Abstract
It is increasingly evident that tumor-associated macrophages (TAMs) play an important role in tumor invasion, proliferation, and metastasis. While delivery of drugs, imaging agents and vaccines to TAMs was achieved by exploiting membrane receptors on TAMs, the uptake by normal macrophages remains an issue. In this communication, we report a PEG-sheddable, mannose-modified nanoparticle platform that can efficiently target TAMs via mannose-mannose receptor recognition after acid-sensitive PEG shedding in the acidic tumor microenvironment, while their uptake by normal macrophages in the mononuclear phagocyte system (MPS) organs was significantly reduced due to effective PEG shielding at neutral pH. These nanoparticles have the potential to target drugs of interest to TAMs, with decreased uptake by normal macrophages.
Keywords: tumor-associated macrophages, nanoparticles, mannose receptor, acid-sensitivity, PEGylation
Compelling evidence from clinical and preclinical studies indicate that tumor-associated macrophages (TAMs) play an important role in tumor cell invasion, proliferation, survival, and metastasis to local and distant sites.1–3 TAM infiltration has been shown to correlate with cancer growth, metastasis, and poor prognosis in a variety of human carcinomas, including breast, prostate, ovarian, and cervical cancers.4, 5 Therefore, TAMs represent an appealing target for cancer therapy. Elevated expression of membrane receptors such as mannose receptors and scavenger receptors has been reported in TAMs,6 which were exploited to facilitate the specific delivery of oligos, DNA, imaging agents, and vaccines to TAMs by modifying the delivery systems with mannose derivatives,7, 8 anti-mannose receptor nanobody9 or galactose derivatives.10 Recently, Dreaden et al. demonstrated that gold nanorods modified with macrolides, a class of structurally homologous antibiotics, also showed high affinity to TAMs.11 In addition, it was reported that folate receptor-beta is expressed by TAMs and constitutes a marker for M2 anti-inflammatory/regulatory macrophages.12, 13 Folate receptor-beta had been exploited to target toxins to TAMs.14, 15 However, these targeted drug delivery systems can be inevitably taken up by the normal macrophages in the mononuclear phagocyte system (MPS) organs after intravenous (i.v.) injection, because normal macrophages generally express similar membrane receptors as TAMs.6, 16 Given the important role of the normal macrophages in the innate and acquired immunity and homeostasis,16, 17 it would be beneficial to target a drug of interest to TAMs, while avoiding or minimizing uptake by normal macrophages. In the present study, we report a mannose-modified, PEG-sheddable nanoparticle platform that can be efficiently taken up by TAMs via ligand-receptor recognition after acid-sensitive shedding of PEG in the acidic tumor microenvironment, while showing decreased accumulation in MPS organs due to successful PEG shielding in normal pH.
The nanoparticles were prepared with poly(lactic-co-glycolic acid) (PLGA), an FDA-approved biodegradable and biocompatible polymer,18–20 following a previously reported nanoprecipitation method (Fig. 1A).21 To quantify the in vitro uptake and to track the in vivo fate of the nanoparticles, PLGA 752H was labeled with fluorescein isothiocyanate (FITC) to give PLGA-FITC (Scheme S1 and Fig. S1 in the Supporting Information), which was blended with unmodified PLGA 752H at a 5:95 weight ratio to prepare the nanoparticles. Mannose was used as a ligand to target the nanoparticles to macrophages, because it was reported that macrophages, especially M2 type macrophages (e.g., TAMs) overexpress mannose receptors.6 To this end, O-stearoyl mannose (M-C18) was synthesized (Fig. 1A, Scheme S2 and Fig. S2 in the Supporting Information) and used to modify the nanoparticles. For sheddable PEGylation in tumor tissues, an acid-sensitive amphiphile, which was previously synthesized in our laboratory by conjugating polyethylene glycol (molecular weight, 2000) (i.e., PEG2000) with stearic hydrazide (C18) using a hydrazone bond (PEG-hydrazone-C18, PHC) (Scheme S3, Figs. S3 and S4 in the Supporting Information),22 was used to modify the nanoparticles. An acid-insensitive counterpart of PHC was also synthesized by conjugating PEG2000-amine with stearoyl chloride (C18) to form PEG-amide-C18 (PAC), which was used as a control.23 When the nanoparticles are modified with both M-C18 and PHC, the long flexible PEG chains are expected to shield the mannose and prevent its interaction with mannose receptors on the surface of macrophages before reaching tumors after i.v. injection. Once the nanoparticles passively accumulate in the tumor tissues by the enhanced permeability and retention (EPR) effect, the acidic extracellular tumor microenvironment (~ pH 6.8) is expected to catalyze the hydrolysis of PHC,24, 25 expose the mannose on the surface of the nanoparticles, and thus allow the uptake of the nanoparticles by TAMs via mannose-mannose receptor recognition.
