Abstract
Purpose
Among cases of visually significant uveitic macular edema (ME), to estimate the incidence of visual improvement and identify predictive factors.
Design
Retrospective cohort study.
Participants
Eyes with uveitis, seen at five academic ocular inflammation centers in the United States, for which ME was documented to be currently present and the principal cause of reduced visual acuity (worse than 20/40).
Methods
Data were obtained by standardized chart review.
Main Outcome Measures
Decrease of at least 0.2 logMAR (base 10 logarithm of visual acuity decimal fraction)-equivalent; risk factors for such visual improvement.
Results
We identified 1,510 eyes (of 1,077 patients) with visual impairment to a level worse than 20/40 attributed to ME. Most patients were female (67%) and white (76%), and had bilateral uveitis (82%). The estimated six-month incidence of at least two lines of visual acuity improvement in affected eyes was 52% (95% confidence interval [CI], 49%–55%). Vision reduced by ME was more likely to improve by two lines in eyes initially with poor visual acuity (20/200 or worse; adjusted hazard ratio [HR] 1.5, 95% CI 1.3–1.7), active uveitis (HR 1.3, 95% CI 1.1–1.5), and anterior uveitis as opposed to intermediate (HR=1.2), posterior (HR=1.3) or panuveitis (HR=1.4) (overall p=0.02). During follow-up, reductions in anterior chamber or vitreous cellular activity or in vitreous haze each led to statistically significant improvements in visual outcome (p<0.001 for each). Conversely, snowbanking (HR 0.7, 95% CI 0.4–0.99), posterior synechiae (HR 0.8, 95% CI 0.6–0.9), and hypotony (HR 0.2, 95% CI 0.06–0.5) each were associated with lower incidence of visual improvement with respect to eyes lacking each of these attributes at a given visit.
Conclusions
These results suggest that many, but not all, patients with ME causing low vision in a tertiary care setting will enjoy meaningful visual recovery in response to treatment. Evidence of significant ocular damage from inflammation (posterior synechiae and hypotony) portends a lower incidence of visual recovery. Better control of anterior chamber or vitreous activity is associated with a higher incidence of visual improvement, supporting an aggressive anti-inflammatory treatment approach for ME cases with active inflammation.
Keywords: uveitis, macular edema
Macular edema (ME) is a common structural ocular complication encountered in patients with uveitis.1,2 Its pathogenesis involves disruption of the blood-retinal barrier (BRB), followed by both intra- and extracellular fluid accumulation within the macular retina.3 ME may persist despite adequate control of uveitis activity, and frequently leads to permanent photoreceptor damage and loss of central visual acuity. It is a frequent complication of uveitis in patients with intermediate uveitis, posterior uveitis, or panuveitis.4,5 Advanced age, active smoking, the presence of an epiretinal membrane, and the absence of a posterior vitreous detachment all have been identified as independent risk factors for uveitic ME.6-8 Epiretinal membrane also has been associated with failure of medical treatment to clear ME.9
ME is the leading cause of visual loss in uveitis.1,10 In one large study from a tertiary uveitis center, ME accounted for 41% of visual impairment and 29% of blindness.4 Few data exist regarding the factors influencing visual recovery in patients with visually significant uveitic ME. One recent study found that younger patients experience more favorable visual outcomes than older patients.11 In this study, we have evaluated the factors associated with visual improvement in a large cohort of uveitic eyes with ME which had been identified as the main cause of decreased vision, followed from the point of initial detection of ME.
METHODS
Study Population
The design of the Systemic Immunosuppressive Therapy for Eye (SITE) Disease Cohort Study has been detailed previously.12 Briefly, the SITE Disease Cohort Study is a retrospective cohort study of patients with inflammatory eye diseases seen at five tertiary academic ocular inflammation centers in the United States. Institutional review board approval was obtained and maintained at all centers. This research adhered to the tenets of the Declaration of Helsinki. Whereas some previous reports refer to random sampling of a subset of patients at one center, the study group subsequently completed data entry for the previously unsampled patients at that center; the complete database was available for this analysis. Whereas some papers have excluded one of the sites because its consultative approach to follow-up biased ascertainment of some outcomes, for this analysis, preliminary evaluation indicated a similar pattern of outcomes for all the centers, so the results of all five centers were retained in the analysis. Patients with infectious uveitis and Human Immunodeficiency Virust (HIV) infection had been excluded from the parent study. For this report, the study period included patient visits spanning from May 18, 1978 to September 25, 2007.
Eyes of patients who presented to the five centers were included if they were diagnosed with ME, had visual acuity worse than 20/40, and had ME identified as the principal cause of visual impairment. Reviewers were instructed to identify the single most important cause of visual impairment for each eye, considering the various complications of inflammatory disease as well as non-inflammatory disease (if the cause could be determined by chart review). At the participating centers, the diagnosis of ME had been established either by clinical exam, or, when indicated and/or available, by fluorescein angiography (FA) or optical coherence tomography (OCT). Longitudinal observations of the cases identified were available from serial clinical examinations over the period retrospectively observed. Improvement in visual acuity was chosen as a clinically meaningful primary outcome in this situation given its explicit link to ME among patients in our cohort, sensitivity to partial (though still meaningful) anatomic resolution of ME, and availability for nearly all follow-up visits. Knowledge of presence or absence of ME was incomplete given the limitations of clinical examinations, non-uniform use of imaging modalities in clinical practice, and missing documentation of macular status in a number of instances. However, visual acuity was seldom missing, visual acuity gain likely closely reflects partial or complete resolution of ME in this cohort of uveitic eyes with visual impairment primarily due to ME.5,13,14
Data Collection
All participant data had been organized using a data entry system with extensive intrinsic quality control measures, requiring correction of errors in real time, as described in previous reports.15-24 The analysis of visual acuity outcome was done by eye. Eye-time at ”risk” of visual improvement was calculated beginning from the time when ME with visual impairment to a level worse than 20/40 first was observed, and ending with either the date at which at least the equivalent of two lines of Early Treatment Diabetic Retinopathy Study (ETDRS) visual acuity— or the analogous Snellen visual acuity (−0.2 logMAR [base 10 logarithm of visual acuity decimal fraction]-equivalent or a more favorable change in visual acuity)—was gained, or the date of the last follow-up visit (for cases in which vision had not improved signficantly).
