all cochlear turns is replaced by a flat epithelium (Fig. 3G-I). Thus, the degeneration of the organ of Corti in the mutant mice is noted as early as 1 month of age and rapidly progresses from base to apex, such that by 6 months of age the auditory epithelium is flat throughout the cochlea.
Figure 3.

Cross sections through the organ of Corti of mutant mice from ears obtained at ages 1 (A-C), 3 (D-F) or 6 months (G-I). (A-B) In the apex (A) and middle (B), the organ of Corti region displays partial degeneration of hair cells and the tunnel of Corti appears poorly formed. Supporting cells remain in a differentiated state. (C) The organ of Corti region in the base is completely replaced by a flat epithelium. (D) The organ of Corti region in the apex is similar to 1 month ears (A), but the organ of Corti region in the middle (E) and base (F) is replaced by flat epithelium. (G-I) At 6 months of age, the organ of Corti region in all turns is lacking sensory cells and differentiated supporting cells. The tissue appears as a flat epithelium throughout the cochlea. Scale bar indicates 50 μm.
3.4. SGN degeneration
Degeneration of SGNs follows the gradient patterns seen in the organ of Corti. In cochleae of wild type mice (Fig. 4) at 1 and 3 months of age, Rosenthal’s canal is fully occupied by the somata of SGNs. Neurons appear normal in all cochlear turns (Fig. 4A-F). At 6 months, the SGN density is slightly reduced in all turns (Fig. 4G-I). This slow age-related reduction in SGN density is typical of the C57BL/6 mouse (Idrizbegovic et al., 2003; Idrizbegovic et al., 2004; Someya et al., 2008). In addition to the quantitative changes of the spiral ganglion in these wild type animals, we also noted that the surviving neuronal somata tended to be clustered in small aggregates, especially in the apex (Fig. 4G, inset). Such clustering of the neurons is also seen in younger animals, but less frequently (3 months, data not shown).
Figure 4.

Photomicrographs of cross sections through Rosenthal’s canal of wild type mice from ears obtained at ages 1 (A-C), 3 (D- F) or 6 months (G-I). (A-F) Rosenthal’s canal is occupied by soma of SGNs in all cochlear turns. Neurons appear normal but occasional clustering is observed. (G-I) The SGN density appears reduced in all cochlear turns. In the apex (G) somata of several SGNs are clustered together. Scale bar indicates 50 μm.
In Gjb2-CKO mouse ears (Fig. 5), Rosenthal’s canal is fully occupied by somata of SGNs in the apex and middle (Fig. 5A-B), but SGN density appears reduced compared to wild types in the base (Fig. 5C). At the 3 month time point, the spiral ganglion still appears normal in the apex (Fig. 5D) but SGN density is reduced markedly from middle (Fig. 5E) to base (Fig. 5F). At 6 months of age, all cochlear turns display severe SGN degeneration (Fig. 5G-I). In the apex, somata of surviving SGNs are often clustered together to form clumps of cells in Rosenthal’s canal. In each cluster of neurons, cells appear to be in close apposition to each other. Nuclei and cytoplasm of the clustered neurons appear normal and are similar to those seen in basal SGNs. In addition, a few Schwann cells are associated with the cluster, but myelin sheaths are not observed (Fig. 5G, inset). In the base, the few SGNs that survive appear shrunken and dysmorphic, and the central and peripheral processes are barely visible (Fig. 5I, inset). We found that SGN degeneration progresses from base to apex in the Gjb2-CKO mice, similar to the degeneration of the organ of Corti.
Figure 5.

Cross sections through Rosenthal’s canal of mutant mice (A-I) from ears obtained at ages 1 (A-C), 3 (D-F) or 6 months (G-I). (A-B) Rosenthal’s canal is fully occupied by soma of SGNs in the apex and middle cochlear turns. (C) The SGN density appears reduced in the base. (D) The SGN density in apex is similar to 1 month ears (A). (E-F) The SGN density is reduced markedly from base (F) to middle (E). (G-I) At 6 months, the SGN density is reduced in all cochlear turns. Soma of surviving SGNs are often clustered together to form isolated islands in Rosenthal’s canal (G). Scale bar indicates 50 μm.
Variability was noted among Gjb2-CKO mice in the degree of degeneration of the neurons. For instance, the density of SGNs in a specific 3 month old animal (Fig. 5E) is lower (worse) than that seen in a specific 6 month old animal (Fig. 5H). However, the average density conformed with a gradual degeneration along with advancing age, as seen in Figure 6, which shows the changes in density of neurons in Rosenthal’s canal graphically. There is little change in SGN density of wild types between 1 and 3 months, and a more substantial change at 6 months. In contrast, Gjb2-CKO mice appear to have a similar pattern only in the apex; the middle and base have larger changes in SGN density between 1 and 3 months than between 3 and 6 months. Also, the basal turn of mutants already has lower SGN density than controls at 1 month.
Figure 6.
Comparison of SGN density between wild type and mutant mice. There is a significant difference in density of SGNs between the ages of 1 month and 6 months. In the mutant mice, these data show that SGN degeneration progressed from base to apex gradually. Data in wild type ears show that SGN degeneration starts to manifest at 6 months in all turns, caused by the age-related hearing loss mutation in C57BL/6 mice. Error bars indicate 1 standard error of the mean.
Statistical analysis of all three Rosenthal’s canal profiles, simultaneously, confirms that there are differences in SGN density among ages and between genotypes (Table 1). This analysis also finds that the interaction term is significant, indicating that the differences in cell counts between wild types and Gjb2-CKO mice differ by age. As Figure 6 demonstrates, only the basal profile of the Gjb2-CKO mice has reduced SGN density at 1 month, but at 6 months, both genotypes have reduced SGN density in all 3 profiles of Rosenthal’s canal.
Table 1.
