Abstract
We employed in vivo microdialysis to characterize the effect of an ethanol challenge injection on endocannabinoid levels in the nucleus accumbens of ethanol-naïve and chronic ethanol-treated rats. Ethanol (0.75 and 2 g/kg, i.p.) dose-dependently increased dialysate 2-arachidonoylglycerol (to a maximum 157 ± 20% of baseline) and decreased anandamide (to a minimum 52 ± 9% of baseline) in ethanol-naïve rats. The endocannabinoid clearance inhibitor N-(4-hydrophenyl) arachidonoylamide (AM404; 3 mg/kg) potentiated ethanol effects on 2-arachidonoylglycerol levels but did not alter ethanol-induced decreases in anandamide. AM404 alone did not alter dialysate levels of either endocannabinoid. Then, we characterized the effect of ethanol challenge on nucleus accumbens endocannabinoid levels in rats previously maintained on an ethanol-containing liquid diet. Ethanol challenge produced a greater and more prolonged increase in 2-arach-idonoylglycerol (to a maximum 394 ± 135% of baseline) in ethanol-experienced than in ethanol-naïve rats. The profile in ethanol-experienced rats was similar to that produced by AM404 pre-treatment in ethanol-naïve rats. AM404 in ethanol-experienced rats led to a further enhancement in the 2-arachidonoylglycerol response to ethanol challenge (to a maximum 704 ± 174% of baseline). Our findings demonstrate that ethanol-induced increases in nucleus accumbens 2-arachidonoylglycerol are potentiated in animals with a history of ethanol consumption.
Keywords: 2-arachidonoylglycerol, AM404, anandamide, in vivo microdialysis
There is compelling evidence that several neurotransmitter systems are involved in the regulation of ethanol (EtOH) intake. EtOH alters the release of excitatory (Weiner and Valenzuela 2006) and inhibitory (Tsai 1998) amino acids, monoamines (Weiss et al. 1993) and neuropeptides (Oswald and Wand 2004) in the central nervous system and alterations in the function of these systems following long-term EtOH exposure are implicated in the development of EtOH dependence (Koob et al. 1998; Johnson et al. 2006).
The endocannabinoid (EC) system is also involved in the behavioral effects of EtOH. Recently, we showed that EtOH self-administration is accompanied by increases in extracellular 2-arachidonoylglycerol (2-AG) in the nucleus accumbens (NAC) shell of rats as measured by in vivo microdialysis and that intra-NAC infusions of the cannabinoid CB1 receptor antagonist SR141716 reduce EtOH self-administration (Caillé et al. 2007). Our data is in agreement with previous observations showing that genetic deletion (Naassila et al. 2004) or pharmacological blockade of CB1 receptors decreases EtOH consumption in mice (Naassila et al. 2004) and rats (Arnone et al. 1997; Gallate and McGregor 1999; Freedland et al. 2001). Conversely, increases in EtOH intake have been observed with administration of CB1 receptor agonists (Gallate and McGregor 1999; Colombo et al. 2002) further suggesting an involvement of EC in EtOH consumption.
Recent studies suggest that chronic EtOH exposure induces neuroadaptations in the EC system. For example, chronic EtOH treatment increases the formation of anandamide (AEA) and 2-AG in SK-N-SH and cerebellar granule neurons (Basavarajappa et al. 1997, 2000, 2003; Basavarajappa and Hungund 1999) and alters EC content in bulk rodent brain tissue in a regionally specific manner (González et al. 2002; Vinod et al. 2006). As a presumed consequence of prolonged elevations in brain EC levels chronic EtOH treatment decreases both CB1 receptor binding (Basavarajappa et al. 1998) and CB1 agonist-induced G-protein activation (Basavarajappa and Hungund 1999). Several lines of evidence suggest these alterations in CB1 receptor influence participate in neural adaptations to chronic EtOH exposure. For example the CB1 receptor modulation of monoamine synthesis is altered following chronic EtOH treatment (Moranta et al. 2006) and EtOH-induced alterations in NMDA and GABAA receptor expression and binding may depend intact CB1 receptor signaling (Warnault et al. 2007). Further, chronic intermittent EtOH exposure is accompanied by reduced EC-mediated hippocampal depolarization-induced suppression of inhibition, a consequence that may contribute to the development of cognitive impairments in alcoholism (Mitrirattanakul et al. 2007). Collectively these and other findings support a hypothesized role for the EC system in the development of EtOH dependence and/or tolerance (Hungund and Basavarajappa 2000; Basavarajappa and Hungund 2005; Nowak et al. 2006).
The evidence reviewed above points to altered brain EC levels and signaling following chronic ethanol exposure. However, there is little information on the EC response to an acute EtOH challenge following chronic EtOH exposure, particularly with regard in extracellular EC levels monitored in vivo. In the present study, we evaluated the effect of chronic EtOH treatment (CET) on the NAC EC response to an acute EtOH challenge. Extracellular EC levels are highly regulated by EC clearance mechanisms, and it is likely these mechanisms dampen the perceived effect of EtOH on brain EC levels as measured by microdialysis sampling. Accordingly we also evaluated the effect of reduced EC clearance [induced by the compound N-(4-hydrophenyl) arachidonoylamide (AM404)] on EtOH-induced alterations in extracellular EC levels. Initial experiments characterized the dose-dependent effects of acute EtOH administration and the relative influence of EC clearance inhibition on the EC response to EtOH administration in EtOH naïve rats. Subsequently the effects of acute EtOH challenge on interstitial NAC EC levels were evaluated in rats maintained on either an EtOH-containing or EtOH-free liquid diet. Our findings show that CET potentiates EtOH-induced increases in NAC 2-AG levels, without altering the AEA response to EtOH challenge.
Materials and methods
Subjects
Male Wistar rats (Charles River, Wilmington, MA, USA) weighing 250–300 g at the beginning of the experiments were housed in groups of 2–3 in a humidity and temperature-controlled (22°C) vivarium on a 12 h light/dark cycle (lights off at 8 AM). Upon arrival in the vivarium, rats were allowed to acclimatize to the new environment for 7 days before any experimental procedure was performed. The rats had ad libitum access to food and water throughout the course of the studies, except during exposure to an EtOH-containing liquid diet. All procedures were conducted in strict adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Drugs
N-(4-hydrophenyl) Arachidonoylamide and (R)-methananandamide (AEA) were obtained from Cayman Chemical (Ann Arbor, MI, USA). EtOH (10% and 20% w/v) was prepared with 95% ethyl alcohol and water.
Surgery
Rats were anesthetized with an isoflurane/oxygen vapor mixture (1.5–2%) and mounted on a stereotaxic apparatus for implantation of a microdialysis guide cannula (19 gauge, 12 mm below pedestal) (Plastics One, Roanoke, VA, USA) aimed at the NAC shell. The stereotaxic coordinates were as follows: relative to Bregma AP + 1.6 mm, ML ± 0.8 mm, DV − 5.7 mm (from dura) (Paxinos and Watson 1998). The guide cannula was lowered 2 mm above of the NAC shell and secured using skull screws and cranioplastic cement. Dummy stylets extending just to the tip of the cannula were inserted to avoid cannula blockade. Rats were allowed to recover from surgery for a minimum of 7 days.
Experiment I: dose-dependent effects of acute EtOH administration on dialysate EC levels
Ethanol-naïve rats were anesthetized (1.5–2% isoflurane) and a dialysis probe was implanted via the previously implanted guide cannula 12–15 h prior to the microdialysis procedure. Following a baseline dialysate collection (60 min), rats were injected with either vehicle (EtOH : emulphor : saline; 1 : 1 : 18) or AM404 (3 mg/kg, i.p.) and dialysates collected for 30 min. Subsequently rats received an intraperitoneal injection of either 0.75 or 2 g/kg EtOH (20%, w/v) or saline followed by an additional 120 min of dialysate collection. The treatment groups were vehicle/EtOH 0.75 g/kg (n = 6), AM404/EtOH 0.75 g/kg (n = 6), vehicle/EtOH 2 g/kg (n = 5), AM404/EtOH 2 g/kg (n = 6), AM404/vehicle (n = 5).
Experiment II: effect of CET on the EC response to EtOH challenge
After post-operative recovery, regular chow diet was removed and replaced by the liquid diet consisting of chocolate flavored Boost liquid nutritional supplement fortified with vitamins and minerals. Rats were separated into two groups; the CET group received a diet containing 10% (w/v) EtOH and the control group received an EtOH-free diet supplemented with sucrose to equalize the caloric intake in both groups. The liquid diet was available 24 h for 14 days.