Potential risk factors for visual improvement included: age, sex, race, smoking status, laterality of uveitis, visual acuity, site of uveitis, overall activity, the presence of snowballs, snowbanking, histories of cataract, glaucoma, or vitreoretinal surgeries, and HLA-B27 status (all at the time of ME diagnosis). The following time-updated variables assessed as possible risk factors included: changes in anterior chamber or vitreous cells or in vitreous haze, the presence of snowballs, snowbanking, posterior synechiae, peripheral anterior synechiae, epiretinal membrane, hypotony, or ocular hypertension, or exposure to topical prostaglandin analogues, carbonic anhydrase inhibitors, oral non-steroidal anti-inflammatory drugs, or oral acetazolamide.
HLA-B27 testing was performed when clinically indicated, and the presence of 23 systemic inflammatory diseases was diagnosed by the study ocular inflammation specialists based on history, examination findings, and review of records. The ocular inflammation specialists who had evaluated the patients at the centers had completed an internal medicine residency, a rheumatology fellowship, and/or an ocular inflammation specialty fellowship training that included systemic inflammatory disease diagnosis as a major objective of training.
Statistical Analysis
Factors potentially predictive of improvement in visual acuity were evaluated on the basis of adjusted hazard ratios (HR, 95% confidence intervals [CIs]) generated using crude and adjusted Cox proportional hazards models with a robust sandwich estimate to account for correlation between the eyes of individual patients.25 The proportion with visual improvement by a given point in time was evaluated by calculating the cumulative incidence of resolution estimated from the crude Cox regression hazard function, which allowed estimates of time-varying risk factors as well as 95% CIs accounting for correlation between eyes of the same patient.
The multiple Cox regression model adjusted for presenting visual acuity, overall activity at the time of ME diagnosis, uveitis anatomic location,26,27 primary uveitis diagnosis, time-updated change in anterior chamber cells from the outset of observation, HLA-B27 status, history of cataract surgery prior to diagnosis of ME, hypotony (intraocular pressure [IOP] <5mmHg), and ocular hypertension (IOP >21mmHg). However, in order to avoid multiple adjustment by highly correlated time-updated inflammatory activity covariates, change in anterior chamber cells was not used to adjust the estimates for change in vitreous cells, change in vitreous haze, and snowbanking; for the same reason only change in anterior chamber cells was used for the final multiple Cox regression model. The data analyses were performed using SAS v9.3 (SAS Inc., Cary, NC).
RESULTS
During the study period, 1,510 eyes of 1,077 patients were diagnosed with ME which also was determined to be the major cause of reduced visual acuity to a level worse than 20/40 at the time of diagnosis. Cases had been followed subsequently for a total of 2,060 eye-years (1,744 person-years) until either VA improved by at least 2 lines or follow-up was completed without such improvement being observed. ME was identified in the majority of eyes based on clinical examination alone (77%), with fewer cases established by FA (5%) or OCT (18%).Table 1 details characteristics for all patients with visually significant ME. The median age was 36 years. The majority of patients were female (67%), white (76%), and nonsmokers (64%). Most patients had bilateral uveitis (82%).
Table 1.
Person-level characteristics at the time of macular edema diagnosis
| Baseline characteristics | Total Macular Edema | |
|---|---|---|
| Sex | Male | 359 (33.3%) |
| Female | 718 (66.7%) | |
| Race category | White | 819 (76.0%) |
| Black | 145 (13.5%) | |
| Hispanic | 53 (4.9%) | |
| Other | 60 (5.6%) | |
| Age at uveitis diagnosis | ≤25 | 313 (29.1%) |
| 26-45 | 419 (38.9%) | |
| 46-55 | 253 (23.5%) | |
| >65 | 92 (8.5%) | |
| Primary Uveitis Diagnosis | Unilateral | 189 (17.5%) |
| Bilateral | 888 (82.5%) | |
| HLA-B27 | No | 1000 (92.9%) |
| Yes | 77 (7.1%) | |
| Systemic Diabetes Mellitus | No | 1036 (96.2%) |
| Yes | 41 (3.8%) | |
| Systemic Hypertension | No | 850 (78.9%) |
| Yes | 227 (21.1%) | |
| Oral NSAID | No | 937 (93.2%) |
| Yes | 68 (6.8%) | |
| Oral Acetazolamide | No | 983 (97.8%) |
| Yes | 22 (2.2%) | |
| Topical PGA in at least one eye | No | 992 (98.7%) |
| Yes | 13 (1.3%) | |
| Topical CAI in at least one eye | No | 944 (93.9%) |
| Yes | 61 (6.1%) | |
| Smoking | Never | 557 (51.7%) |
| Past | 134 (12.4%) | |
| Current | 257 (23.9%) | |
| Unknown | 129 (12.0%) |
Prevalence of systemic inflammatory diseases in this cohort, listed as number of patients (% of cohort): Sarcoidosis: 37 (3.4%); Spondyloarthropathy: 24 (2.2%); Multiple sclerosis: 15 (1.5%); Behçet Syndrome: 14 (1.3%); Rheumatoid Arthritis 14 (1.3%); Inflammatory Bowel Disease: 13 (1.2%); Juvenile Idiopathic Arthritis 11 (1.0%); Systemic Lupus Erythematosus: 7 (0.6%). Regarding medication exposures, only 1 patient was taking oral niacin, and no patients were taking tamoxifen at the initial visit. Abbreviations: NSAID = non-steroidal anti-inflammatory drug; PGA = prostaglandin analogue; CAI = carbonic anhydrase inhibitor.
