Abstract
Purpose
To evaluate the ability of longitudinal frequency doubling technology (FDT) to predict development of glaucomatous visual field loss on standard automated perimetry (SAP) in glaucoma suspects.
Design
Prospective observational cohort study.
Participants
The study included 587 eyes of 367 patients with suspected glaucoma at baseline selected from the Diagnostic Innovations in Glaucoma Study (DIGS) and the African Descent and Glaucoma Evaluation Study (ADAGES). These eyes had an average of 6.7±1.9 FDT tests during a mean follow-up time of 73.1±28.0 months.
Methods
Glaucoma suspects had either intraocular pressure >21mmHg or an optic disc appearance suspicious of glaucoma. All patients had either normal or non-repeatable abnormal SAP at baseline. Humphrey Matrix FDT testing was performed within 6 months of SAP testing. The study endpoint was the development of 3 consecutive abnormal SAP tests. Joint longitudinal survival models were used to evaluate the ability of rates of FDT pattern standard deviation (PSD) change to predict development of visual field loss on SAP, adjusting for confounding variables (baseline age, mean intraocular pressure, corneal thickness, and follow-up measurements of SAP PSD).
Main Outcome Measures
The R2 index was used to evaluate and compare the predictive abilities of the model containing longitudinal FDT PSD data with the model containing only baseline data.
Results
Sixty-three of 587 (11%) eyes developed SAP visual field loss during follow-up. The mean rate of FDT PSD change in eyes that developed SAP visual field loss was 0.07dB/year versus 0.02dB/year in those that did not (P<0.001). Baseline FDT PSD and slopes of FDT PSD change were significantly predictive of progression, with hazard ratios of 1.11 per 0.1dB higher (95% confidence interval [CI]: 1.04 - 1.18; P=0.002) and 4.40 per 0.1dB/year faster (95%CI: 1.08 - 17.96; P=0.04), respectively. The longitudinal model performed significantly better than the baseline model with R2 of 82% (95%CI: 74% - 89%) vs. 11% (95%CI: 2% - 24%), respectively.
Conclusion
Rates of FDT PSD change were highly predictive of development of SAP visual field loss in glaucoma suspects. This finding suggests that longitudinal FDT evaluation may be useful for risk stratification of patients suspected of having glaucoma.
Glaucoma is an optic neuropathy characterized by progressive loss of retinal ganglion cells, which ultimately can lead to functional loss, visual disability and blindness.1 Standard automated perimetry (SAP) using a white stimulus on a white background is the most commonly used method for detection of glaucomatous functional damage. However, histological and clinical studies have shown that visual field defects on SAP often are detectable only after a substantial number of ganglion cells have been lost.2, 3
Frequency doubling technology (FDT) perimetry has been proposed as a test for the early detection of glaucomatous functional damage.4, 5 Testing involves presentation of a frequency-doubling stimulus and the contrast sensitivity of the stimulus is adjusted to determine the limit of detection. Several independent studies have shown that FDT has high sensitivity and specificity for discriminating glaucomatous and healthy subjects.4, 6-12 The 24-2 Matrix FDT (Carl-Zeiss Meditec, Inc.) has been shown to be significantly better than SAP at distinguishing eyes with early glaucoma from healthy, and also offers shorter test duration and less variability in areas of low sensitivity than SAP.8, 10, 13, 14
Baseline functional abnormalities detected by FDT perimetry have been shown to be predictive of future onset and location of SAP visual field loss among glaucoma suspect patients.15-17 However, the predictive ability of information obtained only from the baseline visit is relatively weak.16 Predictive models that take into account longitudinal information as it becomes available during follow up could potentially perform better than those using only baseline information. To the best of our knowledge, no study has yet evaluated the ability of longitudinal FDT data in predicting the development of visual field loss in glaucoma.
The purpose of the current study was to evaluate the ability of longitudinal FDT measurements to predict the development of glaucomatous visual field loss on SAP in a cohort of glaucoma suspects.
METHODS
This was an observational cohort study involving participants from two prospective longitudinal studies designed to evaluate optic nerve structure and visual function in glaucoma: The African Descent and Glaucoma Evaluation Study (ADAGES) and the Diagnostic Innovations in Glaucoma Study (DIGS). The 3-site ADAGES collaboration included the Hamilton Glaucoma Center at the Department of Ophthalmology, University of California, San Diego (UCSD) (data coordinating center); the New York Eye and Ear Infirmary; and the Department of Ophthalmology, University of Alabama, Birmingham. Although the DIGS includes only patients recruited at the UCSD, the protocols of the two studies are identical. Methodological details have been described previously.18
All patients from the DIGS and ADAGES who met the inclusion criteria described below were enrolled in the present study. Informed consent was obtained from all participants. This prospectively designed study received institutional review board approval at each of the involved sites. The methodology adhered to the tenets of the Declaration of Helsinki and to the Health Insurance Portability and Accountability Act.
At each visit during follow-up, subjects underwent a comprehensive ophthalmologic examination, including review of medical history, best-corrected visual acuity, slit-lamp biomicroscopy, intraocular pressure (IOP) measurement, gonioscopy, dilated funduscopic examination and stereoscopic optic disc photography and SAP testing. Central corneal thickness (CCT) was calculated as the average of three measurements obtained during the same visit using an ultrasound pachymeter (Pachette GDH 500; DGH Technology, Inc, Philadelphia, PA, USA). For inclusion, subjects had to have open angles on gonioscopy. Subjects were excluded if they had a best-corrected visual acuity of less than 20/40, spherical refraction outside ± 5.0 diopters, cylinder correction outside 3.0 diopters, or a combination thereof; or any other ocular or systemic disease that could affect the optic nerve or the visual field.
This study included eyes suspected of having glaucoma at the baseline visit. The diagnosis of suspect glaucoma was based on the presence of suspicious appearance of the optic disc (neuroretinal rim thinning, excavation or suspicious RNFL defects) or elevated IOP (>21mmHg). As the study was designed to evaluate the predictive ability of FDT in glaucoma suspects, eyes that had repeatable (at least two consecutive) abnormal SAP tests at baseline were excluded. During follow-up, each patient was treated at the discretion of the attending ophthalmologist.
