Abstract
The level of the renin-angiotensin-aldosterone system (RAAS) activity in kidney transplant recipients has not been extensively studied or serially profiled. To describe this axis and to determine its association with GFR change, interstitial expansion and end-stage renal disease (ESRD) we measured plasma renin activity (PRA) and plasma aldosterone levels annually for 5 years in 153 kidney transplant recipients randomly assigned to losartan or placebo. PRA and plasma aldosterone levels were in the normal range at all times and did not vary by immunosuppression regimen. Those on losartan exhibited higher PRA but similar plasma aldosterone levels. Neither baseline nor serial PRA or plasma aldosterone levels were associated with GFR decline, proteinuria or interstitial expansion. Losartan use, [HR 0.48 (95% CI 0.21–1.0), insignificant], and Caucasian donor, [HR 0.18 (95% CI 0.07–0.4), significant] were associated with less doubling of serum creatinine, death or ESRD. Hypertension, less than 3 HLA-matches, the combination of tacrolimus-rapamycin and acute rejection were associated with more events. Neither PRA nor plasma aldosterone levels were independently associated with this outcome. Higher serial plasma aldosterone levels were associated, however, with a significantly higher risk of ESRD, [HR 1.01 (95% CI 1.00–1.02)]. Thus, systemic RAAS is not overly activated in kidney transplant recipients but this may not reflect the intrarenal system. Importantly, plasma aldosterone levels may be associated with more ESRD.
INTRODUCTION
The benefits of suppressing the renin-angiotensin-aldosterone system (RAAS) is generally considered to extend beyond their antihypertensive properties and RAAS blockers are the preferred agents in established chronic kidney disease (CKD), particularly in those with proteinuria(1–3). Ameliorating hyperfiltration that is mainly driven by the rise in the intra-glomerular pressure has been suggested as a central pathway by which these agents could convey their renoprotection. Their benefit in the setting of kidney transplantation, a possibly more pure form of hyperfiltration considering the reduced renal mass, has been suggested but is far from established (4). We recently tested whether angiotensin II (AII) blockade, compared to placebo, would retard the development of interstitial fibrosis or graft loss when given for the first 5 post-transplant years (5). The result was not statistically significant raising the question of whether RAAS is stimulated in this setting. To address this possibility, we measured plasma renin activity (PRA) and plasma aldosterone levels (Paldo) at baseline and annually in participants of the Angiotensin II Blockade in Chronic Allograft Nephropathy (ABCAN) Trial, Clinical Trials.Gov (NCT 01467895). We assessed predictors of their levels and their responsiveness to angiotensin II blockade. We hypothesized that RAAS is not overly activated in kidney transplant recipients, and that losartan at 100 mg/day would effectively suppress this hormonal axis. We also examined whether higher PRA and Paldo would be associated with inferior allograft function and structure.
RESULTS
Participants
At 58 ± 34 days after kidney transplantation, a total of 153 kidney recipients were randomized to placebo (n=76) or 100 mg losartan daily (n=77). Mean age was 48.7 ± 12.4 years, most recipients were White, received a kidney from a live donor (71%) and a third had diabetes. The characteristics of participants are described in detail in Table 1.
Table 1.
Recipient Characteristics at Randomization (n=153)
| Variable | Mean (SD) or N (%) |
|---|---|
| Age (years) | 48.7 (12.4) |
| Female Recipient | 59 (38.6) |
| Cause of Kidney Disease | |
| Diabetes mellitus | 57 (37.3) |
| Hypertension | 10 (6.5) |
| Polycystic kidney disease | 23 (15.0) |
| Other | 63 (41.2) |
| BMI (kg/m2) | 26.4 (5.1) |
| HLA Matches <3 | 79 (51.6) |
| SBP (mmHg) | 130.5 (18.3) |
| DBP (mmHg) | 73.5 (10.7) |
| Donor Age (years) | 40.3 (12.1) |
| Female Donor | 83 (54.3) |
| Deceased Donor | 45 (29.4) |
| Induction Immunosuppression | |
| Interleukin 2 antagonist | 27 (17.6) |
| Anti-thymocyte globulin | 126 (82.3) |
| Immunosuppression | |
| Tacrolimus + Rapamycin | 21 (13.7) |
| Tacrolimus + MMF | 34 (22.2) |
| Tacrolimus + MMF* + Pred. | 15 (9.8) |
| * CSA | 40 (26.1) |
| CSA + MMF + Pred. | 26 (17.0) |
| Other | 17 (11.1) |
| Loop Diuretics | 39 (25.5) |
| Thiazide Diuretics | 12 (7.8) |
| ACR* (mg/g creatinine) | 75.5 (157.3) |
| Iothalamate GFR (ml/min) | 56.1 (17.0) |
CSA: Cyclosporine;
ACR: Albumin-Creatinine Ratio, MMF: mycophenolate mofeti
RAAS elements at baseline and over time
Baseline PRA was 0.99 ± 1.37 ng/ml/hour and Paldo was 136 ± 9.18 pg/ml in the group as a whole. In the losartan group they were 0.91 ± 1.11 and 138.1 ± 92.8, while in placebo they were 1.07 ± 1.59 and 134.1 ± 91.4, respectively (p values not significant). The mean 24-hour urinary sodium and potassium excretion rates were comparable between the two groups: 171.3 ± 94.3 mEq/day and 49.5 ±26.1 mEq/day in the losartan group and 183 ± 99.2 and 53.2 ± 26.4 in the placebo group, respectively.
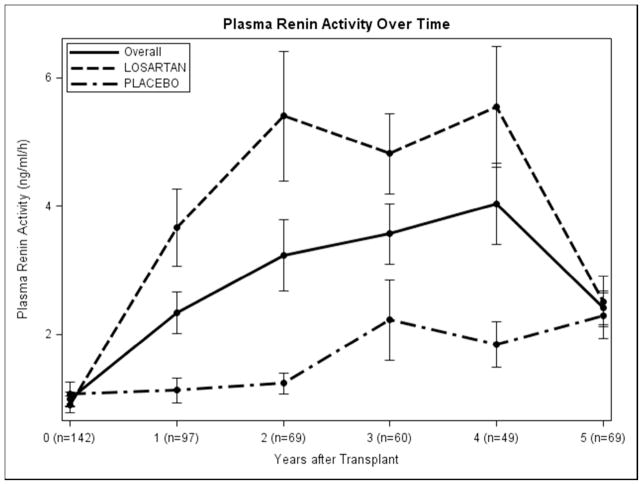
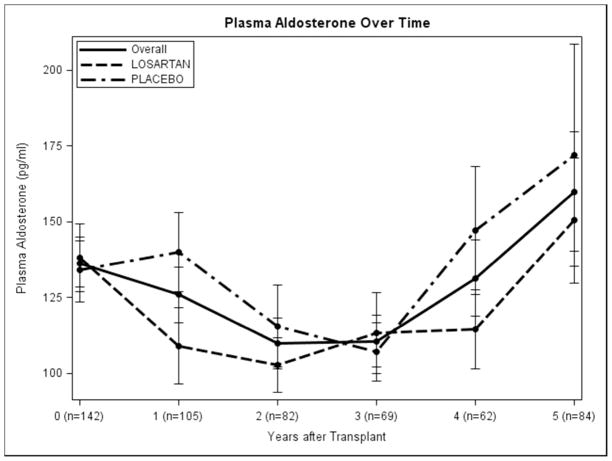
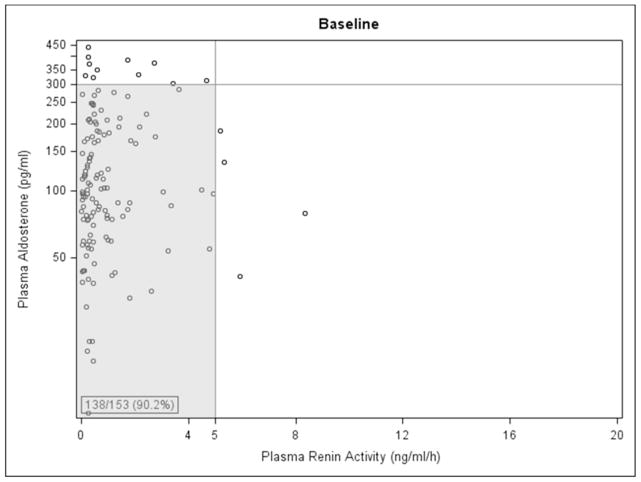
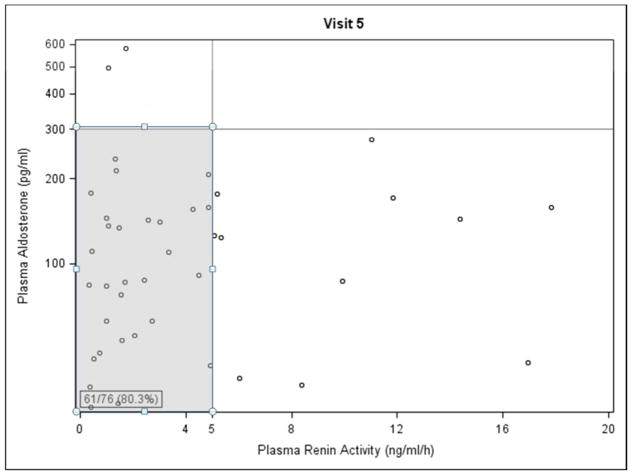
Baseline Paldo was 43.4 pg/ml (p=0.049) lower in living donor transplant recipients, and 117.1 pg/ml higher in those with hyperlipidemia (p=0.04), (Table 2 and Appendix 2). Increased urine sodium excretion rate was associated with decreased Paldo (p=0.01). Female recipients had a lower PRA but, interestingly, those with a kidney from a female donor had PRA levels 0.67 ng/ml/hour higher than those with a male donor (p=0.01). Increased donor age was associated with lower baseline PRA (p=0.01). There was no statistically significant difference in Paldo for those on losartan vs. placebo at any time point. Those on losaratan had significantly higher PRA levels than those on placebo at each annual time point post-transplant in years 1–4 losartan was discontinued 1 month before the year 5 visit (Figure 1A and 1B). Interestingly, 138/153 (90.2%) and 61/76 (80.3%) of participants had PRA and Paldo values that were within the normal range (≤300 pg/ml and ≤5 ng/ml/h, respectively) at these time points (Figure 2A and 2B).