Initial characteristics of the 1,510 eyes with visual acuity reduced to a level worse than 20/40 as a result of ME are given as Table 2. Among these eyes with visual impairment primarily due to ME, 26% had 20/200 or worse visual acuity when ME was first noted. By anatomic site of inflammation, 28% of patients had anterior uveitis only, 35% had intermediate (± anterior) uveitis, 19% had posterior uveitis, and 18% had panuveitis. Twenty-nine per cent of eyes had no uveitis activity, 14% had slight activity, and 57% had clear-cut activity. Eight per cent of eyes had snowballs, 6% had snowbanking, 17% had posterior synechiae, 21% had epiretinal membrane, and 1% had hypotony at the time ME was diagnosed. Regarding previous ocular surgeries, 26% of eyes had undergone cataract surgery (approximately 1/3 of which were left aphakic), 11% had undergone pars plana vitrectomy, and 4% had undergone either filtering or shunting glaucoma surgery.
Table 2.
Eye-level characteristics at the time of macular edema diagnosis
| Baseline characteristics | Total Macular Edema | |
|---|---|---|
| Visual Acuity at Diagnosis of ME | Better than 20/200 | 1122 (74.3%) |
| 20/200 or worse | 388 (25.7%) | |
| Uveitis Category | Anterior | 424 (28.1%) |
| Intermediate | 533 (35.3%) | |
| Posterior | 285 (18.9%) | |
| Panuveitis | 268 (17.7%) | |
| Overall Activity | Inactive | 436 (28.9%) |
| Slightly active | 211 (14.0%) | |
| Active | 863 (57.2%) | |
| Anterior Chamber Cells | Quiet | 720 (47.7%) |
| 0.5+ | 329 (21.8%) | |
| 1+ | 268 (17.8%) | |
| ≥2+ | 191 (12.7%) | |
| Vitreous Cells | Quiet | 536 (36.0%) |
| 0.5+ | 241 (16.2%) | |
| 1+ | 348 (23.4%) | |
| ≥2+ | 364 (24.4%) | |
| Vitreous Haze | Quiet | 1011 (71.8%) |
| 1+ | 289 (20.5%) | |
| ≥2+ | 109 (7.7%) | |
| Snowballs | No | 1389 (92.2%) |
| Yes | 118 (7.8%) | |
| Snowbanking | No | 1412 (93.7%) |
| Yes | 95 (6.3%) | |
| Posterior Synechiae | No | 1255 (83.2%) |
| Yes | 254 (16.8%) | |
| Peripheral Anterior Synechiae | No | 1486 (98.5%) |
| Yes | 23 (1.5%) | |
| Epiretinal Membrane | No | 1195 (79.3%) |
| Yes | 312 (20.7%) | |
| Hypotony (<5 mmHg) | No | 1457 (98.8%) |
| Yes | 18 (1.2%) | |
| Ocular hypertension (>21 mmHg) | No | 1356 (91.9%) |
| Yes | 119 (8.1%) | |
| Cataract Surgery | No | 1114 (73.8%) |
| Aphakic | 135 (8.9%) | |
| Pseudophakic | 261 (17.3%) | |
| YAG Posterior Capsuolotomy | No | 1437 (95.2%) |
| Yes | 73 (4.8%) | |
| Trabeculectomy or Tube Shunt Surgery | No | 2963 (96.2%) |
| Yes | 57 (3.8%) | |
| Pars Plana Vitrectomy | No | 1350 (89.4%) |
| Yes | 160 (10.6%) |
Macular edema was determined to be present by clinical examination, fluorescein angiography and/or optical coherence tomography, and was judged to be the major cause of a reduction in visual acuity to 20/40 or worse (see Methods). Abbreviations: ME = macular edema; YAG = Yttrium aluminium garnet.
The incidence of and predictive factors for gain of at least two lines of ETDRS-equivalent (or equivalent) visual acuity (suggesting improvement of ME) are summarized in Table 3, and more thoroughly detailed in Table 4 (available online at http://aaojournal.org). In a sensitivity analysis in which a three-line threshold for gain in visual acuity was employed, a similar pattern of results was observed (data not shown), except the overall incidence rate was lower corresponding to the higher standard used to define success.
Table 3.
Risk Factors for ≥2 Lines Improvement of Macular Edema-Induced Reduction of Visual Acuity
| Estimated Proportion Resolved (95% Confidence Interval) | Unadjusted | Adjusted** | |||||
|---|---|---|---|---|---|---|---|
| N | 6-Month | Hazard Ratio | p-value | Hazard Ratio | p-value | ||