Statistical analysis of SGN density by age and genotype, evaluating all 3 profiles of Rosenthal’s canal simultaneously by ANOVA.
| Wilks’ Lambda | F | d.f. | p | |
|---|---|---|---|---|
| Age | 0.220 | 8.69 | 6, 46 | <0.00001 |
| Genotype | 0.176 | 35.94 | 3, 23 | <0.00001 |
| Age × Genotype | 0.457 | 3.68 | 6, 46 | 0.00456 |
Both main factors (Age and Genotype) have highly significant effects, as does the interaction term (Age × Genotype). F, F-ratio; d.f., degrees of freedom; p, p-value.
When the profiles of Rosenthal’s canal are analyzed separately (Table 2), it can be seen that age has a significant effect on SGN density in the apex, but genotype does not. Figure 6 shows apical SGN density does not change until 6 months, when it declines in both wild types and Gjb2-CKO mice. The statistical analysis did find a significant interaction effect, which is consistent with the somewhat greater decrease in apical SGN density in the mutants.
Table 2.
Statistical analysis of SGN density by age and genotype, evaluating each profile of Rosenthal’s canal separately by ANOVA.
| Canal profile | SS | d.f. | F | p | |
|---|---|---|---|---|---|
| Apex | Age | 1.36 × 10 −6 | 2 | 14.12 | 0.00008 |
| Genotype | 1.00 × 10 −7 | 1 | 2.07 | 0.16 | |
| Age × Genotype | 5.55 × 10 −7 | 2 | 5.72 | 0.0090 | |
| Residual | 1.21 × 10 −6 | 25 | |||
| Middle | Age | 1.00 × 10 −6 | 2 | 10.48 | 0.00049 |
| Genotype | 1.16 × 10 −6 | 1 | 24.38 | 0.00005 | |
| Age × Genotype | 4.33 × 10 −7 | 2 | 4.54 | 0.021 | |
| Residual | 1.19 × 10 −6 | 25 | |||
| Base | Age | 1.16 × 10 −6 | 2 | 15.54 | 0.00004 |
| Genotype | 3.75 × 10 −6 | 1 | 100.81 | <0.00001 | |
| Age × Genotype | 1.90 × 10 −7 | 2 | 2.55 | 0.098 | |
| Residual | 9.30 × 10 −7 | 25 |
p-values in bold are significant. Age has significant effects in all 3 profiles of Rosenthal’s canal; genotype has a significant effect only in the profiles from the middle and base. The interaction term is significant in the apex and middle but not in the base. SS, sums of squares; d.f., degrees of freedom; F, F-ratio; p, p-value.
SGN densities from the middle profiles of Rosenthal’s canal show significant effects of both age and genotype. As Figure 6 illustrates, both genotypes lose substantial numbers of cells, but the mutants lose them before 3 months and the wild types lose them after that age. The interaction term would be considered significant if it were the only test performed, but after accounting for the number of tests in the table with a sequential Bonferroni criterion, it must be considered not significant.
In the basal region, the effect of age on SGN density is significant, as in the other profiles of Rosenthal’s canal, but unlike the other profiles, genotype has a significant effect, whereas the interaction does not. The plot shows that the wild type mice do not begin to lose cells until after 3 months, but the mutants have already lost a large proportion of cells by 1 month and have lost nearly all SGN by 3 months. The absence of a significant interaction effect is consistent with the difference in the timing of SGN loss in the basal profile.
3.5. SGN protection with Ad.BDNF
To determine the influence of viral-mediated elevation of the neurotrophin BDNF on SGN survival, we investigated the density of SGNs using mid-modiolar cross sections. We compared ears treated with Ad.BDNF (mice were 1 month old when inoculated with Ad.BDNF) to the contralateral ears that received no treatment. Viral vector inoculation was either into the scala tympani perilymph or the scala media endolymph of the basal region, and ears were examined 1 month after inoculation (when mice were 2 months old).
The SGNs in the base of contralateral ears were very sparse as most cells have degenerated by this stage (Fig. 7A). In contrast, in ears treated with Ad.BDNF into the perilymph, SGNs were better preserved than in the contralateral ears, although some variability was found (Fig. 7B). Quantitative analysis shows that BDNF treatment resulted in a significant increase in density of neurons only in the basal turn (Fig. 7C). Endolymph inoculation of the BDNF gene vector into endolymph produced similar results (Fig. 8). Cross sections through Rosenthal’s canal revealed survival enhancing effects of BDNF in the basal turn (Fig. 8 A-B). The diameter of the neurons in BDNF treated ears appeared larger than that seen in controls. Quantitative assessment showed that SGN densities in the middle and the apex of the cochlea were not significantly increased by the neurotrophin treatment via endolymph (Fig. 8C) similar to that seen with perilymphatic inoculation of BDNF (Fig. 7C). Survival of SGNs in the untreated (contralateral) ears did differ slightly between perilymph and endolymph inoculation groups: 2.81 +/- 0.55, (mean +/- sd, cells/10,000 μm2) after perilymph inoculation, and 1.98 +/- 1.24 after endolymph inoculation; however this difference is not significant (p=0.107).
Figure 7.
(A-B): Sections of representative Rosenthal’s canals of 2 month old mice, in ears treated with Ad.BDNF into the perilymph (B) or contralateral ears (A). (A) The SGNs in the base of contralateral ears were very sparse as most cells have degenerated by this stage. Surviving cells appear pyknotic. (B) SGNs in ears treated with Ad.BDNF appeared healthy and occupied most of the space in Rosenthal’s canal. Connective tissue could be observed in the scala tympani (arrowhead). (C) The density of SGNs in Rosenthal’s canal after Ad.BDNF was inoculated into the scala tympani, compared to untreated (contralateral) ears. The density of SGNs in the Ad.BDNF-treated-ears was significantly different from contralateral ears only in the basal profile of Rosenthal’s canal (*, p< 0.05). Bars are standard error of the mean and statistical comparison was done by t-test for paired samples (N=5). Scale bar indicates 50 μm.
Figure 8.