Dialysis probes were implanted 12–15 h prior to the experiment at which time the EtOH-containing liquid diet was replaced by the EtOH-free liquid diet. Following a baseline dialysate collection (60 min), control and CET rats were pre-treated with either vehicle (EtOH : emulphor : saline; 1 : 1 : 18) or AM404 (3 mg/kg, i.p.). Thirty minutes later, rats received an intraperitoneal injection of 2 g/kg EtOH (20%, w/v) followed by an additional 120 min of dialysate collection. The 2 g/kg EtOH dose was chosen based on the results obtained in the experiment testing the dose-dependent effect of EtOH demonstrating significant effects of this dose on dialysate EC levels. The treatment groups were as follows: control rats (vehicle/EtOH, n = 9; AM404/EtOH, n = 10) and CET rats (vehicle/EtOH, n = 6, AM404/EtOH, n = 6).
Blood alcohol assay
Blood samples were collected once per week for a total of two times during maintenance of the EtOH liquid diet. Rats were tail-bled within the first 2–3 h after the beginning of the dark cycle. The method consisted of clipping the tail approximately 5 mm from the tip to collect 100 μL of blood into a microtube containing anticoagulant (4 μL heparin; 1000 USP units/mL). Samples were centrifuged at 2000 g for 10 min. Serum was extracted and assayed for EtOH content using the Alcohol Oxidase method (Analox Instrument LTD, Lunenburg, MA, USA).
In vivo microdialysis
The day before the microdialysis procedure, rats were briefly anesthetized (1.5–2% isoflurane) and a microdialysis probe was inserted and secured to the previously implanted guide cannula. The microdialysis probes (SciPro Inc, Sanborn, NY, USA) had polyethyl sulfone dialysis membranes (15 kDa MW cutoff), with a 2 mm active length. Following probe implantation, rats were individually housed in a standard cage with access to the non-EtOH-containing liquid diet and water bottle. This pre-microdialysis period was implemented to allow (i) rats to habituate to their new environment and (ii) the equilibration of interstitial neurotransmitter levels following tissue disruption by the probe. The microdialysis probe was perfused at 0.2 μL/min with artificial CSF delivered via a liquid swivel. Artificial CSF (pH 7.2–7.4) was composed of 145 mM NaCl, 2.8 mM KCl, 1.2 mM MgCl2, 1.2 mM CaCl2, 5.4 mM D-Glucose, 0.25 mM ascorbic acid.
Twelve to fifteen hours later, the aCSF perfusate was replaced by an aCSF containing 30% (w/v) hydroxypropyl-β-cyclodextrin. As previously described (Walker et al. 1999; Caillé et al. 2007; Ferrer et al. 2007), inclusion of hydroxypropyl-β-cyclodextrin substantially increases the recovery of EC by microdialysis. Immediately after, the perfusion flow rate was increased to 0.6 μL/min for 90 min to reach an interstitial equilibrium before sample collection. Dialysate samples were subsequently collected every 10 min throughout each experiment, and were frozen on dry ice and stored at −70°C until analysis for endocannabinoid content using liquid chromatography coupled with mass spectrometry.
Liquid chromatography coupled with mass spectrometry
Five μL microdialysate aliquots were spiked with 5 μL of 50 nM of S-2 methanandamide as an internal standard. The samples were subsequently loaded onto a pre-column (1.0 ×10 mm, Haisil HL C18 5 μm; Higgins Analytical Inc., Mountain View, CA, USA) using a refrigerated autoinjector and 10% MeOH (v/v) mobile phase delivered at 47 μL/min. Following a 4 min wash period, mobile phase flow through the pre-column was reversed via a switching valve and the EC were delivered to a 0.5 × 150 mm microbore analytical column (Haisil HL C18 3 μm; Higgins Analytical Inc.) using an isocratic mobile phase consisting of 76% MeOH (v/v) delivered at 9 μL/min. The analytical column eluent was delivered via a nanoelectrospray interface into the mass spectrometer (1100MSD) (Agilent Technologies, Santa Clara, CA, USA) that was run in positive Selected Ion Monitoring mode to maximize sensitivity. The following mass/charge (m/z) ratios were used: AEA, 370.3 (M + 1Na); 2-AG and 1-AG, 401.3 (M + 1Na); S-2 methanandamide, 384.3 (M + 1Na). External calibration curves were constructed from a minimum of three standard concentrations (each run in duplicate) and new calibrations were generated daily. Under these conditions the limits of quantitation were approximately 0.1 nM for each analyte.
Histology
Brains were sliced (45 μm) and the placement of microdialysis probes was verified using the atlas of Paxinos and Watson (1998).
Statistical analysis
Between-group differences in baseline dialysate 2-AG and AEA concentrations were first compared by analysis of variance (ANOVA). After of confirmation of no group differences in baseline concentration the data were converted to the percent change from the average baseline concentration obtained prior to pre-treatment. Statistical analysis was performed on the percentage data. Within-and between-groups analysis were performed by either one-way or two-way repeated measures ANOVA, respectively. A two-way multilevel ANOVA was done to determine the relative effect of AM404 pre-treatment on EtOH-induced 2-AG levels between control and CET rats. The control group in experiment II was comprised of both EtOH-naïve rats maintained on control diet and some EtOH-naïve rats from experiment I. There were no significant differences in baseline AEA [t(17) = 1.622, n.s.] or 2-AG [t(17) = 1.789, n.s.] levels in dialysates collected from these two cohorts of EtOH-naïve animals, and care was taken to ensure a similar distribution of these cohorts across the two control groups in experiment II (VEH pre-treatment: five EtOH naïve + four control diet; AM404 pre-treatment: six EtOH naïve + four control diet). Area under the curve (AUC) measures were used for comparison of overall vehicle and AM404 pre-treatment effects on EtOH-induced EC alterations in acute and CET rats. The AUC was calculated for each animal by subtracting 100 from the percent of baseline value for each data point following EtOH administration, and subsequently summing all these data points. Significant differences in AUC measures were determined using standard ANOVA.
Results
Experiment I: dose-dependent effects of acute EtOH administration on dialysate EC levels
The effects of acute EtOH administration on 2-AG levels in NAC dialysates are shown in Fig. 1. The baseline 2-AG concentration for all the groups were as follows: Vehicle/EtOH 0.75 g/kg, 3.7 ± 0.7 nM; Vehicle/EtOH 2 g/kg, 4.5 ± 1.1 nM; AM404/Vehicle, 6.6 ± 1.7 nM; AM404/EtOH 0.75 g/kg, 7.8 ± 1.9 nM; AM404/EtOH 2 g/kg, 5.4 ± 0.6 nM. There were no significant differences in the baseline levels of Vehicle/EtOH 0.75 g/kg vs. Vehicle/EtOH 2 g/kg (t(9) = 0.6475; n.s.); AM404/Vehicle vs. AM404/EtOH 0.75 g/kgvs. AM404/EtOH 2 g/kg (F2, 14 = 0.6965; n.s.); Vehicle/EtOH 0.75 g/kg vs. AM404/EtOH 0.75 g/kg (t(10) = 2.081; n.s.); Vehicle/EtOH 2 g/kg vs. AM404/EtOH 2 g/kg (t(9) = 0.7279; n.s.).
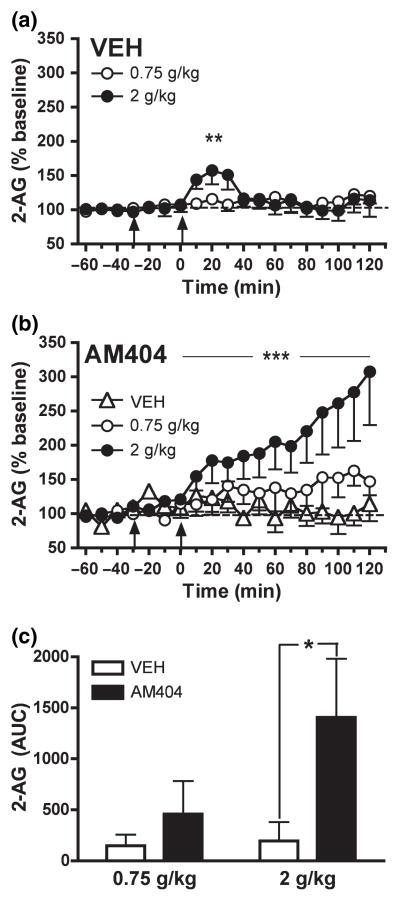
Fig. 1.
Dose-dependent effect of acute EtOH administration (0.75 and 2 g/kg, i.p.) on NAC dialysate 2-AG levels from rats pre-treated with (a) vehicle (n = 11) and (b) AM404 (3 mg/kg, i.p.; n = 17). Data are expressed as the percentage change from baseline levels (mean ± SEM). (c) The AUC data summarize the effect of AM404 pre-treatment on EtOH-induced changes in dialysate 2-AG levels. The EC clearance inhibitor, AM404, potentiated EtOH-induced increases in dialysate NAC 2-AG (see Results section for details). Vehicle and AM404 pre-treatment is indicated by arrow at −30 min. EtOH injection is indicated by arrow at time point zero. *p < 0.05 denote significant differences from vehicle. **p < 0.01 and ***p < 0.001 denotes a significant dose-dependent effect.