| All | All | 1510 | 51.9% (49.0% - 54.6%) | n/a | |||
| Smoking | Never | 759 | 54.2% (50.3% - 57.8%) | 1.00 | 0.02 | 1.00 | 0.75 |
| Past | 188 | 50.3% (44.2% - 55.8%) | 0.90 (0.74 - 1.08) | 1.02 (0.84 - 1.24) | |||
| Current | 382 | 52.7% (47.7% - 57.2%) | 0.96 (0.83 - 1.11) | 0.97 (0.83 - 1.13) | |||
| Unknown | 181 | 42.7% (35.9% - 48.8%) | 0.71 (0.58 - 0.87) | 0.88 (0.70 - 1.10) | |||
| Visual Acuity at Diagnosis of ME | Better than 20/200 | 1122 | 48.9% (45.8% - 51.8%) | 1.00 | <0.001 | 1.00 | <0.001 |
| 20/200 or worse | 388 | 60.9% (55.6% - 65.6%) | 1.40 (1.22 - 1.60) | 1.46 (1.26 - 1.68) | |||
| Site of Uveitis | Anterior | 424 | 60.7% (55.6% - 65.2%) | 1.00 | <0.001 | 1.00 | 0.02 |
| Intermediate | 533 | 50.4% (46.3% - 54.2%) | 0.75 (0.65 - 0.87) | 0.83 (0.70 - 1.00) | |||
| Posterior | 285 | 49.6% (43.6% - 55.0%) | 0.74 (0.61 - 0.88) | 0.76 (0.63 - 0.93) | |||
| Panuveitis | 268 | 44.0% (38.4% - 49.2%) | 0.62 (0.51 - 0.75) | 0.73 (0.59 - 0.89) | |||
| Overall Activity at ME Diagnosis | Inactive | 436 | 46.5% (41.9% - 50.6%) | 1.00 | <0.001 | 1.00 | <0.01 |
| Slightly active | 211 | 41.0% (35.2% - 46.4%) | 0.85 (0.68 - 1.04) | 0.89 (0.70 - 1.13) | |||
| Active | 863 | 57.6% (54.0% - 60.9%) | 1.37 (1.20 - 1.58) | 1.27 (1.08 - 1.50) | |||
| Change in Anterior Chamber Cells | ≥2 steps better (or to 0) | * | 65.2% (60.5% - 69.3%) | 1.59 (1.39 - 1.83) | <0.001 <0.001† |
1.50 (1.29 - 1.74) | <0.001 <0.001† |
| 1 step better | * | 59.4% (49.9% - 67.1%) | 1.36 (1.06 - 1.72) | 1.31 (1.00 - 1.68) | |||
| No change | * | 48.5% (44.9% - 51.8%) | 1.00 | 1.00 | |||
| 1 step worse | * | 38.5% (30.9% - 45.3%) | 0.73 (0.57 - 0.93) | 0.77 (0.60 - 0.97) | |||
| ≥2 steps worse (or to 4+) | * | 35.0% (25.3% - 43.5%) | 0.65 (0.46 - 0.89) | 0.64 (0.45 - 0.87) | |||
| HLA-B27 | No | 1419 | 51.4% (48.5% - 54.1%) | 1.00 | 0.12 | 1.00 | 0.51 |
| Yes | 91 | 60.2% (47.1% - 70.1%) | 1.28 (0.99 - 1.62) | 1.10 (0.85 - 1.42) | |||
| Cataract Surgery prior to ME | No | 1114 | 53.3% (50.1% - 56.3%) | 1.00 | 0.08 | 1.00 | 0.07 |
| Aphakic | 135 | 44.2% (36.1% - 51.4%) | 0.77 (0.61 - 0.96) | 0.75 (0.58 - 0.95) | |||
| Pseudophakic | 261 | 49.9% (44.3% - 55.0%) | 0.91 (0.77 - 1.07) | 0.88 (0.74 - 1.05) | |||
| Hypotony (<5 mmHg) | No | * | 52.2% (49.3% - 54.9%) | 1.00 | <0.01 | 1.00 | <0.01 |
| Yes | * | 14.6% (0.0% - 27.3%) | 0.21 (0.07 - 0.50) | 0.21 (0.06 - 0.49) | |||
| Ocular hypertension (>21 mmHg) | No | * | 51.2% (48.2% - 54.0%) | 1.00 | 0.11 | 1.00 | 0.32 |
| Yes | * | 57.3% (49.3% - 64.0%) | 1.19 (0.97 - 1.44) | 1.11 (0.90 - 1.36) | |||
Time updated covariates vary in their number at risk over time, and therefore cannot be represented by a single number.
Adjusted for all other variables in the table.
Test of linear trend across categories.
Abbreviation: ME = macular edema.
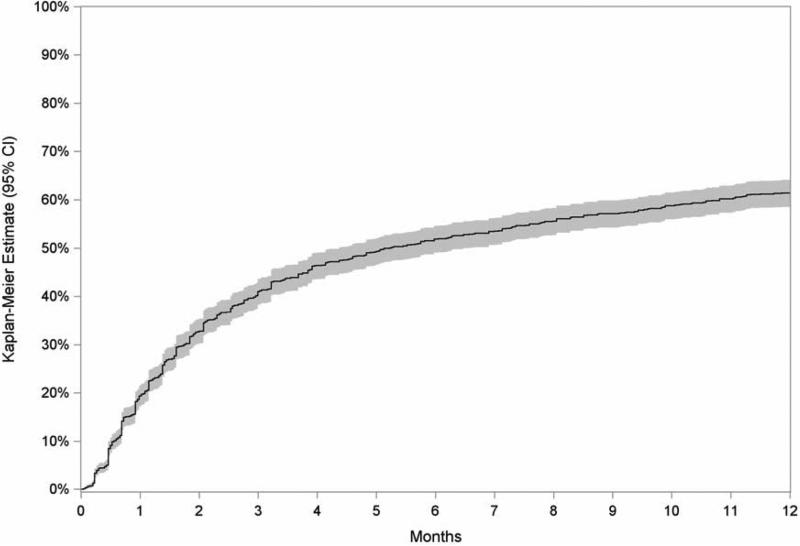
The median eye follow-up time after initial diagnosis was 112 days (interquartile range of 35–463 days). Overall, the proportion with ≥2-line improvement of ME-induced visual impairment was estimated at 41% (CI 38–44%) at 3 months, 52% (CI 49–55%) at 6 months, and 61% (CI 59–64%) at 12 months (see Figure 1). Incidences of visual improvement did not vary significantly across age groups, gender, race, laterality (unilateral versus bilateral uveitis), or smoking status. Neither autoimmune disease nor use of the medications listed in Table 1 were found to significantly alter the course of visual improvement (data not shown).
Figure 1.
Kaplan-Meier estimate of the incidence of at least two lines improvement in visual acuity among eyes with macular edema causing a reduction of visual acuity to worse than 20/40 (shaded area denotes the 95% confidence interval [CI]).