(A-B): Sections of representative profiles of Rosenthal’s canal of 2 month old mice, in ears treated with Ad.BDNF into the endolymph (B) or contralateral ears (A). (A) The SGNs in the basal turn of contralateral ears were very sparse and pyknotic. (B) SGNs in ears treated with Ad.BDNF into the endolymph appeared large and almost fully occupied the canal. Connective tissue could be observed in the scala tympani (arrowhead). (C) Quantitative analyses of the density of SGNs in Rosenthal’s canal after Ad.BDNF inoculation into the scala media compared to untreated (contralateral) ears. The density of SGNs in the Ad.BDNF-treated ears was significantly greater than contralateral ears only in the basal profile of Rosenthal’s canal (*, p< 0.05). Bars are standard error of the mean and statistical comparison was done by t-test for paired samples (N=9). Scale bar indicates 50 μm.
Qualitative observation of surviving SGNs in the base area of ears treated with BDNF showed that somata appeared large following both scala tympani and scala media inoculations (Fig. 7B and 8B) and their perimeter contours were smooth and regular, similar to normal (wild type) SGNs. In some ears treated with Ad.BDNF, we observed connective tissue growing in the scala tympani (Fig. 7B and 8B, arrowheads). Ad.BDNF treatment did not protect the organ of Corti, which appeared similar in both treated and contralateral ears, with no hair cells in the base and the middle, and a flat epithelium in the base (data not shown). Thus, the protective effect of BDNF on the SGNs was not secondary to preservation of hair cells.
4. Discussion
Over 100 mutations in GJB2 have been described in the literature and databases (The Connexin-deafness homepage; http://davinci.crg.es/deafness/; The Deafness Variation Database; http://deafnessvariationdatabase.org). There is a large variability in the audiological phenotypes of patients with different mutations, from mild to profound (Snoeckx et al., 2005). The variability in the auditory phenotype for each of the human mutations led to the hypothesis that modifier genes are in part responsible for the variable expressivity (Hilgert et al., 2009). Unfortunately, correlation of temporal bone histopathology with the type of mutation is still largely unavailable. Here we show that in a mouse model for GJB2 with a deletion of C×26, the organ of Corti degenerates rapidly in the first few months after birth, as do the SGNs in Rosenthal’s canal. Both regions of the cochlea, the organ of Corti and the spiral ganglion, follow a base to apex gradient in their degeneration. We also demonstrate that viral-mediated over-expression of BDNF leads to preservation of the SGNs in the base region and that the same treatment does not rescue the sensory epithelium.
4.1. Comparison between the C×26 mice and the other mutant mice
The C×26 gene is expressed exclusively in non-sensory cells, yet other cell types degenerate in the mutant mice, most notably hair cells and SGNs. It is not immediately clear why a mutation in non-sensory cells leads to degeneration in cells that do not express the gene. Comparisons with other mouse deafness mutations reveal that this finding is not unique to the C×26 mutation. For instance, in Pou4f3 mutant mice, where the mutation leads to hair cell degeneration, SGNs also degenerate and supporting cell morphology is also abnormal (Fukui et al., 2012). The process of SGN degeneration is faster in the Pou4f3 mutants, with all neurons completely degenerated by 1 month of age. Since hair cells are absent in the Pou4f3 mouse, the timing of degeneration suggests a possible relationship to the presence or absence of hair cells in the cochlea.
Another relevant comparison is to a different C×26 mutation in which the pathology seems more severe and starts earlier than in our mice (Sun et al., 2009; Wang et al., 2009). The C×26 mutant described in these two papers exhibits a cochlea in which the tunnel of Corti and the spaces of Nuel never develop, and the organ of Corti degenerates severely in early postnatal days. Similar to our mice, degeneration of the SGNs follows the loss of epithelial structures. Comparing the pathology in the organ of Corti between the different mice (Cohen-Salmon et al., 2002; Kudo et al., 2003; Wang et al., 2009) reveals that the pathology is slowest to be manifested in the Otog-cre mouse (Cohen-Salmon et al., 2002) and fastest to appear in our mouse and that described by Wang et al. and Sun et al. Future GJB2 temporal bone analysis will help correlate mouse mutations with each of the human mutations.
In the apical turn of the 6 month old Gjb2-CKO mice, hair cells are absent and the organ of Corti is flat, indicating that differentiated supporting cells are also missing. Nevertheless, some SGNs survive in the apical area, despite the absence of hair cells and supporting cells. It is unclear why apical neurons can survive longer in these ears. One possibility is that small patches of hair cell or supporting cells exist, but are not included in our sections, and that these patches of epithelial cells contributed to the survival of the neurons.
The surviving apical neurons are often clustered together in groups. To a lesser extent, this was also noted in wild type animals, suggesting that the clustering of surviving apical neurons could be related to other mutations in the C57BL/6 mouse, such as the mitochondrial DNA mutation, PolgD257A. Clumping of SGNs in the apex of the cochlea was also observed by others who performed histology in PolgD257A and PolgD257A/+ mouse ears (Crawley et al., 2011; Willott, 2009), in the Ly5.1 mouse mutant (Jyothi et al., 2010) and in another C×26 mutant described in 2 papers (Sun et al., 2009; Yu et al., 2013). Transmission electron microscopy (TEM) analysis in the latter study showed that clumping SGNs lacked myelin cover on their soma, a feature otherwise known to be exclusive to human auditory neurons (Tylstedt et al., 1997). Aggregates of clumped SGNs were also shown to exist in the apex of the human cochlea (Tylstedt et al., 1997). The reasons for the clumping and for its restriction to the low frequency region remains unclear, although the inability of BDNF to resolve the clumping suggests that lack of neurotrophins may not be the main reason for this phenomenon. Examples of deaf mutants in which SGNs degenerate but clumping has not been observed include the Ahl mutation (Ohlemiller et al., 2004; Someya et al., 2009) as well as others (Fukui et al., 2012; Sato et al., 2006). It therefore appears that the clumping is not a general phenomenon related to survival of apical neurons in the absence of a sensory epithelium. Rather, the clumping of neurons appears to be specific to certain mutations. Further TEM level characterization of the clusters may shed light on the organization of these cells, the possible involvement of Schwann cells in these aggregates, and the reasons for their restricted location in the apex.
