Abstract
A novel Chlamydia muridarum antigen (TC0582) was used to vaccinate BALB/c mice. Mice were also immunized with other components of the ATP synthase complex (TC0580, TC0581, and TC0584), or with the major outer membrane protein (MOMP). TC0582 was also formulated in combination with TC0580, TC0581 or MOMP. TC0582 alone, or in combination with the other antigens, elicited strong Chlamydia-specific humoral and cellular immune responses. Vaccinated animals were challenged intranasally and the course of the infection was followed for 10 days. Based on percentage change in body weight, lung weight, and number of Chlamydia inclusion forming units recovered from the lungs, mice immunized with TC0582, TC0581 or MOMP, as single antigens, showed significant protection. Mice immunized with combinations of two antigens were also protected but the level of protection was not additive. TC0582 has sequence homology with the eukaryotic ATP synthase subunit A (AtpA). Therefore, to determine if immunization with TC0582, or with Chlamydia, elicited antibodies that cross-reacted with the mouse AtpA, the two proteins were printed on a microarray. Sera from mice immunized with TC0582 and/or live Chlamydia, strongly reacted with TC0582 but did not recognize the mouse AtpA. In conclusion, TC0582 may be considered as a Chlamydia vaccine candidate.
Keywords: Chlamydia, vaccine, immunization, mice, ATP synthase subunit A
1. Introduction
Chlamydia trachomatis is worldwide the most common sexually transmitted bacterial pathogen and the causative agent of ocular, gastrointestinal and respiratory infections [1–3]. Attempts to control this organism using antibiotics have failed and therefore, a vaccine program has been considered [4–7]. Using whole inactivated and viable C. trachomatis vaccination trials were performed to protect against trachoma [2, 8, 9]. The protection was found to be short-lived, serovar specific and in some individuals, a hypersensitivity reaction was observed after re-exposure to Chlamydia [2]. The cause of the hypersensitivity reaction is thought to be mediated by a component present in Chlamydia and therefore, efforts are now focused on formulating a subunit vaccine [10, 11].
The major outer membrane protein (MOMP) of C. trachomatis has been tested as a vaccine in several models [5, 6, 12–15]. For example, mice immunized with native MOMP (nMOMP) showed protection against genital and respiratory challenges [14, 16, 17]. The protection elicited by nMOMP was found to be, at least in part, dependent on its native structure [17]. Extraction of the native MOMP cannot be scaled up at a reasonable cost. Furthermore, the serovar specific protection observed during the trachoma vaccine trials was thought to be mediated by MOMP [18]. Therefore, additional antigens need to be identified to formulate a broadly protective vaccine.
By probing a Chlamydia muridarum (previously called C. trachomatis mouse pneumonitis [MoPn] biovar) proteome microarray with sera from mice infected with this pathogen, the protein coded by the open reading frame (ORF) TC0582 was identified as a novel immunodominant antigen [19]. TC0582 is a highly conserved V-type ATP synthase subunit A (AtpA), which is part of the hexamer of three AtpA and three AtpB subunits and has sequence identity with its eukaryotic homologue. The AtpB from different bacteria have been reported as being immunodominant antigens [20]. Recently, TC0582 was found to be preferentially recognized by sera from mice that developed hydrosalpinx following a vaginal infection with C. muridarum and therefore, was considered as a potential pathology-associated antigen [21].
Here, we investigated the protective efficacy of TC0582 and related antigens (TC0580, TC0581, and TC0584), and assessed its potential role in the immunopathogenesis of a chlamydial infection. Our results show that TC0582 can elicit protection against a challenge with Chlamydia and is likely not involved in inducing tissue damage. Therefore, TC0582 should be considered as a potential Chlamydia vaccine candidate.
2. Materials and methods
2.1. Cloning of the C. muridarum TC0580, TC0581, TC0582, TC0584 and MOMP and the Neisseria gonorrhoeae porin B (Ng-rPorB) ORF
C. muridarum (ATCC; Manassas, VA) was grown in HeLa-229 cells and purified elementary bodies (EB) were stored at −70°C [12, 22]. Genomic DNA from C. muridarum and N. gonorrhoeae strain FA 1090 (ATCC) were extracted [23] and the TC0580, TC0581, TC0582, and TC0584 genes were amplified with Pfu Turbo DNA Polymerase (Stratagene, La Jolla, CA) using the following primers: TC0580 F: 5'-GGGGTACCTCTTCACAAATAAAATTAAC-3' and R: 5'-CGGGATCCCTACTCCTTATGCTGCTGAATT; TC0581 F: 5'-GGGGTACCCAAACAATATATACAAGAA-3' and R: 5'-ATAGTTTAGCGGCCGCTTATTTGTGAAGACATGCT-3'; TC0582 F: 5'-CATGCCATGGTAGCAACTTCAAAAGA-3' and R: 5'-ATAGTTTAGCGGCCGCCGTCTGCACCATTTTGC-3'; TC0584 F: 5'-GGGGTACCGCAGATCTCAGCGCTCAGG-3' and R: 5'-CGGGATCCCTAACAAGACTGAAAAATC-3'. TC0580, TC0581, and TC0584 were cloned into the pET-45b vector (Novagen, Gibbstown, NJ). The C. muridarum MOMP and the N. gonorrhoeae porin B (Ng-PorB) genes were amplified without the signal sequence as described [17]. After confirmation by DNA sequencing the proteins were expressed [17].
2.2. Purification of antigens
The TC0580, TC0581, TC0582 and TC0584 His-tagged proteins were extracted from the Escherichia coli inclusion bodies using the Invitrogen ProBond™ (Carlsbad, CA). The MOMP and Ng-rPorB proteins were isolated as described by Marston [24]. Following solubilization, the MOMP and Ng-rPorB proteins were loaded onto a Sephacryl-S-300 column and the peak fractions were pooled [16, 23, 25, 26].
Before immunization all proteins were dialyzed against PBS (pH 7.4) with 0.05% Z3–14 and stored at −80°C [27]. The apparent MW and purity of TC0580, TC0581, TC0582, TC0584, MOMP and Ng-rPorB proteins were determined by 10% tricine-SDS-PAGE [28]. Using the limulus amoebocyte assay (BioWhittaker, Inc., Walkersville, MD), the recombinant antigens were found to have less than 0.05 EU of LPS/mg of protein.
2.3. Immunization protocols
Three-weeks-old female BALB/c (H-2d) mice (Charles River Laboratories; Wilmington, MA) were housed at the University of California, Irvine, Vivarium. The University of California, IACUC approved all animal protocols.
CpG-1826 (TriLink, San Diego, CA; 10 μg/mouse/immunization) and Montanide ISA 720 VG (SEPPIC inc., Fairfield, NJ; 70% of total volume) as adjuvants were directly mixed with single antigens (TC0580, TC0581, TC0582, TC0584, MOMP, Ng-rPorB; 10 μg/mouse/immunization) or antigen combinations (TC0582 + TC0581, TC0582 + TC0580, TC0582 + MOMP; 10 μg of each protein/mouse/immunization) [16, 29].