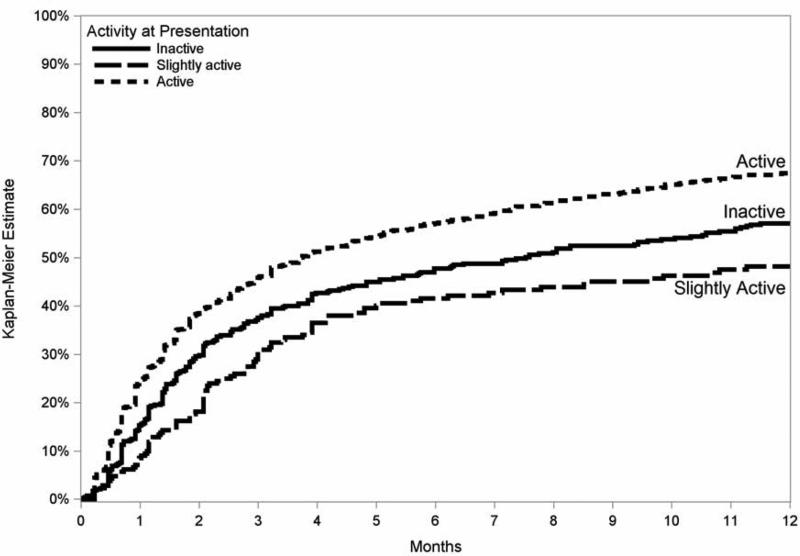
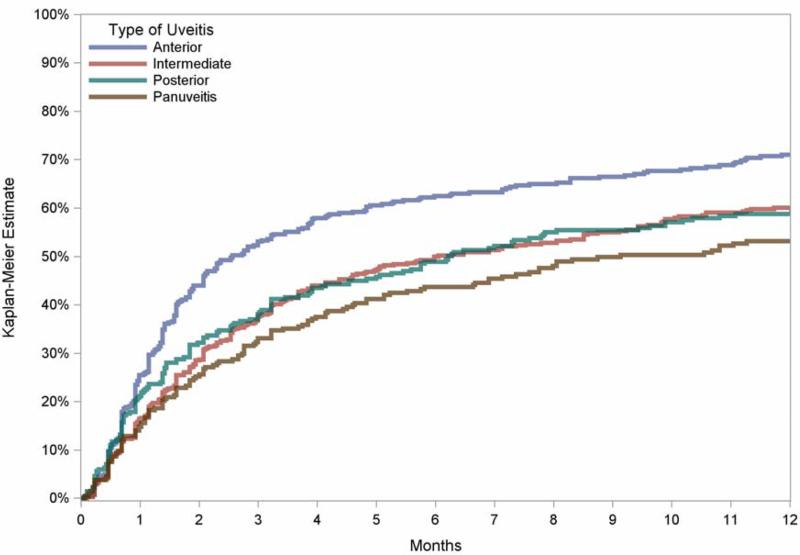
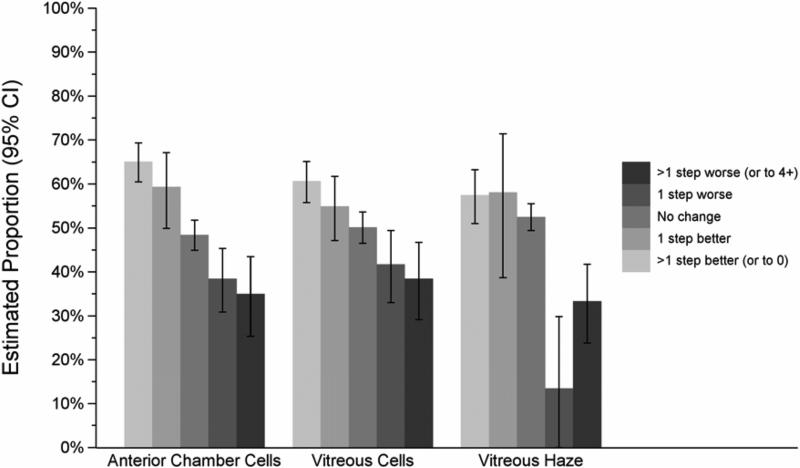
Poor baseline visual acuity (20/200 or worse) was associated with more frequent ≥2-line improvement in visual acuity (HR 1.5, CI 1.3–1.7) than cases with lesser degrees of visual impairment. The category of uveitis also influenced the likelihood of visual improvement, with eyes with only anterior uveitis more readily experiencing gains in vision than those with intermediate (+/− anterior) (HR=1.2), posterior (HR=1.3) or panuveitis (HR=1.4) (overall p=0.02; Figure 2). Clear-cut uveitis activity at baseline, as opposed to slight or absent activity, also was associated positively with visual improvement (overall p=0.02; Figure 3). Improvements in anterior chamber and vitreous cellular activity, as well as in vitreous haze, each demonstrated highly significant step-wise linear correlations with ME resolution (p<0.001 for all three measures; see Figure 4).
Figure 2.
Kaplan-Meier estimates of the incidence of at least two lines improvement in visual acuity among eyes with macular edmea causing reduction of visual acuity to worse than 20/40, comparing estimates of vision gain among those with baseline activity, inactivity, or slight activity.
Figure 3.
Kaplan-Meier estimates of the incidence of at least two lines improvement in visual acuity among eyes with macular edema causing reduction of visual acuity to worse than 20/40, comparing estimates of vision gain among those with anterior uveitis, intermediate uveitis, posterior uveitis, and panuveitis.
Figure 4.
Influence of the change in uveitis activity over six months on the proportion of eyes with macular edema causing visual acuity to worse than 20/40 that gained at least two lines’ improvement in visual acuity by that time. Error bars denote 95% confidence intervals. p<0.01 comparing degrees of step-wise improvements within each category of uveitis activity. CI = confidence interval
Regarding ocular surgeries prior to diagnosis of ME, histories of cataract, glaucoma, or vitreoretinal surgery were not significantly associated with improvement of ME-induced visual decline. Few cataract, glaucoma, or vitreoretinal surgeries were performed during follow-up, limiting statistical confidence of their time-dependent effects on visual outcomes; there was no obvious signal suggesting that the pattern of (non-) association was different than that observed for cases which had undergone each type of surgery prior to ME diagnosis (data not shown).