Visual Field Testing
SAP was obtained using the Humphrey Field Analyzer II (Carl Zeiss Meditec, Dublin, CA, USA) and the 24-2 Swedish Interactive Threshold Algorithm strategy. FDT was performed using the Humphrey Matrix (Carl Zeiss Meditec, Dublin, CA, USA) and the 24-2 threshold strategy. Only reliable SAP and FDT tests were included. A reliable visual field test was defined as ≤ 33% fixation losses and false negatives, and < 15% false positives. All visual fields were evaluated by the UCSD Visual Field Assessment Center (VisFACT).19 Each visual field test was performed twice at baseline within a 3-month period. The examination protocol was repeated annually. When the FDT date did not match the SAP date, the tests were included in the study analysis only if they were performed within six months apart. Visual fields were reviewed for the following artifacts: lid and rim artifacts, fatigue effects, inappropriate fixation, evidence that the visual field results were due to a disease other than glaucoma (such as homonymous hemianopia), and inattention. If an artifact was identified a repeat visual field test was requested.
SAP tests were defined as abnormal if they had a pattern standard deviation (PSD) with P < 0.05 and/or a glaucoma hemifield test (GHT) outside normal limits. Each patient was required to have a minimum of 12 months of follow-up.
Follow-up and Definition of Study Endpoints
The study endpoint was defined as the development of repeatable abnormal SAP defects during follow-up. A repeatable SAP defect was defined by the presence of 3 consecutive abnormal SAP tests during follow-up. Eyes that developed an endpoint during follow-up, i.e., progressed to a SAP defect, were designated “progressors” whereas eyes that did not develop the study endpoint were designated “nonprogressors”. As the purpose of the study was to evaluate the prediction of early SAP visual field loss, the time of progression was defined as the date of the first abnormal SAP test. For progressors, follow-up time was defined as the time between the FDT baseline visit and the date of the first abnormal SAP result (the study endpoint). For nonprogressors, follow-up time was defined as the time between the FDT baseline visit and date of last available follow-up.
In order to evaluate whether FDT measurements were predictive of the study endpoints, only FDT tests acquired before the event date were analyzed in the study. Eyes that did not develop the study endpoint were considered censored at the last follow-up visit. All FDT tests up to the last available follow-up date were analyzed for these eyes.
Statistical Analysis
The primary purpose of the study was to evaluate the ability of longitudinal FDT measurements to predict the development of glaucomatous SAP visual field loss. To assess the relationship between longitudinal FDT measurements and the development of glaucomatous visual field loss on SAP we used a joint model incorporating longitudinal and survival data. The parameter PSD was selected to represent the FDT longitudinal measurements because it has been identified in previous studies as having the best overall performance for early glaucoma diagnosis.12, 20 The joint models of longitudinal and survival data are ideally suited to study the association between changes in a longitudinal marker and the risk for an event, and have been described in detail elsewhere.21 We have previously reported on the use of these models to study the association between glaucoma biomarkers and disease progression.22 In brief, they are composed of a longitudinal submodel and a survival submodel, which are tied together by sharing random effects. The longitudinal submodel was composed of a linear mixed model with the following formulation:
The model specifically accounts for measurement error of the marker by postulating that the observed level of the outcome yi(t), corresponding to the FDT measurements, equals the unobserved true value mi(t) plus a random error term, εi(t). The mixed model assumes random slopes and random intercepts, allowing different rates of change and intercept values for each eye.
To quantify the strength of the association between the longitudinal marker and the risk for the event (development of SAP visual field loss), a survival submodel was used with the form:
In the survival submodel, hi(t) determines the hazard function at time t, h0 denotes the baseline hazard function specified by a Weibull distribution, wi is a vector of baseline covariates with corresponding vector of coefficients (γ1), vi is a vector of time-dependent covariates with corresponding vector of coefficients (γ2). This model was estimated jointly with the longitudinal submodel and allowed an evaluation of the relationship between the true marker values mi(t) and the risk for the event. We were mainly interested whether the slopes of change in the marker (i.e., FDT PSD) were associated with risk of progression. Therefore, mi’ measured the first derivative (slope) of the marker profile and the coefficient α2 measured how strongly associated was the value of the slope of the true longitudinal marker at time t with the risk for an event at the same time point, adjusting for the intercept value and values of other covariates. The interpretation of α2 is straightforward as in regular survival models, with exp(α2) corresponding to the hazard ratio (HR) for a one unit change in the slope of the marker.
The survival model including the longitudinal FDT measurements information (intercepts and slopes) was adjusted for the baseline covariates age, CCT, mean IOP, and for the time-dependent covariate SAP PSD. These variables have been reported to be significantly associated with the risk of development of glaucomatous visual field loss among patients with ocular hypertension or suspected glaucoma.23-25 Mean IOP was calculated as the arithmetic mean of all available IOP measurements per eye.
To assess and compare the importance of variables in determining the outcome, we used an R2 index proposed by Royston.26 The modified R2 index is equivalent to the coefficient of determination of a linear model and measures the amount of variation in the outcome (survival time) explained by the predictor or, in other words, the strength of the relationship between the predictor and the outcome in a survival model. The modified R2 index has been proposed as the best way to assess prognostic information of survival models.27 Confidence intervals (CIs) for the modified R2 indices were obtained by bootstrapping, with 1000 replications.
The mean rates of FDT PSD change of progressors and nonprogressors were compared using generalized estimating equations with robust standard errors to adjust for potential correlations between both eyes of the same individual.
All statistical analyses were performed with commercially available software (STATA, version 12; StataCorp LP). The α level (type I error) was set at 0.05.
RESULTS
The study included 587 eyes of 367 patients classified as glaucoma suspects at the baseline visit. These eyes had an average (median, interquartile range) of 5.8 (6.0, 4.0 to 7.0) FDT tests and 6.0 (6.0, 5.0 to 7.0) SAP tests during a mean (median, interquartile range) follow-up time of 73.1 (83.8, 48.3 to 97.2) months. Sixty-three (11%) eyes progressed and developed repeatable SAP defects during follow-up. Table 1 shows baseline demographic and clinical characteristics of the study patients. For progressors, the mean (median, interquartile range) time until the development of a repeatable SAP defect was 45.5 (37.9, 23.9 to 63.2) months. For nonprogressors, the mean (median, interquartile range) follow-up time was 76.4 (85.6, 49.7 to 97.8) months.
Table 1.