Figure 1.
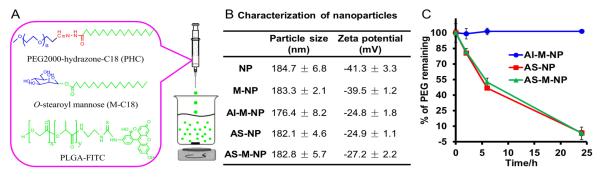
A) Schematic illustration of the preparation of PLGA nanoparticle with various surface modifications. B) Particle sizes and zeta potentials of the PLGA nanoparticles with various modifications (n = 3). C) The percentage of PEG remaining on the surface of various nanoparticles after up to 24 h of incubation in a pH 6.8 buffer (n = 3). (Abbreviations: NP, PLGA-nanoparticles; M-NP, NP surface-modified with mannose (i.e., M-C18); AI-M-NP, NP surface-modified with mannose and acid-insensitive PEG(2000) (i.e., PAC); AS-NP, NP surface-modified acid-sensitive PEG(2000) (i.e., PHC); AS-M-NP, NP surface-modified with mannose and acid-sensitive PEG(2000).
A nanoprecipitation method was used to prepare nanoparticles with various surface modifications:21 unmodified PLGA nanoparticles (NP), M-C18-modified PLGA nanoparticles (M-NP), acid-insensitive PAC- and M-C18-modified PLGA nanoparticles (AI-M-NP), acid-sensitive PHC-modified PLGA nanoparticles (AS-NP) and acid-sensitive PHC- and M-C18-modified PLGA nanoparticles (AS-M-NP) (Fig. 1B). All nanoparticles were around 180 nm, with a negative zeta potential (Fig. 1B). Modification of NP with M-C18 did not significantly affect the zeta potential of the nanoparticles (P > 0.05), while modification with PHC or PAC significantly decreased the zeta potential by 35–40% (P < 0.01). The charge-shielding effect of PEG chains was reported when other nanoparticles or polymers were PEGylated.26, 27 The acid-sensitive shedding of PEG from the nanoparticles was monitored at pH 6.8 using an iodide staining method.28 As seen in Fig. 1C, AI-M-NP did not show any significant PEG shedding even after 24 h of incubation at pH 6.8. In contrast, AS-NP and AS-M-NP that were modified with the acid-sensitive PHC shedded around 20% and 50% of the PEG after 2 h and 6 h of incubation at pH 6.8, about 100% after 24 h. The results of the acid-sensitive PEG shedding was in agreement with the acid-sensitivity of PHC molecule.22 Previously, we have shown that around 40% of PHC was hydrolyzed after 6 h of incubation at pH 6.8, and an almost complete degradation was observed after 24 h.22
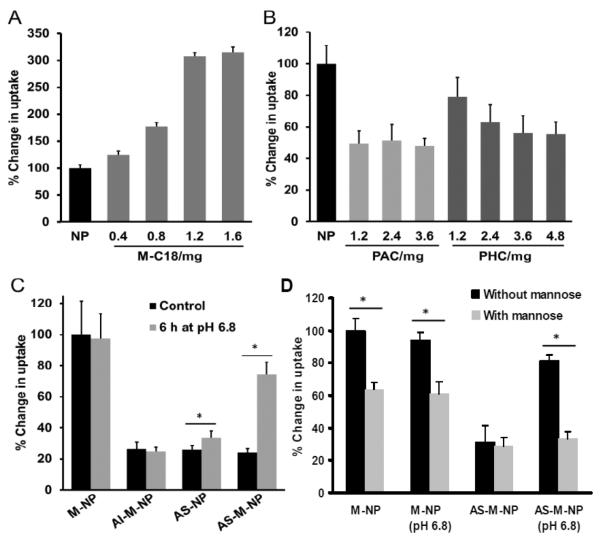
The cellular uptake of the nanoparticles with different surface modifications was evaluated using the murine J774A.1 macrophage cells.29–31 Cells (2 × 105) were incubated with various nanoparticles prepared with the PLGA-FITC (5% of total PLGA, w/w) for 1 h to determine nanoparticle uptake by fluorescence spectrometry. As shown in Fig. 2A, there was a significant increase in the uptake of the PLGA nanoparticles by the J774A.1 cells when 0.4 mg of M-C18 was incorporated into the nanoparticles (P < 0.01). When the amount of M-C18 increased to 1.2 mg, there was a 3.1-fold increase in the cellular uptake of the nanoparticles. Further increasing the amount of M-C18 in the nanoparticles to more than 1.2 mg did not further increase the uptake (Fig. 2A). It is likely that the optimum ligand density for mannose receptor-mediated cellular uptake was achieved when 1.2 mg of M-C18 was incorporated into the nanoparticle formulation. Therefore, the nanoparticle formulation with 1.2 mg of M-C18 was used for further studies.