A pilot study was performed with five mice per group (Supplemental Table 1). Antigens that elicited protection were subsequently tested with additional mice (Tables 1, 2). Mice were immunized three times by the intramuscular plus subcutaneous (i.m./s.c.) routes at 2-week intervals. As positive vaccine controls mice were immunized i.n. once with 104 inclusion forming units (IFU) of C. muridarum while a negative control group was inoculated once i.n. with 20 μl of MEM-0 [29, 30]. Mice were challenged i.n. four weeks after the last immunization.
Table 1.
Serum antibody geometric mean titer (range) the day before the i.n. challenge.
| Vaccine | Anti-C. muridarum EB | Anti-TC0582 | Anti-rMOMP | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| IgG1 | IgG2a | IgG2a/IgG1 | IgA | IgG | IgA | IgG | IgA | |
| TC0582 + CpG + Montanide | <100 | <100 | --- | <100 | 204,800 (204,800–204,800) | 40,637 (25,600–51,200) | <100 | <100 |
| MOMP + CpG + Montanide | 12,800 (3,200–51,200) | 162,550 (102,400–204,800) | 13 | 4,032 (3,200–6,400) | <100 | <100 | 1,638,400 (1,638,400–1,638,400) | 102,400 (102,400–102,400) |
| MOMP + TC0582 + CpG + Montanide | 10,159 (3,200–25,600) | 51,200 (51,200–51,200) | 5 | 635 (400–800) | 204,800 (204,800–204,800) | 40,637 (25,600–51,200) | 409,600 (409,600–409,600) | 40,637 (25,600–51,200) |
| Ng-rPorB + CpG + Montanide | <100 | <100 | --- | <100 | <100 | <100 | <100 | <100 |
| 10,000 live EB | 3,200 (3,200–3,200) | 12,800 (12,800–12,800) | 4 | 504 (400–800) | 566 (400–800) | <100 | 3,200 (3,200–3,200) | 200 (200–200) |
| MEM-0 | <100 | <100 | --- | <100 | <100 | <100 | <100 | <100 |
Table 2.
Disease burden, yields of C. muridarum detected in the lungs and C. muridarum-specific IgA and IFN-γ levels at 10 days p.c.
| Vaccine | Times and routes of immunization | # of mice per group | %BWCa | Lungs weight (g) | # of IFU | IgA in lungs (OD405) | IFN-γ in lungs (pg/ml) |
|---|---|---|---|---|---|---|---|
| Mean ± 1SE | Mean ± 1SE | Median (range) ×106 | Mean ± 1SE | Mean ± 1SE | |||
| TC0582 + CpG + Montanide | 3 × i.m./s.c. | 26 | −7.60 ± 0.99d | 0.30 ± 0.01 | 17 (0.2–2,738) | 0.335 ± 0.011 | 2,611 ± 665 |
| MOMP + CpG + Montanide | 3 × i.m./s.c. | 17 | −5.86 ± 0.95 | 0.23 ±0.01b | 0.6 (0.007 – 6,382)c | 0.536 ± 0.023b | 825 ± 377b |
| MOMP + TC0582 + CpG + Montanide | 3 × i.m./s.c. | 16 | −3.56 ± 0.92b,d | 0.26 ± 0.02b,d | 2.9 (0.003–190)c,e | 0.558 ± 0.071b,d | 1,065 ± 361b,d |
| Ng-PorB + CpG + Montanide | 3 × i.m./s.c. | 18 | −15.45 ± 1.22b | 0.35 ±0.01b | 1,136 (10.3–8,415)c | 0.258 ± 0.012b | 3,317 ± 252 |
| 10,000 live EB | 1 × i.n. | 21 | 1.70 ± 0.44 | 0.20 ± 0.01 | BLDf | 2.552 ± 0.157 | BLDf |
| MEM-0 | 1 × i.n. | 21 | −18.73 ± 1.08b | 0.34 ± 0.01b | 2,168 (17–42,952)c | 0.214 ± 0.020b | 2,970 ± 298 |
BWC, body weight change.
p < 0.05 determined by the Student's t-test in comparison to the group of mice immunized only with TC0582.
p < 0.05 determined by the Mann-Whitney U-test in comparison to the groups of mice immunized only with TC0582.
p > 0.05 determined by the Student's t-test in comparison to the group of mice immunized only with MOMP.
p > 0.05 determined by the Mann-Whitney U-test in comparison to the groups of mice immunized only with MOMP.
BLD, below limit of detection.
2.4. Characterization of the humoral response elicited by vaccination
Blood was collected from each mouse the day before the challenge. To measure Chlamydia-specific, or antigen-specific antibody levels, an ELISA was performed as previously described [30]. Each well of a 96-well plate was coated with 100 μl per well of EB (10 μg/ml of protein) or TC0582 or MOMP protein (1 μg/ml) in PBS. Serial dilutions of serum were added and, following incubation, the plates were washed, horseradish peroxidase-conjugated goat anti-mouse immunoglobulin (Ig)G (KPL, Gaithersburg, MD), IgG1, IgG2a (BD Pharmingen, San Diego, CA), and IgA (ICN Pharmaceuticals, Aurora, Ohio) were added. The binding was measured in an ELISA reader (Labsystem Multiscan; Helsinki, Finland) using 2, 2'-azino-bis-(3-ethylbenzthiazoline-6-sulfonate) as the substrate. The geometric mean titers (GMT) are expressed as the inverse of the highest dilution that gave a positive result [31].
The Western blot was performed as previously described [30]. Approximately 40 mg of purified EB were loaded on a polyacrylamide gel. Following transfer to a nitrocellulose membrane, nonspecific binding was blocked with BLOTTO overnight. Serum samples were added to the membranes and incubated. The membrane was washed and incubated with horseradish peroxidase-conjugated goat anti-mouse antibody, followed by visualization of the bands with 0.01% hydrogen peroxide and 4-chloro-1-naphthol.
2.5. In vitro splenic T-cell responses following vaccination
To assess the T-cell memory response following immunization, an in vitro LPA was performed before the i.n. challenge as previously described [30, 32]. In brief, spleens were harvested and T-cell-enriched lymphocytes (105 cells/well) were cultured with antigen presenting cells (APCs; 1.25 × 105/well), which were prepared by irradiating (3,000 rads; 137Cs) syngeneic unseparated splenocytes, and incubated with UV-inactivated C. muridarum EB at a 1:1 ratio. Concanavalin A (ConA; Sigma-Aldrich, St. Louis, MO) was used as a positive stimulant and tissue culture media as a negative control. After 96 hrs of incubation, 1.0 μCi of [methyl-3H] thymidine (47 Ci/mmol; Amersham, Arlington Heights, IL) was added to each well and the uptake of the [3H] thymidine was measured at 48 hrs.
Levels of IFN-γ in supernatants from the splenic T-cells stimulated with UV-inactivated EB were determined with ELISA kits (BD Pharmingen, San Diego, CA) and measured (Labsystem Multiscan; Helsinki, Finland) at 450nm using tetramethylbenzidine [30].
2.6. Intranasal challenge with C. muridarum
Mice were challenged i.n. with 104 IFU of C. muridarum and for 10 consecutive days xtheir body weights were monitored [30]. At day 10 post-challenge (p.c.) mice were euthanized, their lungs harvested, weighed, homogenized and 10-fold serial dilutions inoculated onto HeLa-229 cells. The chlamydial inclusions were stained with a cocktail of monoclonal antibodies prepared in our laboratory [30]. The limit of detection per mouse was 50 IFU.
