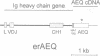
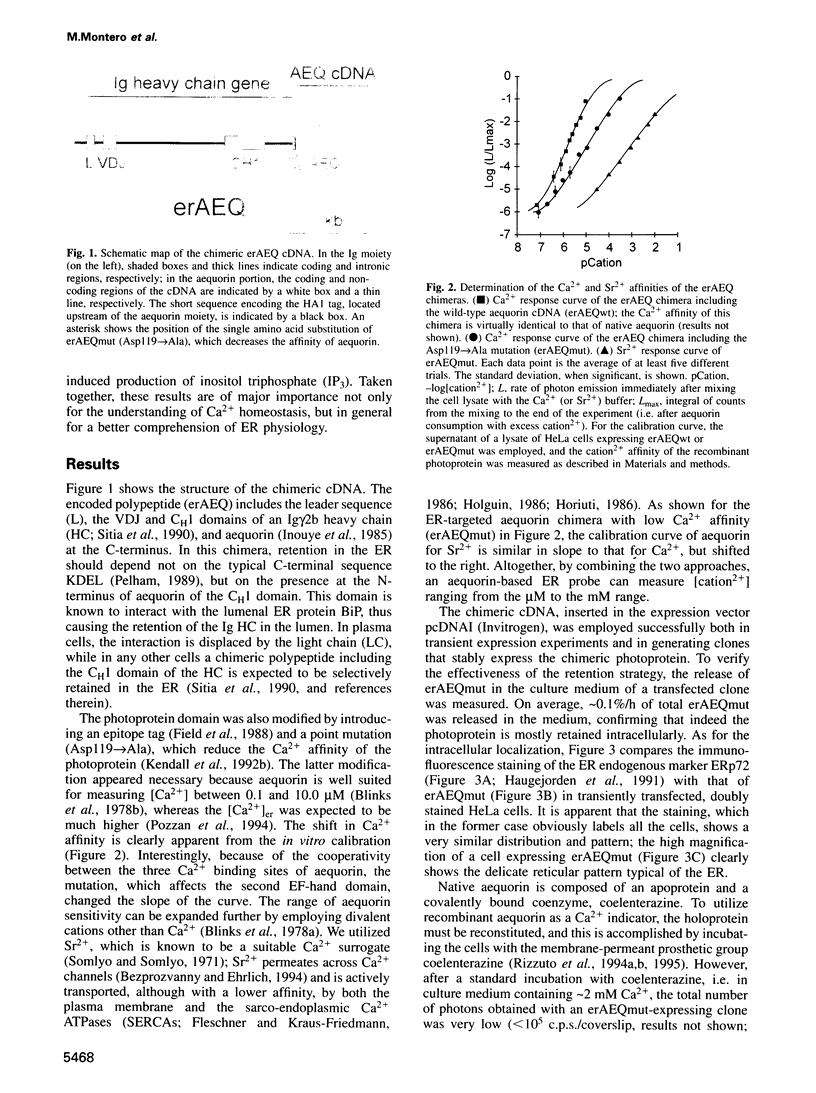
Abstract
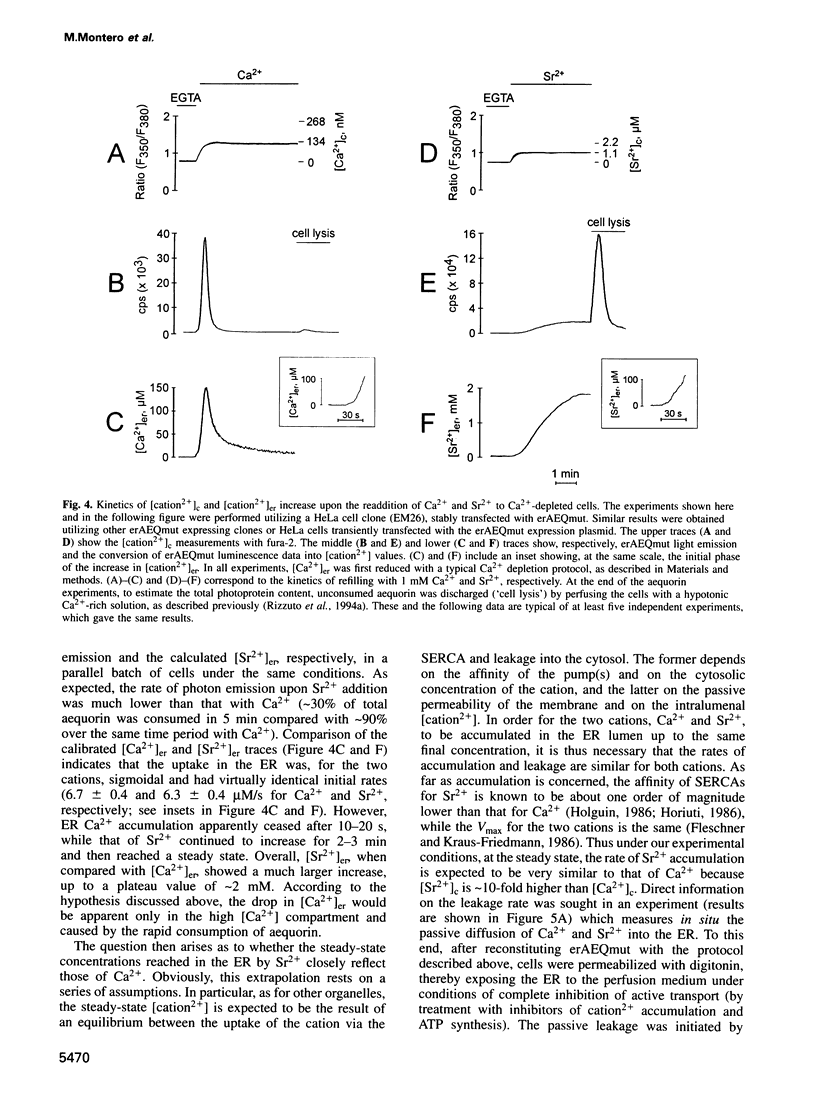
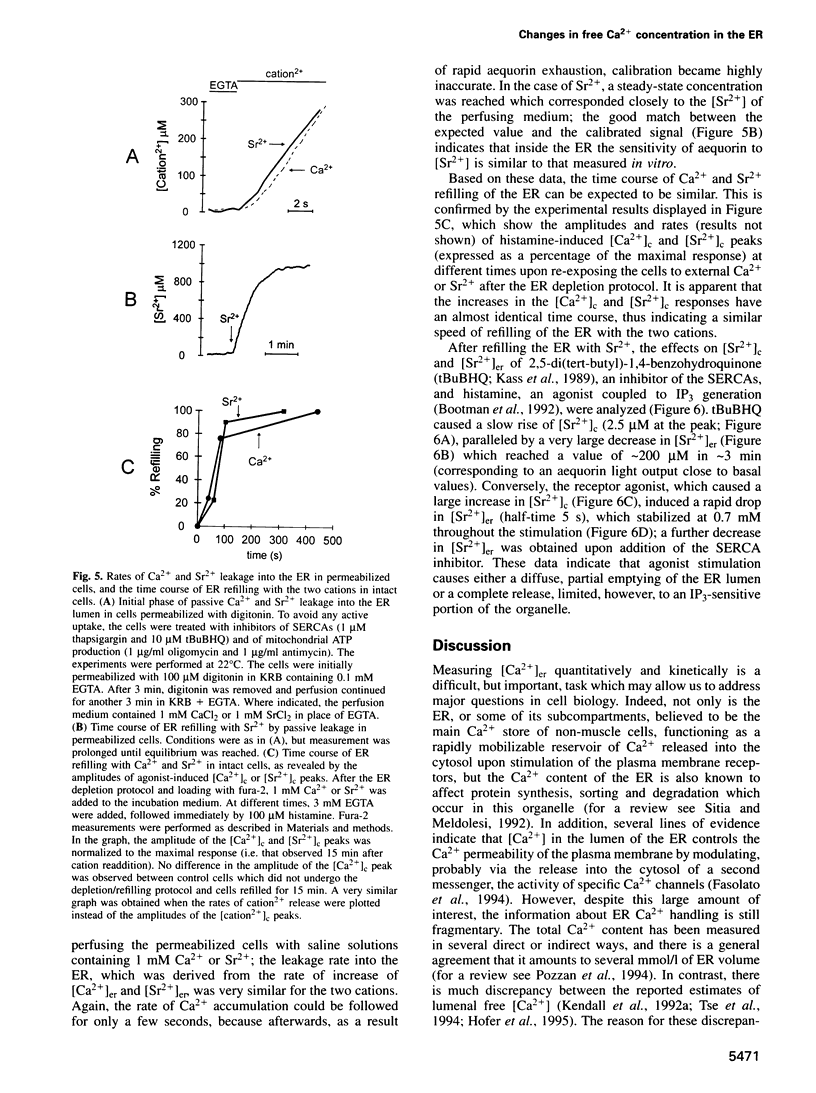
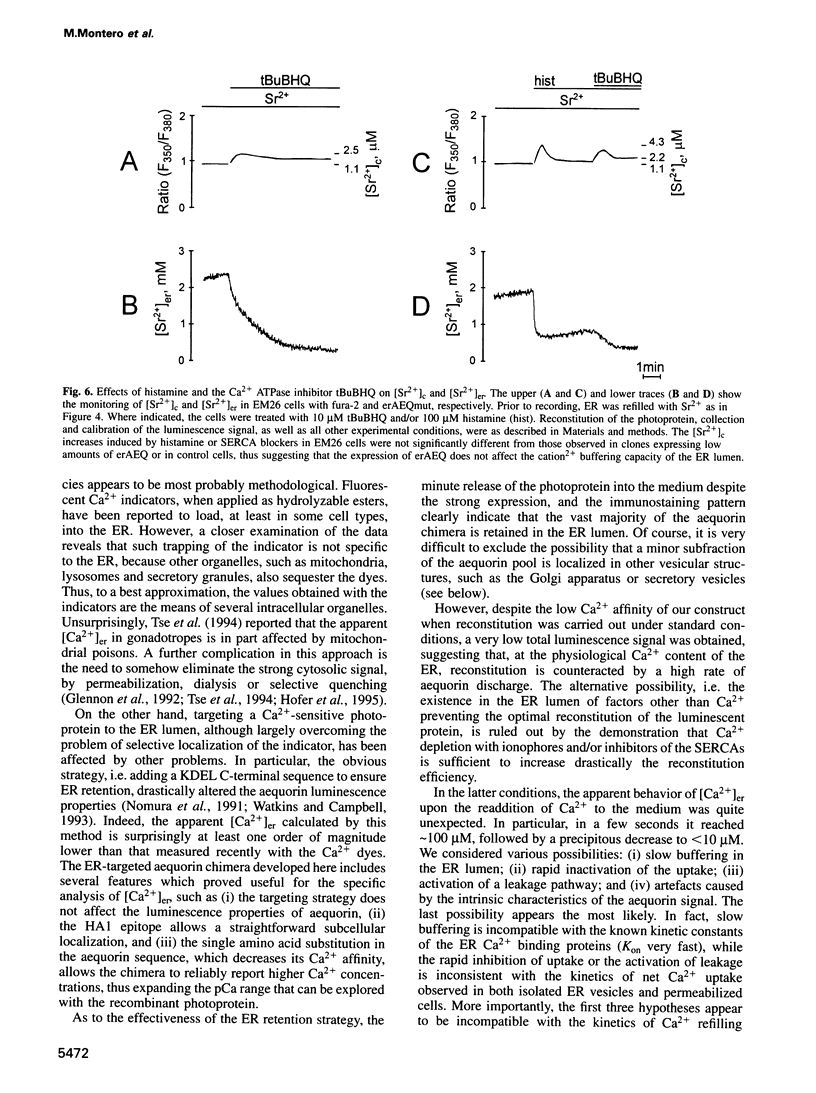
Direct monitoring of the free Ca2+ concentration in the lumen of the endoplasmic reticulum (ER) is an important but still unsolved experimental problem. We have shown that a Ca(2+)-sensitive photoprotein, aequorin, can be addressed to defined subcellular compartments by adding the appropriate targeting sequences. By engineering a new aequorin chimera with reduced Ca2+ affinity, retained in the ER lumen via interaction of its N-terminus with the endogenous resident protein BiP, we show here that, after emptying the ER, Ca2+ is rapidly re-accumulated up to concentrations of > 100 microM, thus consuming most of the reporter photoprotein. An estimate of the steady-state Ca2+ concentration was obtained using Sr2+, a well-known Ca2+ surrogate which elicits a significantly slower rate of aequorin consumption. Under conditions in which the rate and extent of Sr2+ accumulation in the ER closely mimick those of Ca2+, the steady-state mean lumenal Sr2+ concentration ([Sr2+]er) was approximately 2 mM. Receptor stimulation causes, in a few seconds, a 3-fold decrease of the [Sr2+]er, whereas specific inhibition of the ER Ca2+ ATPase leads to an approximately 10-fold drop in a few minutes.