Baseline Demographic and Clinical Characteristics of Study Patients
| Variables | Progressors (n = 63) |
Nonprogressors (n = 524) |
|---|---|---|
| Age at baseline (years) | 61.6 ± 10.0 | 56.1 ±11.4 |
| Gender (% Female) | 74.6 | 65.4 |
| Race | ||
| Caucasian, % | 50.8 | 54.2 |
| African-American, % | 49.2 | 40.8 |
| Spherical equivalent (D) | 0.13 ± 1.8 | −0.41 ± 1.8 |
| IOP (mmHg) | 17.3 ± 4.7 | 18.1 ± 4.8 |
| CCT (μm) | 538.6 ± 38.8 | 546.6 ± 38.7 |
| SAP MD (dB) | −0.61 ± 1.0 | −0.01 ± 1.11 |
| SAP PSD (dB) | 1.83 ± 0.48 | 1.56 ± 0.31 |
| FDT MD (dB) | −2.17 ± 2.96 | −1.19 ± 2.80 |
| FDT PSD (dB) | 3.18 ± 0.73 | 2.85 ± 0.66 |
Abbreviations: D, Diopters; IOP, Intraocular Pressure; CCT, Central Corneal Thickness; SAP, Standard Automated Perimetry; MD, Mean Deviation; PSD, Pattern Standard Deviation; FDT, Frequency Doubling Technology.
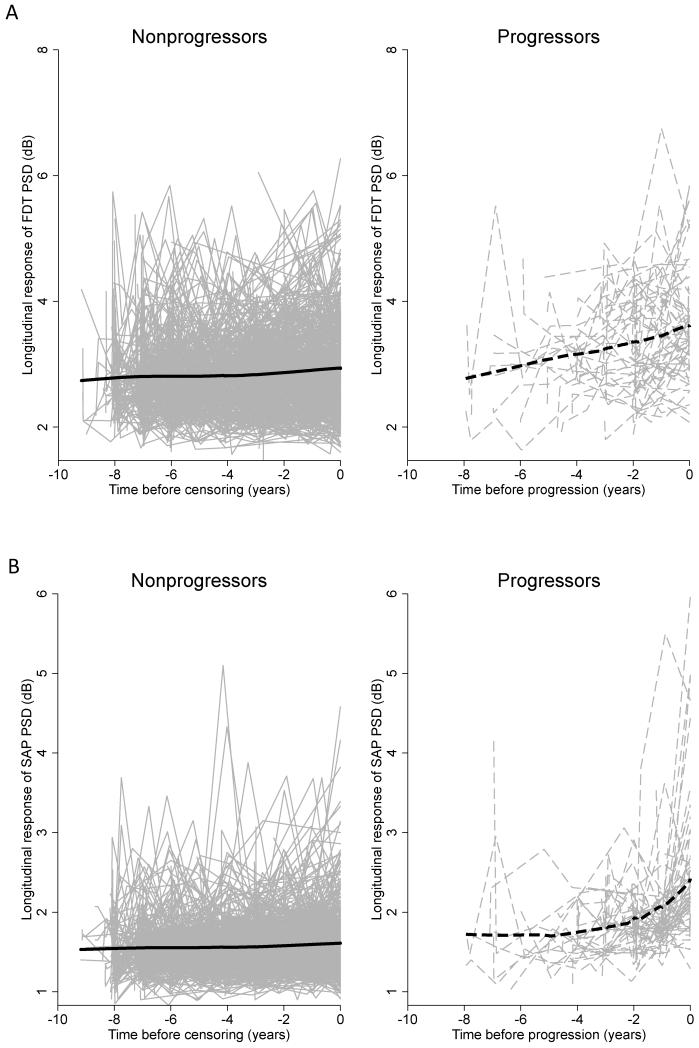
Figure 1A shows the longitudinal trajectories of raw measurements of FDT PSD in nonprogressors and progressors while Figure 1B shows the longitudinal trajectories of raw measurements of SAP PSD in nonprogressors and progressors. Comparison of lowess (locally weighted scatterplot smoothing) curves overlaid to the longitudinal trajectories of these indices for the progressor group suggested that FDT PSD measurements started to increase earlier than SAP PSD measurements.
Figure 1.
A. Longitudinal trajectory of frequency doubling technology (FDT) pattern standard deviation (PSD) raw measurements in progressors (right) and nonprogressors (left). B. Longitudinal trajectory of SAP PSD raw measurements in progressors (right) and nonprogressors (left). SAP = standard automated perimetry
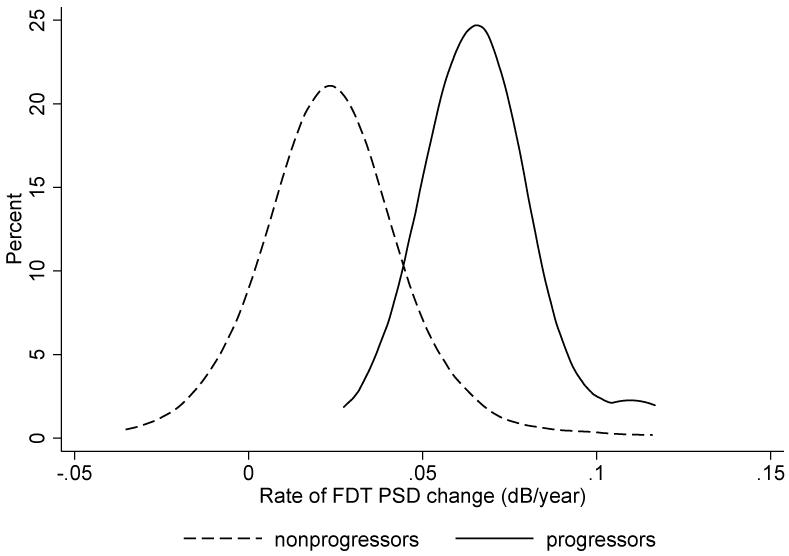
The average rate of FDT PSD change in all eyes was 0.03 dB/year. The mean (± standard deviation) rate of FDT PSD change for progressors was significantly faster than for nonprogressors (0.07 ± 0.02 vs. 0.02 ± 0.02 dB/year, respectively; P < 0.001). Figure 2 illustrates the distribution of slopes of change in the 2 groups.
Figure 2.
Distribution of rates of frequency doubling technology (FDT) pattern standard deviation (PSD) change in eyes that developed standard automated perimetry (SAP) visual field loss versus eyes that did not develop SAP visual field loss. Eyes that developed SAP visual field loss had considerably faster rates of FDT PSD change when compared to eyes that did not developed SAP visual field loss.