To assess the local humoral and cellular immunities in the lungs, the titers of Chlamydia-specific IgA and levels of IFN-γ were determined by an ELISA as described above using a 1:2 dilution of the supernatants of homogenized lungs from each mouse.
2.7. Cross reactivity between TC0582 and the mouse ATP6v1a gene
TC0582 has ~40% amino acid sequence identity with its eukaryotic homolog. In order to determine whether antibodies from mice immunized with TC0582 cross-reacted with the mouse ATP synthetase subunit A protein, sera from immunized mice were tested using a protein microarray chip. The mouse ATP6v1a gene was purchased from OriGene Technologies (Rockville, MD). Mouse ATP6v1a and TC0582, with His and HA tags at the 3' and 5' end, respectively, were cloned into a T7 promoter based plasmid expression vector pET-28b vector (Novagen, Gibbstown, NJ) [33]. The genes were expressed using the RTS 100 E. coli HY kit (Roche, Indianapolis, IN) [33]. The expression of both genes were quality controlled by using anti-His and anti-HA antibodies.
2.8. Characterization of the antibody response to TC0582
To determine the timing and levels of antibody production to TC0582 sera were collected from three strains of mice infected with C. muridarum EB by the i.n. or intravaginal (i.vag.) routes [34]. For i.n. inoculation, 12 BALB/c and C57BL/6 mice received 104 IFU/mouse of MoPn while C3H/HeN mice were infected with 101 IFU. For i.vag. infection mice received 105 IFU/mouse [22, 30, 35–37]. Six months after infection the animals were euthanized and their genital tract inspected for hydrosalpinx. Serum samples were collected every two weeks after infection and probed on the C. muridarum protein array as previously described [33, 38].
Antigen-specific signal intensities were first corrected for background noise by using QuantArray software (Perkin Elmer, Waltham, MA). In addition to the target antigen TC0582, control antigens TC0052 (MOMP), TC0386 (hsp60), and TC0727 (omp2) were evaluated. The data was transformed using the log variant asinh. The antigen signal intensity data were averaged and no DNA control plus two standard deviations was removed. Next, the signal of each antigen the day before infection was subtracted from that specific antigen signal post-infection. The signal intensity from each animal was then transformed using log and then plotted over time.
2.9. Statistical analyses
The two-tailed unpaired Student's t-test, the Mann-Whitney U-test, and repeated measures ANOVA were employed to determine the significance of the differences between groups.
3. Results
3.1. Pilot vaccination experiment
Following the C. muridarum i.n. challenge mice were weighed daily and, at 10 days p.c., they were euthanized, their lungs collected, weighed and the number of IFU determined (Supplemental Table 1). Mice vaccinated with TC0582 had lost least body weight at day 10 p.c. (−3.79 ± 0.87), had the lightest lung weight (0.23 ± 0.02 g) and the lowest number of Chlamydia in the lungs [(median: 1.9; range: 0.8 – 25) × 106 IFU] when compared to the other three groups of mice immunized with a single antigen. The mice immunized with TC0581, when compared to the negative control group, also showed partial protection based on the percentage change in mean body weight, lung weight, and yield of IFU from the lungs, whereas TC0580 and TC0584 were not protected.
To determine if enhanced protection could be obtained by combining two of these antigens mice were immunized with TC0580 plus TC0582 or with TC0581 plus TC0582. As shown in Supplemental Table 1, none of these combinations showed better protection than TC0582 by itself. Based on these results, further testing was performed only with TC0582.
3.2. Humoral immune responses following vaccination
To further assess the protective capacity of T0582 and its ability to enhance the protection elicited by MOMP, mice were vaccinated with these two antigens singly or in combination (Tables 1, 2). Sera from mice immunized with TC0582 showed no reactivity to C. muridarum EB or MOMP but had high IgG (GMT: 204,800; range: 204,800–204,800) and IgA (GMT: 40,637; range: 25,600–51,200) titers to TC0582. When using EB as the antigen, the mice immunized with MOMP showed a strong Th1 biased response with high IgG2a in comparison to IgG1 titers (GMT: IgG1= 12,800 and IgG2a= 162,550; IgG2a/IgG1 ratio: 13). The IgG anti-MOMP titers (GMT= 1,638,400; range: 1,638,400–1,638,400) were high in animals immunized with MOMP. This group of mice had no detectable antibody to TC0582. Mice immunized with TC0582 + MOMP also had Th1 biased immune response (IgG1= 10,159 and IgG2a= 51,200, IgG2a/IgG1= 5) when using EB as the antigen. The IgG antibody titer to TC0582 in this group of mice was not different from that of the group immunized only with TC0582. In contrast, in mice immunized with a combination of MOMP and TC0582 the IgG antibody titer to MOMP (GMT IgG=409,600; range: 409,600–409,600) was lower than in the group only vaccinated with MOMP. As expected, the positive control group immunized with live EB elicited a Th1-biased response with higher EB-specific IgG2a than IgG1 antibody levels (GMT: IgG1= 3,200 and IgG2a= 12,800, IgG2a/IgG1= 4). Positive control mice also had IgG antibodies to MOMP and TC0582 (GMT= 3,200; range: 3,200–3,200; GMT=566; range: 400–800), respectively.
A higher EB-specific IgA titer was detected in mice vaccinated with MOMP (GMT: 4,032; range: 3,200–6,400) when compared to mice immunized with MOMP + TC0582 (GMT: 635; range: 400–800) or with TC0582 alone (<100). In contrast, in mice vaccinated with MOMP + TC0582, or with TC0582 alone, the anti-TC0582 IgA were the same (GMT=40,637; range: 25,600–51,200). The anti-MOMP IgA titers in mice vaccinated with MOMP + TC0582 versus with MOMP only were different (GMT=40,637; range: 25,600–51,200, versus GMT= 102,400; range: 102,400–102,400). No detectable IgG or IgA anti-C. muridarum antibodies were found in mice immunized with Ng-rPorB or MEM-0.
By Western blot mice vaccinated with TC0582 developed antibodies only against this protein (MW 65-kDa; Supplemental Fig. 1). Animals immunized with MOMP had specific antibodies only against this protein (MW 40-kDa). Mice vaccinated with the TC0582 and MOMP combination developed strong specific antibodies against MOMP but the 65-kDa band was fairly weak. Animals immunized i.n. with live C. muridarum produced antibodies against several antigenic components. Sera collected from preimmunized mice and from the mice immunized with Ng-rPorB or MEM-0 were negative by Western blot.