In vehicle-pre-treated rats administration of 0.75 g/kg EtOH did not significantly alter 2-AG levels (F12, 60 = 0.9398; n.s.) (Fig. 1a), though 2 g/kg EtOH produced a moderate and transitory significant increase in 2-AG levels to a maximum of 157.6 ± 20.3% of baseline (F12, 48 = 3.903; p < 0.001) (Fig. 1a). A two-way Repeated Measures ANOVA detected a significant EtOH dose effect on the 2-AG response to EtOH administration (F12, 108 = 2.389; p < 0.01).
The effect of pre-treatment with the EC clearance inhibitor AM404 (3 mg/kg, i.p.) on EtOH-induced alterations in NAC 2-AG are shown in Fig. 1(b). Although AM404 administration itself did not alter dialysate 2-AG levels in rats subsequently administered vehicle (F12, 48 = 0.2656; n.s.), pre-treatment with the EC clearance inhibitor potentiated the effects of EtOH administration on NAC 2-AG. In contrast to effects observed in vehicle-pre-treated rats administration of 0.75 g/kg EtOH significantly increased dialysate 2-AG levels in AM404-pre-treated rats (F12, 60 = 2.467; p < 0.05). However, as evaluated by two-way repeated measures ANOVA there was no significant difference between the effects of 0.75 g/kg EtOH on NAC 2-AG in vehicle- or AM404-pre-treated rats (F12, 120 = 1.139; n.s.). Administration of 2 g/kg EtOH induced a robust and sustained increase in 2-AG levels in rats pre-treated with AM404 (F12, 48 = 6.197; p < 0.001), and this effect was significantly greater than that observed in vehicle-pre-treated rats (F12, 96 = 7.236, p < 0.0001). Similar to that observed in vehicle-pre-treated rats a two-way ANOVA revealed a significant effect of EtOH dose on NAC 2-AG levels in AM404-pre-treated rats (F12, 108 = 4.154, p < 0.0001). However, the dose-dependent effect of EtOH on NAC 2-AG was generally greater in AM404-pre-treated rats as indicated by a significant interaction between EtOH dose and pre-treatment condition in a Three-way ANOVA (F12, 216 = 5.128, p < 0.001). This is easily observed in the AUC measures shown in Fig. 1(c), in which the dose-dependency of EtOH-induced increases in 2-AG are clearly more evident in those animals given AM404 pre-treatment. The apparent lack of EtOH dose-dependency in the AUC measures from vehicle pre-treated rats results from the summation of all data from zero to 120 min, which serves to dilute evidence of the transient increase in 2-AG within the first hour after EtOH administration that was evident with repeated measures ANOVA.
The effects of acute EtOH administration on AEA levels in NAC dialysates are shown in Fig. 2. The baseline AEA concentration for all the groups were as follows: Vehicle/EtOH 0.75 g/kg, 2.0 ± 0.5 nM; Vehicle/EtOH 2 g/kg, 2.1 ± 1.1 nM; AM404/Vehicle, 4.4 ± 0.7 nM; AM404/EtOH 0.75 g/kg, 2.0 ± 0.4 nM; AM404/EtOH 2 g/kg, 3.7 ± 1.7 nM. There were no significant differences in the baseline levels of Vehicle/EtOH 0.75 g/kg vs. Vehicle/EtOH 2 g/kg (t(9) = 0.08246; n.s.); AM404/Vehicle vs. AM404/EtOH 0.75 g/kgvs. AM404/EtOH 2 g/kg (F2, 14) = 1.242; n.s.); Vehicle/EtOH 0.75 g/kg vs. AM404/EtOH 0.75 g/kg (t(10) = 0.06074; n.s.); Vehicle/EtOH 2 g/kg vs. AM404/EtOH 2 g/kg (t(9) = 0.7548; n.s.).
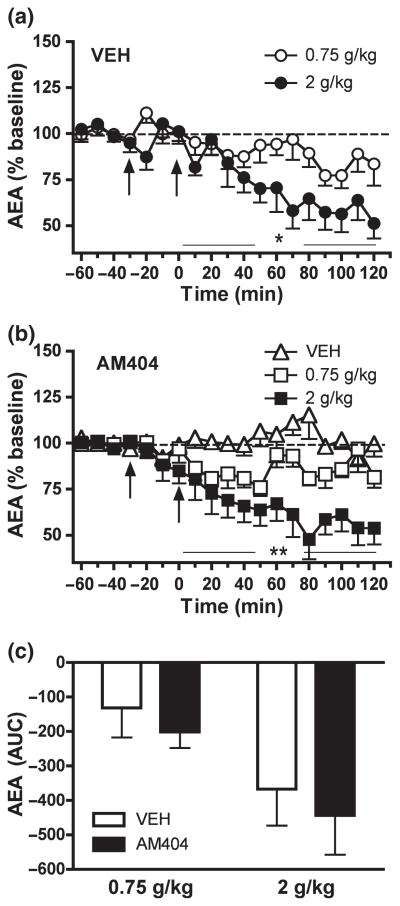
Fig. 2.
Dose-dependent effect of acute EtOH administration (0.75 and 2 g/kg, i.p.) on NAC dialysate AEA levels from rats pre-treated with (a) vehicle (n = 11) and (b) AM404 (3 mg/kg, i.p.; n = 17). Data are expressed as the percentage change from baseline levels (mean ± SEM). (c) The AUC data summarize the effect of AM404 pre-treatment on EtOH-induced changes in dialysate AEA levels. The EC clearance inhibitor, AM404, did not alter EtOH-induced decreases in dialysate AEA (see Results section for details). Vehicle and AM404 pre-treatment is indicated by arrow at −30 min. EtOH injection is indicated by arrow at time point zero. *p < 0.05 and **p < 0.01 denotes a significant dose-dependent effect.
In vehicle-pre-treated rats 0.75 g/kg EtOH induced a non-significant trend toward decreased dialysate AEA levels(F12, 60 = 1.690; n.s.) (Fig. 2a). In comparison, 2 g/kg EtOH induced a more persistent and significant reduction in AEA interstitial levels to approximately 51.2 ± 8.2% of baseline(F12, 48 = 6.505; p < 0.001) (Fig. 2a). A Two-Way Repeated Measures ANOVA showed a significant EtOH dose effect on the AEA response to EtOH administration (F12, 108 = 2.304; p < 0.05).
Pre-treatment with the EC clearance inhibitor AM404 (3 mg/kg, i.p.) did not significantly alter the effect of acute EtOH on NAC AEA levels (Fig. 2b). As was observed in vehicle pre-treated rats, significant reductions in dialysate AEA levels were observed following administration of either 0.75 g/kg EtOH (F12, 60 = 2.218; p < 0.05) or 2 g/kg EtOH (F12, 60 = 4.674, p < 0.001), and magnitude of this effect was found to be EtOH dose-dependent (F12, 120 = 2.713, p < 0.005). There was no significant effect of AM404 administration on dialysate AEA levels in animals subsequently administered vehicle rather than EtOH (F12, 48 = 0.6822; n.s.). Comparison of data gathered in AM404- and vehicle-pre-treated rats revealed no significant effect of AM404 on the AEA response to either 0.75 g/kg EtOH (F12, 120 = 1.178, n.s.) or 2 g/kg EtOH (F12, 108 = 1.414, n.s.) and there was no significant interaction between EtOH dose and pre-treatment condition (F12, 228 = 0.695, n.s.). This relationship is clearly evident in the AUC data presented in Fig. 2(c). AM404 pre-treatment did not alter the AEA response to EtOH 0.75 g/kg (F1, 10 = 0.234, p < 0.05) or EtOH 2 g/kg (F1, 9 = 0.29, n.s.) when compared to the respective vehicle pre-treatment group (Fig. 2c). Dialysis probe placements for rats in experiment I are shown in Fig. 5(a).
Fig. 5.
Schematic representation of the location of dialysis probe membranes within the NAC shell in Experiments 1 (n = 28) (a) and 2 (n = 31) (b). Distances shown are in mm from Bregma (Paxinos and Watson 1998).
Collectively, these data demonstrate that acute EtOH administration induces dose-dependent increases in NAC 2-AG levels and dose-dependent decreases in NAC AEA levels in EtOH-naïve rats. EtOH-induced increases in NAC 2-AG are robustly potentiated by AM404 pre-treatment highlighting a strong influence of EC clearance mechanisms on interstitial 2-AG levels as measured by microdialysis.