Table 2.
Predictors of Plasma Renin Activity (ng/ml/h) and Plasma Aldosterone (pg/ml)
| VvInt/C doubling or ESRD from IF/TA | ||
|---|---|---|
| Variable | OR (95% CI) | P-Value |
| Plasma Aldosterone | 1.01 (1, 1.01) | 0.21 |
| Plasma Renin Activity | 0.91 (0.49, 1.7) | 0.77 |
| Female Recipient | 0.36 (0.12, 1.08) | 0.07 |
| Acute Rejection | 4.62 (1.8, 11.83) | <0.01 |
| <3 HLA Matches | 3.13 (1.08, 9.11) | 0.04 |
| Tacrolimus+ Rapamycin | 3.32 (0.98, 11.27) | 0.05 |
| VvInt/C doubling or any ESRD | ||
| Plasma Aldosterone | 1.01 (1, 1.02) | 0.08 |
| Plasma Renin Activity | 0.47 (0.17, 1.27) | 0.14 |
| Female Recipient | 0.37 (0.12, 1.08) | 0.07 |
| DBP (mmHg) | 0.85 (0.73, 1) | 0.05 |
| Hypertension | 11.99 (1.9, 75.59) | 0.01 |
| Acute Rejection | 8.14 (2.04, 32.38) | <0.01 |
| <3 HLA Matches | 6.17 (1.37, 27.74) | 0.02 |
| Losartan | 0.24 (0.08, 0.76) | 0.02 |
| Loop Diuretics | 3.64 (0.96, 13.77) | 0.06 |
| CSA + MMF | 0.06 (0.01, 0.59) | 0.02 |
| CSA + MMF + Pred. | 0.13 (0.02, 1.06) | 0.06 |
| Creatinine doubling, ESRD or Death | ||
| Plasma Aldosterone | 1 (0.99, 1) | 0.39 |
| Plasma Renin Activity | 0.92 (0.57, 1.47) | 0.73 |
| SBP (mmHg) | 1.04 (1, 1.09) | 0.05 |
| Hypertensive | 2.07 (1.03, 4.18) | 0.04 |
| Acute Rejection | 2.66 (1.36, 5.18) | <0.01 |
| <3 HLA Matches | 1.66 (0.9, 3.04) | 0.10 |
| Caucasian Donor | 0.37 (0.19, 0.73) | <0.01 |
| Losartan | 0.5 (0.27, 0.91) | 0.02 |
| Iothalamate GFR | 1.04 (1.01, 1.08) | 0.02 |
SBP: systolic blood pressure; DBP: diastolic blood pressure; CSA: Cyclosporine, MMF: mycophenolate mofetil, DM: diabetes mellitus, PCKD; polycystic kidney disease, HTN: hypertension, ACR: albumin-creatinine ratio
Figure 1.
Plasma Renin Activity and Plasma Aldosterone over time: A. overall and B. by treatment group). Error bars are ± 1 SE.
Figure 2.
Plasma Renin Activity vs. Plasma Aldosterone at A. baseline and B. visit number 5 (four years post-transplant). Shaded area indicates patients with normal values for both parameters.
Over time, a decrease in urine sodium and an increase in urine potassium excretion rates, diastolic blood pressure (DBP) and body mass index (BMI) were associated with an increase in Paldo. Those subjects with living donors had a Paldo 33.3 pg/ml (p=0.04) lower than those with deceased donors. Examining PRA over time, subjects taking losartan had a PRA 1.61 ng/ml/h (p<0.01) higher than those taking placebo. Subjects with a female donor had a PRA 1.11 ng/ml/hour (p=0.01) higher than those with a male donor, and those with a living donor had a PRA 0.93 ng/ml/hour lower than those with a deceased donor (Table 3 and Appendix 3). An increase in urine sodium and potassium excretion rates and DBP were also associated with a decrease in serial PRA. Subjects whose kidney disease was caused by hypertension had a PRA 1.54 ng/ml/hour (p=0.049) higher than those who did not. There were no differences in PRA or Paldo for the different immunosuppressants’ combinations (cyclosporine + mycophenolate mofetil (MMF), tacrolimus + MMF, or tacrolimus + rapamycin) regardless of steroids use (data not shown). Filtration fraction was consistently lower in the losartan group, except for the last visit when the drug was stopped one month prior. Filtration fraction averaged around 20% in the losartan group and 22% in the placebo group.
Table 3.
Predictors of last VvInt/C, last GFR, last ACR and VvInt/C Change
| Last VvInt/C | ||
|---|---|---|
| Variable | Estimate (95% CI) | P-Value |
| Plasma Aldosterone | 0 (0, 0) | 0.47 |
| Plasma Renin Activity | −0.001 (−0.018, 0.017) | 0.94 |
| Caucasian Donor | 0.044 (−0.004, 0.092) | 0.07 |
| Tacrolimus + Rapamycin | 0.067 (−0.014, 0.148) | 0.10 |
| Last GFR | ||
| Plasma Aldosterone | 0.01 (−0.03, 0.06) | 0.62 |
| Plasma Renin Activity | 0.84 (−3.32, 4.99) | 0.69 |
| Caucasian Recipient | −11.86 (−23.09, −0.64) | 0.04 |
| <3 HLA Matches | −7.74 (−15.68, 0.19) | 0.06 |
| Losartan | −6.78 (−14.95, 1.39) | 0.10 |
| Tacrolimus + Rapamycin | −16.2 (−34.78, 2.37) | 0.09 |
| Last ACR | ||
| Plasma Aldosterone | −0.36 (−1.02, 0.3) | 0.28 |
| Plasma Renin Activity | 18.16 (−47.8, 84.12) | 0.58 |
| Recipient BMI | 17.72 (3.09, 32.36) | 0.02 |
| Tacrolimus + MMF | −322.12 (−612.94, −31.31) | 0.03 |
| Tacrolimus+ MMF + Pred. | −362.28 (−750.67, 26.1) | 0.07 |
| CSA + MMF | −334.44 (−635.84, −33.04) | 0.03 |
| CSA + MMF + Pred. | −401.41 (−715.09, −87.73) | 0.01 |
| VvInt/C Change | ||
| Plasma Aldosterone | 0 (0, 0) | 0.61 |
| Plasma Renin Activity | −0.001 (−0.034, 0.033) | 0.96 |
| Acute Rejection | 0.085 (−0.006, 0.175) | 0.06 |
BMI: body mass index; CSA: Cyclosporine, MMF: mycophenolate mofetil, Pred.: Prednisone.
ACE Gene polymorphism and PRA and Paldo levels
In all, 30% of recipients harbored the deletion/deletion (DD) allele, 52% the deletion/insertion (DI) allele and 18% the II allele. These proportions were 30%, 46% and 23% in the donors, respectively. There were no differences amongst the three allele carriers in PRA or Paldo (Appendix). Importantly, there were no differences in those levels for the nine different possible donor-recipient permutations and there were no statistically significant associations with any of the outcomes studied (Appendix).
Association of PRA and Paldo with final and longitudinal glomerular filtration rate (GFR), albumin-creatinine ratio (ACR), and interstitial expansion
Paldo and PRA, along with other baseline values were used as predictors of the continuous outcomes of the cortical interstitial volume (Vvlnt/C), VvInt/C change, GFR and ACR. We found no significant predictors of last VvInt/C or VvInt/C change (Table 3 and Appendix 3). Greater iothalamate GFR and ACR at baseline were associated with a higher final GFR (p<0.01) and (p=0.02), respectively. White recipients had a lower final GFR than non-whites (p=0.04). An increase in BMI was associated with an increase in final ACR (p=0.02), while MMF-based immunosuppression regimens resulted in a significant decrease.