Table 2 shows the multivariable HRs with 95% CIs for each putative predictive factor obtained with the joint model of longitudinal FDT PSD evaluation and time until development of a repeatable SAP defect. In the multivariable survival model, slopes of FDT PSD change were significantly associated with risk of developing a SAP defect. Each 0.1 dB/year faster rate of FDT PSD change was associated with four times higher risk of developing a SAP defect (adjusted HR: 4.40; 95% CI = 1.08 – 17.96; P = 0.04). The baseline FDT PSD measurement (intercept) was also a significant predictive factor for developing a SAP defect in the multivariable model. Each 0.1 dB larger FDT PSD at baseline was associated with 11% higher risk of developing a glaucomatous SAP defect (adjusted HR: 1.11; 95% CI = 1.04 – 1.18; P = 0.002). Other significant predictive factors for developing a SAP defect in the multivariable model were SAP PSD, mean IOP and CCT.
Table 2.
Results of the joint longitudinal survival model investigating the effect of longitudinal changes in frequency doubling technology pattern standard deviation in predicting the risk of development of visual field loss, while adjusting for confounding factors
| Longitudinal submodel | |||
|---|---|---|---|
| Parameter | Coefficient | 95% confidence interval |
P |
| Constant | 2.85 | 2.81 – 2.90 | <0.001 |
| Time | 0.029 | 0.018 – 0.039 | <0.001 |
| Survival submodel | ||||
|---|---|---|---|---|
| Parameter | Coefficient | 95% confidence interval |
P | Hazard Ratio |
| Slope of FDT PSD, per 0.1dB/year higher | 1.48 | 0.08 – 2.89 | 0.039 | 4.40 |
| Intercept (Baseline FDT PSD), 0.1dB higher | 0.10 | 0.04 – 0.17 | 0.002 | 1.11 |
| Age, per decade older | 0.22 | −0.06 – 0.49 | 0.119 | 1.25 |
| Mean IOP, per 1mmHg higher | 0.08 | 0.008 – 0.15 | 0.029 | 1.08 |
| CCT, per 40μm thinner | 0.40 | 0.10 – 0.70 | 0.009 | 1.50 |
| SAP PSD * , per 0.1dB higher | 0.11 | 0.09 – 0.14 | <0.001 | 1.12 |
Abbreviations: FDT, Frequency Doubling Technology; PSD, Pattern Standard Deviation; SAP, Standard Automated Perimetry; IOP, Intraocular Pressure; CCT, Central Corneal Thickness.
Time-dependent variable
The R2 index was used to evaluate and compare the predictive abilities of the model containing only longitudinal FDT PSD data with the model containing only FDT baseline data. The longitudinal model performed significantly better than the baseline model, presenting R2 of 82% (95% CI = 74% - 89%), vs. 11% (95% CI = 2% - 24%), respectively.
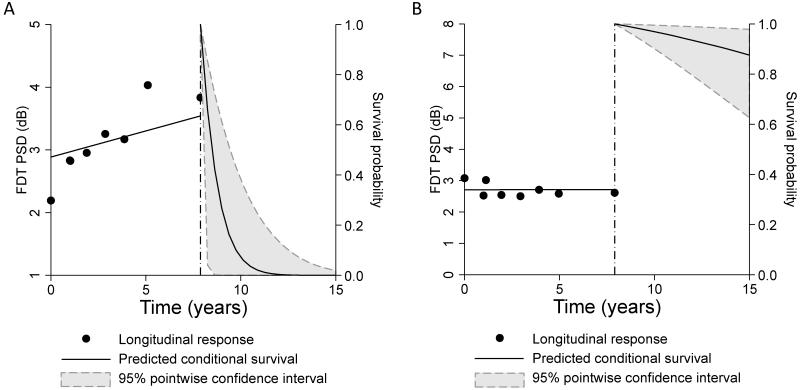
Results of the joint model allowed survival predictions for individual eyes to be obtained. The survival probability indicated the probability of retaining a normal SAP visual field during follow-up, i.e., not developing a glaucomatous defect. Figure 3 shows 2 cases of suspected glaucoma with different outcomes predicted by the longitudinal evaluation of FDT PSD. Figure 3A shows a case of an eye with increasing values of FDT PSD over time. Predicted survival probabilities for this eye were very low, indicating a high chance of developing visual field loss. This eye in fact showed glaucoma progression during follow-up with development of SAP visual field defect. Figure 3B shows an eye with stable FDT PSD and high predicted survival probabilities. This eye did not show progression based on SAP during the follow-up time of the study.
Figure 3.
Predicted survival probabilities for two eyes, one that showed a relatively fast rate of frequency doubling technology (FDT) pattern standard deviation (PSD) change during follow-up (A) and another that showed stable measurements over time (B). A comparison of the predicted survival probabilities shows that the eye with fast progression had much lower predicted probabilities of survival, i.e., retaining a normal standard automated perimetry (SAP) visual field. The former in fact showed development of visual field loss during follow-up whereas the eye with stable FDT PSD measurements did not develop any SAP field defect.