Full text
PDF
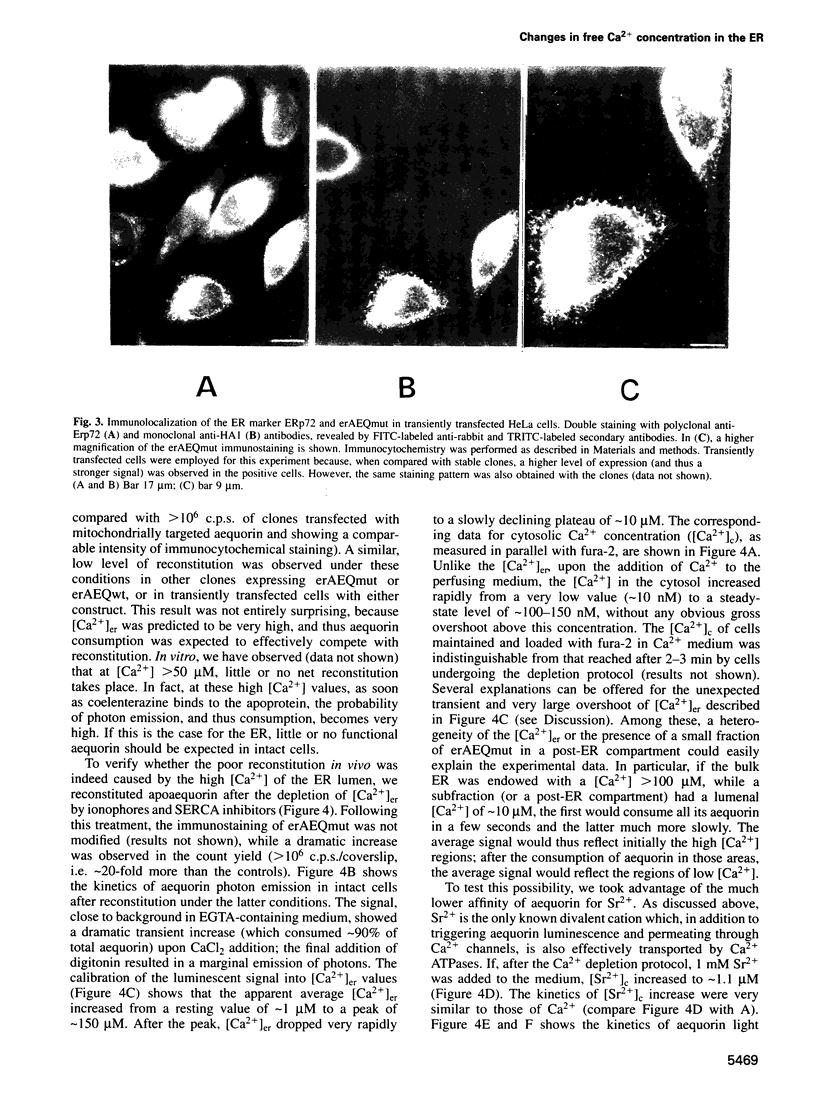



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. C., Ridgway E. B. Simultaneous recording of membrane potential, calcium transient and tension in single muscle fibers. Nature. 1968 Sep 14;219(5159):1168–1169. doi: 10.1038/2191168a0. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Ehrlich B. E. Inositol (1,4,5)-trisphosphate (InsP3)-gated Ca channels from cerebellum: conduction properties for divalent cations and regulation by intraluminal calcium. J Gen Physiol. 1994 Nov;104(5):821–856. doi: 10.1085/jgp.104.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Allen D. G., Prendergast F. G., Harrer G. C. Photoproteins as models of drug receptors. Life Sci. 1978 Apr 3;22(13-15):1237–1244. doi: 10.1016/0024-3205(78)90092-9. [DOI] [PubMed] [Google Scholar]
- Bootman M. D., Berridge M. J., Taylor C. W. All-or-nothing Ca2+ mobilization from the intracellular stores of single histamine-stimulated HeLa cells. J Physiol. 1992 May;450:163–178. doi: 10.1113/jphysiol.1992.sp019121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brini M., Marsault R., Bastianutto C., Alvarez J., Pozzan T., Rizzuto R. Transfected aequorin in the measurement of cytosolic Ca2+ concentration ([Ca2+]c). A critical evaluation. J Biol Chem. 1995 Apr 28;270(17):9896–9903. doi: 10.1074/jbc.270.17.9896. [DOI] [PubMed] [Google Scholar]
- Brini M., Murgia M., Pasti L., Picard D., Pozzan T., Rizzuto R. Nuclear Ca2+ concentration measured with specifically targeted recombinant aequorin. EMBO J. 1993 Dec;12(12):4813–4819. doi: 10.1002/j.1460-2075.1993.tb06170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W. P., Nickoloff J. A. Site-directed mutagenesis of virtually any plasmid by eliminating a unique site. Anal Biochem. 1992 Jan;200(1):81–88. doi: 10.1016/0003-2697(92)90280-k. [DOI] [PubMed] [Google Scholar]
- Fasolato C., Innocenti B., Pozzan T. Receptor-activated Ca2+ influx: how many mechanisms for how many channels? Trends Pharmacol Sci. 1994 Mar;15(3):77–83. doi: 10.1016/0165-6147(94)90282-8. [DOI] [PubMed] [Google Scholar]
- Field J., Nikawa J., Broek D., MacDonald B., Rodgers L., Wilson I. A., Lerner R. A., Wigler M. Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol Cell Biol. 1988 May;8(5):2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleschner C. R., Kraus-Friedmann N. The effect of Mg2+ on hepatic microsomal Ca2+ and Sr2+ transport. Eur J Biochem. 1986 Jan 15;154(2):313–320. doi: 10.1111/j.1432-1033.1986.tb09399.x. [DOI] [PubMed] [Google Scholar]
- Glennon M. C., Bird G. S., Takemura H., Thastrup O., Leslie B. A., Putney J. W., Jr In situ imaging of agonist-sensitive calcium pools in AR4-2J pancreatoma cells. Evidence for an agonist- and inositol 1,4,5-trisphosphate-sensitive calcium pool in or closely associated with the nuclear envelope. J Biol Chem. 1992 Dec 15;267(35):25568–25575. [PubMed] [Google Scholar]