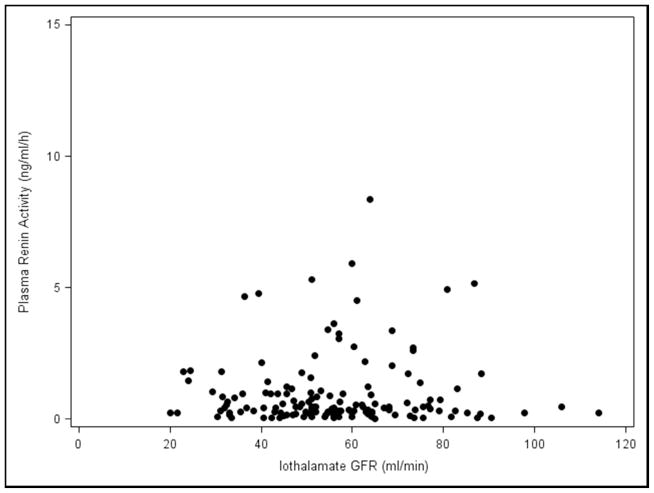
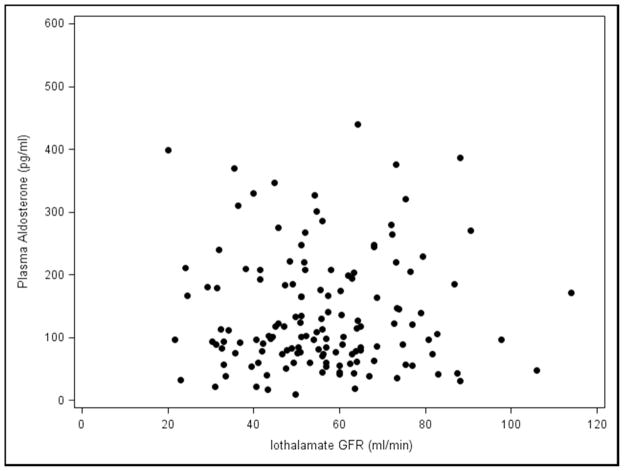
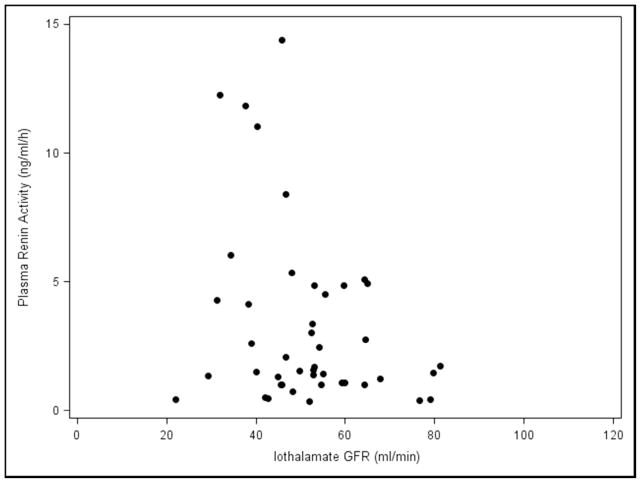
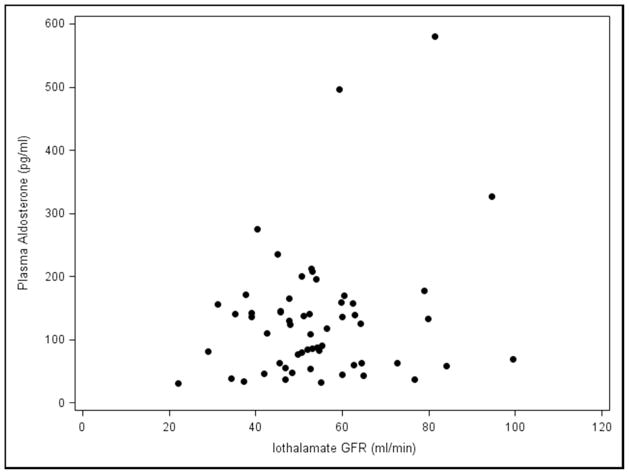
The effects of Paldo, PRA and other covariates over time on serial GFR and serial ACR are shown in (Table 4 and Appendix 4). Again, neither serial PRA nor serial Paldo were associated with serial GFR or ACR over time. Recipients who were white (estimate=−6.78, p=0.049), had older donors (p=0.01) or a higher serial ACR (estimate=−0.01, p=0.01) had a significantly lower final GFR. Those with a higher serial GFR (p=0.02) or cause of kidney disease listed as other (p=0.03) had a significantly lower final ACR. Subjects who had acute rejection (p=0.01) or a higher SBP (p<0.01) had a significantly higher final ACR. Whether recipients with low GFR may have elevated PRA and/or Paldo levels by virtue of possible increased production from scarred areas in the setting of chronic allograft dysfunction was then examined. There was no relationship between the iothalamate GFR values and PRA and/or Paldo at baseline (Figure 3A and 3B) or visit 5 (Figure 3C and 3D). Furthermore, when we divided PRA and Paldo into quartiles and related them to GFR there was no evident pattern or a statistical correlation (data not shown).
Table 4.
Predictors of Serial GFR and urinary albumin-creatinine ratio (ACR)
| GFR | ||
|---|---|---|
| Variable | Estimate (95% CI) | P-Value |
| Plasma Aldosterone | 0 (−0.02, 0.01) | 0.45 |
| Plasma Renin Activity | −0.13 (−0.61, 0.34) | 0.58 |
| Caucasian Recipient | −6.78 (−13.55, −0.01) | 0.05 |
| Donor Age (years) | −0.28 (−0.49, −0.07) | 0.01 |
| Female Donor | −4.02 (−8.86, 0.81) | 0.10 |
| CSA + MMF | −9.35 (−18.71, 0) | 0.05 |
| ACR | ||
| Plasma Aldosterone | 0.07 (−0.06, 0.2) | 0.30 |
| Plasma Renin Activity | −2.23 (−7.19, 2.74) | 0.38 |
| SBP (mmHg) | 2.07 (0.72, 3.42) | <0.01 |
| Acute Rejection | 66.42 (16.03, 116.81) | 0.01 |
| Cause of Kidney Disease | ||
| Other (vs. DM) | −57.85 (−108.47, −7.23) | 0.03 |
SBP: systolic blood pressure; CSA: Cyclosporine, MMF: mycophenolate mofetil, DM: diabetes mellitus
Figure 3.
Plasma Renin Activity and Plasma Aldosterone levels vs. Iothalamate GFR values A., B. baseline and C., D. visit number 5.
Association of PRA and Paldo with interstitial fibrosis/tubular atrophy (IF/TA) and non-IF/TA related ESRD
The following dichotomous outcomes were considered: doubling of VvInt/C or ESRD from IF/TA, doubling of VvInt/C and all-cause ESRD and the more conventional endpoint of doubling of serum creatinine, ESRD or death (Table 5 and Appendix 5). PRA and Paldo were not statistically significantly associated with these outcomes. Subjects who experienced acute rejection [OR=4.62 (95% CI 1.8–11.83), p<0.01)] or had <3 HLA matches [OR=3.13 (95% CI 1.08–9.11) p=0.04)] had higher risks of doubling of VvInt/C or ESRD from IF/TA. Subjects taking losartan [OR=0.24 (95% CI 0.08–0.76), p=0.02)], having lower DBP [OR=0.85 (95% CI 0.73–0.99), p=0.049] or on CSA + MMF without prednisone [OR=0.06 (95% CI 0.01–0.59), p=0.02] had lower risks of doubling of VvInt/C. Subjects with <3 HLA matches [OR=6.17 (95% CI 1.37–27.74), p=0.02], hypertension [OR=11.99 (95% CI 1.9–75.5), p=0.01] or who had an acute rejection [OR=8.14 (95% CI 2.04–32.38), p<0.01] were also at higher risk for these outcomes. Subjects on losartan [OR=0.50 (95% CI 0.27–0.91), p=0.02] or who had a white donor [OR=0.37 (95% CI 0.19–0.77), p<0.01] were at lower risk of doubling of serum creatinine, all-cause ESRD or death. In contrast, having a higher iothalamate GFR [OR=1.04 (95% CI 1.01–1.08), p=0.02], hypertension [OR=2.07 (95% CI 1.03–4.18), p=0.04] or acute rejection [OR=2.66 (95% CI 1.36–5.18), p<0.01] resulted in a higher risk of doubling, death or ESRD (Table 5 and Appendix 5).
Table 5.
Predictors of Interstitial expansion and ESRD
| VvInt/C doubling or ESRD from IF/TA | ||
|---|---|---|
| Variable | OR (95% CI) | P-Value |
| Plasma Aldosterone | 1.01 (1, 1.01) | 0.21 |
| Plasma Renin Activity | 0.91 (0.49, 1.7) | 0.77 |
| Female Recipient | 0.36 (0.12, 1.08) | 0.07 |
| Acute Rejection | 4.62 (1.8, 11.83) | <0.01 |
| <3 HLA Matches | 3.13 (1.08, 9.11) | 0.04 |
| Tacrolimus+ Rapamycin | 3.32 (0.98, 11.27) | 0.05 |
| VvInt/C doubling or any ESRD | ||
| Plasma Aldosterone | 1.01 (1, 1.02) | 0.08 |
| Plasma Renin Activity | 0.47 (0.17, 1.27) | 0.14 |
| Female Recipient | 0.37 (0.12, 1.08) | 0.07 |
| DBP (mmHg) | 0.85 (0.73, 1) | 0.05 |
| Hypertension | 11.99 (1.9, 75.59) | 0.01 |
| Acute Rejection | 8.14 (2.04, 32.38) | <0.01 |
| <3 HLA Matches | 6.17 (1.37, 27.74) | 0.02 |
| Losartan | 0.24 (0.08, 0.76) | 0.02 |
| Loop Diuretics | 3.64 (0.96, 13.77) | 0.06 |
| CSA + MMF | 0.06 (0.01, 0.59) | 0.02 |
| CSA + MMF + Pred. | 0.13 (0.02, 1.06) | 0.06 |
| CR doubling, ESRD or Death | ||
| Plasma Aldosterone | 1 (0.99, 1) | 0.39 |
| Plasma Renin Activity | 0.92 (0.57, 1.47) | 0.73 |
| SBP (mmHg) | 1.04 (1, 1.09) | 0.05 |
| Hypertensive | 2.07 (1.03, 4.18) | 0.04 |
| Acute Rejection | 2.66 (1.36, 5.18) | <0.01 |
| <3 HLA Matches | 1.66 (0.9, 3.04) | 0.10 |
| Caucasian Donor | 0.37 (0.19, 0.73) | <0.01 |
| Losartan | 0.5 (0.27, 0.91) | 0.02 |
| Iothalamate GFR | 1.04 (1.01, 1.08) | 0.02 |
DBP: diastolic blood pressure; SBP: systolic blood pressure; CSA: Cyclosporine, MMF: mycophenolate mofetil; Pred.: Prednisone.