Experiment II: effect of CET on the EC response to EtOH challenge
The average daily EtOH intake by CET rats during the 14 days maintenance on EtOH-containing liquid diet was 13.3 ± 2.1 g/kg, resulting in blood alcohol concentrations of 193.6 ± 30.2 mg%. The effects of CET on EtOH-induced alterations in NAC 2-AG levels are shown in Fig. 3. Baseline 2-AG concentrations in NAC shell dialysates were as follows: control-Vehicle/EtOH 2 g/kg 10.1 ± 2.9 nM; control-AM404/EtOH 2 g/kg 4.4 ± 0.6 nM; CET-Vehicle/EtOH 2 g/kg 8.0 ± 2.1 nM; CET-AM404/EtOH 2 g/kg 11.0 ± 5.1 nM. There were no significant differences in the baseline 2-AG concentrations of the following group comparisons: control-Vehicle/EtOH 2 g/kg vs. CET-Vehicle/EtOH 2 g/kg (t(13) = 0.5389; n.s.); control-AM404/EtOH 2 g/kg vs. CET/AM404/EtOH 2 g/kg (t(14) = 1.653; n.s.); control-Vehicle/EtOH 2 g/kg vs. control-AM404/EtOH 2 g/kg (t(17) = 2.059; n.s.); CET-Vehicle/EtOH 2 g/kg vs. CET-AM404/EtOH 2 g/kg (t(10) = 0.5314; n.s.).
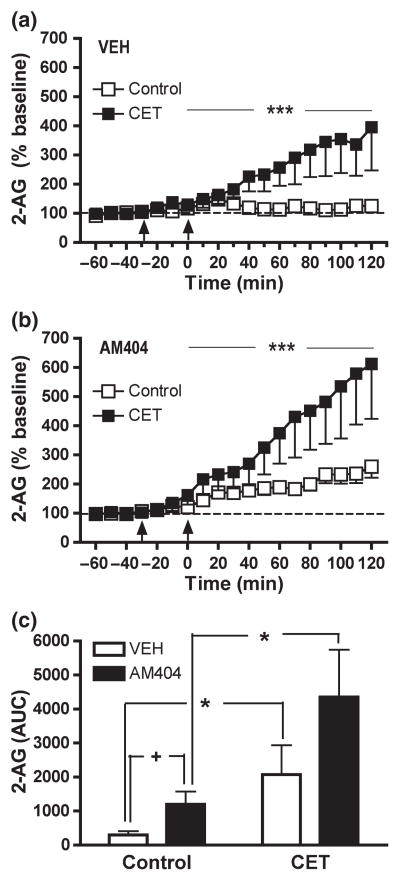
Fig. 3.
CET significantly enhanced EtOH-induced increases in NAC dialysate 2-AG as compared to EtOH-naïve control rats (control: n = 9; CET: n = 6) (a). Inhibition of EC clearance mechanisms by AM404 potentiated EtOH-induced increases in dialysate 2-AG levels in control (n = 10) and CET (n = 6) (b). Data are expressed as the percentage change from baseline levels (mean ± SEM). (c) The AUC data summarize the effect of AM404 pre-treatment on the 2-AG response to EtOH challenge in control and CET rats. CET does not alter the effect of AM404 on EtOH-induced increases in NAC 2-AG (see Results section for details). Vehicle and AM404 pre-treatment is indicated by arrow at −30 min. EtOH injection is indicated by arrow at time point zero. +p < 0.05 denotes significant differences from vehicle. *p < 0.05 denotes a significant effect of EtOH history in vehicle-pre-treated rats. **p < 0.01 denotes a significant effect of EtOH history in AM404-pre-treated rats. ***p < 0.001 denotes a significant effect of EtOH history in vehicle- and AM404-pre-treated rats as determined by Two-Way Repeated measures ANOVA.
In control rats, acute EtOH challenge (2 g/kg, i.p.) resulted in a subtle but significant increase in dialysate 2-AG levels (F12, 168 = 2.095, p < 0.05) (Fig. 3a). This same EtOH dose induced a more robust and prolonged increase in NAC 2-AG in rats with a history of EtOH exposure (F12, 168 = 3.203, p < 0.005) (Fig. 3a). A Two-Way Repeated Measures ANOVA revealed a significant influence of EtOH history on the 2-AG response to EtOH challenge (F12, 156 = 5.252, p < 0.001).
Figure 3(b) shows the effect of the EC clearance inhibitor AM404 (3 mg/kg, i.p.) on acute EtOH-induced 2-AG alterations in control and CET rats. EtOH challenge significantly increased dialysate 2-AG levels in both control(F12, 180 = 5.164, p < 0.001) and CET (F12, 180 = 9.714, p < 0.0001) groups. However, the effect of EtOH challenge on dialysate 2-AG was significantly greater in CET versus control rats (F12, 168 = 8.217, p < 0.001). Comparison of the response to EtOH following vehicle versus AM404 pre-treatment revealed that AM404 significantly enhanced EtOH-induced increases in NAC 2-AG in control (F12, 216 = 2.561, p < 0.005) but not in CET (F12, 120 = 1.449, n.s.) rats. Three- way ANOVA revealed no significant interaction between EtOH history and pre-treatment condition on EtOH-induced increases in NAC 2-AG (F12, 336 = 0.986, n.s.).
As shown in Fig. 3(c) AUC analyses showed a significant effect of EtOH history (control vs. CET) on the 2-AG response to EtOH challenge in both vehicle- (F1, 13 = 7.672, p < 0.05) and AM404- pre-treated (F1, 14 = 8.755, p < 0.01) rats. Compared to vehicle, AM404 pre-treatment significantly potentiated EtOH-induced 2-AG levels in EtOH-naïve control diet rats (F1, 17 = 5.326, p < 0.05). While a similar effect of AM404 on the 2-AG response to EtOH was evident in CET rats this pre-treatment effect did not reach statistical significance (F1, 10 = 2.338, n.s.).
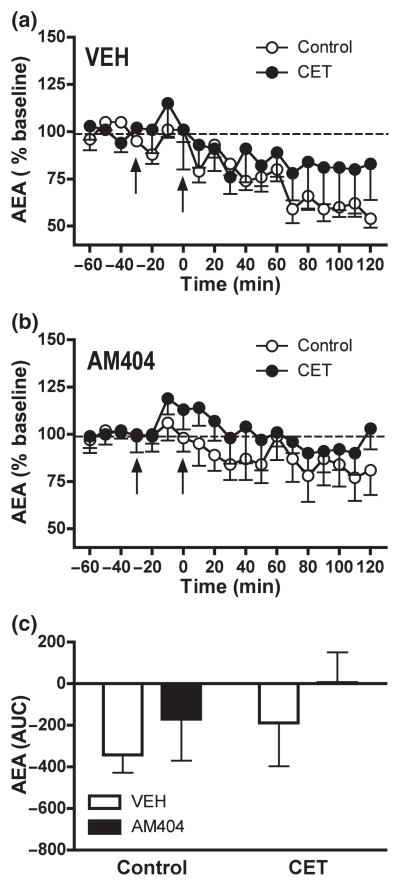
The effects of CET on EtOH-induced alterations in NAC AEA levels are shown in Fig. 4. Baseline AEA concentrations in NAC shell dialysates were as follows: control-Vehicle/EtOH 2 g/kg 1.7 ± 0.6 nM; control-AM404/EtOH 2 g/kg 2.6 ± 1.1 nM; CET-Vehicle/EtOH 2 g/kg 1.6 ± 0.3 nM; CET-AM404/EtOH 2 g/kg 1.8 ± 0.4 nM. There were not significant differences in the baseline AEA concentrations of the following group comparisons: control-Vehicle/EtOH 2 g/kg vs. CET-Vehicle/EtOH 2 g/kg (t(13) = 0.1210; n.s.); control-AM404/EtOH 2 g/kg vs. CET/AM404/EtOH 2 g/kg (t(14) = 0.5363; n.s.); control-Vehicle/EtOH 2 g/kg vs. control-AM404/EtOH 2 g/kg (t(17) = 0.7319; n.s.); CET-Vehicle/EtOH 2 g/kg vs. CET-AM404/EtOH 2 g/kg (t(10) = 0.5380; n.s.).
Fig. 4.
CET did not significantly alter EtOH-induced decreases in NAC dialysate AEA observed in control rats (control: n = 9; CET: n = 6) (a). EtOH challenge did not significantly alter NAC AEA levels in either control (n = 10) or CET (n = 6) rats pre-treated with AM404 (b). Data are expressed as the percentage change from baseline levels (mean ± SEM). (c) The AUC data summarize the effect of AM404 pre-treatment on the 2-AG response to EtOH challenge in control and CET rats. Vehicle and AM404 pre-treatment is indicated by arrow at −30 min. EtOH injection is indicated by arrow at time point zero.
In control rats AEA levels were significantly reduced following EtOH challenge lasting for at least 120 min (F12, 168 = 6.534, p < 0.0001) (Fig. 4a). CET rats displayed a less robust and non-significant AEA response to EtOH challenge (F12, 168 = 0.993, n.s.). A Two-Way Repeated Measures ANOVA revealed a no significant influence of EtOH history on the AEA response to EtOH challenge (F12, 156 = 1.593, n.s.).