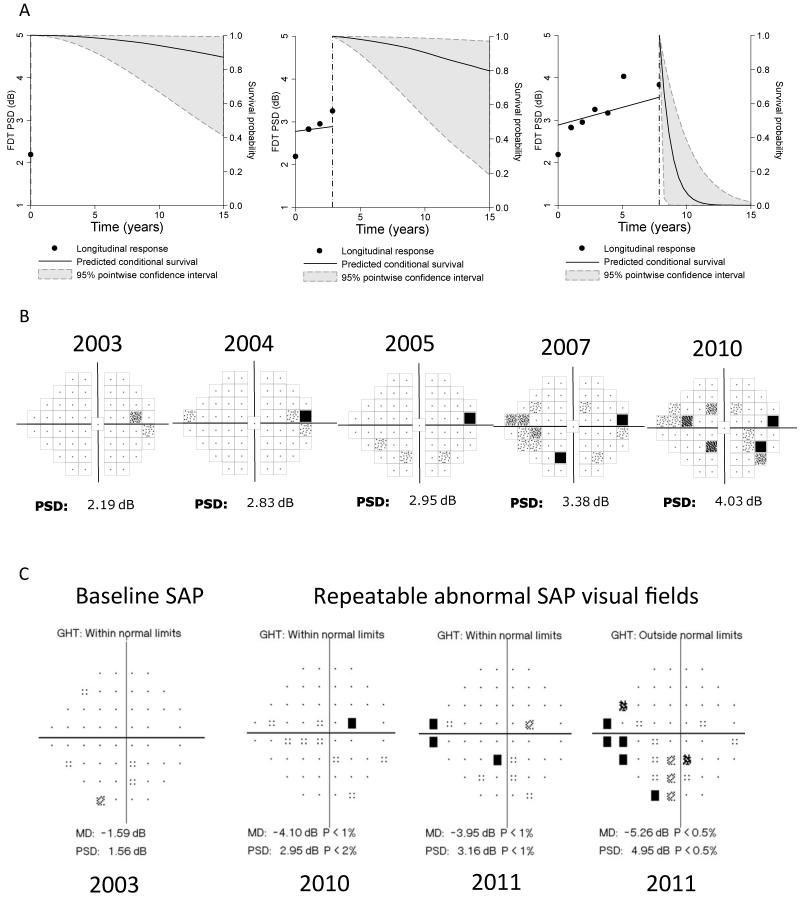
Figure 4 shows how survival probabilities can be continuously updated during follow-up as more information becomes available. The time course of FDT PSD changes, as seen in the pattern deviation plots for the same eye, is also shown. The predicted survival probabilities were relatively high when only baseline measurements were considered. As more information became available and a clear trend of FDT PSD increase was observed, the model estimated much lower probabilities of survival. The reported eye later developed a SAP visual field defect.
Figure 4.
Example of how survival probabilities can be updated as more information on predictive factors becomes available during follow-up. A. Left panel shows survival probabilities after considering only the baseline data. The model estimated that the probability of retaining a normal standard automated perimetry (SAP) visual field over time was relatively high. As more information became available (middle and right panels), the survival probabilities were updated. The estimated survival probabilities became much lower as the result of progressive increase of frequency doubling technology (FDT) pattern standard deviation (PSD) over time. B. Corresponding FDT pattern deviation plot showing progressive deterioration. C. SAP visual fields for the same eye showing development of a repeatable defect at the end of follow-up.
The predictive abilities of baseline and longitudinal models including FDT mean deviation (MD) were also tested. However they did not show statistically significant predictive abilities after adjustment for age, mean IOP, CCT and time-dependent SAP PSD (P > 0.05 for both models).
DISCUSSION
This study demonstrated that longitudinal evaluation of patients with suspected glaucoma using FDT perimetry was useful for predicting future development of SAP visual field loss. Subjects with faster slopes of change in FDT PSD had a greater risk of developing functional glaucomatous damage on SAP than those with relatively slower changes. In addition, models including longitudinal FDT PSD measurements were better predictors of future SAP defects than models including only baseline information. These findings may have significant implications for the use of FDT perimetry in clinical practice and for evaluation of glaucoma progression.
As SAP is the current standard for glaucoma diagnosis and is widely available in clinical practice, this study was designed to assess whether the longitudinal evaluation of FDT perimetry could provide any additional benefit for early diagnosis by predicting future development of glaucomatous defects on SAP. To account for the likely scenario of FDT being used to supplement rather than replace SAP, the FDT predictive models were adjusted with SAP measurements. As shown by the multivariable analyses, FDT perimetry still provided additional independent information. The results of this study therefore suggest that there may be value to including FDT in the assessment of glaucoma suspects.
Eyes that developed SAP visual field defects during follow-up had significantly faster rates of change in the FDT PSD parameter compared to eyes that did not develop visual field defects. The mean rate of FDT PSD change for eyes that progressed was 0.07 dB/year, which was over 3 times faster than for eyes that did not show visual field loss by SAP (0.02 dB/year). This finding suggests that FDT PSD measurements could be used for monitoring glaucoma suspect patients with normal SAP and is in agreement with previous studies that demonstrated FDT abnormalities preceding SAP abnormalities.28-30 Bayes and Erb28 reported that C-20 FDT progression preceded SAP progression in 74% of open angle glaucoma patients. Nakagawa et al29 showed progressive C-20 FDT abnormality occurring on normal SAP hemifield of normal tension glaucoma patients. However they did not report whether subsequent SAP loss developed in patients with progressive FDT defect. Haymes et al30 demonstrated C-20 FDT progressive abnormalities in open angle glaucoma patients, but also did not report rates of change, nor whether the FDT abnormalities preceded SAP loss. Differences in study features such as design, selection criteria and methods of analysis hindered further comparison of these studies with ours. The present study was the first to report the relation between the rates of FDT measurements change and the risk for development of visual field defect on SAP in a cohort of glaucoma suspects.
The use of a joint longitudinal survival model allowed us to quantify the ability of rates of change in FDT PSD to predict the risk of developing a SAP defect, while also taking into account the censored aspect of the data and adjusting for the effect of confounding variables. Each 0.1 dB/year faster rate of FDT PSD change corresponded to 4.4 times higher risk of progression over time in a multivariable model adjusting for age, CCT, mean IOP, and longitudinal SAP PSD measurements. The predictive ability of the longitudinal model was significantly better than the model including only baseline information. The longitudinal model including baseline and rates of change in FDT PSD measurements had an R2 of 82%, i.e., this model was able to explain 82% of the variation in the outcome defined by SAP visual field loss. The model including only baseline FDT PSD data performed worse than the longitudinal model, with an R2 of only 11%. This result suggests that the longitudinal evaluation of FDT PSD measurements seem to provide additional information that can help to identify those glaucoma suspects more likely to develop SAP visual field defects. This result was expected since the longitudinal analysis evaluates all test results available during the follow-up period of interest. The longitudinal approach can provide estimates of rates of visual field progression over time allowing the identification of patients with higher risk of developing functional impairment as a result of the disease.