Figure 2.
Cellular uptake of the nanoparticles by J774A.1 murine macrophages in culture. A) The effect of the amount of M-C18 in the nanoparticle formulation on the uptake of the nanoparticles by J774A.1 cells (n = 6). J774A.1 cells (2 × 105) were incubated with various nanoparticles prepared with PLGA-FITC for 1 h before measuring the fluorescence intensity. B) The effect of the amount of PAC or PHC in the nanoparticle formulations on the cellular uptake of the nanoparticles (n = 6). C) The effect of PEGylation on the uptake of M-NP and the effect of pre-incubation of the nanoparticles at pH 6.8 for 6 h on their uptake by the J774A.1 cells (n = 5). D) The effect of pre-incubating the J774A.1 cells with mannose on the uptake of the M-NP and AS-M-NP that were pre-incubated at pH 6.8 for 6 h to facilitate the shedding of the PEG chains (n = 5). In A–B, all values were standardized to the fluorescence intensity of the cells incubated with NP; in C and D, all values were standardized to the fluorescence intensity of the M-NP. (* P < 0.01).
PEGylation is well recognized to decrease cellular uptake because the hydrophilic PEG chains serve as a barrier, preventing interactions between nanoparticles and cells.32, 33 The effect of PEGylation on the cellular uptake was studied in the absence of M-C18 modification first. As seen in Fig. 2B, incorporation of 1.2 mg of PAC or PHC into the nanoparticles significantly decreased the cellular uptake to 49.4% (P < 0.01) and 79.0% (P < 0.05), respectively. The ability for PAC and PHC to decrease the cellular uptake appeared to be different, possibly because the PHC (MW2527.2) (Fig. S4 in the Supporting Information) has slightly longer PEG chains than PAC (MW2279.2).23 Moreover, it is possible that the PHC was incorporated into the nanoparticles to a less extent than PAC due to its relatively higher hydrophilicity. Further increasing the amount of PAC in the formulation did not further decrease the cellular uptake, indicating that 1.2 mg of PAC sufficiently covered the nanoparticle surface and prevented the interaction between the nanoparticles and macrophages. On the other hand, at least 3.6 mg of PHC was needed to effectively prevent the uptake of the nanoparticles by macrophages (Fig. 2B). Therefore, for further studies, the amounts of PAC and PHC that were incorporated into the nanoparticle formulations were 1.2 mg and 3.6 mg, respectively.
We then tested whether the PEGylation of the M-C18-modified nanoparticles (M-NP) can effectively inhibit their uptake by macrophages and whether the acid-sensitive shedding of PHC will restore the uptake of the nanoparticles by macrophages. As shown in Fig. 2C, PEGylation of M-NP with PAC or PHC significantly decreased the cellular uptake by 75% (Fig. 2B), indicating the mannose on the surface of the M-NP was shielded by the hydrophilic PEG chains at neutral pH. The PEG has an average molecular weight of around 2000, which is much larger than that of mannose. Previously, PEG2000 had also been reported to successfully shield a cell penetrating peptide (TAT) with a molecular weight of 1560.34 Preincubation of the nanoparticles at pH 6.8 significantly affected the uptake of the nanoparticles by J774A.1 cells. For example, the uptake of AS-NP and AS-M-NP was increased by 1.3-fold and 3.1-fold, respectively, whereas the uptake of the AI-M-NP was not affected by preincubation at pH 6.8. It is likely that, after preincubation at pH 6.8 for 6 h, the PEG chains were shedded from AS-M-NP, exposing the mannose for binding to the mannose receptors on the surface of the macrophages.
To verify that the uptake of the mannose-modified nanoparticles by the J774A.1 macrophages was mediated by mannose-mannose receptor interaction, the uptakes of M-NP and AS-M-NP by J774A.1 cells were evaluated when cells were preincubated with free mannose to competitively inhibit the mannose receptors. As shown in Fig. 2D, preincubation of J774A.1 cells with mannose (2 mg/ml for 45 min prior to the addition of the nanoparticles) significantly decreased the uptake of M-NP, but not the uptake of AS-M-NP, clearly demonstrating that the uptake of M-NP by J774A.1 cells was via mannose receptors on J774A.1 cells and that the PEGylation of the M-NP (e.g., the AS-M-NP) shielded the M-NP from being taken up by the macrophages. In addition, the uptake of the AS-M-NP that were preincubated at pH 6.8 for 6 h to facilitate the shedding of the PEG chains was significantly inhibited by preincubating the cells with mannose (Fig. 2D), again indicating that the acid-sensitive shedding of the PEG chains from the AS-M-NP exposed the mannose on the AS-M-NP to interact with mannose receptors on the J774A.1 macrophages.