4.2. Correlation between the hair cells, neuronal death and neurotrophins
Loss of neurons secondary to hair cell degeneration has been shown in several types of hereditary and environmental pathologies in experimental animals (Jyung et al., 1989; Kanzaki et al., 2002; Koitchev et al., 1982; Webster et al., 1978). The causative link between loss of hair cells and SGN degeneration is thought to be loss of neurotrophic support, based on the ability of neurotrophins to rescue the neurons (Agterberg et al., 2008; Bowers et al., 2002; Shibata et al., 2010; Wise et al., 2010; Wise et al., 2011), although a role for supporting cells in maintenance of the neurons has also been shown (Sugawara et al., 2005; Zilberstein et al., 2012). In the C×26 mutation studied here, the primary pathology is in the non-sensory cells, yet both hair cells and supporting cells eventually degenerate. Our model is not helpful in determining whether the basal neurons degenerate due to loss of hair cells or supporting cells, but it does demonstrate that forced expression of BDNF can rescue SGNs in the base of the cochlea.
The cause for hair cell loss due to a non-sensory cell mutation is not well understood. At this point we can only speculate on the causes for hair cell loss. One possible explanation is that defective potassium recycling generates excessive potassium levels around the hair cells, leading to their demise. Alternatively, it is possible that pathological non-sensory cells are more likely to also involve the hair cells in the degenerative process, either by providing signals leading to their death, or by phagocytosing the hair cells, as normal supporting cells have been shown to do following noise exposure and ototoxic lesions (Abrashkin et al., 2006).
4.3. The influence of neurotrophin therapy is restricted to the base
We assessed the response of cochleae of Gjb2-CKO mice 1 month after inoculating with Ad.BDNF at the age of 1 month. Based on previous work, the duration of exogenous neurotrophin delivery by a mini osmotic pump is finite, and the protective effects with neurotrophins alone had not been shown beyond 2 weeks after cessation of neurotrophin administration (Agterberg et al., 2009; Gillespie et al., 2003), suggesting that a long term source is needed for prolonged neural survival. Adenoviral vector expression is transient, providing elevated levels of transgenes over several weeks, such that introduction of neurotrophin genes with adenovectors into the deafened guinea pig cochlea promoted SGN survival for up to 4-8 weeks after treatment (Atkinson et al., 2012; Chikar et al., 2008; Nakaizumi et al., 2004). Similar to the previous studies, we found the most significant rescue of neurons in the base of the cochlea. In the middle and apex of the cochlea, the survival of SGNs was not significantly enhanced by the BDNF treatment. This is likely due to higher concentrations of BDNF closer to the site of viral vector inoculation, where more cells are likely to take up the adenovirus and express the transgene. However, the base of the cochlea is also where the effect of the mutation is earliest and most severe, and therefore, so is the potential for rescue. Flow of fluids in the cochlea is also a factor that limits the spread of the viral vector and the secreted neurotrophin towards more apical sites (Salt et al., 2009). In a recent study that assessed neurotrophin (NT-3, BDNF and TrkB receptor agonists) treatment in developing ears (P2) of a different C×26 null mouse, protective effects of were observed in the base and middle regions of the cochlea, but not in the apex (Yu et al., 2013). Starting the treatment during the developmental period may have contributed to the more efficient protection beyond the basal turn.
The SGN density in the base of the contralateral ears in Figure 7C (perilymphatic inoculation) was greater than the SGN density in the base of the contralateral ears in Figure 8C (endolymphatic inoculation). While this difference is not significant, it is large enough to suggest that in some perilymph inoculated animals, a fraction of the virus injected into the scala tympani may have filtered into the contralateral ears, raising important considerations. A possible reason for this finding is that viral vector or BDNF produced by the transgene remains restricted to the endolymph after scala media inoculation, whereas perilymphatic inoculation has been shown to lead to a contralateral effect. The contralateral effect is thought to occur due to draining of perilymph via the cochlear aqueduct, leading to the ventricle of the brain and from there, via the contralateral aqueduct, to the base of the contralateral cochlea (Kho et al., 2000; Stöver et al., 2000). Such ear to ear transfer of the viral vector or the transgenic BDNF may account for the observed preservation of contralateral SGNs in the base following perilymphatic inoculation.
4.4. Comparison of the mouse mutation to the human mutation
The degeneration time course of SGNs ranges from a few weeks to years, depending on the species (Jyung et al., 1989; Leake et al., 1988; Otte et al., 1978). The loss of hair cells results in loss of most of the SGNs in a few weeks in mice, rats, cats, and guinea pigs deafened by ototoxic drugs (Dodson et al., 2000; Hardie et al., 1999; Leake et al., 1988). The secondary degeneration of SGNs in humans appears to be much slower (Linthicum et al., 1991) and in some cases neurons do not degenerate in ears with severe pathology in the organ of Corti.
In human ears with a GJB2 mutation, no temporal bone anomalies were found by computerized tomography (Denoyelle et al., 1999). Histological examination of postmortem cochlear specimens obtained from individuals in their 50s have shown a complete degeneration of the hair cells and the stria vascularis, whereas the cochlear ganglion appeared to be preserved (Jun et al., 2000). It therefore appears that the degenerative progression and the involvement of different tissues differ from human to mouse. The reasons for these differences are currently unclear. Despite the better survival of SGNs in most human ears, there are cases where patients exhibit a phase of rapid deterioration in their hearing thresholds and these changes could be related to degeneration of SGNs, for which neurotrophin therapy could be a remedy. Our data are also applicable to other mutations where the neural substrate of the cochlea degenerates secondary to the effects of a mutation in another cell type.