Figure 4(b) depicts the effect of the EC clearance inhibitor, AM404 (3 mg/kg, i.p.) on acute EtOH-induced AEA alterations in control and CET rats. EtOH challenge did not significantly alter NAC AEA levels in either control (F12, 180 = 1.701, n.s.) or CET (F12, 180 = 1.701, n.s.) rats pre-treated with AM404. A Two-Way Repeated Measures ANOVA showed no significant influence of EtOH history on the response of AEA levels to EtOH challenge (F12, 168 = 0.531, n.s.). Comparison of the response to EtOH following vehicle versus AM404 pre-treatment revealed that AM404 significantly attenuated EtOH-induced decreases in NAC AEA in control (F12, 204 = 2.336, p < 0.01) but not in CET (F12, 120 = 0.278, n.s.) rats. Three-way ANOVA revealed no significant interaction between EtOH history and pre-treatment condition on EtOH-induced alterations in NAC AEA (F12, 324 = 1.135, n.s.).
As shown in Fig. 3(c) AUC analyses revealed a no significant effect of EtOH history (control vs. CET) on the AEA response to EtOH challenge in vehicle- (F1, 14 = 0.757, n.s.) and AM404-pre-treated (F1, 14 = 0.429, n.s.) rats (Fig. 4c). AM404 pre-treatment did not alter AEA response neither in control (F1, 17 = 0.717, n.s.) nor CET (F1, 10 = 0.701, n.s.) rats when compared to their respective vehicle group (Fig. 4c). Dialysis probe placements for rats in experiment II are shown in Fig. 5(b).
Collectively, these data demonstrate that chronic EtOH exposure significantly enhances the effect of acute EtOH challenge on extracellular 2-AG levels in the NAC without significantly altering the effect of EtOH on NAC AEA levels.
Discussion
The present data demonstrate that acute systemic administration of EtOH dose-dependently increases dialysate 2-AG levels in the NAC of EtOH-naïve rats and that this effect is potentiated and prolonged in rats with a history of EtOH consumption. In EtOH-naïve rats the 2-AG response to EtOH challenge is enhanced following inhibition of EC clearance mechanisms by AM404, resulting in a profile similar to that observed in CET rats.
Interstitial EC levels in the NAC are altered by acute non-contingent EtOH administration to drug-naïve rats
In the present study, acute non-contingent EtOH administration dose-dependently increased interstitial NAC shell 2-AG levels. These results are in agreement with our previous observations demonstrating that voluntary operant EtOH self-administration increases interstitial 2-AG levels in the NAC shell (Caillé et al. 2007). However, there are some notable differences between the effects of non-contingent EtOH challenge and voluntary EtOH self-administration. In the present study, non-contingent intraperitoneal administration of 2 g/kg EtOH produced a moderate and transitory increase in 2-AG levels whereas we previously observed a stronger and more prolonged increase in NAC 2-AG following voluntary oral self-administration of only 0.4 g/kg EtOH (Caillé et al. 2007). It is possible that the differential effect of EtOH observed in these two studies is related to the relatively greater neurochemical effects produced in response to volitional drug self-administration versus response-independent forced drug administration (Hemby et al. 1997; Mark et al. 1999; Jacobs et al. 2003; Lecca et al. 2007; You et al. 2007). Alternately, it is possible that the greater 2-AG response to EtOH observed during EtOH self-administration relates in part to the prior history of EtOH consumption associated with self-administration training prior to microdialysis testing. This would be consistent with our present observation of greater EtOH-induced increases in NAC 2-AG following a period of EtOH consumption (see below).
Non-contingent EtOH challenge and voluntary EtOH self-administration also appear to produce distinct effects on NAC AEA levels. In the present study AEA dialysate levels were dose-dependently reduced by non-contingent EtOH administration and this is in agreement with our previous observations (Ferrer et al. 2007). However, no changes in NAC dialysate AEA levels are evident either during or after voluntary EtOH self-administration (Caillé et al. 2007). As suggested above for 2-AG it is possible that this differential AEA response to EtOH is related in part to prior drug history. The initial EtOH-induced reduction in AEA levels could result from the inhibitory effects of acute EtOH on glutamatergic transmission. For example, acute EtOH exposure significantly reduces NAC glutamate levels following a profile similar to that of AEA (Ferrer et al. 2007) and also inhibits NMDA receptor function (Lovinger et al. 1989; Hoffman et al. 1990; Woodward 2000). Both of these effects may lead to reductions in AEA, though this would also be expected to reduce 2-AG formation (Hansen et al. 1997, 2001). In contrast, repeated EtOH exposure results in enhanced EtOH-induced glutamate release (Szumlinski et al. 2007; Kapasova and Szumlinski 2008) and increased NMDA receptor expression (Hoffman et al. 1990) and this may ameliorate or even reverse the inhibitory effects of EtOH challenge on AEA formation. Consistent with this is our present observation that EtOH-induced deficits in NAC AEA levels are less pronounced in CET versus EtOH-naïve control rats (Fig. 4). The stress response induced by acute EtOH injection (Rivier 1993) may also influence the effects of intraperitoneal EtOH administration on EC formation, as stress is known to alter brain EC levels (Gorzalka et al. 2008). In fact, acute stress induces selective reductions in mouse brain AEA, but not 2-AG content in regions such as the amygdala and prefrontal cortex (Rademacher et al. 2008).
EtOH-induced increases in NAC 2-AG levels are enhanced by EC clearance inhibition
Endocannabinoid clearance occurs in part by reuptake into cells via a carrier-mediated facilitated diffusion process and/or enzymatic hydrolysis (Giuffrida et al. 2001; Glaser et al. 2005; Hermann et al. 2006). Inhibitors of the EC reuptake include AM404, which blocks AEA and 2-AG transport with similar IC50 values in the μM range (Beltramo et al. 1997; Beltramo and Piomelli 2000; Bisogno et al. 2001; Hájos et al. 2004). Depending on the cell type and assay conditions, AM404 can also inhibit fatty acid amide hydrolase (Fowler et al. 2004), the primary metabolic enzyme for clearance of AEA. Systemic AM404 administration increases AEA levels in rat plasma and bulk levels in tissue from some brain areas, with lesser increases in brain 2-AG levels (Beltramo et al. 1997; Beltramo and Piomelli 2000; Giuffrida et al. 2000; Fegley et al. 2004; Di et al. 2005; Bortolato et al. 2006). However, in the present study, acute AM404 administration on its own did not alter dialysate 2-AG or AEA levels though it did substantially potentiate EtOH-induced increases in NAC 2-AG. This is consistent with the hypothesis that tonic EC formation is low and may not be largely affected by clearance inhibition under unperturbed conditions. However, the impact of reduced EC clearance becomes readily apparent under circumstances that stimulate EC formation (EtOH challenge). This is consistent with our previous observations that monoacylglycerol lipase inhibition does not induce robust alterations in baseline dialysate 2-AG levels though this manipulation substantially enhances depolarization-induced increases in dialysate 2-AG (Long et al. 2009). The lack of AM404-induced alterations in the EtOH-induced reductions in NAC AEA levels is also consistent with the theory that EC clearance mechanisms exert greater influence on stimulated versus basal EC formation in light of evidence that EtOH either reduces (Ferrer et al. 2007) or does not alter NAc AEA levels (Caille et al. 2007).
Baseline NAC EC levels are unaltered following CET
Our data show that continuous CET (via a liquid diet) enhanced the EC response to an EtOH challenge but did not alter baseline dialysate EC levels collected from the NAC. In contrast, previous studies have shown that chronic EtOH exposure alters EC levels in bulk tissue. For example, chronic EtOH exposure via liquid diet for 15 days reduced 2-AG and AEA levels in the midbrain and increased AEA levels in the limbic forebrain (González et al. 2002). Additional studies suggest that total tissue EC content is transiently altered as a function abstinence period (González et al. 2004; Mitrirattanakul et al. 2007). In the present study baseline dialysate samples were collected 12–15 h into EtOH abstinence, and as such it is possible alterations in basal EC levels present in the early hours of abstinence may have dissipated (González et al. 2004). It is also possible that the duration of ethanol exposure or the pattern of ethanol exposure affects EtOH influences on basal EC levels in brain. It is also worth noting that brain tissue EC levels can be affected by a rapid post-mortem increases in EC formation (Bazinet et al. 2005; Patel et al. 2005) that may lead to differences in estimates of EtOH-induced changes in brain EC levels as determined by post-mortem tissue analyses versus in vivo microdialysis. Further more parametric evaluations are necessary to characterize the effect of long-term EtOH exposure on baseline EC levels in brain.