3.3. Cell mediated immune responses (CMI) following vaccination
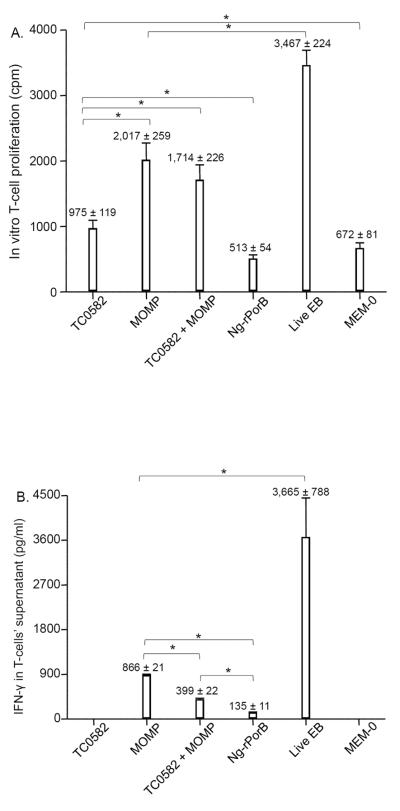
Mice immunized with TC0582 showed weaker T-cell proliferative response (975 ± 119 cpm) in comparison to animals vaccinated with MOMP (2,017 ± 259 cpm), or mice immunized with TC0582 + MOMP (1,714 ± 226 cpm, p < 0.05 respectively) (Fig. 1A). However, the proliferative response with TC0582 was stronger than the control mice immunized with Ng-rPorB (513 ± 54 cpm) or with MEM-0 (672 ± 81 cpm, p < 0.05 respectively). The most robust lymphoproliferative response was observed in control mice inoculated i.n. with live EB (3,467 ± 224 cpm; p < 0.05; compared to any other vaccinated group).
Fig. 1. In vitro splenic T-cell proliferative responses following immunization.
A. Splenic T-cells collected the day before the i.n. C. muridarum challenge were stimulated with UV-treated EB and the proliferative responses were determined (*, p < 0.05, by the two-tailed unpaired Student's t-test).
B. IFN-γ levels detected in the supernatants of proliferating splenic T-cells stimulated with UV-treated EB (*, p < 0.05, by the two-tailed unpaired Student's t-test).
No detectable IFN-g levels were found in mice vaccinated with TC0582 (Fig. 1B). Significantly higher levels of IFN-γ were obtained in the group of mice vaccinated with MOMP (886 ± 21 pg/ml) in comparison to the group immunized with TC0582 + MOMP (399 ± 22 pg/ml; p < 0.05). The highest level of IFN-γ was detected in the supernatants from mice vaccinated with MoPn EB (3,665 ± 788 pg/ml; p < 0.05, in comparison to any other group). Low levels of IFN-γ were found in mice immunized with Ng-rPorB or MEM-0.
3.4. Changes in mean body weight of mice following the i.n. challenge
Except animals immunized i.n. with live EB, all other groups of mice rapidly lost weight from days 2 to 4 p.c. (Fig. 2A). Subsequently, mice vaccinated with MOMP maintained their weight up to 10 days p.c. Mice immunized with TC0582 + MOMP gained more weight during the 10 days in comparison with mice immunized with MOMP (p < 0.05 by Repeated Measured ANOVA). Mice vaccinated with TC0582, Ng-rPorB or MEM-0 lost body weight during most of the 10 days.
Fig. 2. Disease burden following the i.n. challenge with C. muridarum.
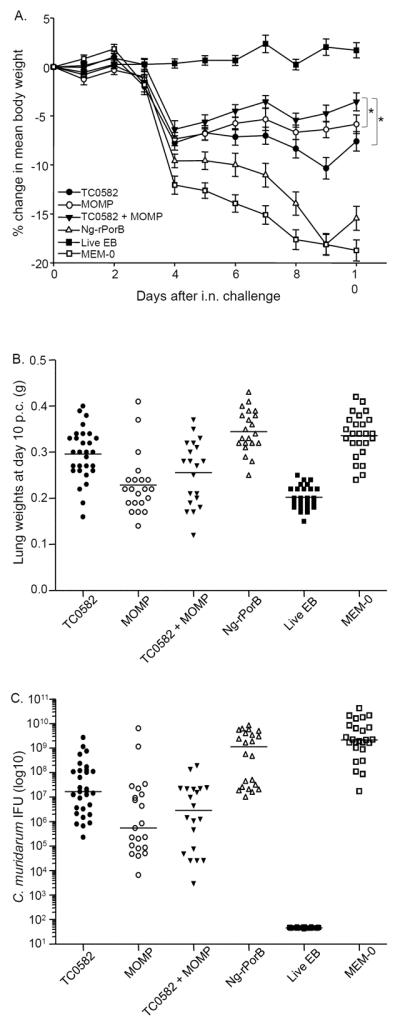
A. Daily percentage change in mean body weight following the i.n. challenge (*, p < 0.05 by the Repeated Measures ANOVA).
B. Lung weights (g) at day 10 after the i.n. challenge. The mean is shown as a horizontal line. Each symbol represents a single animal.
C. Number of Chlamydia IFU recovered from the lungs at day 10 after the i.n. challenge. The median is shown as a horizontal line. Each symbol represents a single animal.
As shown in Supplemental Fig. 2 and Table 2, at day 10 p.c. mice vaccinated with TC0582 + MOMP showed the least body weight loss (−3.56 ± 0.92%) in comparison to any other group of mice, except the control immunized i.n. with live EB which gained some weight by day 10 p.c. (+1.7 ± 0.44%). The body weight loss in the TC0582 + MOMP group was significantly less than in the group immunized with TC0582 (−7.6 ± 0.99%, p < 0.05). However, no significant difference was found between the TC0582 + MOMP group in comparison to mice immunized with MOMP (−5.86 ± 0.95%, p > 0.05). The negative control mice inoculated with MEM-0 (−18.73 ± 1.08%), or Ng-rPorB (−15.45 ± 1.22%), lost more body weight than any of the other groups (p < 0.05).
3.5. Burden of C. muridarum infection in the lungs
The mean weight of the lungs, used as a parameter of the local inflammatory response, from mice vaccinated with TC0582 was 0.30 ± 0.01 g, that was lighter than the negative control mice immunized with Ng-rPorB (0.35 ± 0.01 g) and the animals inoculated i.n. with MEM-0 (0.34 ± 0.01 g; p < 0.05) (Fig. 2B and Table 2). In contrast, the lung weight in these mice was significantly heavier than that of the group vaccinated with MOMP (0.23 ± 0.01 g) or the mice immunized with TC0582 + MOMP (0.26 ± 0.02 g; p < 0.05, respectively). There was no significant difference between the groups of mice vaccinated with MOMP or with TC0582 + MOMP (p > 0.05). The mean weight of the lungs of the positive control mice vaccinated with EB was 0.20 ± 0.01 g, significantly less than any other group (p < 0.05).
The median (range) number of IFU recovered from the lungs of mice vaccinated with TC0582 was 17 (0.2 – 2,738) × 106 IFU (Fig. 2C and Table 2). This was significantly less than the number of IFU from the negative controls immunized with Ng-rPorB [1,136 (10.3 – 8,415) × 106 IFU; p < 0.05], or inoculated i.n. with MEM-0 [2,168 (17 – 42,952) × 106 IFU; p < 0.05]. The number of IFU recovered from the TC0582 immunized group however, was significantly higher than the number of IFU from mice vaccinated with MOMP [0.6 (0.007 – 6,382) × 106 IFU; p < 0.05] or with TC0582 + MOMP [2.9 (0.003 – 190) × 106 IFU; p < 0.05]. There was no significant difference in the number of IFU recovered from mice vaccinated with MOMP versus the group immunized with TC0582 + MOMP (p > 0.05). The number of IFU recovered from the lungs of mice vaccinated with EB was below the level of detection (< 50 IFU/mouse).