Lastly, Table 6 shows the results of Cox proportional hazards regression (utilizing time dependent variables for two outcomes: 1) all-cause ESRD and 2) doubling of serum creatinine, all-cause ESRD or death. An increase in Paldo, [HR=1.01 (95% CI 1.00–1.02), p=0.02] at any time during the study was associated with an increased risk of ESRD, while each additional year spent in the study was associated with a lower risk [HR=0.95 (95% CI 0.89–0.99), p=0.04]. Use of loop diuretics [HR=4.72 (95% CI 1.56–25.8), p=0.049], <3 HLA matches [HR=6.35 (95% CI 1.56–25.8), p=0.03], non-white donors [HR=3.85 (95% CI 1.25–12.5), p=0.03] and acute rejection [HR=12.91 (95% CI 2.6–61.8), p<0.01] was associated with an increased risk of ESRD. Having a white donor [HR=0.18 (95% CI 0.07–0.4), p<0.01] was associated with a decrease in the risk of doubling of serum creatinine, all-cause ESRD or death. Having <3 HLA matches [HR=3.79 (95% CI 1.56–9.1), p<0.01], being hypertensive [HR=6.97 (95% CI 2.23–21.7), p<0.01] or having an acute rejection [HR=3.98 (95% CI 1.80–8.8), p<0.01] resulted in an increased risk.
Table 6.
Cox proportional hazards regression with time-dependent covariates
| All-Cause ESRD | Creatinine Doubling, All-Cause ESRD or Death | ||||
|---|---|---|---|---|---|
| Variable | HR (95% CI) | P-Value | Variable | HR (95% CI) | P-Value |
| Plasma Aldosterone | 1.01 (1.00, 1.0) | 0.02 | Plasma Aldosterone | 0.998 (0.99, 1.003) | 0.43 |
| Plasma Renin Activity | 0.82 (0.56, 1.1) | 0.29 | Plasma Renin Activity | 1.02 (0.90, 1.1) | 0.74 |
| Age at Visit (years) | 0.95 (0.89, 0.99) | 0.04 | Losartan | 0.48 (0.21, 1.0) | 0.07 |
| SBP | 1.03 (0.998, 1.0) | 0.07 | Tacrolimus + Rapamycin | 2.37 (0.98, 5.7) | 0.05 |
| Loop diuretics | 4.72 (1.01, 22.1) | 0.05 | Caucasian donor | 0.18 (0.07, 0.4) | <0.01 |
| Caucasian donor | 0.26 (0.08, 0.8) | 0.03 | <3 HLA Matches | 3.79 (1.56, 9.1) | <0.01 |
| <3 HLA matches | 6.35 (1.56, 25.8) | 0.01 | Hypertension | 6.97 (2.23, 21.7) | <0.01 |
| Hypertension | 7.49 (0.85, 66.3) | 0.07 | Acute rejection (ever) | 3.98 (1.80, 8.8) | <0.01 |
See separate file for Appendix.
DISCUSSION
Our results indicate that: 1). PRA and Paldo levels are not elevated during the first 5 years after kidney transplantation; 2). angiotensin II blockade is effective in suppressing the RAAS as evident by a lower PRA in the placebo group, 3). the DD allele of the ACE gene polymorphism does not convey increased risk for worse allograft function or structure, 4). a higher Paldo at any time during the study was associated with increased risk of ESRD and 5). receiving a kidney from a White donor was associated with a decrease in the risk of doubling serum creatinine, all-cause ESRD and death. On the other hand, having less than 3 HLA matches, being hypertensive, having had an acute rejection episode or receiving tacrolimus - rapamycin based regimen were associated with increased risk.
Angiotensin II has been singled out as a major mediator in initiation and progression of native kidney disease (6). Multiple studies have demonstrated superior efficacy of RAAS blockers in retarding the progression of chronic kidney disease particularly in those with proteinuria (1–3). The presence of such strong evidence to the benefit of these agents is lacking in the kidney transplant arena. A systematic review of 21 randomized kidney trials that included 1549 patients with a median follow-up of 27 months demonstrated that the use of these agents is associated with a significant reduction in GFR, reduction in proteinuria of roughly 500 mg/day but there was insufficient evidence to determine the effects on patient or graft survival (4). Not included in this systematic review is our recent demonstration that angiotensin II blocker when compared to placebo was associated with statistically insignificant (p=0.08) benefit in the primary outcome of retarding the development of interstitial fibrosis and graft loss from IF/TA. However, losartan was associated with a reduction in the the secondary outcome of doubling of interstitium from baseline to 5 year biopsies or all-cause ESRD, [odds ratio of 0.36 (95%CI 0.13–0.99), p= 0.05] (5). Hernandez et al. recently published the results of a longitudinal cohort study of 414 patients who received ACE inhibitors or angiotensin-receptor blockers with a follow-up that ranged between 6 and 40 months and demonstrated that the use of these agents was associated with a reduction in the risk of mortality but they were not associated with a significant improvement of allograft survival (7). On the other hand, the evidence regarding the impact of the ACE gene polymorphisms on kidney transplant function has been mixed. Siekierka-Harreis et al. showed that the DD genotype of the ACE gene was associated with significantly reduced kidney transplant function during the first 18 to 30 months after transplantation (8). However, in this study only the ACE gene polymorphism in the recipient was analyzed. To our knowledge, the data we present herein are unique in that they provide not only ACE gene polymorphism in the recipient but also in the donor and their possible permutations. A large scale genetic polymorphism analysis would be needed to settle this issue. We also recognize that the power to detect important treatment effects and associations is limited by sample size in our study. For the longitudinal data, it is worth to note that there were also some missing samples.
Obviously, given the lack of a large randomized trial with hard clinical endpoints in the setting of kidney transplantation, there is no conclusive evidence that these agents are beneficial beyond their antihypertensive properties. One has to consider, however, whether the renin-angiotensin-aldolsterone system is as amenable to interruption in this patient population as it is in persons with their native kidneys. There has been very little published on the activity of this system in the transplant population and we believe this is the first effort that has an a priori secondary outcome, serial RAAS measurement and testings of the impact RAAS blockade on multiple clinical parameters, including hard endpoints. Bantle et al. studied cyclosporine treated kidney transplant recipients and compared them to those maintained on azathioprine based immunosuppression 7 to 20 months after kidney transplantation (9). After stimulation by a low sodium diet and furosemide, cyclosporine treated patients demonstrated lower PRA and Paldo levels than their azathioprine treated counterparts. In the case of PRA, azathioprine treated participants had 4 times higher and a two-fold increase in their Paldo levels but the latter difference did not reach statistical significance. These findings are consistent with data from Beckerhoff et al. who measured PRA and Paldo in 19 recipients in the first 30 days after kidney transplantation and found these to be in the normal range (10). Notably shortly after transplant (in the first week) both of these parameters were elevated. Our results suggest that the type of calcineurin inhibitor or anti-metabolite used does not affect PRA or Paldo and neither does steroid use as described by others (11). In the non-renal transplant setting, Julien et al. studied 21 heart and 12 liver transplant recipients on a normal salt diet and compared them to 19 age-matched normotensive controls (12). Supine and upright PRA values tended to be higher in hypertensive transplant recipients than in healthy volunteers but that difference was not significant. Moreover, Paldo levels were within the normal range in controls and also liver transplant recipients and it did not correlate with PRA values. Taken together, the available evidence does not suggest that PRA or Paldo are stimulated in the setting of kidney transplantation or non-renal solid organ transplantation. The data we present here are consistent with these older, smaller and short-term follow-up studies.
The findings of normal PRA in the setting of kidney transplantation is not different than one would see as well in native kidney disease as patients with chronic native kidney disease tend to have normal PRA (13). Rosenberg and his colleagues argued that PRA is paradoxically normal in the setting of volume expansion that frequently accompanies CKD (13). They, moreover, argued that it is the local intra-renal activity of the renin-angiotensin system that might be more critical than the systemic state (14). In our study, the 24-hour urine sodium excretion rates were approximately 170 mEq/day. One may argue that this somewhat generous sodium intake should have suppressed PRA further. It is worthwhile to point to a potential limitation of our study: the performed assays for PRA and Paldo do not provide information about local RAAS activity in the kidney allograft that could be driving some of the pathological processes despite the lack of systemic over-activity. In animal studies, local RAAS (in renal tubular cells) may operate independent from its systemic counterpart (15). Intraluminal angiotensinogen may be converted in the distal tubules to angiotensin II and may cause up-regulation of sodium channels independent of systemic aldosterone levels(16). It was also observed that complete systemic inhibition of angiotensin II formation by ACE inhibitors is not accompanied by a significantly reduced intrarenal angiotensin II production (17). However, proximal tubular cells also could take up renin and angiotensinogen from the circulation, indicating a close interaction with the systemic RAAS. In all, there is still scarcity of human data about the roles of local RASS in kidney disease.
The issue of normal Paldo however is intriguing. There is a progressive rise in aldosterone plasma levels as native kidney function declines (18, 19). This was not demonstrated in our study as Paldo was constantly in the normal range throughout the five years of the study. A possible explanation for this phenomenon is the impressively low potassium intake in this population as evidenced by the 24-hour urine potassium that averaged around 50 mEq/day. Not only angiotensin II, but also increased dietary potassium intake, is a powerful stimulus for aldosterone.
Despite the lack of strong clinical data supporting the early use of RAAS blockers following kidney transplantation, the theoretical rationale for such a strategy can be justified in certain conditions, such as diabetes mellitus and heart failure (20, 21). Moreover, there is some anecdotal evidence about the role angiotensin-receptor blockers in treating refractory acute vascular rejection mediated by preformed non-HLA angiotensin II type 1-receptor-activating antibodies (22). Our results also confirm that hypertension, acute rejection episodes, less than 3 HLA matches and tacrolimus -rapamycin based regimen are associated with doubling of serum creatinine, ESRD and death, as has been previously described by others (23–25).