Several eye-specific factors, if present during follow-up, portended worse visual prognoses. The presence of snowbanking—but not snowballs—was associated with more refractory ME-induced reduction in visual acuity (HR 0.67, CI 0.43–0.99). Eyes with posterior synechiae (HR 0.78, CI 0.64–0.94), and especially those with hypotony (HR 0.21, CI 0.06–0.49), also were less likely to experience visual improvement.
A sensitivity analysis of initial characteristics and outcomes involving only the 343 eyes diagnosed by FA or OCT was conducted. The results were largely similar. The only inconsistencies observed were that intermediate (+/− anterior uveitis) was associated with a similar rather than a less favorable outcome compared with anterior uveitis alone (HR 1.3, CI 0.9–2.0), and that a history of pars plana vitrectomy was associated with a more pronounced relationship with the outcome, with reduced incidence of improvement (HR 0.53, CI 0.27–0.95). To test for possible influences of evolving treatment paradigms over time, sensitivity analyses were performed in which the year of the visit was included either as a continuous variable, or categorized by decade. None of the major results of the study were influenced by this analysis (data not shown).
DISCUSSION
The observation that 52% of eyes gained at least two lines of visual acuity in this cohort by 6 months suggests that a substantial number, but not nearly all, of eyes with uveitic ME causing vision loss have an important degree of visual acuity recovery while receiving tertiary uveitis care. The rate of gaining at least two lines of visual acuity appears to be higher during the first few months of treatment, and then diminishes thereafter.
A common early step in the formation of ME is the production of inflammatory mediators, including prostaglandins, histamine, and vascular endothelial growth factor.28 Disruption of the inner BRB of the retinal vascular endothelia by activated T cells, combined with impaired retinal pigment epithelial pump function, then leads to fluid leakage into the extracellular matrix.29 Retinal Müller cells act as metabolic pumps to dehydrate the macula, but eventually fluid overwhelms and accumulates within the Müller cells themselves. Restoration of normal BRB integrity and removal of intraretinal fluid can often restore vision.
We identified active uveitis at the time of presentation with ME as a favorable prognostic factor, suggesting that cases where active inflammation was driving ME and vision loss were susceptible to treatment of the inflammation, which presumably interrupted this pathogenic pathway. Improvements in anterior chamber or vitreous cells and in vitreous haze over time also strongly correlated with visual improvement (see Fig 2). In such cases, visual improvement was likely driven by improvement in ME, as well as by reduction in anterior and vitreous haze. In our patient population, poor initial visual acuity also portended a better prognosis for visual improvement, likely reflecting that the most severely affected cases had the most room for improvement. These cases likely required less ME clearance in order to achieve a two line improvement in visual acuity than those cases with less extensive visual loss.
The observed heterogeneity in visual improvement reflects the variable processes leading both to ME and vision loss in these settings. Nearly one-half of patients were still experiencing decreased visual acuity 6 months after initial detection of ME despite treatment per best medical judgment. Visually significant ME was diagnosed despite baseline overall inactivity in 29% of cases, confirming that ME may evolve or persist despite control or remission of inflammation. Cases where ME was present despite inactivity of uveitis at the initial visit, or perhaps originated from some other cause, appear less likely to regain vision. The early period of rapid visual recovery already may have passed for these individuals prior to the beginning of observation. A more posterior location of inflammation was predictive of less improvement; results were inconsistent in the sensitivity analysis for intermediate uveitis, but for posterior and panuveitis this may have reflected a greater likelihood of chronic macular injury perhaps related to increased incidence of subsequent macular scarring or greater severity of disease. Clinical findings generally indicative of more severe disease, including posterior synechiae and hypotony, also were predictive of a lower incidence of visual improvement. The presence of snowbanking was also associated with a lower incidence of recovery of visual acuity. The retrospective dataset did not distinguish between active exudates and residual fibrosis from past pars planitis; thus, it is unclear whether this result reflects cases with greater severity of past inflammation (reflected by residual fibrosis) or cases where control of activity was not achieved (active exudates) or both. Chronic or severe ME ultimately cause retinal thinning and fibrosis, thus preventing a return to normal vision even once the edema is effectively treated, which likely contributed to the substantial minority which failed to make substantial gains in visual acuity. Co-existing reasons for visual loss might also have limited potential visual recovery in some cases. The relative contributions of cataract and other pathologies to decreased vision cannot be defined well in a retrospective study generally, and furthermore the SITE database allows for only one principal cause of visual impairment to be identified. Moreover, visually significant epiretinal membranes and vitreomacular traction might have been underdetected during the study period, as the majority of patients were managed before the advent of optical coherence tomography
In our cohort, a history of vitreoretinal, cataract, or glaucoma surgery prior to the time of ME diagnosis was not consistently associated with substantially altered visual outcomes. This study could not adequately address whether vitrectomy is an effective strategy to treat existing ME, due to sample size constraints and missing details about the indications for vitrectomy surgery. Current smoking—which has been observed to increase the risk of relapse of ocular inflammation in this and other cohorts,16,30 and has been associated with a greater risk of ME in uveitis cases7,31—was not associated with worse visual outcomes in our population, suggesting that smoking may affect the risk of ME to a greater degree than it affects the prognosis of established ME. With respect to age, there was no measured difference in visual gain comparing younger and older patients. This finding differs from a smaller study that found less visual improvement among elderly patients with uveitis.11 Finally, no systemic autoimmune disease was found to influence the rate of vision gain in this study, nor did we observe any association between use of systemic and topical medications potentially implicated in causing ME (listed in Table 1) and the incidence of visual improvement.