EtOH-induced increases in NAC 2-AG formation are enhanced following CET
Although the mechanism(s) contributing to the potentiation of EtOH-induced increases in NAC 2-AG following CET are not known, some hypotheses can be forwarded based on the mechanisms influencing 2-AG formation and the known physiologic effects of chronic EtOH exposure. 2-AG formation is induced by increases in intracellular Ca2+ concentrations (Stella et al. 1997) that can result from stimulation of ionotropic glutamate (e.g., NMDA receptors) (Smothers et al. 1997; Cebere et al. 1999; Stella and Piomelli 2001; Ohno-Shosaku et al. 2007) and/or group I metabotropic glutamate receptors (Jung et al. 2005). Several lines of evidence indicate that glutamatergic transmission is generally up-regulated following CET. For example, baseline and EtOH-induced increases in extracellular glutamate levels in NAC dialysates are enhanced in EtOH-experienced mice (Szumlinski et al. 2007; Kapasova and Szumlinski 2008), and cellular physiology and microdialysis studies have found evidence of increased glutamate transmission in the central and basolateral nuclei of the amygdala following CET (Roberto et al. 2004; Läck et al. 2007). In addition to increased pre-synaptic glutamate release, there is also substantial evidence of increased NMDA receptor expression and influence following chronic EtOH exposure (Hoffman et al. 1990). Thus, it is conceivable that enhanced EtOH-induced glutamate responses underlie the presently observed enhancement of EtOH-induced EC formation following CET.
It is also possible that the apparent enhancement of EtOH on NAC 2-AG levels actually reflects reduced 2-AG clearance following CET. Indeed, chronic EtOH exposure has been shown to result in decreased EC clearance in primary cultures of cerebellar granule neurons (Basavarajappa et al. 2003) and the profile of EtOH-induced increases in NAC 2-AG in rats with a history of EtOH exposure is remarkably similar to that observed in EtOH-naïve rats pre-treated with the EC clearance inhibitor AM404 (Figs 1 and 3). However, it would be expected that the relative effects of EC clearance inhibition by AM404 would be reduced in CET animals if these clearance mechanisms are already suppressed though we found no evidence of reduced AM404 efficacy in CET rats in the present experiments (Fig. 3b). Thus our preliminary findings suggest that EC clearance mechanisms are not substantially altered following CET, consistent with recent findings by others (Rubio et al. 2009).
Potential consequences of enhanced EtOH-induced 2-AG increases
Several lines of evidence suggest that increased interstitial EC levels could promote EtOH intake through CB1 receptor activation. For example, EtOH intake is increased by stimulation of CB1 receptors either by synthetic agonists (Gallate and McGregor 1999; Colombo et al. 2002) or enhanced EC transmission resulting from fatty acid amide hydrolase inhibition (Basavarajappa et al. 2006; Blednov et al. 2007; Malinen and Hyytiä 2008; Vinod et al. 2008). In contrast, genetic (Naassila et al. 2004) and/or pharmacological (Arnone et al. 1997; Gallate and McGregor 1999; Freedland et al. 2001) inactivation of CB1 receptors is accompanied by reductions in EtOH consumption in non-dependent and EtOH-dependent rats (Rodriguez de Fonseca et al. 1999). In addition, CB1 receptors in the NAC participate in the regulation of EtOH intake since EtOH self-administration is reduced by intra-NAC CB1 antagonist administration and is increased by intra-NAC CB1 agonist administration (Caillé et al. 2007; Malinen and Hyytiä 2008; Alvarez et al., unpublished observations). Collectively, these findings suggest that EtOH-induced increases in 2-AG may promote EtOH consumption through agonistic effects at CB1 receptors.
However, it is known that 2-AG also interacts with other G protein-coupled receptors including CB2 (Sugiura et al. 2006) and GPR55 (Ryberg et al. 2007) receptors. Ryberg et al. (2007) have shown that 2-AG has a greater potency as an agonist at GPR55 (EC50 = 3 nM) than CB1 (EC50 = 519 nM) or CB2 (EC50 = 618 nM) receptors. While the CB1 receptor influence on EtOH intake has been relatively well characterized, the influence of these other receptor systems is largely unexplored. Moreover, in addition to clearance by monoacylglycerol lipase, 2-AG is metabolized by enzymes such as cyclooxygenases and lipoxygenases (Kozak et al. 2000, 2002). Some of the metabolites produced through these pathways are bioactive and may trigger effects opposite to that produced by CB1 receptor stimulation. For example, 2-AG is metabolized by cyclooxygenase-2 to produce prostaglandin ester-2 (Kozak et al. 2000). Prostaglandin ester-2 increases the frequency of miniature inhibitory post-synaptic currents (mIPSCs) (Sang et al. 2006) and miniature excitatory post-synaptic currents (mEPSCs) (Sang et al. 2007) in primary culture hippocampal neurons via non-CB1 receptor mechanisms, effects that are opposite to those produced by 2-AG through CB1 receptor stimulation. In this regard it is interesting that chronic EtOH exposure results in increased cyclooxygenase-2 expression and function in the rat cerebral cortex (Vallés et al. 2004).
In summary, our findings show that acute intraperitoneal EtOH administration dose-dependently increases dialysate 2-AG levels in the NAC of EtOH-naïve rats and that chronic EtOH exposure significantly enhances this effect. In contrast, acute EtOH challenge dose-dependently reduces AEA levels in NAC dialysates and this effect is slightly attenuated following chronic EtOH exposure. This is consistent with previous observations indicating a differential EtOH influence on extracellular 2-AG and AEA levels in the NAC. Inhibition of EC clearance mechanisms by AM404 potentiates EtOH-induced increases in NAC 2-AG in both EtOH-naïve rats and to a lesser degree in CET rats. AM404 pre-treatment produces only modest alterations in EtOH-induced reductions in dialysate AEA levels in either EtOH-naïve or chronic EtOH treated rats, and does not substantially alter dialysate EC levels in the absence of EtOH challenge. From these observations we hypothesize that AM404-induced enhancements in extracellular EC levels are largely reliant on drug administration or other perturbations that increase EC formation.
Acknowledgments
This study was supported by NIAAA grants AA014619 and AA06420. This is publication 20094 from the Scripps Research Institute. The authors listed on this manuscript (L. Alvarez-Jaimes, D.G. Stouffer and L.H. Parsons) do not have any potential conflicts of interest related to the subject of this report. Further, each of the authors on this manuscript is supported in full by NIH research grants and have not received, and do not anticipate receiving, any compensation for professional services from any source outside of the NIH.
Abbreviations used
- 2-AG
2-arachidonoylglycerol
- AEA
anandamide
- AM404
N-(4-hydrophenyl) arachidonoylamide
- AUC
area under the curve
- CET
chronic ethanol treated/treatment
- EC
endocannabinoid(s)
- EtOH
ethanol
- NAC
nucleus accumbens
References
- Arnone M, Maruani J, Chaperon F, Thiébot M, Poncelet M, Soubrie P, Le Fur G. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology. 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Hungund LH. Chronic ethanol increases the cannabinoid receptor agonist anandamide and its precursor N-Arachidonoylphosphatidylethanolamine in SK-N-SH cells. J Neurochem. 1999;72:522–528. doi: 10.1046/j.1471-4159.1999.0720522.x. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Hungund BL. Role of the endocannabinoid system in the development of tolerance to alcohol. Alcohol Alcohol. 2005;40:15–24. doi: 10.1093/alcalc/agh111. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Saito M, Cooper TB, Basalingappa LH. Activation of arachidonic acid-specific phospholipase A2 in human neuroblastoma cells after chronic alcohol exposure: prevention by GM1 ganglioside. Alcohol Clin Exp Res. 1997;21:1199–1203. [PubMed] [Google Scholar]