After confirming the in vitro stability of these nanoparticles in the presence of fetal bovine serum (Table S1 in the Supporting Information), we injected the nanoparticles into healthy C57BL/6 mice via the tail vein to examine whether the shielding of mannose by PEGylation can effectively decrease the accumulation of the NP in major MPS organs such as liver and spleen.35 All nanoparticle preparations have similar fluorescence intensities (Table S2 in the Supporting Information). Mice were euthanized 6 h after the injection to collect the liver and spleen for cryosectioning. As seen in Fig. 3, M-NP showed a significantly higher accumulation than the unmodified NP in the liver and spleen, likely due to the presence of liver Kupffer cells and splenic macrophages in these organs, which express high level of mannose receptors.36 In fact, the mannosylated nanoparticles have been explored to target the liver and spleen.37, 38 AI-M-NP and AS-M-NP showed a less extent of accumulation in mouse liver and spleen, as compared to M-NP (Fig. 3), indicating that PEGylation of the mannose-modified nanoparticles effectively shielded the mannose and reduced their uptake in the MPS. The PEGylated nanoparticles (AI-M-NP and AS-M-NP) also showed less accumulation in liver and spleen than the unmodified NP (Fig. 3), likely because the hydrophilic PEG chains on them prevented the opsonization of nanoparticles during circulations, and thus decreased their accumulation in the liver and spleen.32 Recently, an antineoplastic agent Yondelis39 and a bisphosphonate clodronate40 were used to deplete TAMs for cancer therapy, but they were also non-selectively cytotoxic to normal macrophages.41, 42
Figure 3.
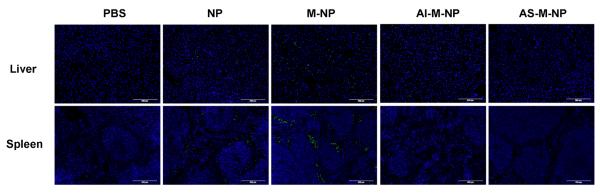
Liver and spleen accumulation of various nanoparticles 6 h after i.v. injection in tumor-free mice. C57BL/6 mice (n = 3) were i.v. injected with AS-M-NP and euthanized 6 h later to collect their liver and spleen, which were then cryosectioned, counterstained with DAPI (blue) to examine the distribution of the FITC-labeled nanoparticles (green) under a fluorescence microscope. As controls, mice were also i.v. injected with AI-M-NP, M-NP, NP, or sterile PBS. Micrographs shown are representative images from 3 slides per mouse (bar, 200 μm).
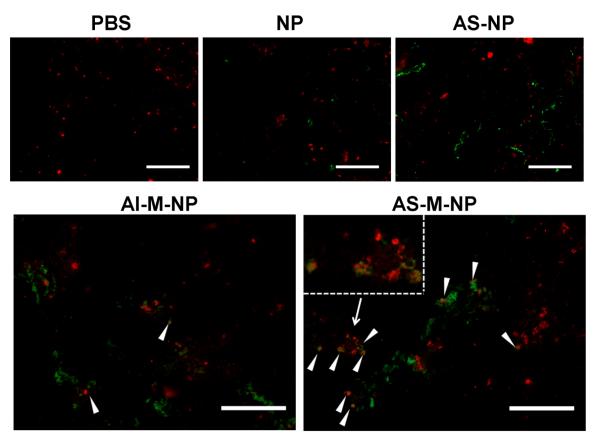
The accumulation of the nanoparticles in tumor tissues and their ability to target TAMs in vivo were of major interest to us. To preliminarily examine whether our acid-sensitive PEG-sheddable, mannose-modified nanoparticles (i.e., AS-M-NP) can target TAMs, we established B16-F10 mouse melanoma tumors subcutaneously in C57BL/6 mice. When tumors reached 6–8 mm in diameter, mice were injected with the nanoparticles via the tail vein. As controls, mice were also i.v. injected with sterile PBS, NP, AS-NP, or AI-M-NP. Tumor tissues were collected 12 h after the injection, cryosectioned, and stained with phycoerythrin (PE)-conjugated rat anti-mouse CD68 antibody as a marker of TAMs.43 As shown in Fig. 4, 12 h after i.v. injection, the PEGylated nanoparticles, including AS-NP, AI-M-NP and AS-M-NP, showed a higher tumor accumulation than the unmodified NP, likely because PEGylation decreased the opsonization of the nanoparticles, prolonged their circulation, and favored tumor accumulation. Importantly, the localizations of these PEGylated nanoparticles in the tumor tissues appeared to be different (Fig. 4). A clear colocalization of the