The association of Paldo with higher ESRD incidence that we described herein, raises an important question of whether aldosterone inhibition alone or in combination with other agents that inhibit the RAAS would be effective in halting renal allograft fibrosis and preventing progression to ESRD. There has been some animal data supporting this approach. However, we are cautious about universally recommending aldosterone inhibition to all kidney transplant recipients, since we believe that the potential benefit from aldosterone inhibition should be weighed against the significant rise in serum potassium. Of concern is the potential for life-threatening hyperkalemia, particularly in kidney transplant recipients with some degree of renal insufficiency especially that this population has a predisposition to hyperkalemia with the concomitant use of calcineurin inhibitors. Moreover, our findings as well as the benefits of aldosterone inhibition in kidney transplant recipients need to be validated in future larger clinical trials.
In summary, we found PRA and Paldo to be normal in the first 5 years after kidney transplantation and that Angiotensin II blockade suppresses this system effectively. Other than the association of serial plasma aldosterone over time with ESRD, we could not demonstrate a relationship between PRA and Paldo on GFR decline, proteinuria or interstitial expansion.
METHODS
The Angiotensin II Blockade in Chronic Allograft Nephropathy (ABCAN) Trial was a 5-year National Institutes of Health sponsored trial that randomized 153 kidney transplant recipients to losartan 100 mg/day (n=77) or placebo (n=76) shortly after transplantation. Participants underwent intra-operative or peri-transplant kidney biopsy and a repeat biopsy 5 years later. The main hypothesis of the trial was that abrogating the fibrogenic and deleterious hemodynamic effects of AII and its downstream mediators will reduce the occurrence of the composite of doubling of the (Vvint/C) from baseline to 5 years biopsies and graft loss from IF/TA by 60% when compared to placebo. At the end of the trial and utilizing the intention to treat analysis 6/47 losartan treated participants and 12/44 placebo treated ones reached the primary endpoint, O.R. 0.39 (95% CI 0.13–1.15), p=0.08 (5).
Annually, participants underwent iothalamate GFR measurement using 5 timed urine and plasma collections. The coefficient of variation (CV) of the iothalamate GFR was < 8%. Concomitantly with iothalamate, para-aminohippuric acid was administered, to measure renal blood flow, effective renal plasma flow (ERPF) and from the ratio of GFR/ERPF, the filtration fraction was calculated in order to gain indirect insight regarding the hydrostatic pressure in the glomerulus. Urinary protein measurement, PRA and Paldo were collected at the annual GFR visit. In addition, 24 hour urine collections were obtained for assessment of urinary sodium and potassium. DNA was collected from recipients and donors (blood in the case of live donors and tissue such as spleen from deceased donors). ACE gene polymorphism (DD, ID, II) were determined. The hypothesis here was that the DD allele would be associated with activated RAAS components and disconcordance between donors and recipients, particularly receiving a DD kidney in a recipient harboring the DD allele, would be associated with worse outcomes.
(VvInt/C) was estimated by point counting, as reported previously (26). In brief, the number of points falling on the interstitium relative to those falling on cortical tissue was counted. The interstitium space was defined as the area of cortex not containing glomeruli, tubules, or blood vessels larger than the average tubular diameter. Plasma used to measure PRA was obtained from blood collected in EDTA tubes. Appropriate steps were taken to keep plasma samples between 2–4°C during the collection and centrifugation processes. All plasma samples were stored at −70°C. Plasma used for aldosterone measurements was obtained in a similar fashion except heparinized tubes were used. I125 radioimmunoassay kits GammaCoat® by DiaSorin and Coat-A- Count® by Siemens were used to measure PRA and Paldo concentration, respectively.
Statistical Analysis
Categorical and continuous variables were compared using chi-square and t-tests, respectively. Utilizing baseline variables, a linear model was used to predict (VvInt/C) measured on the 5 year biopsy, last GFR, last ACR and (VvInt/C) change from baseline to five years. Also using baseline variables, a logistic model was constructed to predict the following three outcomes: 1) doubling of (VvInt/C) or ESRD from IF/TA, 2) doubling of (VvInt/C) or any ESRD, and 3) doubling of serum creatinine, all- cause ESRD or death. A linear mixed model was used to predict serial GFR and serial ACR. In all models the following covariates were considered: Paldo, PRA, losartan/placebo, recipient sex, age, race, BMI, GFR, ACR, SBP, DBP, thiazide or loop diuretic use, immunosuppressive regimen, donor age, gender, source and ethnicity, HLA matches (<3 vs. 3+), cause of kidney disease, hypertension and episodes of acute rejection (ever). A similar set of predictors were used to predict Paldo and PRA, both at baseline and serially. Cox proportional hazards regression with both fixed and time-dependent covariates was utilized to assess their effect on two dichotomous outcomes: all-cause ESRD and doubling of serum creatinine, all-cause ESRD or death. Backwards selection was utilized, only keeping Paldo, PRA, and covariates with p<0.10. All analyses were performed in SAS version 9.3 (Cary, NC). Results are expressed as mean ± SD, unless otherwise specified.
Acknowledgments
Sources of Support: This work was supported by NIDDK, U01 DK060706-09, and Merck Inc. donated losartan and the placebo.
APPENDIX
Appendix 1. ACE Gene Analysis
We analyzed polymorphism data for the 153 ABCAN trial participants and their corresponding donors. An ANOVA was performed to see if the value of either plasma aldosterone or plasma renin activity (PRA) depended on the designation of the donor or recipient ACE gene. The mean, sample size and overall F-test are given below. Since no tests were significant, individual pairwise comparisons were not performed.
| ACE Category | Paldo (pg/ml) | F-test p-value | PRA (ng/ml/h) | F-test p-value |
|---|---|---|---|---|
|
| ||||
| Recipient | ||||
| D/D (30%) | 241.9 | 0.59 | ||
| D/I (52%) | 132.3 | 0.43 | 0.94 | 0.28 |
| I/I (18%) | 165.0 | 1.11 | ||
|
| ||||
| Donor | ||||
| D/D (30%) | 283.7 | 0.87 | ||
| D/I (46%) | 129.1 | 0.32 | 0.93 | 0.73 |
| I/I (23%) | 144.6 | 0.68 | ||
We then ran a second ANOVA with the same outcomes but considered status (donor or recipient) as a covariate along with ACE allele. In both cases we did not find evidence of a significant interaction, i.e. the effect of ACE on the outcome did not depend on whether the measurement was taken from a donor or recipient (p=0.92 and p=0.37 for plasma aldosterone and PRA respectively).