4.5. Value for enhancing cochlear implant therapy
The substantial loss of SGNs below a critical level is likely to reduce the benefits of the cochlear implant, which could result in poor performance in patients (Clopton et al., 1980; Incesulu et al., 1998). The degeneration of significant amounts of SGNs also diminishes the hope for developing future treatments that are based on the regeneration of hair cells. Thus, preventing the loss of SGNs may considerably enhance the benefits of the cochlear implant, which may lead to better language acquisition and speech perception for at least a significant subset of patients. The use of adenovirus, as shown here, restricts the duration of gene expression and does not permit long-term elevation of the neurotrophin. However, the ability to introduce viral vectors such as Adeno-Associated Virus (AAVs) for long term gene expression at the time of the cochlear implant insertion surgery is a practical possibility for providing this type of therapy.
4.6. Conclusions
We have characterized degenerative changes in the inner ear of a mouse model for GJB2 with a C×26 mutation. Our data show that the organ of Corti and SGNs degenerate with a progression from base to apex. The introduction of Ad.BDNF via the scala media or scala tympani therapy can rescue the degeneration of the SGNs in the base of the cochlea. Surviving neurons appeared to have a well preserved morphology. Our data suggest that neurotrophin gene therapy can be used to further refine cochlear implant treatment in ears of GJB2 patients.
We present a mouse model for the common mutation connexin 26, GJB2 in humans.
The model is based on cre-Sox10 driven excision of the C×26 gene from supporting cells of the auditory epithelium.
These mice lack C×26 in the auditory epithelium and show progressive degeneration of the organ of Corti and auditory neurons.
Adenovirus mediated BDNF gene therapy rescued auditory neurons in the basal turn.
Neurotrophin gene therapy can be considered for improving the outcome of cochlear implant therapy in GJB2 patients.
Acknowledgments
We would like to thank Hideto Fukui, Yong Ho Park and Hiu Tung (Candy) Wong for assistance and helpful comments on this paper. This work was supported by the Berte and Alan Hirschfield Foundation (Y.R. and K.B.A.), the R. Jamison and Betty Williams Professorship (Y.R.), I-CORE Gene Regulation in Complex Human Disease Center No. 41/11 (K.B.A.), and by NIH/NIDCD Grants R01-DC010412, R01-DC007634, P30-DC05188 (Y.R.) and R01-DC011835 (K.B.A.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarnisalo AA, Pirvola U, Liang XQ, Miller J, Ylikoski J. Apoptosis in auditory brainstem neurons after a severe noise trauma of the organ of Corti: intracochlear GDNF treatment reduces the number of apoptotic cells. ORL. J. Otorhinolaryngol. Relat. Spec. 2000;62:330–4. doi: 10.1159/000027764. [DOI] [PubMed] [Google Scholar]
- Abrashkin KA, Izumikawa M, Miyazawa T, Wang CH, Crumling MA, Swiderski DL, Beyer LA, Gong TW, Raphael Y. The fate of outer hair cells after acoustic or ototoxic insults. Hear. Res. 2006;218:20–9. doi: 10.1016/j.heares.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Agterberg MJ, Versnel H, van Dijk LM, de Groot JC, Klis SF. Enhanced survival of spiral ganglion cells after cessation of treatment with brain-derived neurotrophic factor in deafened guinea pigs. J Assoc Res Otolaryngol. 2009;10:355–67. doi: 10.1007/s10162-009-0170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agterberg MJ, Versnel H, de Groot JC, Smoorenburg GF, Albers FW, Klis SF. Morphological changes in spiral ganglion cells after intracochlear application of brain-derived neurotrophic factor in deafened guinea pigs. Hear. Res. 2008;244:25–34. doi: 10.1016/j.heares.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Anselmi F, Hernandez VH, Crispino G, Seydel A, Ortolano S, Roper SD, Kessaris N, Richardson W, Rickheit G, Filippov MA, Monyer H, Mammano F. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc. Natl. Acad. Sci. U. S. A. 2008;105:18770–5. doi: 10.1073/pnas.0800793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson PJ, Wise AK, Flynn BO, Nayagam BA, Hume CR, O'Leary SJ, Shepherd RK, Richardson RT. Neurotrophin gene therapy for sustained neural preservation after deafness. PLoS One. 2012;7:e52338. doi: 10.1371/journal.pone.0052338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers WJ, Chen X, Guo H, Frisina DR, Federoff HJ, Frisina RD. Neurotrophin-3 transduction attenuates Cisplatin spiral ganglion neuron ototoxicity in the cochlea. Mol Ther. 2002;6:12–8. doi: 10.1006/mthe.2002.0627. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, White TW, Paul DL. Connections with connexins: the molecular basis of direct intercellular signaling. Eur. J. Biochem. 1996;238:1–27. doi: 10.1111/j.1432-1033.1996.0001q.x. [DOI] [PubMed] [Google Scholar]
- Chikar JA, Colesa DJ, Swiderski DL, Polo AD, Raphael Y, Pfingst BE. Over-expression of BDNF by adenovirus with concurrent electrical stimulation improves cochlear implant thresholds and survival of auditory neurons. Hear. Res. 2008;245:24–34. doi: 10.1016/j.heares.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clopton BM, Spelman FA, Miller JM. Estimates of essential neural elements for stimulation through a cochlear prosthesis. Ann. Otol. Rhinol. Laryngol. Suppl. 1980;89:5–7. doi: 10.1177/00034894800890s202. [DOI] [PubMed] [Google Scholar]