3.6. Local humoral and cellular immune responses in the lungs at day 10 p.c.
The mean OD405 value of Chlamydia-specific IgA in the lungs of mice vaccinated with TC0582 (0.335 ± 0.011) was significantly higher than that of the negative control mice immunized with Ng-rPorB (0.258 ± 0.012), or with MEM-0 (0.214 ± 0.020) (p < 0.05, respectively), but lower than those of mice immunized with MOMP (0.536 ± 0.023) or with TC0582 + MOMP (0.558 ± 0.071; p < 0.05; Fig. 3A and Table 2). No significant difference in the levels of Chlamydia-specific IgA were detected between the mice immunized with MOMP versus TC0582 + MOMP (p > 0.05). As expected, the mice inoculated with EB had the highest Chlamydia-specific IgA levels (2.552 ± 0.157) in the lungs and that was significantly higher than in any other group (p < 0.05).
Fig. 3. Titers of C. muridarum-specific IgA and levels of IFN-γ present in the lungs.
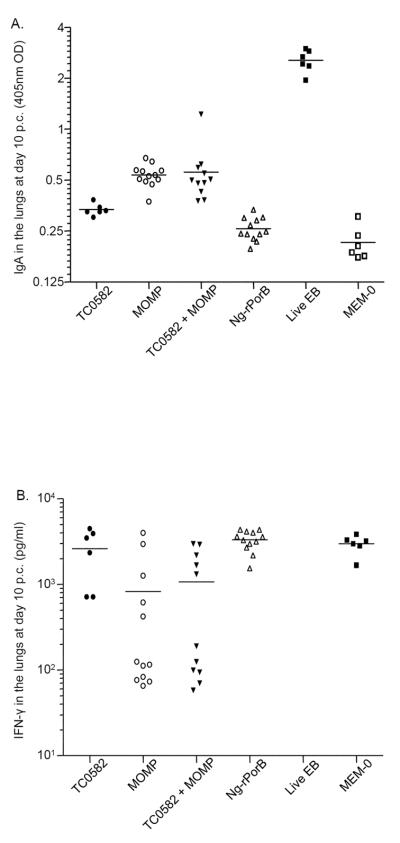
A. Levels of C. muridarum-specific IgA (OD405) detected in the lungs at day 10 following the i.n. challenge. The mean is shown as a horizontal line. Each symbol represents a single animal.
B. Levels of IFN-γ (pg/ml) detected in the lungs at day 10 after the i.n. challenge. The mean is shown as a horizontal line. Each symbol represents a single animal.
The mean level of IFN-γ in the lungs of mice vaccinated with TC0582 was 2,611 ± 665 pg/ml (Fig. 3B and Table 2). This was not significantly different than in the negative control animals immunized with Ng-rPorB (3,317 ± 252 pg/ml) or inoculated with MEM-0 (2,970 ± 298 pg/ml; p > 0.05, respectively). In contrast, the mean level of IFN-γ measured in the lungs of the TC0582 vaccinated group was significantly higher than that of mice immunized with MOMP (825 ± 377 pg/ml) or TC0582 + MOMP (1,065 ± 361 pg/ml; p < 0.05). There was no significant difference in the levels of IFN-γ detected in mice immunized with MOMP versus TC0582 + MOMP (p > 0.05). Undetectable levels of IFN-γ were found in the lungs of mice inoculated with EB.
3.7. Cross reactivity between the C. muridarum TC0582 and the mouse ATP6v1a
To determine if immunization with TC0582 elicited antibodies that cross-reacted with the mouse V-type ATP synthetase subunit A (AtpA), the ORF of both proteins were expressed and printed on the same microarray chip (Supplemental Fig. 3). All serum samples from mice immunized with TC0582, or infected i.n. with EB, reacted with the TC0582 protein. In contrast, none of these serum samples reacted with the mouse V-type ATP synthase subunit A protein, indicating that there is no cross-reactivity.
3.8. Antibody response to TC0582
To determine if the antibody response to TC0582 was an indicator of upper genital tract long-term sequelae, three strains of mice BALB/c, C3H/HeN and C57BL/6 were infected i.vag. or i.n. with C. muridarum. At six months post infection the mice were euthanized and their genital tract inspected for the presence of hydrosalpinx. Serum samples were collected every two weeks over a period of six months and tested for their ability to react with TC0582 and three other MoPn proteins used as controls, TC0052 (MOMP), TC0386 (60-kDa hsp) and TC0727 (60-kDa crp) (Fig. 4).
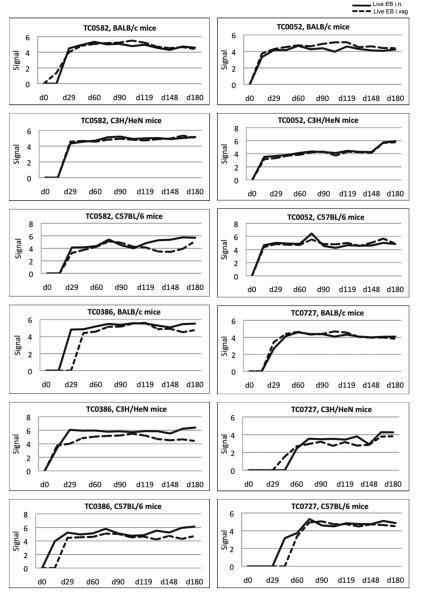
Fig. 4. Reactivity of serum samples to the TC0582, TC0052, TC0386 and TC0727 proteins, from mice infected i.n. or i.vag. with C. muridarum.
Signal intensity of TC0582, TC0052, TC0386, and TC0727 proteins tested with sera from three strains (BALB/c, C3H/HeN, and C57BL/6) infected i.n. (solid line) or by the i.vag. route (dashed line) with MoPn.
Antibodies to TC0582 were detected at 14 days p.i. in BALB/c mice infected i.vag. and at 28 days following the i.n. infection. In both C3H/HeN and C57BL/6 mice a positive signal was detected by day 28 independently of the route of infection. Antibodies to the TC0052 protein were detected at 14 days p.i. in all three strains of mice independently of the route of infection. Reactivity to the TC0386 was first detected at 14 days p.i. in C3H/HeN mice inoculated i.vag or i.n and in the C57BL/6 infected i.n. The most delayed reaction (day 42) was observed in BALB/c mice inoculated i.vag. Antibodies to the TC0727 were overall detected later than to the other three antigens. In BALB/c mice, by day 28 p.i., TC0727 reacted with sera from mice infected i.n. or i.vag. In C3H/HeN and C57BL/6 mice antibodies to the TC0727 were first observed at day 42 and 56 p.i., respectively.
Mice were euthanized at the end of the experiment and their genital tracts inspected for the presence of hydrosalpinx. None of the mice infected i.n. had hydrosalpinx. All BALB/c and C3H/HeN mice and 75% of the C57BL/6 mice infected i.vag., had hydrosalpinx.