- Basavarajappa BS, Cooper TB, Basalingappa LH. Chronic ethanol administration down-regulates cannabinoid receptors in mouse brain synaptic plasma membrane. Brain Res. 1998;793:212–218. doi: 10.1016/s0006-8993(98)00175-9. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Saito M, Cooper TB, Basalingappa LH. Stimulation of cannabinoid receptor agonist 2-arachidonylglycerol by chronic ethanol and its modulation by specific neuromodulators in cerebellar granule neurons. Biochem Biophys Acta. 2000;1535:78–86. doi: 10.1016/s0925-4439(00)00085-5. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Saito M, Cooper TB, Hungund BL. Chronic ethanol inhibits the anandamide transport and increases extracellular anandamide levels in cerebellar granule neurons. Eur J Pharmacol. 2003;466:73–83. doi: 10.1016/s0014-2999(03)01557-7. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Yalamanchili R, Cravatt BF, Cooper TB, Hungund BL. Increased ethanol consumption and preference and decreased ethanol sensitivity in female FAAH knockout mice. Neuropharmacology. 2006;50:834–844. doi: 10.1016/j.neuropharm.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Bazinet RP, Lee HJ, Felder CC, Porter AC, Rapoport SI, Rosenberg TA. Rapid high-energy microwave fixation is required to determine the anandamide (N-arachidonoylethanolamine) concentration of rat brain. Neurochem Res. 2005;30:597–601. doi: 10.1007/s11064-005-2746-5. [DOI] [PubMed] [Google Scholar]
- Beltramo M, Piomelli D. Carrier-mediated transport and enzymatic hydrolysis of the endogenous cannabinoid 2-arachidonoylglycerol. Neuroreport. 2000;11:1231–1235. doi: 10.1097/00001756-200004270-00018. [DOI] [PubMed] [Google Scholar]
- Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, Piomelli D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science. 1997;277:1094–1097. doi: 10.1126/science.277.5329.1094. [DOI] [PubMed] [Google Scholar]
- Bisogno T, MacCarrone M, De Petrocellis L, Jarrahian A, Finazzi-Agrò A, Hillard C, Di Marzo V. The uptake by cells of 2-arachidonoylglycerol, an endogenous agonist of cannabinoid receptors. Eur J Biochem. 2001;268:1982–1999. doi: 10.1046/j.1432-1327.2001.02072.x. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Cravatt BF, Boehm SL, II, Walker D, Harris RA. Role of endocannabinoids in alcohol consumption and intoxication: studies of mice lacking fatty acid amide hydrolase. Neuropsychopharmacology. 2007;32:1570–1582. doi: 10.1038/sj.npp.1301274. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Campolongo P, Mangieri RA, et al. Anxiolytic-like properties of the anandamide transport inhibitor AM404. Neuropsychopharmacology. 2006;31:2652–2659. doi: 10.1038/sj.npp.1301061. [DOI] [PubMed] [Google Scholar]
- Caillé S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci. 2007;27:3695–3702. doi: 10.1523/JNEUROSCI.4403-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebere A, Cebers G, Liljequist S. Enhancement of NMDA-induced functional responses without concomitant NMDA receptor changes following chronic ethanol exposure in cerebellar granule cells. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:623–632. doi: 10.1007/s002109900133. [DOI] [PubMed] [Google Scholar]
- Colombo G, Serra S, Brunetti G, Gomez R, Melis S, Vacca G, Carai MAM, Gessa GL. Stimulation of voluntary ethanol intake by cannabinoid receptor agonists in ethanol-preferring sP rats. Psychopharmacology. 2002;159:181–187. doi: 10.1007/s002130100887. [DOI] [PubMed] [Google Scholar]
- Di S, Boudaba C, Popescu IR, Weng F, Harris C, Marcheselli VL, Bazan NG, Tasker JG. Activity-dependent release and actions of endocannabinoids in the rat hypothalamic supraoptic nucleus. J Physiol. 2005;569:751–760. doi: 10.1113/jphysiol.2005.097477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fegley D, Kathuria S, Mercier R, Li C, Goutopoulos A, Makriyannis A, Piomelli D. Anandamide transport is independent of fatty-acid amide hydrolase activity and is blocked by the hydrolysis-resistant inhibitor AM1172. Proc Natl Acad Sci USA. 2004;101:8756–8761. doi: 10.1073/pnas.0400997101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer B, Bermúdez-Silva FJ, Bilbao A, et al. Regulation of brain anandamide by acute administration of ethanol. Biochem J. 2007;404:97–104. doi: 10.1042/BJ20061898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CJ, Tiger G, Ligresti A, López-Rodríguez ML, Di Marzo V. Selective inhibition of anandamide cellular uptake versus enzymatic hydrolysis-a difficult issue to handle. Eur J Pharmacol. 2004;492:1–11. doi: 10.1016/j.ejphar.2004.03.048. [DOI] [PubMed] [Google Scholar]
- Freedland CS, Sharpe AL, Samson HH, Porrino LJ. Effects of SR141716A on Ethanol and sucrose self-administration. Alcohol Clin Exp Res. 2001;25:277–282. [PubMed] [Google Scholar]
- Gallate JE, McGregor IS. The motivation for beer in rats: effects of ritanserin, naloxone and SR 141716. Psychopharmacology. 1999;142:302–308. doi: 10.1007/s002130050893. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Rodriguez de Fonseca F, Nava F, Loubet-Lescoulié P, Piomelli D. Elevated circulating levels of anandamide after administration of the transport inhibitor, AM404. Eur J Pharmacol. 2000;408:161–168. doi: 10.1016/s0014-2999(00)00786-x. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Massimiliano B, Piomelli D. Mechanisms of endocannabinoid inactivation: biochemistry and pharmacology. J Pharmacol Exp Ther. 2001;298:7–14. [PubMed] [Google Scholar]
- Glaser ST, Kaczocha M, Deutsch DG. Anandamide transport: a critical review. Life Sci. 2005;77:1584–1604. doi: 10.1016/j.lfs.2005.05.007. [DOI] [PubMed] [Google Scholar]
- González S, Cascio MG, Fernández-Ruiz J, Fezza F, Di Marzo V, Ramos JA. Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain Res. 2002;954:73–81. doi: 10.1016/s0006-8993(02)03344-9. [DOI] [PubMed] [Google Scholar]
- González S, Valenti M, de Miguel R, Fezza F, Fernández-Ruiz J, Di Marzo V, Ramos JA. Changes in endocannabinoid contents in reward-related brain regions of alcohol-exposed rats, and their possible relevance to alcohol relapse. Br J Pharmacol. 2004;143:455–464. doi: 10.1038/sj.bjp.0705963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorzalka BB, Hill MN, Hillard CJ. Regulation of endocannabinoid signaling by stress: implications for stress-related affective disorders. Neurosci Biobehav Rev. 2008;32:1152–1160. doi: 10.1016/j.neubiorev.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Hájos N, Kathuria S, Dinh T, Piomelli D, Freund TF. Endocannabinoid transport tightly controls 2-arachidonoyl glycerol actions in the hippocampus: effects of low temperature and the transport inhibitor AM404. Eur J Neurosci. 2004;19:2991–2996. doi: 10.1111/j.0953-816X.2004.03433.x. [DOI] [PubMed] [Google Scholar]
- Hansen HS, Lauritzen L, Strand AM, Vinggaard AM, Frandsen A, Schousboe A. Characterization of glutamate-induced formation of N-acylphosphatidylethanolamine and N-acylethanolamine in cultured neocortical neurons. J Neurochem. 1997;69:753–761. doi: 10.1046/j.1471-4159.1997.69020753.x. [DOI] [PubMed] [Google Scholar]
- Hansen HH, Schmid PC, Bittigau P, Lastres-Becker I, Berrendero F, Manzanares J, Ikonomidou C, Schmid HH, Fernández-Ruiz JJ, Hansen HS. Anandamide, but not 2-arachidonoylglycerol, accumulates during in vivo neurodegeneration. J Neurochem. 2001;78:1415–1427. doi: 10.1046/j.1471-4159.2001.00542.x. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology. 1997;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- Hermann A, Kaczocha M, Deutsch DG. 2-Arachidonoyl-glycerol (2-AG) membrane transport: history and outlook. AAPS J. 2006;8:E409–E412. doi: 10.1007/BF02854913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman PL, Rabe CS, Grant KA, Valverius P, Hudspith M, Tabakoff B. Ethanol and the NMDA receptor. Alcohol. 1990;7:229–231. doi: 10.1016/0741-8329(90)90010-a. [DOI] [PubMed] [Google Scholar]
- Hungund BL, Basavarajappa BS. Are anandamide and cannabinoid receptors involved in ethanol tolerance? A review of the evidence. Alcohol Alcohol. 2000;35:126–133. doi: 10.1093/alcalc/35.2.126. [DOI] [PubMed] [Google Scholar]
- Jacobs EH, Smit AB, de Vries TJ, Schoffelmeer AN. Neuroadaptive effects of active versus passive drug administration in addiction research. Trends Pharmacol Sci. 2003;24:566–573. doi: 10.1016/j.tips.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Koob GF, Schuckit MA, Mason BJ, Ait-Daoud N. Understanding and treating alcohol dependence. Alcohol Clin Exp Res. 2006;30:567–584. doi: 10.1111/j.1530-0277.2005.00066.x. [DOI] [PubMed] [Google Scholar]
- Jung KM, Mangieri R, Stapleton C, Kim J, Fegley D, Wallace M, Mackie K, Piomelli D. Stimulation of endocannabinoid formation in brain slice cultures through activation of group I metabotropic glutamate receptors. Mol Pharmacol. 2005;68:1196–1202. doi: 10.1124/mol.105.013961. [DOI] [PubMed] [Google Scholar]
- Kapasova Z, Szumlinski KK. Strain differences in alcohol-induced neurochemical plasticity: a role for accumbens glutamate in alcohol intake. Alcohol Clin Exp Res. 2008;32:617–631. doi: 10.1111/j.1530-0277.2008.00620.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, Merlo-Pich E, Weiss F. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res. 1998;22:3–9. [PubMed] [Google Scholar]