- Cohen-Salmon M, Ott T, Michel V, Hardelin JP, Perfettini I, Eybalin M, Wu T, Marcus DC, Wangemann P, Willecke K, Petit C. Targeted ablation of connexin26 in the inner ear epithelial gap junction network causes hearing impairment and cell death. Curr. Biol. 2002;12:1106–11. doi: 10.1016/s0960-9822(02)00904-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley BK, Keithley EM. Effects of mitochondrial mutations on hearing and cochlear pathology with age. Hear. Res. 2011;280:201–8. doi: 10.1016/j.heares.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Crispino G, Di Pasquale G, Scimemi P, Rodriguez L, Galindo Ramirez F, De Siati RD, Santarelli RM, Arslan E, Bortolozzi M, Chiorini JA, Mammano F. BAAV mediated GJB2 gene transfer restores gap junction coupling in cochlear organotypic cultures from deaf Cx26Sox10Cre mice. PLoS One. 2011;6:e23279. doi: 10.1371/journal.pone.0023279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen RD, Buchman CA, Brown CJ, Copeland BJ, Zdanski C, Pillsbury HC, 3rd, Shores CG. Cochlear implantation for children with GJB2-related deafness. Laryngoscope. 2004;114:1415–9. doi: 10.1097/00005537-200408000-00019. [DOI] [PubMed] [Google Scholar]
- Denoyelle F, Marlin S, Weil D, Moatti L, Chauvin P, Garabedian EN, Petit C. Clinical features of the prevalent form of childhood deafness, DFNB1, due to a connexin-26 gene defect: implications for genetic counselling. Lancet. 1999;353:1298–303. doi: 10.1016/S0140-6736(98)11071-1. [DOI] [PubMed] [Google Scholar]
- Di Polo A, Aigner LJ, Dunn RJ, Bray GM, Aguayo AJ. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Muller cells temporarily rescues injured retinal ganglion cells. Proc. Natl. Acad. Sci. U. S. A. 1998;95:3978–83. doi: 10.1073/pnas.95.7.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson HC, Mohuiddin A. Response of spiral ganglion neurones to cochlear hair cell destruction in the guinea pig. J. Neurocytol. 2000;29:525–37. doi: 10.1023/a:1007201913730. [DOI] [PubMed] [Google Scholar]
- Duan M, Agerman K, Ernfors P, Canlon B. Complementary roles of neurotrophin 3 and a N-methyl-D-aspartate antagonist in the protection of noise and aminoglycoside-induced ototoxicity [see comments] Proc. Natl. Acad. Sci. U. S. A. 2000;97:7597–602. doi: 10.1073/pnas.97.13.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Pirvola U, Ylikoski J. Making and breaking the innervation of the ear: neurotrophic support during ear development and its clinical implications. [Review] [97 refs] Cell & Tissue Research. 1999;295:369–82. doi: 10.1007/s004410051244. [DOI] [PubMed] [Google Scholar]
- Fukui H, Wong HT, Beyer LA, Case BG, Swiderski DL, Di Polo A, Ryan AF, Raphael Y. BDNF gene therapy induces auditory nerve survival and fiber sprouting in deaf Pou4f3 mutant mice. Sci Rep. 2012;2:838. doi: 10.1038/srep00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie LN, Clark GM, Bartlett PF, Marzella PL. BDNF-induced survival of auditory neurons in vivo: Cessation of treatment leads to accelerated loss of survival effects. J. Neurosci. Res. 2003;71:785–90. doi: 10.1002/jnr.10542. [DOI] [PubMed] [Google Scholar]
- Hardie NA, Shepherd RK. Sensorineural hearing loss during development: morphological and physiological response of the cochlea and auditory brainstem. Hear. Res. 1999;128:147–65. doi: 10.1016/s0378-5955(98)00209-3. [DOI] [PubMed] [Google Scholar]
- Hilgert N, Huentelman MJ, Thorburn AQ, Fransen E, Dieltjens N, Mueller-Malesinska M, Pollak A, Skorka A, Waligora J, Ploski R, Castorina P, Primignani P, Ambrosetti U, Murgia A, Orzan E, Pandya A, Arnos K, Norris V, Seeman P, Janousek P, Feldmann D, Marlin S, Denoyelle F, Nishimura CJ, Janecke A, Nekahm-Heis D, Martini A, Mennucci E, Toth T, Sziklai I, Del Castillo I, Moreno F, Petersen MB, Iliadou V, Tekin M, Incesulu A, Nowakowska E, Bal J, Van de Heyning P, Roux AF, Blanchet C, Goizet C, Lancelot G, Fialho G, Caria H, Liu XZ, Xiaomei O, Govaerts P, Gronskov K, Hostmark K, Frei K, Dhooge I, Vlaeminck S, Kunstmann E, Van Laer L, Smith RJ, Van Camp G. Phenotypic variability of patients homozygous for the GJB2 mutation 35delG cannot be explained by the influence of one major modifier gene. Eur. J. Hum. Genet. 2009;17:517–24. doi: 10.1038/ejhg.2008.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochman JB, Stockley TL, Shipp D, Lin VY, Chen JM, Nedzelski JM. Prevalence of Connexin 26 (GJB2) and Pendred (SLC26A4) mutations in a population of adult cochlear implant candidates. Otol Neurotol. 2010;31:919–22. doi: 10.1097/MAO.0b013e3181e3d324. [DOI] [PubMed] [Google Scholar]
- Idrizbegovic E, Bogdanovic N, Viberg A, Canlon B. Auditory peripheral influences on calcium binding protein immunoreactivity in the cochlear nucleus during aging in the C57BL/6J mouse. Hear. Res. 2003;179:33–42. doi: 10.1016/s0378-5955(03)00076-5. [DOI] [PubMed] [Google Scholar]
- Idrizbegovic E, Bogdanovic N, Willott JF, Canlon B. Age-related increases in calcium-binding protein immunoreactivity in the cochlear nucleus of hearing impaired C57BL/6J mice. Neurobiol. Aging. 2004;25:1085–93. doi: 10.1016/j.neurobiolaging.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Incesulu A, Nadol JB., Jr. Correlation of acoustic threshold measures and spiral ganglion cell survival in severe to profound sensorineural hearing loss: implications for cochlear implantation. Ann. Otol. Rhinol. Laryngol. 1998;107:906–11. doi: 10.1177/000348949810701102. [DOI] [PubMed] [Google Scholar]