| Recipient | |||
|---|---|---|---|
|
| |||
| Donor | D/D | D/I | I/I |
|
| |||
| D/D | 2,2,6 | 1,3,6 | 0,0,1 |
|
| |||
| Plasma | 103.2 (65.3) | 138.4 (79.5) | 171.7 (34.6) (5) |
| Aldosterone | (13) | (15) | 1.3 (2.2) (5) |
| PRA | 1.2 (1.7) (13) | 0.79 (0.72) (14) | |
|
| |||
| D/I | 2,2,3 | 4,5,6 | 0,0,3 |
| Plasma | 129.2 (82.8) | 126.7 (94.0) | 133.5 (71.4) |
| Aldosterone | (13) | (30) | (10) |
| PRA | 0.7 (0.8) (14) | 1.3 (1.7) (29) | 0.5 (0.4) (10) |
|
| |||
| I/I | 0,0,0 | 2,3,4 | 1,1,0 |
| Plasma | 165.6 (108.8) | 117.0 (61.6) | 174.4 (107.7) |
| Aldosterone | (7) | (14) | (8) |
| PRA | 0.3 (0.2) (7) | 0.8 (1.2) (12) | 1.5 (1.7) (8) |
Values in each cell are Mean (SD) (N) or # of events (Doubling of interstitium or ESRD from IF/TA, Doubling of interstitium or any ESRD, Doubling of serum creatinine, all-cause ESRD or Death)
| Counts at Baseline | Losartan (N) (%) | Placebo (N) (%) |
|---|---|---|
|
| ||
| Donor ACE | ||
| D/D | 21 (32.3) | 17 (28.9) |
| D/I | 29 (50.9) | 28 (47.5) |
| I/I | 15 (23.1) | 14 (23.7) |
|
| ||
| Recipient ACE | ||
| D/D | 26 (34.2) | 20 (26.3) |
| D/I | 36 (47.4) | 43 (56.6) |
| I/I | 14 (18.4) | 13 (17.1) |
| Donor ACE = Recipient ACE | No Event | Event | Total |
|---|---|---|---|
| Yes | 51 | 19 | 70 |
| No | 39 | 14 | 53 |
| Total | 90 | 33 | 123 |
Chi-square p-value=0.93
Appendix 2. Predictors of Plasma Renin Activity (ng/ml/h) and Plasma Aldosterone (pg/ml)
| Variable | At Basline | Longitudinally | ||||||
|---|---|---|---|---|---|---|---|---|
| Plasma Renin Activity | Plasma Aldosterone | Plasma Renin Activity | Plasma Aldosterone | |||||
| Estimate (95% CI) | P-Value | Estimate (95% CI) | P-Value | Estimate (95% CI) | P-Value | Estimate (95% CI) | P-Value | |
| Recipient Age (years) | 0.004 (−0.12, 0.03) | 0.75 | −0.47 (−2.11, 1.17) | 0.57 | −0.02 (−0.05, 0.01) | 0.25 | −0.25 (−1.47, 0.97) | 0.69 |
| Female Recipient | −0.81 (−1.35, −0.28) | <0.01 | −36.90 (−73.97, 0.16) | 0.05 | −0.65 (−1.39, 0.10) | 0.09 | −25.59 (−52.62, 1.43) | 0.06 |
| Caucasian Recipient | 0.60 (−0.15, 1.35) | 0.12 | 11.15 (−39.95, 62.26) | 0.67 | 0.38 (−0.70, 1.47) | 0.49 | −6.69 (−46.52, 33.15) | 0.74 |
| Recipient BMI (kg/m2) | 0.003 (−0.05, 0.06) | 0.91 | 0.14 (−3.55, 3.83) | 0.94 | 0.03 (−0.04, 0.11) | 0.37 | 3.15 (0.37, 5.93) | 0.03 |
| SBP (mmHg) | −0.005 (−0.024, 0.013) | 0.56 | −0.37 (−.164, 0.90) | 0.57 | 0.006 (−0.02, 0.03) | 0.63 | −0.32 (−1.15, 0.51) | 0.45 |
| DBP (mmHg) | −0.01 (−0.05, 0.02) | 0.44 | −0.51 (−3.05, 2.03) | 0.69 | −0.05 (−0.09, −0.004) | 0.03 | 1.74 (0.27, 3.21) | 0.02 |
| Live donor | −0.48 (−1.10, 0.13) | 0.13 | −43.41 (−86.80, −0.03) | 0.05 | −0.93 (−1.81, −0.05) | 0.04 | −33.3 (−65.35, −1.25) | 0.04 |
| Donor Age (years) | −0.03 (−0.05, −0.01) | 0.01 | 0.01 (−1.53, 1.54) | 0.99 | −0.02 (−0.05, 0.01) | 0.21 | −0.32 (−1.48, 0.85) | 0.59 |
| Female donor | 0.66 (0.14, 1.17) | 0.01 | 15.82 (−20.15, 51.79) | 0.39 | 1.11 (0.36, 1.86) | <0.01 | 16.59 (−10.96, 44.15) | 0.24 |
| Caucasian donor | −0.26 (−0.86, 0.34) | 0.39 | −35.39 (−76.65, 5.87) | 0.09 | 0.44 (−0.44, 1.31) | 0.32 | −10.34 (−42.56, 21.87) | 0.53 |
| Cause of Kidney Disease | ||||||||
| DM | reference | reference | reference | reference | ||||
| HTN | −0.28 (−1.47, 0.90) | 0.64 | −23.08 (−111.87, 65.70) | 0.61 | 1.54 (0.02, 3.05) | 0.05 | −17.96 (−75.43, 39.51) | 0.54 |
| PCKD | −0.12 (−0.96, 0.72) | 0.78 | −3.39 (−63.60, 56.81) | 0.91 | −0.36 (−1.48, 0.77) | 0.53 | 7.74 (−34.11, 49.59) | 0.72 |
| Other | 0.57 (−0.03, 1.17) | 0.06 | 18.50 (−22.98, 59.97) | 0.38 | 0.65 (−0.23, 1.52) | 0.15 | 5.09 (−26.77, 36.94) | 0.75 |
| Smoking | 0.26 (−0.47, 1.00) | 0.48 | 20.89 (−29.17, 70.95) | 0.41 | −0.96 (−1.95, 0.03) | 0.06 | −20.37 (−58.06, 17.31) | 0.29 |
| Hyperlipidemia | −0.24 (−1.88, 1.40) | 0.77 | 117.12 (3.32, 230.92) | 0.04 | −0.62 (−2.34, 1.11) | 0.48 | 46.25 (−20.40, 112.89) | 0.17 |
| Losartan | −0.11 (−0.59, 0.37) | 0.66 | 5.59 (−27.99, 39.17) | 0.74 | 1.61 (0.90, 2.31) | <0.01 | −14.73 (−40.57, 11.11) | 0.26 |
| Thiazides | 0.75 (−0.18, 1.68) | 0.11 | 44.59 (−20.48, 109.66) | 0.18 | −0.003 (−1.29, 1.29) | 0.99 | 24.21 (−23.88, 72.29) | 0.32 |
| Loop diuretics | 0.04 (−0.57, 0.66) | 0.89 | −6.96 (−48.44, 34.52) | 0.74 | 0.17 (−0.68, 1.02) | 0.69 | −25.64 (−56.97, 5.69) | 0.11 |
| Tacrolimus + Rapamycin | 0.13 (−0.89, 1.16) | 0.80 | 7.65 (−65.62, 80.93) | 0.84 | 0.57 (−0.95, 2.09) | 0.46 | 1.98 (−54.92, 58.88) | 0.95 |
| Tacrolimus + MMF | −0.37 (−1.33, 0.60) | 0.45 | 0.65 (−65.87, 67.16) | 0.98 | −0.05 (−1.43, 1.34) | 0.95 | −1.27 (−52.26, 49.73) | 0.96 |
| Tacrolimus + MMF + Pred. | −0.79 (−1.99, 0.40) | 0.19 | 30.04 (−52.28, 112.36) | 0.47 | 0.23 (−1.59, 2.04) | 0.81 | 47.03 (−19.81, 113.86) | 0.17 |
| CSA + MMF | −0.46 (−1.44, 0.53) | 0.36 | 1.13 (−67.19, 69.44) | 0.97 | 0.76 (−0.66, 2.18) | 0.29 | −27.39 (−79.82, 25.04) | 0.30 |
| CSA + MMF + Pred. | −0.74 (−1.79, 0.31) | 0.16 | −22.45 (−94.30, 49.39) | 0.54 | 0.66 (−0.87, 2.18) | 0.40 | −18.64 (−75.46, 38.18) | 0.52 |
- SBP: systolic blood pressure; DBP: diastolic blood pressure; CSA: Cyclosporine, MMF: mycophenolate mofetil, DM: diabetes mellitus, PCKD; polycystic kidney disease, HTN: hypertension, ACR: albumin-creatinine ratio
Appendix 3. Predictors of last VvInt/C, last GFR, last ACR and VvInt/C Change
| Variable | Last VvInt/C | Last GFR | Last ACR | VvInt/C Change | ||||
|---|---|---|---|---|---|---|---|---|
| Estimate (95% CI) | P-Value | Estimate (95% CI) | P-Value | Estimate (95% CI) | P-Value | Estimate (95% CI) | P-Value | |
| Plasma Aldosterone | 0 (0, 0) | 0.47 | 0.01 (−0.03, 0.06) | 0.62 | −0.36 (−1.02, 0.3) | 0.28 | 0 (0, 0) | 0.61 |
| Plasma Renin Activity | −0.001 (−0.018, 0.017) | 0.94 | 0.84 (−3.32, 4.99) | 0.69 | 18.16 (−47.8, 84.12) | 0.58 | −0.001 (−0.034, 0.033) | 0.96 |
| Recipient Age (years) | −0.001 (−0.003, 0.001) | 0.19 | −0.07 (−0.46, 0.32) | 0.71 | 1.98 (−4.16, 8.13) | 0.52 | −0.001 (−0.004, 0.002) | 0.41 |
| Female Recipient | 0.03 (−0.011, 0.071) | 0.15 | −4.97 (−14.58, 4.63) | 0.30 | 16.01 (−137.8, 169.81) | 0.84 | 0.022 (−0.054, 0.099) | 0.55 |
| Caucasian Recipient | −0.014 (−0.068, 0.04) | 0.61 | −11.86 (−23.09, −0.64) | 0.04 | −63.26 (−254.15, 127.64) | 0.51 | −0.016 (−0.107, 0.076) | 0.73 |
| Recipient BMI | 0.001 (−0.003, 0.006) | 0.56 | −0.47 (−1.34, 0.41) | 0.29 | 17.72 (3.09, 32.36) | 0.02 | 0.002 (−0.006, 0.01) | 0.55 |
| SBP (mmHg) | 0 (−0.001, 0.002) | 0.65 | −0.11 (−0.41, 0.19) | 0.47 | 2.22 (−2.83, 7.27) | 0.38 | 0 (−0.003, 0.003) | 1.00 |
| DBP (mmHg) | 0 (−0.003, 0.003) | 0.95 | 0.36 (−0.24, 0.97) | 0.23 | −5.96 (−15.96, 4.04) | 0.24 | −0.002 (−0.006, 0.003) | 0.53 |
| Hypertension | 0.01 (−0.028, 0.049) | 0.60 | 0.53 (−8.02, 9.09) | 0.90 | 24.01 (−114.56, 162.59) | 0.73 | 0.043 (−0.022, 0.107) | 0.19 |
| Acute Rejection | 0.033 (−0.019, 0.085) | 0.20 | −8.12 (−20.49, 4.25) | 0.19 | 147.27 (−59.74, 354.28) | 0.16 | 0.085 (−0.006, 0.175) | 0.06 |