nanoparticles with TAMs (red) were observed in tumors in mice that were injected with AS-M-NP, while very limited colocalization was observed in the tumors in mice that were injected with AS-NP or AI-M-NP, indicating that both PEG shedding and the exposure of mannose are necessary for targeting the nanoparticles to TAMs. Reversible PEG shielding had been used to protect nanoparticles from elimination due to antibody recognition, electrostatic interaction, and ligand-receptor recognition, while allowing the specific interaction of the nanoparticles with the target cells after PEG shedding.34, 44–46 To the best of our knowledge, this is the first report that the acid-sensitive PEG shedding strategy is used to specifically target TAMs. Our acid-sensitive PHC- and M-C18-modified PLGA nanoparticles may represent a platform technology to target drugs such as the previously used Yondelis and bisphosphonate clodronate to TAMs, while decreasing or minimizing the cytotoxicity of them to macrophages that are not tumor-associated. In addition, TAMs are M2 type macrophages, which express a higher level of mannose receptors than the M1 type macrophages.47, 48 This differential expression of mannose receptors by M1 and M2 macrophages will likely also help decrease the effect of drugs carried by our AS-M-NP on normal macrophages. Finally, in the present study we used the pan-macrophage marker CD68 to label TAMs following a recent publication by Kluza et al.,43 because we used the same tumor model (i.e., B16-F10 murine melonoma model in C57BL/6 mice) as they did. In fact, CD68 has been used by others to stain TAMs as well.49–51 In future studies, we will use a marker or combination of markers to more specifically stain TAMs.
Figure 4.
Accumulation and distribution of nanoparticles in tumor tissue 12 h after i.v. injection into B16-F10 tumor-bearing mice. C57BL/6 mice (female, 6–8 weeks, n = 3) were s.c. injected with B16-F10 tumor cells (5 × 105). When tumors reached 6–8 mm in diameter, mice were i.v. injected with AS-M-NP. As controls, mice were also i.v. injected with AI-M-NP, AS-NP, NP, and sterile PBS. Mice were euthanized 12 h later to collect tumor tissues, which were cryosectioned, stained with PE-labeled rat anti-mouse CD68 antibody and then examined under a fluorescence microscope to localize the FITC-labeled nanoparticles (green) and macrophages (red). White arrow heads indicate the colocalization of the nanoparticles with TAMs. (Inset in AS-M-NP: representative colocalization of the nanoparticles with TAMs shown at a higher magnification). Micrographs shown are representative images from 3 slides per mouse (bar = 200 μm).
In conclusion, we have developed a novel PEG-sheddable, mannose-modified PLGA nanoparticle platform that can efficiently target TAMs after acid-sensitive PEG shedding in the acidic tumor microenvironment, with decreased accumulation in MPS organs due to successful PEG shielding in normal physiological pH (pH 7.4). This nanoparticle platform may be used to carry drugs or macrophage modulatory agents to TAMs for tumor therapeutic purposes. The strategy of conditional PEG shedding to expose TAM-specific ligands on the nanoparticles may provide a useful tool to achieve targeted drug delivery to TAMs.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part by a National Cancer Institute grant (CA135274) to ZC and a Cancer Prevention and Research Institute of Texas (CPRIT) Postdoctoral Training Grant (RP101501) to SZ.