- Jun AI, McGuirt WT, Hinojosa R, Green GE, Fischel-Ghodsian N, Smith RJ. Temporal bone histopathology in connexin 26-related hearing loss. Laryngoscope. 2000;110:269–75. doi: 10.1097/00005537-200002010-00016. [DOI] [PubMed] [Google Scholar]
- Jyothi V, Li M, Kilpatrick LA, Smythe N, LaRue AC, Zhou D, Schulte BA, Schmiedt RA, Lang H. Unmyelinated auditory type I spiral ganglion neurons in congenic Ly5.1 mice. J. Comp. Neurol. 2010;518:3254–71. doi: 10.1002/cne.22398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyung RW, Miller JM, Cannon SC. Evaluation of eighth nerve integrity by the electrically evoked middle latency response. Otolaryngology - Head & Neck Surgery. 1989;101:670–82. doi: 10.1177/019459988910100610. [DOI] [PubMed] [Google Scholar]
- Kanzaki S, Stover T, Kawamoto K, Prieskorn DM, Altschuler RA, Miller JM, Raphael Y. Glial cell line-derived neurotrophic factor and chronic electrical stimulation prevent VIII cranial nerve degeneration following denervation. J. Comp. Neurol. 2002;454:350–60. doi: 10.1002/cne.10480. [DOI] [PubMed] [Google Scholar]
- Kho ST, Pettis RM, Mhatre AN, Lalwani AK. Safety of adeno-associated virus as cochlear gene transfer vector: analysis of distant spread beyond injected cochleae. Mol Ther. 2000;2:368–73. doi: 10.1006/mthe.2000.0129. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Adams JC, Miyabe Y, So E, Kobayashi T. Potassium ion recycling pathway via gap junction systems in the mammalian cochlea and its interruption in hereditary nonsyndromic deafness. Med Electron Microsc. 2000;33:51–6. doi: 10.1007/s007950070001. [DOI] [PubMed] [Google Scholar]
- Koitchev K, Guilhaume A, Cazals Y, Aran JM. Spiral ganglion changes after massive aminoglycoside treatment in the guinea pig. Counts and ultrastructure. Acta Otolaryngol. 1982;94:431–8. doi: 10.3109/00016488209128931. [DOI] [PubMed] [Google Scholar]
- Kong Y, Liu S, Wang SJ, Li SJ, Liang S. Cochlear implantation effect on deaf children with gap junction protein beta 2 gene mutation. Chin. Med. J. (Engl) 2013;126:1298–301. [PubMed] [Google Scholar]
- Kudo T, Kure S, Ikeda K, Xia AP, Katori Y, Suzuki M, Kojima K, Ichinohe A, Suzuki Y, Aoki Y, Kobayashi T, Matsubara Y. Transgenic expression of a dominant-negative connexin26 causes degeneration of the organ of Corti and non-syndromic deafness. Hum. Mol. Genet. 2003;12:995–1004. doi: 10.1093/hmg/ddg116. [DOI] [PubMed] [Google Scholar]
- Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84:381–8. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT. Cochlear pathology of long term neomycin induced deafness in cats. Hear. Res. 1988;33:11–33. doi: 10.1016/0378-5955(88)90018-4. [DOI] [PubMed] [Google Scholar]
- Linthicum FH, Jr., Anderson W. Cochlear implantation of totally deaf ears. Histologic evaluation of candidacy. Acta Otolaryngol. 1991;111:327–31. doi: 10.3109/00016489109137395. [DOI] [PubMed] [Google Scholar]
- Liu XZ, Xu LR, Hu Y, Nance WE, Sismanis A, Zhang SL, Xu Y. Epidemiological studies on hearing impairment with reference to genetic factors in Sichuan, China. Ann. Otol. Rhinol. Laryngol. 2001;110:356–63. doi: 10.1177/000348940111000412. [DOI] [PubMed] [Google Scholar]
- Matsuoka T, Ahlberg PE, Kessaris N, Iannarelli P, Dennehy U, Richardson WD, McMahon AP, Koentges G. Neural crest origins of the neck and shoulder. Nature. 2005;436:347–55. doi: 10.1038/nature03837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaizumi T, Kawamoto K, Minoda R, Raphael Y. Adenovirus-mediated expression of brain-derived neurotrophic factor protects spiral ganglion neurons from ototoxic damage. Audiol. Neurootol. 2004;9:135–43. doi: 10.1159/000077264. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Gagnon PM. Apical-to-basal gradients in age-related cochlear degeneration and their relationship to "primary" loss of cochlear neurons. J. Comp. Neurol. 2004;479:103–16. doi: 10.1002/cne.20326. [DOI] [PubMed] [Google Scholar]
- Otte J, Schunknecht HF, Kerr AG. Ganglion cell populations in normal and pathological human cochleae. Implications for cochlear implantation. Laryngoscope. 1978;88:1231–46. doi: 10.1288/00005537-197808000-00004. [DOI] [PubMed] [Google Scholar]
- Salt AN, Plontke SK. Principles of local drug delivery to the inner ear. Audiol. Neurootol. 2009;14:350–60. doi: 10.1159/000241892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Doi K, Taniguchi M, Yamashita T, Kubo T, Tohyama M. Progressive hearing loss in mice carrying a mutation in the p75 gene. Brain Res. 2006;1091:224–34. doi: 10.1016/j.brainres.2005.12.104. [DOI] [PubMed] [Google Scholar]