The study was not designed to evaluate the various treatment strategies for ME. As difficult-to-control inflammation requires escalated therapy, a direct comparison of treatment modalities would have been substantially biased in this non-randomized, retrospective study. However, regarding the issue of anti-inflammatory treatment, the benefit associated with control of inflammation suggests that treatments that successfully control inflammation are salutory. The strengths of this study include the large number of patients, which allowed for reasonable precision in estimating risk and associations. Our efforts to optimize the quality of retrospective data collection included a standardized protocol for chart reviews supported by detailed study documentation and site visiting for protocol enforcement. The limitations of this study arise from its retrospective nature. Although the problem of incomplete follow-up was addressed in our statistical analysis by using survival analysis, if subjects with resolution of ME and resultant improvement of vision were less likely to return for follow-up visits our overall resolution rate would be underestimated. Another limitation was potential referral bias, in that cases may have been especially severe and perhaps more refractory to clinical management than would be seen in a general ophthalmology setting. If so, our estimates of the likelihood of regaining vision would be lower than would be observed in a non-tertiary care practice, but should be representative of tertiary uveitis practices like ours. Finally, many cases were diagnosed based on clinical examination alone, reflecting in part the unavailability of OCT for much of the study period. However, there was a similar distribution of characteristics among those diagnosed by clinical or ancillary testing methods, and outcomes comparing those diagnosed by FA or OCT versus clinical exam alone were similar.
In summary, our cohort of cases of visually significant uveitic ME presenting to tertiary uveitis centers had a reasonably favorable prognosis for meaningful visual improvement over time. Cases with active inflammation at the time of developing ME, and those that developed improvement in inflammatory signs during follow-up tended to improve more, likely because anti-inflammatory treatment was effective in removing the insult leading to macular edema. In contrast, those cases that did not experience improvement in inflammation, those with ME despite quiescent inflammation initially, and those with markers of a more severe inflammatory process than average (such as posterior synechiae and hypotony), were less likely to experience improvement in vision. These findings may be useful to counsel patients regarding the visual prognosis of this condition, support the concept that aggressive suppression of inflammation improves outcomes, and provide guidance as to which cases require early treatment directed specifically against ME rather than just anti-inflammatory treatment. Further studies would be required to evaluate the relative merits of different therapeutic regimens for treating ME.
Supplementary Material
Précis.
Among 1510 uveitic eyes with macular edema reducing visual acuity ≤20/40, ~52% gained ≥2 lines in ≤6 months. Initial activity, anteriorly located uveitis and lack of hypotony, snowbanking, and/or posterior synechiae were favorable prognostic factors.
Acknowledgments
Financial Support: Supported primarily by National Eye Institute Grant EY014943 (Dr. Kempen). Additional support was provided by Research to Prevent Blindness and the Paul and Evanina Mackall Foundation. During part of the conduct of this project, Dr. Levin was a Heed Ophthalmic Society Fellowship recipient, Dr. Kempen was a Research to Prevent Blindness James S. Adams Special Scholar Award recipient, Dr. Thorne was a Research to Prevent Blindness Harrington Special Scholar Award recipient, and Drs. Jabs and Rosenbaum were Research to Prevent Blindness Senior Scientific Investigator Award recipients. Dr. Suhler receives support from the Veteran's Affairs Administration. Dr. Levy-Clarke was previously supported by and Dr. Nussenblatt continues to be supported by intramural funds of the National Eye Institute. The funding organizations had no role in the design or conduct of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was first presented as a paper at the International Symposium on Uveitis, Halkidiki, Greece, October 2012.
Financial Disclosures: Proprietary or commercial disclosures may be found after the references.
Financial Disclosure(s): The author(s) have made the following disclosure(s): C. Stephen Foster – Equity owner – Eyegate; Consultant, Lecturer – Allergan, Bausch & Lomb; Consultant – Sirion; Lecturer – Alcon, Inspire, Ista, Centocor.
Douglas A. Jabs – Consultant – Roche, Genzyme Corporation, Novartis, Allergan, Glaxo Smith Kline, Applied Genetic Technologies, The Emmes Corporation, The Johns Hopkins Dana Center for Preventative Ophthalmology.
John H. Kempen – Consultant – Alcon, Allergan, Can-Fite, Clearside, Lux Biosciences, Sanofi-Pasteur, Xoma; Support – EyeGate Eric B. Suhler – Support – Eyegate, Lux Biosciences, Genentech, Celgene, Abbott.
Jennifer Thorne – Consultant – Allergan, Horon Evidence Development.
Grace Levy-Clarke – Employee – Johnson & Johnson.
REFERENCES
- 1.Lardenoye CW, van Kooji B, Rothova A. Impact of macular edema on visual acuity in uveitis. Ophthalmology. 2006;113:1446–9. doi: 10.1016/j.ophtha.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 2.Malinowski SM, Pulido JS, Folk JC. Long-term visual outcome and complications associated with pars planitis. Ophthalmology. 1993;100:818–24. doi: 10.1016/s0161-6420(93)31567-8. [DOI] [PubMed] [Google Scholar]
- 3.Yanoff M, Fine BS, Brucker AJ, Eagle RC., Jr Pathology of human cystoid macular edema. Surv Ophthalmol. 1984;28(suppl):505–11. doi: 10.1016/0039-6257(84)90233-9. [DOI] [PubMed] [Google Scholar]
- 4.Rothova A, Suttorp-van Schulten MS, Frits Treffers W, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. 1996;80:332–6. doi: 10.1136/bjo.80.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugar EA, Jabs DA, Altaweel MM, et al. Multicenter Uveitis Steroid Treatment (MUST) Trial Research Group Identifying a clinically meaningful threshold for change in uveitic macular edema evaluated by optical coherence tomography. Am J Ophthalmol. 2011;152:1044–52. doi: 10.1016/j.ajo.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hikichi T, Trempe CL. Role of the vitreous in the prognosis of peripheral uveitis. Am J Ophthalmol. 1993;116:401–5. doi: 10.1016/s0002-9394(14)71395-9. [DOI] [PubMed] [Google Scholar]