Footnotes
Supporting Information Available Synthesis and characterization of PLGA-FITC, O-stearoyl mannose, and PEG-hydrazone-C18 (PHC), preparation and characterization of nanoparticles, in vitro stability study (of nanoparticles), fluorescence intensities of nanoparticles, in vitro cellular uptake, accumulation of nanoparticles in liver and spleen, and colocalization of nanoparticles with TAMs. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coffelt SB, Hughes R, Lewis CE. Tumor-associated macrophages: effectors of angiogenesis and tumor progression. Biochim. Biophy. Acta. 2009;1796(1):11–8. doi: 10.1016/j.bbcan.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nature reviews. Cancer. 2004;4(1):71–8. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 4.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J. Pathol. 2002;196(3):254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 5.Condeelis J, Pollard JW. Macrophages: Obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 7.Yu WY, Liu CX, Liu Y, Zhang N, Xu WF. Mannan-modified solid lipid nanoparticles for targeted gene delivery to alveolar macrophages. Pharm. Res. 2010;27(8):1584–1596. doi: 10.1007/s11095-010-0149-z. [DOI] [PubMed] [Google Scholar]
- 8.Locke LW, Mayo MW, Yoo AD, Williams MB, Berr SS. PET imaging of tumor associated macrophages using mannose coated Cu-64 liposomes. Biomaterials. 2012;33(31):7785–7793. doi: 10.1016/j.biomaterials.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Movahedi K, Schoonooghe S, Laoui D, Houbracken I, Waelput W, Breckpot K, Bouwens L, Lahoutte T, De Baetselier P, Raes G, Devoogdt N, Van Ginderachter JA. Nanobody-based targeting of the macrophage mannose receptor for effective in vivo imaging of tumor-associated macrophages. Cancer Res. 2012;72(16):4165–4177. doi: 10.1158/0008-5472.CAN-11-2994. [DOI] [PubMed] [Google Scholar]
- 10.Huang Z, Zhang ZP, Jiang YC, Zhang DC, Chen JN, Dong L, Zhang JF. Targeted delivery of oligonucleotides into tumor-associated macrophages for cancer immunotherapy. J. Controlled Release. 2012;158(2):286–292. doi: 10.1016/j.jconrel.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Dreaden EC, Mwakwari SC, Austin LA, Kieffer MJ, Oyelere AK, El-Sayed MA. Small molecule-gold nanorod conjugates selectively target and induce macrophage cytotoxicity towards breast cancer cells. Small. 2012;8(18):2819–2822. doi: 10.1002/smll.201200333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurahara H, Takao S, Kuwahata T, Nagai T, Ding Q, Maeda K, Shinchi H, Mataki Y, Maemura K, Matsuyama T, Natsugoe S. Clinical significance of folate receptor beta-expressing tumor-associated macrophages in pancreatic cancer. Ann. Surg. Oncol. 2012;19(7):2264–71. doi: 10.1245/s10434-012-2263-0. [DOI] [PubMed] [Google Scholar]
- 13.Puig-Kroger A, Sierra-Filardi E, Dominguez-Soto A, Samaniego R, Corcuera MT, Gomez-Aguado F, Ratnam M, Sanchez-Mateos P, Corbi AL. Folate receptor beta is expressed by tumor-associated macrophages and constitutes a marker for M2 anti-inflammatory/regulatory macrophages. Cancer Res. 2009;69(24):9395–403. doi: 10.1158/0008-5472.CAN-09-2050. [DOI] [PubMed] [Google Scholar]
- 14.Nagai T, Tanaka M, Tsuneyoshi Y, Xu B, Michie SA, Hasui K, Hirano H, Arita K, Matsuyama T. Targeting tumor-associated macrophages in an experimental glioma model with a recombinant immunotoxin to folate receptor beta. Cancer Immunol. Immunother.: CII. 2009;58(10):1577–86. doi: 10.1007/s00262-009-0667-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagayoshi R, Nagai T, Matsushita K, Sato K, Sunahara N, Matsuda T, Nakamura T, Komiya S, Onda M, Matsuyama T. Effectiveness of anti-folate receptor beta antibody conjugated with truncated Pseudomonas exotoxin in the targeting of rheumatoid arthritis synovial macrophages. Arth. Rheum. 2005;52(9):2666–75. doi: 10.1002/art.21228. [DOI] [PubMed] [Google Scholar]
- 16.Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu. Rev. Immunol. 2005;23:901–944. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- 17.Twigg HL. Macrophages in innate and acquired immunity. Sem. Resp. Crit. Care Med. 2004;25(1):21–31. doi: 10.1055/s-2004-822302. [DOI] [PubMed] [Google Scholar]
- 18.Lim YT, Noh YW, Han JH, Cai QY, Yoon KH, Chung BH. Biocompatible polymer-nanoparticle-based bimodal imaging contrast agents for the labeling and tracking of dendritic cells. Small. 2008;4(10):1640–1645. doi: 10.1002/smll.200800582. [DOI] [PubMed] [Google Scholar]