Among progressors, FDT PSD measurements started to increase earlier than the corresponding SAP PSD measurements, whereas no differences were seen for nonprogressors (Figure 1). Importantly, FDT measurements were still predictive of progression despite inclusion of longitudinal SAP PSD measurements in the same model. This result supports the concept that the FDT perimetry may be valuable for the detection of early glaucomatous damage.7, 16, 20, 31-34 However, the biological basis for this finding is still unclear. The frequency doubling illusion phenomenon was initially thought to be mediated by a subset of magnocellular retinal ganglion cells,6 which have been proposed to be relatively more vulnerable to early glaucomatous damage.35 However, selective loss of magnocellular over the parvocellular function in glaucoma has not been consistently demonstrated in previous studies.36-38 In fact, recent research has suggested that the origins of the response to the frequency doubling illusion to be most likely cortical, rather than ocular.39, 40 Whatever the mechanism underlying detection of the FDT stimulus might be, our results support the importance of this test as an additional tool for detection of early functional losses in glaucoma. A possible explanation for finding that additive ability of FDT to predict the future development of SAP defects is that the test stimuli of FDT perimetry and SAP have different spatial and temporal characteristics, and cover different receptive fields. Moreover, FDT and SAP use different threshold strategies and different decibel scales.41 Differences in FDT and SAP test results are therefore to be expected and have been found in previous studies. For example, although the global indices of SAP and second generation FDT perimetry are highly correlated, FDT has been found to produce narrower test-retest intervals at test locations with lower sensitivity, which could provide advantages for the monitoring of patients with glaucoma.13, 41
Although the present study found the longitudinal evaluation of FDT to be a useful predictor of the development of a SAP defect, it is possible that any worsening perimetric test may be able to predict the development of a defect in another perimetric device. We therefore also investigated whether longitudinal measurements of SAP PSD would predict glaucoma progression based on FDT perimetry, defined as the development of a repeatable FDT defect (PSD < 5% and/or GHT outside normal limits), in a cohort of patients without repeatable abnormal FDT at baseline. The analysis revealed that although the baseline values of SAP PSD were significant predictors of FDT progression (HR: 1.10 per 0.1 dB higher; 95% CI = 1.03 – 1.18; P = 0.004), the rates of SAP PSD change were not significant predictors of FDT progression during follow-up (HR per 0.1dB/year higher: 0.93; 95% CI = 0.46 – 1.89; P = 0.85).
The joint longitudinal survival model presented in our study also allowed estimation of individual survival probabilities over time. Using this model, the risk of development of SAP visual field loss can be updated as information on predictive factors is made continuously available over time. Such approach offers significant advantages over currently available predictive models or risk calculators designed to estimate risk of glaucoma development.42, 43 Currently available risk calculators use only baseline information on predictive factors which has limited value in predicting outcomes. As Figure 4 illustrates, the probabilities of progression can be continuously updated as more information becomes available, resulting in more effective use of clinical information. Similarly, the two eyes shown on Figure 3 had similar baseline FDT PSD values, but their risks of progression were very different when longitudinal information was incorporated into the model.
In contrast to models including FDT PSD, those including baseline and longitudinal FDT MD were not significantly predictive of the development of a SAP defect. Baseline FDT MD has been reported to be a poor predictor of progression in a previous study of glaucoma suspects, using an older version of FDT perimetry.16 The Ocular Hypertension Treatment Study also found that PSD, not MD, was a predictor of glaucoma development.23, 42 These studies suggest that PSD is an important index for detecting early disease. MD, as a center-weighted average of decibel deviation, is useful for staging visual loss; however, PSD better reflects the presence of focal visual field defects which are common in early stages of disease.44, 45 MD is also less specific for glaucomatous damage and affected more by media opacities; common in the glaucomatous population. A disadvantage of PSD is that it decreases in moderate to advanced disease as areas of localized loss progress to become diffuse. For this reason the optimal use of FDT PSD, as found in this study, is likely to be in detecting early functional damage.
This study has limitations. PSD is a global visual field index that numerically describes the uniformity of the visual field and reflects the contour of the island of vision. Although its increase is generally related to the development or worsening of a true visual field defect, PSD could also increase due to artifacts; for example, if the corrective lens is poorly position, or if there is eyelid ptosis, refractive scotoma or patient inattention. In order to avoid such confounding effects, visual fields were reviewed for the presence of artifacts and abnormalities due to diseases other than glaucoma and the repetition of such tests was requested when necessary. Another limitation of the study is that FDT tests were only repeated annually. It is possible that more frequent tests could potentially improve the predictive ability of FDT. Finally, we evaluated the ability of FDT in predicting SAP visual field defects. Although SAP defects have been shown to be associated with quality of life measures in glaucoma patients,46 future studies should evaluate whether FDT tests are predictive of vision-related quality of life outcomes in glaucoma.
It is important to note that clinicians frequently face constraints about the number of tests that can be performed on an individual patient in clinical practice. Therefore, before recommending including FDT tests in clinical practice, it will be important to assess their benefit in relation to the actual number of other tests that can be performed under realistic scenarios. Future studies should evaluate issues of cost-benefit analysis of including multiple structural and functional tests for glaucoma management.
In conclusion, the results of this study demonstrate that baseline and longitudinal evaluation of FDT perimetry are able to predict development of glaucomatous visual field loss on SAP in patients with suspected glaucoma. Furthermore, longitudinal evaluation of FDT PSD rates of change was better than baseline data at predicting which glaucoma suspect eyes develop functional glaucoma progression during follow-up. These findings suggest that the addition of longitudinal evaluation of FDT may be advantageous as a clinical tool for risk stratification of patients suspected of having glaucoma.