- 7.Thorne JE, Daniel E, Jabs DA, et al. Smoking as a risk factor for cystoids macular edema complicating intermediate uveitis. Am J Ophthalmol. 2008;145:841–6. doi: 10.1016/j.ajo.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Kooij B, Probst K, Fijnheer R, et al. Risk factors for cystoid macular oedema in patients with uveitis. Eye (Lond) 2008;22:256–60. doi: 10.1038/sj.eye.6702595. [DOI] [PubMed] [Google Scholar]
- 9.Markomichelakis NN, Halkiadakis I, Partelia E, et al. Course of macular edema in uveitis under medical treatment. Ocul Immunol Inflamm. 2007;15:71–9. doi: 10.1080/09273940701244509. [DOI] [PubMed] [Google Scholar]
- 10.Durrani OM, Tehrani NM, Marr JE, et al. Degree, duration, and causes of visual loss in uveitis. Br J Ophthalmol. 2004;88:1159–62. doi: 10.1136/bjo.2003.037226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tranos PG, Tsaousis KT, Vakalis AN, et al. Long-term follow-up of inflammatory cystoid macular edema. Retina. 2012;32:1624–8. doi: 10.1097/IAE.0b013e3182483348. [DOI] [PubMed] [Google Scholar]
- 12.Kempen JH, Daniel E, Gangaputra S, et al. Methods for identifying long-term adverse effects of treatment in patients with eye diseases: the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study. Ophthalmic Epidemiol. 2008;15:47–55. doi: 10.1080/09286580701585892. [DOI] [PubMed] [Google Scholar]
- 13.Taylor SR, Lightman SL, Sugar EA, et al. The impact of macular edema on visual function in intermediate, posterior, and panuveitis. Ocul Immunol Inflamm. 2012;20:171–81. doi: 10.3109/09273948.2012.658467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tran TH, de Smet MD, Bodaghi B, et al. Uveitic macular oedema: correlation between optical coherence tomorgraphy patterns with visual acuity and fluorescein angiography. Br J Ophthalmol. 2008;92:922–7. doi: 10.1136/bjo.2007.136846. [DOI] [PubMed] [Google Scholar]
- 15.Daniel E, Pistilli M, Pujari SS, et al. Risk of hypotony in noninfectious uveitis. Ophthalmology. 2012;119:2377–85. doi: 10.1016/j.ophtha.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galor A, Feuer W, Kempen JH, et al. Adverse effects of smoking on patients with ocular inflammation. Br J Ophthalmol. 2010;94:848–53. doi: 10.1136/bjo.2009.174466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gangaputra S, Newcomb CW, Liesegang TL, et al. ystemic Immunosuppressive Therapy for Eye Diseases Cohort Study Methotrexate for ocular inflammatory dieseases. Ophthalmology. 2009;116:2188–98. doi: 10.1016/j.ophtha.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grunwald L, Newcomb CW, Daniel E, et al. Systemic Immunosuppressive Therapy for Eye Diseases Cohort Study Risk of relapse in primary acuite anterior uveitis. Ophthalmology. 2011;118:1911–5. doi: 10.1016/j.ophtha.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kacmaz RO, Kempen JH, Newcomb C, et al. Systemic Immunosuppressive Therapy for Eye Diseases Cohort Study Ocular inflammation in Behçet disease: incidence of ocular complications and of loss of visual acuity. Am J Ophthalmol. 2008;146:828–36. doi: 10.1016/j.ajo.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kacmaz RO, Kempen JH, Newcomb C, et al. Cyclosporine for ocular inflammatory diseases. Ophthalmology. 2010;117:576–84. doi: 10.1016/j.ophtha.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kempen JH, Daniel E, Dunn JP, et al. Overall and cancer related mortality among patients with ocular inflammation treated with immunosuppressive drugs: retrospective cohort study [report online]. [September 9, 2013];BMJ. 2009 339:b2480. doi: 10.1136/bmj.b2480. Available at: http://www.bmj.com/content/339/bmj.b2480?view=long&pmid=19578087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasadhika S, Kempen JH, Newcomb CW, et al. Azathioprine for ocular inflammatory diseases. Am J Ophthalmol. 2009;148:500–9. doi: 10.1016/j.ajo.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Udoetuk JD, Dai Y, Ying GS, et al. Systemic Immunosuppressive Therapy for Eye Diseases Cohort Study Research Group Risk of corticosteroid-induced hyperglycemia requiring medical therapy among patients with inflammatory eye diseases. Ophthalmology. 2012;119:1569–74. doi: 10.1016/j.ophtha.2012.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaidi A, Ying GS, Daniel E, et al. Systemic Immunosuppressive Therapy for Eye Diseases Cohort Study Research Group Hypopyon in patients with uveitis. Ophthalmology. 2010;117:366–72. doi: 10.1016/j.ophtha.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by using the marginal distribution. J Am Stat Assoc. 1989;84:1065–73. [Google Scholar]
- 26.Bloch-Michel E, Nussenblatt R. International Uveitis Study Group recommendations for the evaluation of intraocular inflammatory disease. Am J Ophthalmol. 1987;103:234–5. doi: 10.1016/s0002-9394(14)74235-7. [DOI] [PubMed] [Google Scholar]
- 27.Standardization of Uveitis Nomenclature (SUN) Working Group Standardization of uveitis nomenclature for reporting clinical data: results of the First International Workshop. Am J Ophthalmol. 2005;140:509–16. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fine HF, Baffi J, Reed GF, et al. Aqueous humor and plasma vascular endothelial growth factor in uveitis-associated cystoid macular edema. Am J Ophthalmol. 2001;132:794–6. doi: 10.1016/s0002-9394(01)01103-5. [DOI] [PubMed] [Google Scholar]
- 29.Lightman S, Greenwood J. Effect of lymphocytic infiltration of the blood-retinal barrier in experimental autoimmune uveoretinitis. Clin Exp Immunol. 1992;88:473–7. doi: 10.1111/j.1365-2249.1992.tb06474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin P, Loh AR, Margolis TP, Acharya NR. Cigarette smoking as a risk factor for uveitis. Ophthalmology. 2010;117:585–90. doi: 10.1016/j.ophtha.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roesel M, Ruttig A, Schumacher C, et al. Risk factors for the development of macular edema in noninfectious uveitis. Eur J Ophthalmol. 2011;21:625–30. doi: 10.5301/ejo.2011.6297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.