- 19.Luo RC, Neu B, Venkatraman SS. Surface functionalization of nanoparticles to control cell interactions and drug release. Small. 2012;8(16):2585–2594. doi: 10.1002/smll.201200398. [DOI] [PubMed] [Google Scholar]
- 20.Woodrow KA, Cu Y, Booth CJ, Saucier-Sawyer JK, Wood MJ, Saltzman WM. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nat. Mater. 2009;8(6):526–533. doi: 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong YC, Feng SS. Methoxy poly(ethylene glycol)-poly(lactide) (MPEG-PLA) nanoparticles for controlled delivery of anticancer drugs. Biomaterials. 2004;25(14):2843–2849. doi: 10.1016/j.biomaterials.2003.09.055. [DOI] [PubMed] [Google Scholar]
- 22.Zhu SJ, Wonganan P, Lansakara-P DS, O'Mary HL, Li Y, Cui ZR. The effect of the acid-sensitivity of 4-(N)-stearoyl gemcitabine-loaded micelles on drug resistance caused by RRM1 overexpression. Biomaterials. 2013;34(9):2327–2339. doi: 10.1016/j.biomaterials.2012.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu SJ, Lansakara-P DS, Li XR, Cui ZR. Lysosomal delivery of a lipophilic gemcitabine prodrug using novel acid-sensitive micelles improved its antitumor activity. Bioconjugate Chem. 2012;23(5):966–980. doi: 10.1021/bc2005945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engin K, Leeper DB, Cater JR, Thistlethwaite AJ, Tupchong L, Mcfarlane JD. Extracellular pH distribution in human tumors. Int. J. Hyperther. 1995;11(2):211–216. doi: 10.3109/02656739509022457. [DOI] [PubMed] [Google Scholar]
- 25.Kim D, Lee ES, Oh KT, Gao ZG, Bae YH. Doxorubicin-loaded polymeric micelle overcomes multidrug resistance of cancer by double-targeting folate receptor and early endosomal pH. Small. 2008;4(11):2043–2050. doi: 10.1002/smll.200701275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He QJ, Zhang JM, Shi JL, Zhu ZY, Zhang LX, Bu WB, Guo LM, Chen Y. The effect of PEGylation of mesoporous silica nanoparticles on nonspecific binding of serum proteins and cellular responses. Biomaterials. 2010;31(6):1085–1092. doi: 10.1016/j.biomaterials.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 27.Zhu SJ, Hong MH, Tang GT, Qian LL, Lin JY, Jiang YY, Pei YY. Partly PEGylated polyamidoamine dendrimer for tumor-selective targeting of doxorubicin: The effects of PEGylation degree and drug conjugation style. Biomaterials. 2010;31(6):1360–1371. doi: 10.1016/j.biomaterials.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 28.Bazile D, Prud'homme C, Bassoullet MT, Marlard M, Spenlehauer G, Veillard M. Stealth Me.PEG-PLA nanoparticles avoid uptake by the mononuclear phagocytes system. J. Pharm. Sci. 1995;84(4):493–8. doi: 10.1002/jps.2600840420. [DOI] [PubMed] [Google Scholar]
- 29.Nahar M, Jain NK. Preparation, characterization and evaluation of targeting potential of amphotericin B-loaded engineered PLGA nanoparticles. Pharm. Res. 2009;26(12):2588–2598. doi: 10.1007/s11095-009-9973-4. [DOI] [PubMed] [Google Scholar]
- 30.Kaindl T, Oelke J, Pasc A, Kaufmann S, Konovalov OV, Funari SS, Engel U, Wixforth A, Tanaka M. Regulation of adhesion behavior of murine macrophage using supported lipid membranes displaying tunable mannose domains. J. Phys.: Condens. Matter. 2010;22(28) doi: 10.1088/0953-8984/22/28/285102. [DOI] [PubMed] [Google Scholar]
- 31.Singodia D, Verma A, Verma RK, Mishra PR. Investigations into an alternate approach to target mannose receptors on macrophages using 4-sulfated N-acetyl galactosamine more efficiently in comparison with mannose-decorated liposomes: an application in drug delivery. Nanomed. Nanotechnol. 2012;8(4):468–477. doi: 10.1016/j.nano.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Owens DE, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharmaceut. 2006;307(1):93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Sloat BR, Sandoval MA, Li D, Chung WG, Lansakara DSP, Proteau PJ, Kiguchi K, DiGiovanni J, Cui ZR. In vitro and in vivo anti-tumor activities of a gemcitabine derivative carried by nanoparticles. Int. J. Pharmaceut. 2011;409(1–2):278–288. doi: 10.1016/j.ijpharm.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sawant RM, Hurley JP, Salmaso S, Kale A, Tolcheva E, Levchenko TS, Torchilin VP. “SMART” drug delivery systems: Double-targeted pH-responsive pharmaceutical nanocarriers. Bioconjugate Chem. 2006;17(4):943–949. doi: 10.1021/bc060080h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cavadas M, Gonzalez-Fernandez A, Franco R. Pathogen-mimetic stealth nanocarriers for drug delivery: a future possibility. Nanomed. Nanotechnol. 2011;7(6):730–743. doi: 10.1016/j.nano.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Kelly C, Jefferies C, Cryan SA. Targeted liposomal drug delivery to monocytes and macrophages. J. Drug Del. 2011;2011:727241. doi: 10.1155/2011/727241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Un K, Kawakami S, Suzuki R, Maruyama K, Yamashita F, Hashida M. Development of an ultrasound-responsive and mannose-modified gene carrier for DNA vaccine therapy. Biomaterials. 2010;31(30):7813–26. doi: 10.1016/j.biomaterials.2010.06.058. [DOI] [PubMed] [Google Scholar]