- Kozak KR, Rowlinson SW, Marnett LJ. Oxygenation of the endocannabinoid, 2-arachidonoylglycerol, to glyceryl prostaglandins by cyclooxygenase-2. J Biol Chem. 2000;275:33744–33749. doi: 10.1074/jbc.M007088200. [DOI] [PubMed] [Google Scholar]
- Kozak KR, Gupta RA, Moody JS, Ji C, Boeglin WE, DuBois RN, Brash AR, Marnett LJ. 15-Lipoxygenase metabolism of 2-arachidonoylglycerol. Generation of a peroxisome proliferator-activated receptor alpha agonist. J Biol Chem. 2002;277:23278–23286. doi: 10.1074/jbc.M201084200. [DOI] [PubMed] [Google Scholar]
- Läck AK, Diaz MR, Chappell A, DuBots DW, McCool BA. Chronic ethanol and withdrawal differentially modulate pre- and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. J Neurophysiol. 2007;98:3185–3196. doi: 10.1152/jn.00189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca D, Valentini V, Cacciapaglia F, Acquas E, Di Chiara G. Reciprocal effects of response contingent and noncontingent intravenous heroin on in vivo nucleus accumbens shell versus core dopamine in the rat: a repeated sampling microdialysis study. Psychopharmacology. 2007;194:103–116. doi: 10.1007/s00213-007-0815-y. [DOI] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, et al. Selective blockade 0f 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Malinen H, Hyytiä P. Ethanol self-administration is regulated by CB1 receptors in the nucleus accumbens and ventral tegmental area in alcohol-preferring AA rats. Alcohol Clin Exp Res. 2008;32:1976–1983. doi: 10.1111/j.1530-0277.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- Mark GP, Hajnal A, Kinney AE, Keys AS. Self-administration of cocaine increases the release of acetylcholine to a greater extent than response-independent cocaine in the nucleus accumbens of rats. Psychopharmacology. 1999;143:47–53. doi: 10.1007/s002130050918. [DOI] [PubMed] [Google Scholar]
- Mitrirattanakul S, López-Valdés HE, Liang J, Matsuka Y, Mackie K, Faull KF, Spigelman I. Bidirectional alterations of hippocampal cannabinoid 1 receptors and their endogenous ligands in a rat model of alcohol withdrawal and dependence. Alcohol Clin Exp Res. 2007;31:855–867. doi: 10.1111/j.1530-0277.2007.00366.x. [DOI] [PubMed] [Google Scholar]
- Moranta D, Esteban S, García-Sevilla JA. Ethanol desensitizes cannabinoid CB1 receptors modulating monoamine synthesis in the rat brain in vivo. Neurosci Lett. 2006;392:58–61. doi: 10.1016/j.neulet.2005.08.061. [DOI] [PubMed] [Google Scholar]
- Naassila M, Pierrefiche O, Ledent C, Daoust M. Decreased alcohol self-administration and increased alcohol sensitivity and withdrawal in CB1 receptor knockout mice. Neuropharmacology. 2004;46:243–253. doi: 10.1016/j.neuropharm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Nowak KL, Vinod KY, Hungund BL. Pharmacological manipulation of CB1 receptor function alters development of tolerance to alcohol. Alcohol Alcohol. 2006;41:24–32. doi: 10.1093/alcalc/agh217. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Hashimotodani Y, Ano M, Takeda S, Tsubokawa H, Kano M. Endocannabinoid signaling triggered by NMDA receptor-mediated calcium entry into rat hippocampal neurons. J Physiol. 2007;584:407–418. doi: 10.1113/jphysiol.2007.137505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald LM, Wand GS. Opioids and alcoholism. Physiol Behav. 2004;81:339–358. doi: 10.1016/j.physbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Patel S, Carrier EJ, Ho WS, Rademacher DJ, Cunningham S, Reddy DS, Falck JR, Cravatt BF, Hillard CJ. The postmortal accumulation of brain N-arachidonylethanolamine (anandamide) is dependent upon fatty acid amide hydrolase activity. J Lipid Res. 2005;46:342–349. doi: 10.1194/jlr.M400377-JLR200. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- Rademacher DJ, Meier SE, Shi L, Ho WS, Jarrahian A, Hillard CJ. Effects of acute and repeated restraint stress on endocannabinoid content in the amygdala, ventral striatum, and medial prefrontal cortex in mice. Neuropharmacology. 2008;54:108–116. doi: 10.1016/j.neuropharm.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Rivier C. Acute interactions between cytokines and alcohol on ACTH and corticosterone secretion in the rat. Alcohol Clin Exp Res. 1993;17:946–950. doi: 10.1111/j.1530-0277.1993.tb05646.x. [DOI] [PubMed] [Google Scholar]
- Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis. J Neurosci. 2004;24:1594–1603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Roberts AJ, Bilbao A, Koob GF, Navarro M. Cannabinoid receptor antagonist SR 141716A decreases operant ethanol self-administration in rats exposed to ethanol-vapor chambers. Acta Pharmacol Sinica. 1999;20:1109–1114. [PubMed] [Google Scholar]
- Rubio M, de Miguel R, Fernández-Ruiz J, Gutiérrez-López D, Carai MAM, Ramos JA. Effects of a short-term exposure to alcohol in rats on FAAH enzyme and CB1 receptor in different brain areas. Drug Alcohol Depend. 2009;99:354–358. doi: 10.1016/j.drugalcdep.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson NO, Leo-nova J, Elebring T, Nilsson K, Drmota T, Greasley PJ. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang N, Zhang J, Chen C. PGE2 glycerol ester, a COX-2 oxidative metabolite of 2-arachidonoylglycerol, modulates inhibitory synaptic transmission in mouse hippocampal neurons. J Physiol. 2006;572:735–745. doi: 10.1113/jphysiol.2006.105569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang N, Zhang J, Chen C. COX-2 oxidative metabolite of endocannabinoid 2-AG enhances excitatory glutamatergic synaptic transmission and induces neurotoxicity. J Neurochem. 2007;102:1966–1977. doi: 10.1111/j.1471-4159.2007.04668.x. [DOI] [PubMed] [Google Scholar]
- Smothers CT, Mrotek JJ, Lovinger DM. Chronic ethanol exposure leads to a selective enhancement of N-methyl-D-aspartate receptor function in cultured hippocampal neurons. J Pharmacol Exp Ther. 1997;283:1214–1222. [PubMed] [Google Scholar]
- Stella N, Piomelli D. Receptor-dependent formation of endogenous cannabinoids in cortical neurons. Eur J Pharmacol. 2001;425:189–196. doi: 10.1016/s0014-2999(01)01182-7. [DOI] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kishimoto S, Oka S, Gokoh M. Biochemistry, pharmacology and physiology of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Prog Lipid Res. 2006;45:405–446. doi: 10.1016/j.plipres.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Diab ME, Friedman R, Henze LM, Lominac KD, Bowers MS. Accumbens neurochemical adaptations produced by binge-like alcohol consumption. Psychopharmacology. 2007;190:415–431. doi: 10.1007/s00213-006-0641-7. [DOI] [PubMed] [Google Scholar]
- Tsai G. Glutamatergic transmission in alcoholism. J Biomed Sci. 1998;5:309–320. doi: 10.1007/BF02253441. [DOI] [PubMed] [Google Scholar]
- Vallés SL, Blanco AM, Pasqual M, Guerri C. Chronic ethanol treatment enhances inflammatory mediators and cell death in the brain and astrocytes. Brain Pathol. 2004;14:365–371. doi: 10.1111/j.1750-3639.2004.tb00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinod KY, Yalamanchili R, Xie S, Cooper TB, Hungund BL. Effect of chronic ethanol exposure and its withdrawal on the endocannabinoid system. Neurochem Int. 2006;49:619–625. doi: 10.1016/j.neuint.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Sangunio E, Yalamanchili R, Manzanares J, Hungund BL. Manipulation of fatty acid amide hydrolase functional activity alters sensitivity and dependence to ethanol. J Neurochem. 2008;104:233–243. doi: 10.1111/j.1471-4159.2007.04956.x. [DOI] [PubMed] [Google Scholar]
- Walker JM, Huang SM, Strangman NM, Tsou K, Sanudo-Pena MC. Pain modulation by release of the endogenous cannabinoid anandamide. Proc Natl Acad Sci USA. 1999;96:12198–12203. doi: 10.1073/pnas.96.21.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnault V, Houchi H, Barbier E, Pierrefiche O, Vilpoux C, Ledent C, Daoust M, Naassila M. The lack of CB1 receptors prevents neuroadaptations of both NMDA and GABA(A) receptors after chronic ethanol exposure. J Neurochem. 2007;102:741–752. doi: 10.1111/j.1471-4159.2007.04577.x. [DOI] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GAB-Aergic transmission: the view from the slice. Pharmacol Ther. 2006;111:533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- Woodward JJ. Ethanol and NMDA receptor signaling. Crit Rev Neurobiol. 2000;14:69–89. doi: 10.1080/08913810008443548. [DOI] [PubMed] [Google Scholar]
- You ZB, Wang B, Zitzman D, Azari S, Wise RA. A role for conditioned ventral tegmental glutamate release in cocaine seeking. J Neurosci. 2007;27:10546–10555. doi: 10.1523/JNEUROSCI.2967-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]