- Haugejorden S. M., Srinivasan M., Green M. Analysis of the retention signals of two resident luminal endoplasmic reticulum proteins by in vitro mutagenesis. J Biol Chem. 1991 Apr 5;266(10):6015–6018. [PubMed] [Google Scholar]
- Hirose K., Iino M. Heterogeneity of channel density in inositol-1,4,5-trisphosphate-sensitive Ca2+ stores. Nature. 1994 Dec 22;372(6508):791–794. doi: 10.1038/372791a0. [DOI] [PubMed] [Google Scholar]
- Hofer A. M., Machen T. E. Technique for in situ measurement of calcium in intracellular inositol 1,4,5-trisphosphate-sensitive stores using the fluorescent indicator mag-fura-2. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2598–2602. doi: 10.1073/pnas.90.7.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer A. M., Schlue W. R., Curci S., Machen T. E. Spatial distribution and quantitation of free luminal [Ca] within the InsP3-sensitive internal store of individual BHK-21 cells: ion dependence of InsP3-induced Ca release and reloading. FASEB J. 1995 Jun;9(9):788–798. doi: 10.1096/fasebj.9.9.7601343. [DOI] [PubMed] [Google Scholar]
- Holguín J. A. Cooperative effects of Ca2+ and Sr2+ on sarcoplasmic reticulum adenosine triphosphatase. Arch Biochem Biophys. 1986 Nov 15;251(1):9–16. doi: 10.1016/0003-9861(86)90045-7. [DOI] [PubMed] [Google Scholar]
- Horiuti K. Some properties of the contractile system and sarcoplasmic reticulum of skinned slow fibres from Xenopus muscle. J Physiol. 1986 Apr;373:1–23. doi: 10.1113/jphysiol.1986.sp016032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Noguchi M., Sakaki Y., Takagi Y., Miyata T., Iwanaga S., Miyata T., Tsuji F. I. Cloning and sequence analysis of cDNA for the luminescent protein aequorin. Proc Natl Acad Sci U S A. 1985 May;82(10):3154–3158. doi: 10.1073/pnas.82.10.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass G. E., Duddy S. K., Moore G. A., Orrenius S. 2,5-Di-(tert-butyl)-1,4-benzohydroquinone rapidly elevates cytosolic Ca2+ concentration by mobilizing the inositol 1,4,5-trisphosphate-sensitive Ca2+ pool. J Biol Chem. 1989 Sep 15;264(26):15192–15198. [PubMed] [Google Scholar]
- Kendall J. M., Dormer R. L., Campbell A. K. Targeting aequorin to the endoplasmic reticulum of living cells. Biochem Biophys Res Commun. 1992 Dec 15;189(2):1008–1016. doi: 10.1016/0006-291x(92)92304-g. [DOI] [PubMed] [Google Scholar]
- Kendall J. M., Sala-Newby G., Ghalaut V., Dormer R. L., Campbell A. K. Engineering the CA(2+)-activated photoprotein aequorin with reduced affinity for calcium. Biochem Biophys Res Commun. 1992 Sep 16;187(2):1091–1097. doi: 10.1016/0006-291x(92)91309-e. [DOI] [PubMed] [Google Scholar]