- 38.Kawakami S, Sato A, Nishikawa M, Yamashita F, Hashida M. Mannose receptor-mediated gene transfer into macrophages using novel mannosylated cationic liposomes. Gene Ther. 2000;7(4):292–299. doi: 10.1038/sj.gt.3301089. [DOI] [PubMed] [Google Scholar]
- 39.Allavena P, Signorelli M, Chieppa M, Erba E, Bianchi G, Marchesi F, Olimpio CO, Bonardi C, Garbi A, Lissoni A, de Brand F, Jimeno J, D'Incalci M. Anti-inflammatory properties of the novel antitumor agent yondelis (Trabectedin): Inhibition of macrophage differentiation and cytokine production. Cancer Res. 2005;65(7):2964–2971. doi: 10.1158/0008-5472.CAN-04-4037. [DOI] [PubMed] [Google Scholar]
- 40.Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjallman AHM, Ballmer-Hofer K, Schwendener RA. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br. J. Cancer. 2006;95(3):272–281. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sturm E, Havinga R, Baller JFW, Wolters H, van Rooijen N, Kamps JAAM, Verkade HJ, Karpen SJ, Kuipers F. Kupffer cell depletion with liposomal clodronate prevents suppression of Ntcp expression in endotoxin-treated rats. J. Hepatol. 2005;42(1):102–109. doi: 10.1016/j.jhep.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 42.Vanrooijen N, Kors N, Vanderende M, Dijkstra CD. Depletion and repopulation of macrophages in spleen and liver of rat after intravenous treatment with liposome-encapsulated dichloromethylene diphosphonate. Cell Tissue Res. 1990;260(2):215–222. doi: 10.1007/BF00318625. [DOI] [PubMed] [Google Scholar]
- 43.Kluza E, Yeo SY, Schmid S, van der Schaft DW, Boekhoven RW, Schiffelers RM, Storm G, Strijkers GJ, Nicolay K. Anti-tumor activity of liposomal glucocorticoids: The relevance of liposome-mediated drug delivery, intratumoral localization and systemic activity. J. Controlled Release. 2011;151(1):10–7. doi: 10.1016/j.jconrel.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 44.Romberg B, Hennink WE, Storm G. Sheddable coatings for long-circulating nanoparticles. Pharm. Res. 2008;25(1):55–71. doi: 10.1007/s11095-007-9348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker GF, Fella C, Pelisek J, Fahrmeir J, Boeckle S, Ogris M, Wagner E. Toward synthetic viruses: Endosomal pH-triggered deshielding of targeted polyplexes greatly enhances gene transfer in vitro and in vivo. Mol. Ther. 2005;11(3):418–425. doi: 10.1016/j.ymthe.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 46.Li WM, Mayer LD, Bally MB. Prevention of antibody-mediated elimination of ligand-targeted liposomes by using poly(ethylene glycol)-modified lipids. J. Pharmacol. Exp. Ther. 2002;300(3):976–983. doi: 10.1124/jpet.300.3.976. [DOI] [PubMed] [Google Scholar]
- 47.Schmieder A, Michel J, Schonhaar K, Goerdt S, Schledzewski K. Differentiation and gene expression profile of tumor-associated macrophages. Sem. Cancer Biol. 2012;22(4):289–97. doi: 10.1016/j.semcancer.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 48.Jager NA, Teteloshvili N, Zeebregts CJ, Westra J, Bijl M. Macrophage folate receptor-beta (FR-beta) expression in auto-immune inflammatory rheumatic diseases: a forthcoming marker for cardiovascular risk? Autoimmun. Rev. 2012;11(9):621–6. doi: 10.1016/j.autrev.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 49.Ryder M, Ghossein RA, Ricarte-Filho JC, Knauf JA, Fagin JA. Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocrine-relat. Cancer. 2008;15(4):1069–74. doi: 10.1677/ERC-08-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liou P, Bader L, Wang A, Yamashiro D, Kandel JJ. Correlation of tumor-associated macrophages and clinicopathological factors in Wilms tumor. Vascular Cell. 2013;5(1):5. doi: 10.1186/2045-824X-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo Y, Zhou H, Krueger J, Kaplan C, Lee SH, Dolman C, Markowitz D, Wu W, Liu C, Reisfeld RA, Xiang R. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J. Clin. Invest. 2006;116(8):2132–2141. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.