4. Discussion
In spite of significant efforts we still do not have a vaccine to protect against C. trachomatis infections. Here, to establish the ability of the C. muridarum TC0582 protein to elicit protection, BALB/c mice were vaccinated with this antigen formulated with adjuvants that induce Th1 responses. Mice immunized with TC0582 did not developed detectable levels of antibodies when using EB as the antigen in an ELISA. This was not unexpected since TC0582 is not a surface exposed antigen and furthermore, it is likely present at low concentrations in EB [21]. This was confirmed when the same sera were tested by ELISA using TC0582 as the antigen. In this case, high IgG and IgA titers were detected and the specificity of the reaction was confirmed by Western blot. Moreover, T-cells from mice immunized with TC0582 proliferated when stimulated in vitro with EB. To identify antigens that elicit antibodies, Finco et al. [39] utilized a panel of 120 C. trachomatis serovar D recombinant proteins to screen sera from Chlamydia infected patients. The 79 proteins that gave positive results were then used to stimulate splenocytes from C. trachomatis-infected mice. Five of the proteins (CT119, CT372, CT443, CT681 and CT823), also induced CD4+/IFN-γ, indicative of cell-mediated immune responses. Based on our results, TC0582 (CT308) can now be added to the list of chlamydial antigens that, at least in mice, can elicit both humoral and cell mediated immune responses. Mice vaccinated with TC0582 were i.n. challenged and, as determined by changes in body weight, lung weight and number of Chlamydia IFU recovered from the lungs, it induced an immune response that provided statistically significant protection.
TC0582 has homology to the eukaryotic V-type ATP synthase subunit A [21]. The eukaryotic ATP synthase complex is made up of multiple subunits. In C. muridarum TC0580, TC0581, TC0582 and TC0584, may code for proteins that are part of the ATP synthase complex. To determine if we could identify additional protective proteins from the same complex mice were immunized with TC0580 (subunit D), TC0581 (subunit B) or TC0584 (subunit E). TC0581 elicited moderate protection while, in mice vaccinated with TC0580 or TC0584, no protection was observed.
Neutral, additive, synergistic and subtractive effects have been observed when formulating vaccines with several antigens [39–41]. For example, Finco et al. [39] reported an additive effect by combining partially protective, or not-protective, chlamydial antigens. Yu et al. [40] on the other hand, observed neutral effects with combinations of Chlamydia antigens. Therefore, to determine if we could enhance protection using two antigens, mice were immunized with vaccines formulated with TC0582 and TC0581 or TC0582 and TC0580. None of the two combinations conferred enhanced protection when compared to TC0582 alone. Differences in the ability of antigen combinations to enhance a protective immune response may depend on several factors including the antigens and adjuvants utilized, the genetic background of the animal and/or the experimental model.
In the trachoma vaccination trials, using live or inactivated C. trachomatis as the antigen, the protection was observed to be serovar or serogroup specific [2, 9, 42]. DNA sequencing demonstrated that the serovar classification of C. trachomatis was likely dependent on the amino acid sequence of the variable domains of MOMP [43]. The serovar-specificity of the MOMP-induced protection could be a limitation for using this protein as the only vaccine antigen [44]. Well-conserved C. trachomatis antigens could, in principle, elicit a broad-protection. Therefore, we also tested a combination of TC0582, an antigen with more than 99.5% sequence identity among all the C. trachomatis serovars, plus MOMP.
Mice immunized with TC0582 plus MOMP had higher antibody titers to EB than those vaccinated only with TC0582 but lower than the group immunized only with MOMP. Antibody titers to MOMP were also lower in the mice vaccinated with the combination than in animals immunized only with MOMP. Interestingly, antibody levels to TC0582 were the same in both groups. The T-cell lymphoproliferative responses and levels of IFN-γ were higher in mice immunized only with MOMP than in those vaccinated with TC0582 plus MOMP. Following the i.n. challenge, mice vaccinated with TC0582 and MOMP maintained their body weight better than those immunized only with TC0582 or MOMP. However, by day 10 p.c., mice immunized with the combination of TC0582 plus MOMP had a body weight not significantly different from mice vaccinated only with MOMP. Similarly, neither the lung weight nor the number of IFU, were significantly different between the two groups. Furthermore levels of Chlamydia-specific IgA, a protective parameter and IFN-γ, an inflammatory marker, in the lungs at day 10 p.c., were not significantly different between mice vaccinated with TC0582 plus MOMP and those immunized with only MOMP. Therefore, our results combining TC0582 with other subunits of the ATP synthetase complex, or with MOMP, parallel those of Yu et al. [40] that reported that vaccination with Chlamydia antigen combinations only protected as well as the best individual protein in the mixture. We can thus conclude that, at least in this model using the homologous isolate to challenge, the MOMP and TC0582 combination does not offer an advantage over using MOMP as the only antigen.
TC0582 has ~40% amino acid sequence identity with the eukaryotic V-type ATP synthase subunit A. Zeng et al. [21] proposed that the hydrosalpinx that develops in mice after C. muridarum intravaginal infection, correlates with TC0582 antigen-specific antibody responses. They hypothesized that TC0582 contributed to chlamydial pathogenesis by mechanisms similar to those proposed for hsp60 (TC0386) [21]. In women infected with C. trachomatis, significantly elevated anti-hsp60 antibodies have been detected in patients with tubal factor infertility [11, 45, 46]. The anti-hsp60 antibody responses, due to the high level of amino acid sequence conservation between the chlamydial and human hsp60s, are thought to contribute to the damage in the upper genital tract [46]. However, in spite of this perceived limitation, heat shock proteins have been tested as vaccine candidates with no negative outcomes [47].
To determine whether cross-reactive antibodies are generated by vaccination with TC0582, we expressed the ATP6v1a mouse gene and, using a protein microarray, we tested mouse sera. Sera from mice vaccinated with TC0582, or with EB, gave strong signals with TC0582 but did not react with the mouse AtpA protein. To further assess the possibility that antibodies to TC0582 were associated with hydrosalpinx formation, using a C. muridarum protein microarray, we tested sera from mice infected i.vag. or i.n. with MoPn. Our results showed that specific anti-TC0582 antibodies are elicited following i.vag. or i.n. inoculation with live EB. The antibody response to TC0582 paralleled that elicited by TC0052 (MOMP) and TC0386 (hsp60). Only antibodies to TC0727 (omp2), an antigen also considered to be associated with hydrosalpinx and cardiac pathology [48–50], were delayed by 2–4 weeks. Therefore, since mice immunized multiple times with TC0582, or EB, do not produce cross-reactive antibodies to the eukaryotic ATP6v1a and animals infected i.n. with live EB, that do not develop hydrosalpinx, have antibodies levels to TC0582 like those inoculated i.vag., it is unlikely that this antigen plays a role in the upper genital pathology resulting from a chlamydial vaginal infection [35]. Furthermore, in women, the C. trachomatis orthologue of this protein, (CT308), has not been found to be associated with chlamydial tubal factor infertility [48, 49].
In conclusion, immunization with TC0582 elicited statistically significant protection in BALB/c mice against a challenge with C. muridarum. TC0582, in combination with other components of the ATP synthase subunits, or with MOMP, did not confer enhanced protection when compared to itself, or to MOMP alone. However, TC0582 is highly conserved among all the human C. trachomatis serovars and therefore, it may be a good antigen to include in a vaccine to elicit broad cross-serovar protection.