| <3 HLA Matches | 0.018 (−0.018, 0.054) | 0.33 | −7.74 (−15.68, 0.19) | 0.06 | 62.23 (−66.96, 191.43) | 0.34 | −0.004 (−0.064, 0.055) | 0.89 |
| Live Donor | −0.01 (−0.057, 0.036) | 0.65 | −3.14 (−13.56, 7.28) | 0.55 | −79.6 (−247.76, 88.57) | 0.35 | 0.011 (−0.061, 0.083) | 0.76 |
| Donor Age (years) | 0 (−0.001, 0.002) | 0.61 | −0.03 (−0.45, 0.39) | 0.87 | 0.02 (−6.39, 6.44) | 0.99 | −0.001 (−0.003, 0.002) | 0.70 |
| Female Donor | −0.006 (−0.045, 0.034) | 0.77 | −4.29 (−12.88, 4.3) | 0.32 | −2.03 (−146.53, 142.46) | 0.98 | −0.006 (−0.071, 0.059) | 0.85 |
| Caucasian Donor | 0.044 (−0.004, 0.092) | 0.07 | 6.59 (−4.72, 17.89) | 0.25 | 103.56 (−78.51, 285.63) | 0.26 | 0.067 (−0.019, 0.153) | 0.12 |
| Cause of Kidney Disease | ||||||||
| DM | reference | reference | reference | reference | ||||
| HTN | −0.051 (−0.126, 0.024) | 0.18 | 7.66 (−8.42, 23.73) | 0.34 | −107.73 (−382.49, 167.04) | 0.43 | −0.052 (−0.173, 0.069) | 0.38 |
| PCKD | −0.001 (−0.061, 0.059) | 0.97 | 2.03 (−10.54, 14.6) | 0.75 | 100.06 (−114.75, 314.87) | 0.35 | 0.053 (−0.052, 0.159) | 0.31 |
| Other | −0.03 (−0.082, 0.022) | 0.25 | 8.25 (−3.08, 19.58) | 0.15 | 16.01 (−169.33, 201.35) | 0.86 | −0.039 (−0.127, 0.049) | 0.37 |
| Losartan | −0.01 (−0.047, 0.027) | 0.60 | −6.78 (−14.95, 1.39) | 0.10 | 96.32 (−39.7, 232.33) | 0.16 | −0.05 (−0.114, 0.013) | 0.11 |
| Thiazides | −0.016 (−0.085, 0.052) | 0.63 | 7.15 (−7.38, 21.67) | 0.33 | −77.25 (−326.01, 171.51) | 0.54 | −0.048 (−0.181, 0.086) | 0.47 |
| Loop Diuretics | 0 (−0.051, 0.051) | 0.99 | 2.9 (−7.14, 12.93) | 0.56 | 66.74 (−107.71, 241.2) | 0.45 | −0.016 (−0.098, 0.066) | 0.69 |
| Tacrolimus + Rapamycin | 0.067 (−0.014, 0.148) | 0.10 | −16.2 (−34.78, 2.37) | 0.09 | −137.41 (−445.59, 170.78) | 0.37 | 0.049 (−0.072, 0.171) | 0.41 |
| Tacrolimus + MMF | 0.02 (−0.061, 0.101) | 0.62 | 3.37 (−15.05, 21.78) | 0.71 | −322.12 (−612.94, −31.31) | 0.03 | −0.003 (−0.147, 0.14) | 0.96 |
| Tacrolimus+ MMF + Pred. | −0.001 (−0.112, 0.111) | 0.99 | −6.91 (−30.12, 16.3) | 0.55 | −362.28 (−750.67, 26.1) | 0.07 | −0.127 (−0.343, 0.088) | 0.24 |
| CSA + MMF | 0.01 (−0.076, 0.096) | 0.82 | −5.42 (−24.32, 13.48) | 0.57 | −334.44 (−635.84, −33.04) | 0.03 | −0.088 (−0.224, 0.049) | 0.20 |
| CSA + MMF + Pred. | 0.009 (−0.081, 0.099) | 0.85 | 3.62 (−15.74, 22.98) | 0.71 | −401.41 (−715.09, −87.73) | 0.01 | −0.04 (−0.186, 0.106) | 0.58 |
SBP: systolic blood pressure; DBP: diastolic blood pressure; CSA: Cyclosporine, MMF: mycophenolate mofetil, DM: diabetes mellitus, PCKD: polycystic kidney disease, HTN: hypertension, ACR: albumin creatinine ratio
Appendix 4. Predictors of Serial GFR and urinary albumin-creatinine ratio (ACR)
| Variable | GFR | ACR | ||
|---|---|---|---|---|
| Estimate (95% CI) | P-Value | Estimate (95% CI) | P-Value | |
| Plasma Aldosterone | 0 (−0.02, 0.01) | 0.45 | 0.07 (−0.06, 0.2) | 0.30 |
| Plasma Renin Activity | −0.13 (−0.61, 0.34) | 0.58 | −2.23 (−7.19, 2.74) | 0.38 |
| Recipient Age (years) | −0.01 (−0.22, 0.19) | 0.89 | −0.39 (−2.18, 1.41) | 0.67 |
| Female Recipient | −2.8 (−7.41, 1.81) | 0.23 | 2.25 (−37.64, 42.14) | 0.91 |
| Caucasian Recipient | −6.78 (−13.55, −0.01) | 0.05 | −3.68 (−62.07, 54.71) | 0.90 |
| Recipient BMI | −0.13 (−0.62, 0.36) | 0.59 | −2.35 (−6.6, 1.89) | 0.27 |
| SBP (mmHg) | 0.03 (−0.11, 0.16) | 0.69 | 2.07 (0.72, 3.42) | <0.01 |
| DBP (mmHg) | −0.01 (−0.25, 0.23) | 0.94 | −0.99 (−3.39, 1.4) | 0.41 |
| Hypertensive | 2.37 (−2.69, 7.42) | 0.36 | 32.57 (−10.89, 76.04) | 0.14 |
| Acute Rejection | −1.11 (−6.89, 4.67) | 0.71 | 66.42 (16.03, 116.81) | 0.01 |
| <3 HLA Matches | −3.29 (−8.01, 1.44) | 0.17 | −24.64 (−65.65, 16.37) | 0.24 |
| Live Donor | 2.66 (−3.12, 8.44) | 0.36 | −29.16 (−79.16, 20.83) | 0.25 |
| Donor Age (years) | −0.28 (−0.49, −0.07) | 0.01 | 1.49 (−0.34, 3.32) | 0.11 |
| Female Donor | −4.02 (−8.86, 0.81) | 0.10 | 19.53 (−22.46, 61.53) | 0.36 |
| Caucasian Donor | 4.32 (−1.21, 9.85) | 0.12 | 9.57 (−38.63, 57.77) | 0.70 |
| Cause of Kidney Disease | ||||
| DM | reference | reference | ||
| HTN | −0.79 (−10.64, 9.06) | 0.87 | −38.75 (−123.41, 45.91) | 0.37 |
| PCKD | 0.53 (−6.88, 7.93) | 0.37 | −12.6 (−77.3, 52.1) | 0.70 |
| Other | 2.59 (−3.15, 8.33) | 0.89 | −57.85 (−108.47, −7.23) | 0.03 |
| Losartan | −1.6 (−6.21, 3) | 0.49 | 3.43 (−36.8, 43.67) | 0.87 |
| Thiazides | 0.44 (−7.81, 8.7) | 0.92 | −14.99 (−85.67, 55.7) | 0.68 |
| Loop Diuretics | −2.05 (−7.51, 3.42) | 0.46 | −28.15 (−75.47, 19.16) | 0.24 |
| Tacrolimus + Rapamycin | −2.7 (−12.46, 7.06) | 0.59 | −6.12 (−90.92, 78.69) | 0.89 |
| Tacrolimus + MMF + Pred. | −5.23 (−14.13, 3.66) | 0.25 | −37.23 (−114.55, 40.1) | 0.34 |
| Tacrolimus + MMF + Pred. | −5.17 (−16.9, 6.56) | 0.38 | −24.16 (−126.86, 78.55) | 0.64 |
| CSA + MMF | −9.35 (−18.71, 0) | 0.05 | −8.69 (−90.61, 73.22) | 0.83 |
| CSA + MMF + Pred. | −7.66 (−17.53, 2.22) | 0.13 | −14.26 (−100.15, 71.63) | 0.74 |
SBP: systolic blood pressure; DBP: diastolic blood pressure; CSA: Cyclosporine
Appendix 5. Predictors of Interstitial expansion and ESRD
| Variable | VvInt/C doubling or ESRD from IF/TA | VvInt/C doubling or any ESRD | Cr Doubling, ESRD or Death | |||
|---|---|---|---|---|---|---|
|
| ||||||
| OR (95% CI) | P-Value | OR (95% CI) | P-Value | OR (95% CI) | P-Value | |
| Plasma Aldosterone | 1.01 (1, 1.01) | 0.21 | 1.01 (1, 1.02) | 0.08 | 1 (0.99, 1) | 0.39 |
| Plasma Renin Activity | 0.91 (0.49, 1.7) | 0.77 | 0.47 (0.17, 1.27) | 0.14 | 0.92 (0.57, 1.47) | 0.73 |
| Recipient Age (years) | 1 (0.93, 1.07) | 0.90 | 1 (0.92, 1.08) | 0.96 | 1.02 (0.97, 1.07) | 0.46 |
| Female Recipient | 0.36 (0.12, 1.08) | 0.07 | 0.37 (0.12, 1.08) | 0.07 | 0.93 (0.51, 1.69) | 0.80 |
| Caucasian Recipient | 0.9 (0.29, 2.75) | 0.85 | 0.43 (0.11, 1.69) | 0.23 | 1.86 (0.81, 4.27) | 0.14 |
| Recipient BMI | 1.07 (0.89, 1.28) | 0.46 | 1.1 (0.89, 1.37) | 0.38 | 1.07 (0.95, 1.22) | 0.27 |
| SBP (mmHg) | 0.95 (0.88, 1.02) | 0.16 | 1.02 (0.96, 1.08) | 0.57 | 1.04 (1, 1.09) | 0.05 |
| DBP (mmHg) | 1.03 (0.91, 1.17) | 0.65 | 0.85 (0.73, 1) | 0.05 | 0.96 (0.88, 1.04) | 0.33 |
| Hypertension | NA* | 11.99 (1.9, 75.59) | 0.01 | 2.07 (1.03, 4.18) | 0.04 | |
| Acute Rejection | 4.62 (1.8, 11.83) | <0.01 | 8.14 (2.04, 32.38) | <0.01 | 2.66 (1.36, 5.18) | <0.01 |
| <3 HLA Matches | 3.13 (1.08, 9.11) | 0.04 | 6.17 (1.37, 27.74) | 0.02 | 1.66 (0.9, 3.04) | 0.10 |
| Live Donor | 0.43 (0.13, 1.39) | 0.16 | 0.69 (0.22, 2.15) | 0.52 | 0.71 (0.36, 1.4) | 0.32 |
| Donor Age (years) | 1.02 (0.95, 1.1) | 0.55 | 0.96 (0.86, 1.07) | 0.48 | 1 (0.95, 1.06) | 0.92 |
| Female Donor | 1.01 (0.41, 2.49) | 0.98 | 0.68 (0.18, 2.48) | 0.56 | 1.56 (0.85, 2.86) | 0.15 |
| Caucasian Donor | 1.29 (0.52, 3.16) | 0.59 | 1.12 (0.37, 3.35) | 0.84 | 0.37 (0.19, 0.73) | <0.01 |
| Cause of Kidney Disease | ||||||
| DM | reference | reference | reference | |||
| HTN | 1.31 (0.13, 13.37) | 0.82 | 0.25 (0, 42.24) | 0.59 | 3.38 (0.59, 19.23) | 0.17 |
| PCKD | 0.93 (0.1, 8.29) | 0.95 | 2.65 (0.08, 83.56) | 0.58 | 0.51 (0.11, 2.36) | 0.39 |
| Other | 0.84 (0.18, 3.92) | 0.83 | 3.53 (0.33, 37.52) | 0.30 | 0.57 (0.18, 1.77) | 0.33 |