Acknowledgments
Supported in part by National Institutes of Health/National Eye Institute grants EY021818 (F.A.M.), EY11008 (L.M.Z.), EY14267 (L.M.Z.), EY019869 (L.M.Z.); Brazilian National Research Council-CNPq grant 200178/2012-1 (D.M.F); an unrestricted grant from Research to Prevent Blindness (New York, NY); grants for participants’ glaucoma medications from Alcon, Allergan, Pfizer, Merck and Santen.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure(s):
The author(s) have made the following disclosure(s):
Daniel Meira-Freitas – None
Andrew J. Tatham - None
Renato Lisboa – None
Tung-Mei Kuang – None
Linda M. Zangwill – financial support from Carl-Zeiss Meditec, and Heidelberg Engineering
Robert N. Weinreb – financial support from Alcon, Allergan, Merck, Bausch & Lomb, Carl-Zeiss Meditec, Optovue, Novartis, Nidek, Topcon, and Aeries
Jeffrey M. Liebmann – financial support from Carl-Zeiss Meditec, and Heidelberg Engineering Christopher A. Girkin – financial support from Carl-Zeiss Meditec
Felipe A. Medeiros – Financial support from Alcon, Allergan, Merck, Bausch & Lomb, Nidek, Carl-Zeiss Meditec, Heidelberg Engineering
REFERENCES
- 1.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–20. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 2.Harwerth RS, Quigley HA. Visual field defects and retinal ganglion cell losses in patients with glaucoma. Arch Ophthalmol. 2006;124:853–9. doi: 10.1001/archopht.124.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quigley HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol. 1989;107:453–64. doi: 10.1016/0002-9394(89)90488-1. [DOI] [PubMed] [Google Scholar]
- 4.Landers J, Goldberg I, Graham S. A comparison of short wavelength automated perimetry with frequency doubling perimetry for the early detection of visual field loss in ocular hypertension. Clin Experiment Ophthalmol. 2000;28:248–52. doi: 10.1046/j.1442-9071.2000.00318.x. [DOI] [PubMed] [Google Scholar]
- 5.Sample PA, Bosworth CF, Blumenthal EZ, et al. Visual function-specific perimetry for indirect comparison of different ganglion cell populations in glaucoma. Invest Ophthalmol Vis Sci. 2000;41:1783–90. [PubMed] [Google Scholar]
- 6.Johnson CA, Samuels SJ. Screening for glaucomatous visual field loss with frequency-doubling perimetry. Invest Ophthalmol Vis Sci. 1997;38:413–25. [PubMed] [Google Scholar]
- 7.Cello KE, Nelson-Quigg JM, Johnson CA. Frequency doubling technology perimetry for detection of glaucomatous visual field loss. Am J Ophthalmol. 2000;129:314–22. doi: 10.1016/s0002-9394(99)00414-6. [DOI] [PubMed] [Google Scholar]
- 8.Medeiros FA, Sample PA, Zangwill LM, et al. A statistical approach to the evaluation of covariate effects on the receiver operating characteristic curves of diagnostic tests in glaucoma. Invest Ophthalmol Vis Sci. 2006;47:2520–7. doi: 10.1167/iovs.05-1441. [DOI] [PubMed] [Google Scholar]
- 9.Wadood AC, Azuara-Blanco A, Aspinall P, et al. Sensitivity and specificity of frequency-doubling technology, tendency-oriented perimetry, and Humphrey Swedish interactive threshold algorithm-fast perimetry in a glaucoma practice. Am J Ophthalmol. 2002;133:327–32. doi: 10.1016/s0002-9394(01)01424-6. [DOI] [PubMed] [Google Scholar]
- 10.Liu S, Lam S, Weinreb RN, et al. Comparison of standard automated perimetry, frequency-doubling technology perimetry, and short-wavelength automated perimetry for detection of glaucoma. Invest Ophthalmol Vis Sci. 2011;52:7325–31. doi: 10.1167/iovs.11-7795. [DOI] [PubMed] [Google Scholar]
- 11.Leeprechanon N, Giangiacomo A, Fontana H, et al. Frequency-doubling perimetry: comparison with standard automated perimetry to detect glaucoma. Am J Ophthalmol. 2007;143:263–71. doi: 10.1016/j.ajo.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 12.Clement CI, Goldberg I, Healey PR, Graham S. Humphrey matrix frequency doubling perimetry for detection of visual-field defects in open-angle glaucoma. Br J Ophthalmol. 2009;93:582–8. doi: 10.1136/bjo.2007.119909. [DOI] [PubMed] [Google Scholar]
- 13.Artes PH, Hutchison DM, Nicolela MT, et al. Threshold and variability properties of matrix frequency-doubling technology and standard automated perimetry in glaucoma. Invest Ophthalmol Vis Sci. 2005;46:2451–7. doi: 10.1167/iovs.05-0135. [DOI] [PubMed] [Google Scholar]
- 14.Wall M, Woodward KR, Doyle CK, Artes PH. Repeatability of automated perimetry: a comparison between standard automated perimetry with stimulus size III and V, matrix, and motion perimetry. Invest Ophthalmol Vis Sci. 2009;50:974–9. doi: 10.1167/iovs.08-1789. [DOI] [PubMed] [Google Scholar]
- 15.Landers JA, Goldberg I, Graham SL. Detection of early visual field loss in glaucoma using frequency-doubling perimetry and short-wavelength automated perimetry. Arch Ophthalmol. 2003;121:1705–10. doi: 10.1001/archopht.121.12.1705. [DOI] [PubMed] [Google Scholar]
- 16.Medeiros FA, Sample PA, Weinreb RN. Frequency doubling technology perimetry abnormalities as predictors of glaucomatous visual field loss. Am J Ophthalmol. 2004;137:863–71. doi: 10.1016/j.ajo.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Kamantigue ME, Joson PJ, Chen PP. Prediction of visual field defects on standard automated perimetry by screening C-20-1 frequency doubling technology perimetry. J Glaucoma. 2006;15:35–9. doi: 10.1097/01.ijg.0000196621.41991.ff. [DOI] [PubMed] [Google Scholar]
- 18.Sample PA, Girkin CA, Zangwill LM, et al. ADGES Study Group The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol. 2009;127:1136–45. doi: 10.1001/archophthalmol.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Racette L, Liebmann JM, Girkin CA, et al. ADAGES Group African Descent and Glaucoma Evaluation Study (ADAGES): III. Ancestry differences in visual function in healthy eyes. Arch Ophthalmol. 2010;128:551–9. doi: 10.1001/archophthalmol.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cellini M, Toschi PG, Strobbe E, et al. [Accessed September 8, 2013];Frequency doubling technology, optical coherence technology and pattern electroretinogram in ocular hypertension. BMC Ophthalmol [serial online] 2012 12:33. doi: 10.1186/1471-2415-12-33. Available at: http://www.biomedcentral.com/1471-2415/12/33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wulfsohn MS, Tsiatis AA. A joint model for survival and longitudinal data measured with error. Biometrics. 1997;53:330–9. [PubMed] [Google Scholar]