Supplementary Material
Acknowledgments
Supported was provided by grants R41AI072847, R42AI072847 and RO1AI067888 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Miller WC, Ford CA, Morris M, Handcock MS, Schmitz JL, Hobbs MM, Cohen MS, Harris KM, Udry JR. Prevalence of chlamydial and gonococcal infections among young adults in the United States. Jama. 2004;291:2229–2236. doi: 10.1001/jama.291.18.2229. [DOI] [PubMed] [Google Scholar]
- [2].Schachter J, Dawson CR. Human chlamydial infections. PSG Pub. Co.; Littleton, Mass.: 1978. [Google Scholar]
- [3].Grayston JT, Wang S. New knowledge of chlamydiae and the diseases they cause. J Infect Dis. 1975;132:87–105. doi: 10.1093/infdis/132.1.87. [DOI] [PubMed] [Google Scholar]
- [4].Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005;5:149–161. doi: 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- [5].de la Maza LM, Peterson EM. Vaccines for Chlamydia trachomatis infections. Curr Opin Investig Drugs. 2002;3:980–986. [PubMed] [Google Scholar]
- [6].Farris CM, Morrison RP. Vaccination against Chlamydia genital infection utilizing the murine C. muridarum model. Infect Immun. 2011;79:986–996. doi: 10.1128/IAI.00881-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Igietseme JU, Black CM, Caldwell HD. Chlamydia vaccines: strategies and status. BioDrugs. 2002;16:19–35. doi: 10.2165/00063030-200216010-00003. [DOI] [PubMed] [Google Scholar]
- [8].Grayston JT, Wang SP. The potential for vaccine against infection of the genital tract with Chlamydia trachomatis. Sex Transm Dis. 1978;5:73–77. doi: 10.1097/00007435-197804000-00011. [DOI] [PubMed] [Google Scholar]
- [9].Taylor HR. Trachoma: a blinding scourge from the Bronze Age to the twenty-first century. Haddington Press Pry Ltd; Victoria, Australia: 2008. [Google Scholar]
- [10].Morrison RP, Lyng K, Caldwell HD. Chlamydial disease pathogenesis. Ocular hypersensitivity elicited by a genus-specific 57-kD protein. J Exp Med. 1989;169:663–675. doi: 10.1084/jem.169.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Arno JN, Yuan Y, Cleary RE, Morrison RP. Serologic responses of infertile women to the 60-kd chlamydial heat shock protein (hsp60) Fertil Steril. 1995;64:730–735. doi: 10.1016/s0015-0282(16)57847-9. [DOI] [PubMed] [Google Scholar]
- [12].Caldwell HD, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang J, Zhang Y, Lu C, Lei L, Yu P, Zhong G. A genome-wide profiling of the humoral immune response to Chlamydia trachomatis infection reveals vaccine candidate antigens expressed in humans. J Immunol. 2010;185:1670–1680. doi: 10.4049/jimmunol.1001240. [DOI] [PubMed] [Google Scholar]
- [14].Farris CM, Morrison SG, Morrison RP. CD4+ T cells and antibody are required for optimal major outer membrane protein vaccine-induced immunity to Chlamydia muridarum genital infection. Infect Immun. 2010;78:4374–4383. doi: 10.1128/IAI.00622-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kari L, Whitmire WM, Crane DD, Reveneau N, Carlson JH, Goheen MM, Peterson EM, Pal S, de la Maza LM, Caldwell HD. Chlamydia trachomatis native major outer membrane protein induces partial protection in nonhuman primates: implication for a trachoma transmission-blocking vaccine. J Immunol. 2009;182:8063–8070. doi: 10.4049/jimmunol.0804375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pal S, Peterson EM, de la Maza LM. Vaccination with the Chlamydia trachomatis major outer membrane protein can elicit an immune response as protective as that resulting from inoculation with live bacteria. Infect Immun. 2005;73:8153–8160. doi: 10.1128/IAI.73.12.8153-8160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sun G, Pal S, Weiland J, Peterson EM, de la Maza LM. Protection against an intranasal challenge by vaccines formulated with native and recombinant preparations of the Chlamydia trachomatis major outer membrane protein. Vaccine. 2009;27:5020–5025. doi: 10.1016/j.vaccine.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang S-P, Grayston J. Microimmunofluorescence serology of Chlamydia trachomatis. In: de la Maza LM, Peterson EM, editors. Medical Virology III. Elsevier Science Pub.; New York: 1984. pp. 87–118. [Google Scholar]
- [19].Molina DM, Pal S, Kayala MA, Teng A, Kim PJ, Baldi P, Felgner PL, Liang X, de la Maza LM. Identification of immunodominant antigens of Chlamydia trachomatis using proteome microarrays. Vaccine. 2010;28:3014–3024. doi: 10.1016/j.vaccine.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Delvecchio VG, Connolly JP, Alefantis TG, Walz A, Quan MA, Patra G, Ashton JM, Whittington JT, Chafin RD, Liang X, Grewal P, Khan AS, Mujer CV. Proteomic profiling and identification of immunodominant spore antigens of Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis. Appl Environ Microbiol. 2006;72:6355–6363. doi: 10.1128/AEM.00455-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zeng H, Gong S, Hou S, Zou Q, Zhong G. Identification of antigen-specific antibody responses associated with upper genital tract pathology in mice infected with Chlamydia muridarum. Infect Immun. 2012;80:1098–1106. doi: 10.1128/IAI.05894-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pal S, Hui W, Peterson EM, de la Maza LM. Factors influencing the induction of infertility in a mouse model of Chlamydia trachomatis ascending genital tract infection. J Med Microbiol. 1998;47:599–605. doi: 10.1099/00222615-47-7-599. [DOI] [PubMed] [Google Scholar]
- [23].Sun G, Pal S, Sarcon AK, Kim S, Sugawara E, Nikaido H, Cocco MJ, Peterson EM, de la Maza LM. Structural and functional analyses of the major outer membrane protein of Chlamydia trachomatis. J Bacteriol. 2007;189:6222–6235. doi: 10.1128/JB.00552-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Marston FA. The purification of eukaryotic polypeptides synthesized in Escherichia coli. Biochem J. 1986;240:1–12. doi: 10.1042/bj2400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Blake MS, Gotschlich EC. Purification and partial characterization of the opacity-associated proteins of Neisseria gonorrhoeae. J Exp Med. 1984;159:452–462. doi: 10.1084/jem.159.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Qi HL, Tai JY, Blake MS. Expression of large amounts of neisserial porin proteins in Escherichia coli and refolding of the proteins into native trimers. Infect Immun. 1994;62:2432–2439. doi: 10.1128/iai.62.6.2432-2439.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pal S, Theodor I, Peterson EM, de la Maza LM. Immunization with the Chlamydia trachomatis mouse pneumonitis major outer membrane protein can elicit a protective immune response against a genital challenge. Infect Immun. 2001;69:6240–6247. doi: 10.1128/IAI.69.10.6240-6247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schagger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- [29].Cheng C, Jain P, Bettahi I, Pal S, Tifrea D, de la Maza LM. A TLR2 agonist is a more effective adjuvant for a Chlamydia major outer membrane protein vaccine than ligands to other TLR and NOD receptors. Vaccine. 2011;29:6641–6649. doi: 10.1016/j.vaccine.2011.06.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pal S, Fielder TJ, Peterson EM, de la Maza LM. Protection against infertility in a BALB/c mouse salpingitis model by intranasal immunization with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect Immun. 1994;62:3354–3362. doi: 10.1128/iai.62.8.3354-3362.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cheng C, Cruz-Fisher MI, Tifrea D, Pal S, Wizel B, de la Maza LM. Induction of protection in mice against a respiratory challenge by a vaccine formulated with the Chlamydia major outer membrane protein adjuvanted with IC31(R) Vaccine. 2011;29:2437–2443. doi: 10.1016/j.vaccine.2011.01.031. [DOI] [PubMed] [Google Scholar]