| Losartan | 0.62 (0.28, 1.37) | 0.24 | 0.24 (0.08, 0.76) | 0.02 | 0.5 (0.27, 0.91) | 0.02 |
| Thiazides | 0.75 (0.17, 3.23) | 0.70 | 0.79 (0.16, 3.96) | 0.77 | 0.75 (0.26, 2.16) | 0.59 |
| Loop Diuretics | 1.09 (0.41, 2.86) | 0.87 | 3.64 (0.96, 13.77) | 0.06 | 1.03 (0.55, 1.93) | 0.92 |
| Tacrolimus+ Rapamycin | 3.32 (0.98, 11.27) | 0.05 | 0.87 (0.18, 4.24) | 0.86 | 1.64 (0.52, 5.14) | 0.40 |
| Tacrolimus + MMF | 1.59 (0.57, 4.44) | 0.38 | 0.36 (0.07, 1.89) | 0.23 | 1.55 (0.53, 4.53) | 0.42 |
| Tacrolimus + MMF + Pred. | 2.62 (0.66, 10.33) | 0.17 | 0.68 (0.1, 4.54) | 0.69 | 1.41 (0.38, 5.25) | 0.60 |
| CSA + MMF | NA* | 0.06 (0.01, 0.59) | 0.02 | 0.58 (0.16, 2.09) | 0.41 | |
| CSA + MMF + Pred. | NA* | 0.13 (0.02, 1.06) | 0.06 | 1.11 (0.31, 3.96) | 0.88 | |
| Iothalamate GFR | 1 (0.95, 1.05) | 0.95 | 0.97 (0.91, 1.03) | 0.32 | 1.04 (1.01, 1.08) | 0.02 |
excluded due to collinearity
SBP: systolic blood pressure; DBP: diastolic blood pressure; CSA: Cyclosporine, MMF: mycophenolate mofetil, DM: diabetes mellitus, PCKD: polycystic kidney disease, HTN: hypertension, ACR: albumin-creatinine ratio
Footnotes
DISCLOSURE
The authors have no conflicts of interest to disclose.
References
- 1.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 2.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 3.Turner JM, Bauer C, Abramowitz MK, et al. Treatment of chronic kidney disease. Kidney Int. 2012;81:351–362. doi: 10.1038/ki.2011.380. [DOI] [PubMed] [Google Scholar]
- 4.Hiremath S, Fergusson D, Doucette S, et al. Renin angiotensin system blockade in kidney transplantation: a systematic review of the evidence. Am J Transplant. 2007;7:2350–2360. doi: 10.1111/j.1600-6143.2007.01928.x. [DOI] [PubMed] [Google Scholar]
- 5.Ibrahim HN, Jackson S, Connaire J, et al. Angiotensin II Blockade in Kidney Transplant Recipients. J Am Soc Nephrol. 2013;24:320–327. doi: 10.1681/ASN.2012080777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg ME, Correa-Rotter R, Inagami T, et al. Glomerular renin synthesis and storage in the remnant kidney in the rat. Kidney Int. 1991;40:677–683. doi: 10.1038/ki.1991.260. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez D, Muriel A, Abraira V, et al. Renin-angiotensin system blockade and kidney transplantation: a longitudinal cohort study. Nephrol Dial Transplant. 2012;27:417–422. doi: 10.1093/ndt/gfr276. [DOI] [PubMed] [Google Scholar]
- 8.Siekierka-Harreis M, Kuhr N, Willers R, et al. Impact of genetic polymorphisms of the renin-angiotensin system and of non-genetic factors on kidney transplant function--a single-center experience. Clin Transplant. 2009;23:606–615. doi: 10.1111/j.1399-0012.2009.01033.x. [DOI] [PubMed] [Google Scholar]
- 9.Bantle JP, Nath KA, Sutherland DE, et al. Effects of cyclosporine on the renin-angiotensin-aldosterone system and potassium excretion in renal transplant recipients. Arch Intern Med. 1985;145:505–508. [PubMed] [Google Scholar]
- 10.Beckerhoff R, Uhlschmid G, Vetter W, et al. Plasma renin and aldosterone after renal transplantation. Kidney Int. 1974;5:39–46. doi: 10.1038/ki.1974.5. [DOI] [PubMed] [Google Scholar]
- 11.Nieszporek T, Grzeszczak W, Kokot F, et al. Does the kind of immunosuppressive therapy influence plasma renin activity, aldosterone and vasopressin in patients with a kidney transplant? Int Urol Nephrol. 1989;21:233–240. doi: 10.1007/BF02550813. [DOI] [PubMed] [Google Scholar]
- 12.Julien J, Farge D, Kreft-Jais C, et al. Cyclosporine-induced stimulation of the renin-angiotensin system after liver and heart transplantation. Transplantation. 1993;56:885–891. doi: 10.1097/00007890-199310000-00022. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg ME, Smith LJ, Correa-Rotter R, Hostetter TH. The paradox of the renin-angiotensin system in chronic renal disease. Kidney Int. 1994;45:403–410. doi: 10.1038/ki.1994.52. [DOI] [PubMed] [Google Scholar]
- 14.Ponda MP, Hostetter TH. Aldosterone antagonism in chronic kidney disease. Clin J Am Soc Nephrol. 2006;1:668–677. doi: 10.2215/CJN.00120106. [DOI] [PubMed] [Google Scholar]
- 15.Nishiyama A, Seth DM, Navar LG. Renal interstitial fluid concentrations of angiotensins I and II in anesthetized rats. Hypertension. 2002;39:129–134. doi: 10.1161/hy0102.100536. [DOI] [PubMed] [Google Scholar]
- 16.Beutler KT, Masilamani S, Turban S, et al. Long-term regulation of ENaC expression in kidney by angiotensin II. Hypertension. 2003;41:1143–1150. doi: 10.1161/01.HYP.0000066129.12106.E2. [DOI] [PubMed] [Google Scholar]
- 17.Nishiyama A, Seth DM, Navar LG. Renal interstitial fluid angiotensin I and angiotensin II concentrations during local angiotensin-converting enzyme inhibition. J Am Soc Nephrol. 2002;13:2207–2212. doi: 10.1097/01.asn.0000026610.48842.cb. [DOI] [PubMed] [Google Scholar]
- 18.Hene RJ, Boer P, Koomans HA, Mees EJ. Plasma aldosterone concentrations in chronic renal disease. Kidney Int. 1982;21:98–101. doi: 10.1038/ki.1982.14. [DOI] [PubMed] [Google Scholar]
- 19.Ibrahim HN, Hostetter TH. Role of dietary potassium in the hyperaldosteronism and hypertension of the remnant kidney model. J Am Soc Nephrol. 2000;11:625–631. doi: 10.1681/ASN.V114625. [DOI] [PubMed] [Google Scholar]
- 20.Opelz G, Zeier M, Laux G, et al. No improvement of patient or graft survival in transplant recipients treated with angiotensin-converting enzyme inhibitors or angiotensin II type 1 receptor blockers: a collaborative transplant study report. J Am Soc Nephrol. 2006;17:3257–3262. doi: 10.1681/ASN.2006050543. [DOI] [PubMed] [Google Scholar]
- 21.Shihab FS, Bennett WM, Tanner AM, Andoh TF. Angiotensin II blockade decreases TGF-beta1 and matrix proteins in cyclosporine nephropathy. Kidney Int. 1997;52:660–673. doi: 10.1038/ki.1997.380. [DOI] [PubMed] [Google Scholar]
- 22.Dragun D, Muller DN, Brasen JH, et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352:558–569. doi: 10.1056/NEJMoa035717. [DOI] [PubMed] [Google Scholar]
- 23.Opelz G, Wujciak T, Ritz E. Association of chronic kidney graft failure with recipient blood pressure. Collaborative Transplant Study. Kidney Int. 1998;53:217–222. doi: 10.1046/j.1523-1755.1998.00744.x. [DOI] [PubMed] [Google Scholar]
- 24.Mizutani K, Terasaki P, Rosen A, et al. Serial ten-year follow-up of HLA and MICA antibody production prior to kidney graft failure. Am J Transplant. 2005;5:2265–2272. doi: 10.1111/j.1600-6143.2005.01016.x. [DOI] [PubMed] [Google Scholar]
- 25.Cortazar F, Molnar MZ, Isakova T, et al. Clinical outcomes in kidney transplant recipients receiving long-term therapy with inhibitors of the mammalian target of rapamycin. Am J Transplant. 2012;12:379–387. doi: 10.1111/j.1600-6143.2011.03826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fioretto P, Steffes MW, Sutherland DE, Mauer M. Sequential renal biopsies in insulin-dependent diabetic patients: structural factors associated with clinical progression. Kidney Int. 1995;48:1929–1935. doi: 10.1038/ki.1995.493. [DOI] [PubMed] [Google Scholar]