- Nomura M., Inouye S., Ohmiya Y., Tsuji F. I. A C-terminal proline is required for bioluminescence of the Ca(2+)-binding photoprotein, aequorin. FEBS Lett. 1991 Dec 16;295(1-3):63–66. doi: 10.1016/0014-5793(91)81385-l. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- Pozzan T., Rizzuto R., Volpe P., Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev. 1994 Jul;74(3):595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Bastianutto C., Brini M., Murgia M., Pozzan T. Mitochondrial Ca2+ homeostasis in intact cells. J Cell Biol. 1994 Sep;126(5):1183–1194. doi: 10.1083/jcb.126.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R., Brini M., Bastianutto C., Marsault R., Pozzan T. Photoprotein-mediated measurement of calcium ion concentration in mitochondria of living cells. Methods Enzymol. 1995;260:417–428. doi: 10.1016/0076-6879(95)60155-4. [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Brini M., Murgia M., Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993 Oct 29;262(5134):744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Brini M., Pozzan T. Targeting recombinant aequorin to specific intracellular organelles. Methods Cell Biol. 1994;40:339–358. doi: 10.1016/s0091-679x(08)61121-8. [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Simpson A. W., Brini M., Pozzan T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature. 1992 Jul 23;358(6384):325–327. doi: 10.1038/358325a0. [DOI] [PubMed] [Google Scholar]
- Sitia R., Meldolesi J. Endoplasmic reticulum: a dynamic patchwork of specialized subregions. Mol Biol Cell. 1992 Oct;3(10):1067–1072. doi: 10.1091/mbc.3.10.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitia R., Neuberger M., Alberini C., Bet P., Fra A., Valetti C., Williams G., Milstein C. Developmental regulation of IgM secretion: the role of the carboxy-terminal cysteine. Cell. 1990 Mar 9;60(5):781–790. doi: 10.1016/0092-8674(90)90092-s. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Somlyo A. P. Strontium accumulation by sarcoplasmic reticulum and mitochondria in vascular smooth muscle. Science. 1971 Nov 26;174(4012):955–958. doi: 10.1126/science.174.4012.955. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Tse F. W., Tse A., Hille B. Cyclic Ca2+ changes in intracellular stores of gonadotropes during gonadotropin-releasing hormone-stimulated Ca2+ oscillations. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9750–9754. doi: 10.1073/pnas.91.21.9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins N. J., Campbell A. K. Requirement of the C-terminal proline residue for stability of the Ca(2+)-activated photoprotein aequorin. Biochem J. 1993 Jul 1;293(Pt 1):181–185. doi: 10.1042/bj2930181. [DOI] [PMC free article] [PubMed] [Google Scholar]