- [32].Cheng C, Bettahi I, Cruz-Fisher MI, Pal S, Jain P, Jia Z, Holmgren J, Harandi AM, de la Maza LM. Induction of protective immunity by vaccination against Chlamydia trachomatis using the major outer membrane protein adjuvanted with CpG oligodeoxynucleotide coupled to the nontoxic B subunit of cholera toxin. Vaccine. 2009;27:6239–6246. doi: 10.1016/j.vaccine.2009.07.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Davies DH, Liang X, Hernandez JE, Randall A, Hirst S, Mu Y, Romero KM, Nguyen TT, Kalantari-Dehaghi M, Crotty S, Baldi P, Villarreal LP, Felgner PL. Profiling the humoral immune response to infection by using proteome microarrays: high-throughput vaccine and diagnostic antigen discovery. Proc Natl Acad Sci U S A. 2005;102:547–552. doi: 10.1073/pnas.0408782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Teng A, Cruz-Fisher MI, Cheng C, Pal S, Sun G, Ralli-Jain P, Molina DM, Felgner PL, Liang X, de la Maza LM. Proteomic identification of immunodominant chlamydial antigens in a mouse model. J proteomics. 2012;77:176–186. doi: 10.1016/j.jprot.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].de la Maza LM, Pal S, Khamesipour A, Peterson EM. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect Immun. 1994;62:2094–2097. doi: 10.1128/iai.62.5.2094-2097.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Morrison SG, Morrison RP. Resolution of secondary Chlamydia trachomatis genital tract infection in immune mice with depletion of both CD4+ and CD8+ T cells. Infect Immun. 2001;69:2643–2649. doi: 10.1128/IAI.69.4.2643-2649.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tuffrey M, Taylor-Robinson D. Progesterone as a key factor in the development of a mouse model for genital-tract infection with Chlamydia trachomatis. FEMS Microbiol Letters. 1981;12:111–115. [Google Scholar]
- [38].Cruz-Fisher MI, Cheng C, Sun G, Pal S, Teng A, Molina DM, Kayala MA, Vigil A, Baldi P, Felgner PL, Liang X, de la Maza LM. Identification of immunodominant antigens by probing a whole Chlamydia trachomatis open reading frame proteome microarray using sera from immunized mice. Infect Immun. 2011;79:246–257. doi: 10.1128/IAI.00626-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Finco O, Frigimelica E, Buricchi F, Petracca R, Galli G, Faenzi E, Meoni E, Bonci A, Agnusdei M, Nardelli F, Bartolini E, Scarselli M, Caproni E, Laera D, Zedda L, Skibinski D, Giovinazzi S, Bastone R, Ianni E, Cevenini R, Grandi G, Grifantini R. Approach to discover T- and B-cell antigens of intracellular pathogens applied to the design of Chlamydia trachomatis vaccines. Proc Natl Acad Sci USA. 2011;108:9969–9974. doi: 10.1073/pnas.1101756108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yu H, Jiang X, Shen C, Karunakaran KP, Jiang J, Rosin NL, Brunham RC. Chlamydia muridarum T-cell antigens formulated with the adjuvant DDA/TDB induce immunity against infection that correlates with a high frequency of gamma interferon (IFN-gamma)/tumor necrosis factor alpha and IFN-gamma/interleukin-17 double-positive CD4+ T cells. Infect Immun. 2010;78:2272–2282. doi: 10.1128/IAI.01374-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Qiu S, Zhang J, Tian Y, Yang Y, Huang H, Yang D, Lu M, Xu Y. Reduced antigenicity of naturally occurring hepatitis B surface antigen variants with substitutions at the amino acid residue 126. Intervirology. 2008;51:400–406. doi: 10.1159/000205265. [DOI] [PubMed] [Google Scholar]
- [42].Wang SP, Grayston JT, Alexander ER. Trachoma vaccine studies in monkeys. Am J Ophthalmol. 1967;63(Suppl):1615–1630. doi: 10.1016/0002-9394(67)94155-4. [DOI] [PubMed] [Google Scholar]
- [43].Stephens RS, Sanchez-Pescador R, Wagar EA, Inouye C, Urdea MS. Diversity of Chlamydia trachomatis major outer membrane protein genes. J Bacteriol. 1987;169:3879–3885. doi: 10.1128/jb.169.9.3879-3885.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tifrea DF, Ralli-Jain P, Pal S, de la Maza LM. Vaccination with the recombinant major outer membrane protein elicits antibodies to the constant domains and induces cross-serovar protection against intranasal challenge with Chlamydia trachomatis. Infect Immun. 2013;81:1741–1750. doi: 10.1128/IAI.00734-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Toye B, Laferriere C, Claman P, Jessamine P, Peeling R. Association between antibody to the chlamydial heat-shock protein and tubal infertility. J Infect Dis. 1993;168:1236–1240. doi: 10.1093/infdis/168.5.1236. [DOI] [PubMed] [Google Scholar]
- [46].Ault KA, Statland BD, King MM, Dozier DI, Joachims ML, Gunter J. Antibodies to the chlamydial 60 kilodalton heat shock protein in women with tubal factor infertility. Infect Dis Obstet Gynecol. 1998;6:163–167. doi: 10.1002/(SICI)1098-0997(1998)6:4<163::AID-IDOG5>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Motin VL, de la Maza LM, Peterson EM. Immunization with a peptide corresponding to chlamydial heat shock protein 60 increases the humoral immune response in C3H mice to a peptide representing variable domain 4 of the major outer membrane protein of Chlamydia trachomatis. Clin Diagn Lab Immunol. 1999;6:356–363. doi: 10.1128/cdli.6.3.356-363.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Budrys NM, Gong S, Rodgers AK, Wang J, Louden C, Shain R, Schenken RS, Zhong G. Chlamydia trachomatis antigens recognized in women with tubal factor infertility, normal fertility, and acute infection. Obste Gynecol. 2012;119:1009–1016. doi: 10.1097/AOG.0b013e3182519326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Rodgers AK, Budrys NM, Gong S, Wang J, Holden A, Schenken RS, Zhong G. Genome-wide identification of Chlamydia trachomatis antigens associated with tubal factor infertility. Fertil Steril. 2011;96:715–721. doi: 10.1016/j.fertnstert.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bachmaier K, Neu N, de la Maza LM, Pal S, Hessel A, Penninger JM. Chlamydia infections and heart disease linked through antigenic mimicry. Science. 1999;283:1335–1339. doi: 10.1126/science.283.5406.1335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.