- 22.Medeiros FA, Lisboa R, Zangwill L, et al. Evaluation of progressive neuroretinal rim loss as a surrogate endpoint for development of visual field loss in glaucoma. Ophthalmology. doi: 10.1016/j.ophtha.2013.06.026. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon MO, Beiser JA, Brandt JD, et al. Ocular Hypertension Treatment Study Group The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–20. doi: 10.1001/archopht.120.6.714. discussion 829-30. [DOI] [PubMed] [Google Scholar]
- 24.European Glaucoma Prevention Study (EGPS) Group Predictive factors for open-angle glaucoma among patients with ocular hypertension in the European Glaucoma Prevention Study. Ophthalmology. 2007;114:3–9. doi: 10.1016/j.ophtha.2006.05.075. [DOI] [PubMed] [Google Scholar]
- 25.Medeiros FA, Zangwill LM, Bowd C, et al. Evaluation of retinal nerve fiber layer, optic nerve head, and macular thickness measurements for glaucoma detection using optical coherence tomography. Am J Ophthalmol. 2005;139:44–55. doi: 10.1016/j.ajo.2004.08.069. [DOI] [PubMed] [Google Scholar]
- 26.Royston P. Explained variation for survival models. Stata J. 2006;6:83–96. [Google Scholar]
- 27.Choodari-Oskooei B, Royston P, Parmar MK. A simulation study of predictive ability measures in a survival model I: explained variation measures. Stat Med. 2012;31:2627–43. doi: 10.1002/sim.4242. [DOI] [PubMed] [Google Scholar]
- 28.Bayer AU, Erb C. Short wavelength automated perimetry, frequency doubling technology perimetry, and pattern electroretinography for prediction of progressive glaucomatous standard visual field defects. Ophthalmology. 2002;109:1009–17. doi: 10.1016/s0161-6420(02)01015-1. [DOI] [PubMed] [Google Scholar]
- 29.Nakagawa S, Murata H, Saito H, et al. Frequency doubling technology for earlier detection of functional damage in standard automated perimetry-normal hemifield in glaucoma with low-to-normal pressure. J Glaucoma. 2012;21:22–6. doi: 10.1097/IJG.0b013e318202777e. [DOI] [PubMed] [Google Scholar]
- 30.Haymes SA, Hutchison DM, McCormick TA, et al. Glaucomatous visual field progression with frequency-doubling technology and standard automated perimetry in a longitudinal prospective study. Invest Ophthalmol Vis Sci. 2005;46:547–54. doi: 10.1167/iovs.04-0973. [DOI] [PubMed] [Google Scholar]
- 31.Brusini P, Busatto P. Frequency doubling perimetry in glaucoma early diagnosis. Acta Ophthalmol Scand Suppl. 1998;(227):23–4. doi: 10.1111/j.1600-0420.1998.tb00869.x. [DOI] [PubMed] [Google Scholar]
- 32.Burnstein Y, Ellish NJ, Magbalon M, Higginbotham EJ. Comparison of frequency doubling perimetry with Humphrey visual field analysis in a glaucoma practice. Am J Ophthalmol. 2000;129:328–33. doi: 10.1016/s0002-9394(99)00364-5. [DOI] [PubMed] [Google Scholar]
- 33.Giuffre I. [Accessed September 8, 2013];Frequency doubling technology vs standard automated perimetry in ocular hypertensive patients. Open Ophthalmol J [serial online] 2009 3:6–9. doi: 10.2174/1874364100903010006. Available at: http://www.benthamscience.com/open/toophtj/articles/V003/6TOOPHTJ.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu LL, Suzuki Y, Kunimatsu S, et al. Frequency doubling technology and confocal scanning ophthalmoscopic optic disc analysis in open-angle glaucoma with hemifield defects. J Glaucoma. 2001;10:256–60. doi: 10.1097/00061198-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Glovinsky Y, Quigley HA, Pease ME. Foveal ganglion cell loss is size dependent in experimental glaucoma. Invest Ophthalmol Vis Sci. 1993;34:395–400. [PubMed] [Google Scholar]
- 36.Harwerth RS, Crawford ML, Frishman LJ, et al. Visual field defects and neural losses from experimental glaucoma. Prog Retin Eye Res. 2002;21:91–125. doi: 10.1016/s1350-9462(01)00022-2. [DOI] [PubMed] [Google Scholar]
- 37.Morgan JE. Retinal ganglion cell shrinkage in glaucoma. J Glaucoma. 2002;11:365–70. doi: 10.1097/00061198-200208000-00015. [DOI] [PubMed] [Google Scholar]
- 38.Martin L, Wanger P, Vancea L, Gothlin B. Concordance of high-pass resolution perimetry and frequency-doubling technology perimetry results in glaucoma: no support for selective ganglion cell damage. J Glaucoma. 2003;12:40–4. doi: 10.1097/00061198-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 39.White AJ, Sun H, Swanson WH, Lee BB. An examination of physiological mechanisms underlying the frequency-doubling illusion. Invest Ophthalmol Vis Sci. 2002;43:3590–9. [PubMed] [Google Scholar]
- 40.Quaid PT, Simpson T, Flanagan JG. Monocular and dichoptic masking effects on the frequency doubling illusion. Vision Res. 2004;44:661–7. doi: 10.1016/j.visres.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 41.Sun H, Dul MW, Swanson WH. Linearity can account for the similarity among conventional, frequency-doubling, and Gabor-based perimetric tests in the glaucomatous macula. Optom Vis Sci. 2006;83:455–65. doi: 10.1097/01.opx.0000225103.18087.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medeiros FA, Weinreb RN, Sample PA, et al. Validation of a predictive model to estimate the risk of conversion from ocular hypertension to glaucoma. Arch Ophthalmol. 2005;123:1351–60. doi: 10.1001/archopht.123.10.1351. [DOI] [PubMed] [Google Scholar]
- 43.Ocular Hypertension Treatment Study Group. European Glaucoma Prevention Study Group Validated prediction model for the development of primary open-angle glaucoma in individuals with ocular hypertension. Ophthalmology. 2007;114:10–9. doi: 10.1016/j.ophtha.2006.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ang GS, Shunmugam M, Azuara-Blanco A. Effect of cataract extraction on the glaucoma progression index (GPI) in glaucoma patients. J Glaucoma. 2010;19:275–8. doi: 10.1097/IJG.0b013e3181b21fb9. [DOI] [PubMed] [Google Scholar]
- 45.Sample PA, Medeiros FA, Racette L, et al. Identifying glaucomatous vision loss with visual-function-specific perimetry in the Diagnostic Innovations in Glaucoma Study. Invest Ophthalmol Vis Sci. 2006;47:3381–9. doi: 10.1167/iovs.05-1546. [DOI] [PubMed] [Google Scholar]
- 46.Patino CM, Varma R, Azen SP, et al. Los Angeles Latino Eye Study Group The impact of change in visual field on health-related quality of life the Los Angeles Latino Eye Study. Ophthalmology. 2011;118:1310–7. doi: 10.1016/j.ophtha.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]