- Shibata SB, Cortez SR, Beyer LA, Wiler JA, Di Polo A, Pfingst BE, Raphael Y. Transgenic BDNF induces nerve fiber regrowth into the auditory epithelium in deaf cochleae. Exp. Neurol. 2010;223:464–72. doi: 10.1016/j.expneurol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoeckx RL, Huygen PL, Feldmann D, Marlin S, Denoyelle F, Waligora J, Mueller-Malesinska M, Pollak A, Ploski R, Murgia A, Orzan E, Castorina P, Ambrosetti U, Nowakowska-Szyrwinska E, Bal J, Wiszniewski W, Janecke AR, Nekahm-Heis D, Seeman P, Bendova O, Kenna MA, Frangulov A, Rehm HL, Tekin M, Incesulu A, Dahl HH, du Sart D, Jenkins L, Lucas D, Bitner-Glindzicz M, Avraham KB, Brownstein Z, del Castillo I, Moreno F, Blin N, Pfister M, Sziklai I, Toth T, Kelley PM, Cohn ES, Van Maldergem L, Hilbert P, Roux AF, Mondain M, Hoefsloot LH, Cremers CW, Lopponen T, Lopponen H, Parving A, Gronskov K, Schrijver I, Roberson J, Gualandi F, Martini A, Lina-Granade G, Pallares-Ruiz N, Correia C, Fialho G, Cryns K, Hilgert N, Van de Heyning P, Nishimura CJ, Smith RJ, Van Camp G. GJB2 mutations and degree of hearing loss: a multicenter study. Am. J. Hum. Genet. 2005;77:945–57. doi: 10.1086/497996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Yamasoba T, Kujoth GC, Pugh TD, Weindruch R, Tanokura M, Prolla TA. The role of mtDNA mutations in the pathogenesis of age-related hearing loss in mice carrying a mutator DNA polymerase gamma. Neurobiol. Aging. 2008;29:1080–92. doi: 10.1016/j.neurobiolaging.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Xu J, Kondo K, Ding D, Salvi RJ, Yamasoba T, Rabinovitch PS, Weindruch R, Leeuwenburgh C, Tanokura M, Prolla TA. Age-related hearing loss in C57BL/6J mice is mediated by Bak-dependent mitochondrial apoptosis. Proc. Natl. Acad. Sci. U. S. A. 2009;106:19432–7. doi: 10.1073/pnas.0908786106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staecker H, Kopke R, Malgrange B, Lefebvre P, Van de Water TR. NT-3 and/or BDNF therapy prevents loss of auditory neurons following loss of hair cells. Neuroreport. 1996;7:889–94. doi: 10.1097/00001756-199603220-00011. [DOI] [PubMed] [Google Scholar]
- Stöver T, Yagi M, Raphael Y. Transduction of the contralateral ear after adenovirus-mediated cochlear gene transfer. Gene Ther. 2000;7:377–83. doi: 10.1038/sj.gt.3301108. [DOI] [PubMed] [Google Scholar]
- Sugawara M, Corfas G, Liberman MC. Influence of supporting cells on neuronal degeneration after hair cell loss. J Assoc Res Otolaryngol. 2005;6:136–47. doi: 10.1007/s10162-004-5050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Tang W, Chang Q, Wang Y, Kong W, Lin X. Connexin30 null and conditional connexin26 null mice display distinct pattern and time course of cellular degeneration in the cochlea. J. Comp. Neurol. 2009;516:569–79. doi: 10.1002/cne.22117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkan O, Sari P, Demirhan O, Kiroglu M, Tuncer U, Surmelioglu O, Ozdemir S, Yilmaz MB, Kara K. Connexin 26 and 30 mutations in paediatric patients with congenital, non-syndromic hearing loss treated with cochlear implantation in Mediterranean Turkey. J. Laryngol. Otol. 2013;127:33–7. doi: 10.1017/S0022215112002587. [DOI] [PubMed] [Google Scholar]
- Tylstedt S, Kinnefors A, Rask-Andersen H. Neural interaction in the human spiral ganglion: a TEM study. Acta Otolaryngol. 1997;117:505–12. doi: 10.3109/00016489709113429. [DOI] [PubMed] [Google Scholar]
- Van De Water TR, Staecker H, Ernfors P, Moonen G, Lefebvre PP. Neurotrophic factors as pharmacological agents for the treatment of injured auditory neurons. [Review] [38 refs] Ciba Found. Symp. 1996;196:149–62. doi: 10.1002/9780470514863.ch11. discussion 162-6. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chang Q, Tang W, Sun Y, Zhou B, Li H, Lin X. Targeted connexin26 ablation arrests postnatal development of the organ of Corti. Biochem. Biophys. Res. Commun. 2009;385:33–7. doi: 10.1016/j.bbrc.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster DB, Webster M. Cochlear nerve projections following organ of corti destruction. Otolaryngology. 1978;86:ORL342–53. doi: 10.1177/019459987808600228. [DOI] [PubMed] [Google Scholar]
- Willott JF. Effects of sex, gonadal hormones, and augmented acoustic environments on sensorineural hearing loss and the central auditory system: insights from research on C57BL/6J mice. Hear. Res. 2009;252:89–99. doi: 10.1016/j.heares.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise AK, Hume CR, Flynn BO, Jeelall YS, Suhr CL, Sgro BE, O'Leary SJ, Shepherd RK, Richardson RT. Effects of localized neurotrophin gene expression on spiral ganglion neuron resprouting in the deafened cochlea. Mol Ther. 2010;18:1111–22. doi: 10.1038/mt.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise AK, Tu T, Atkinson PJ, Flynn BO, Sgro BE, Hume C, O'Leary SJ, Shepherd RK, Richardson RT. The effect of deafness duration on neurotrophin gene therapy for spiral ganglion neuron protection. Hear. Res. 2011;278:69–76. doi: 10.1016/j.heares.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylikoski J, Pirvola U, Virkkala J, Suvanto P, Liang XQ, Magal E, Altschuler R, Miller JM, Saarma M. Guinea pig auditory neurons are protected by glial cell line-derived growth factor from degeneration after noise trauma. Hear. Res. 1998;124:17–26. doi: 10.1016/s0378-5955(98)00095-1. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Takahashi H, Kanda Y, Usami S. Long term speech perception after cochlear implant in pediatric patients with GJB2 mutations. Auris. Nasus. Larynx. 2013;40:435–9. doi: 10.1016/j.anl.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Yu Q, Chang Q, Liu X, Wang Y, Li H, Gong S, Ye K, Lin X. Protection of Spiral Ganglion Neurons from Degeneration Using Small-Molecule TrkB Receptor Agonists. J. Neurosci. 2013;33:13042–52. doi: 10.1523/JNEUROSCI.0854-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein Y, Liberman MC, Corfas G. Inner hair cells are not required for survival of spiral ganglion neurons in the adult cochlea. J. Neurosci. 2012;32:405–10. doi: 10.1523/JNEUROSCI.4678-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]