Abstract
Transport of aminopeptidase I (API) to the vacuole appears to be insensitive to blockage of the secretory pathway. Here we show that the N-terminal extension of the 61 kDa precursor of API (pAPI) is proteolytically processed in two sequential steps. The first step involves proteinase A (PrA) and produces a 55 kDa unstable intermediate (iAPI). The second step involves proteinase B (PrB) and converts iAPI into the 50 kDa stable, mature enzyme (mAPI). Reversion of the cup1 growth phenotype by a pAPI-CUP1 chimera indicates that pAPI is transported to the vacuole by a post-translational mechanism. Deletion of the first 16 amino acids results in accumulation of the truncated protein in the cytosol, indicating that pAPI is actively transported to the vacuole. The chimera pAPI-myc, constructed by fusing a myc tag to the C-terminus of pAPI, was exploited to dissect the mechanism of pAPI transport. Cell fractionation studies show the presence of iAPI-myc and mAPI in a fraction of vacuoles purified by density centrifugation. This and the sequential conversion of pAPI-myc into iAPI-myc and mAPI lacking the myc tag is consistent with insertion of pAPI into the vacuolar membrane through its N-terminal extension. The specific mechanism of API sorting demonstrates a new pathway of protein transport in vacuolar biogenesis.
Full text
PDF
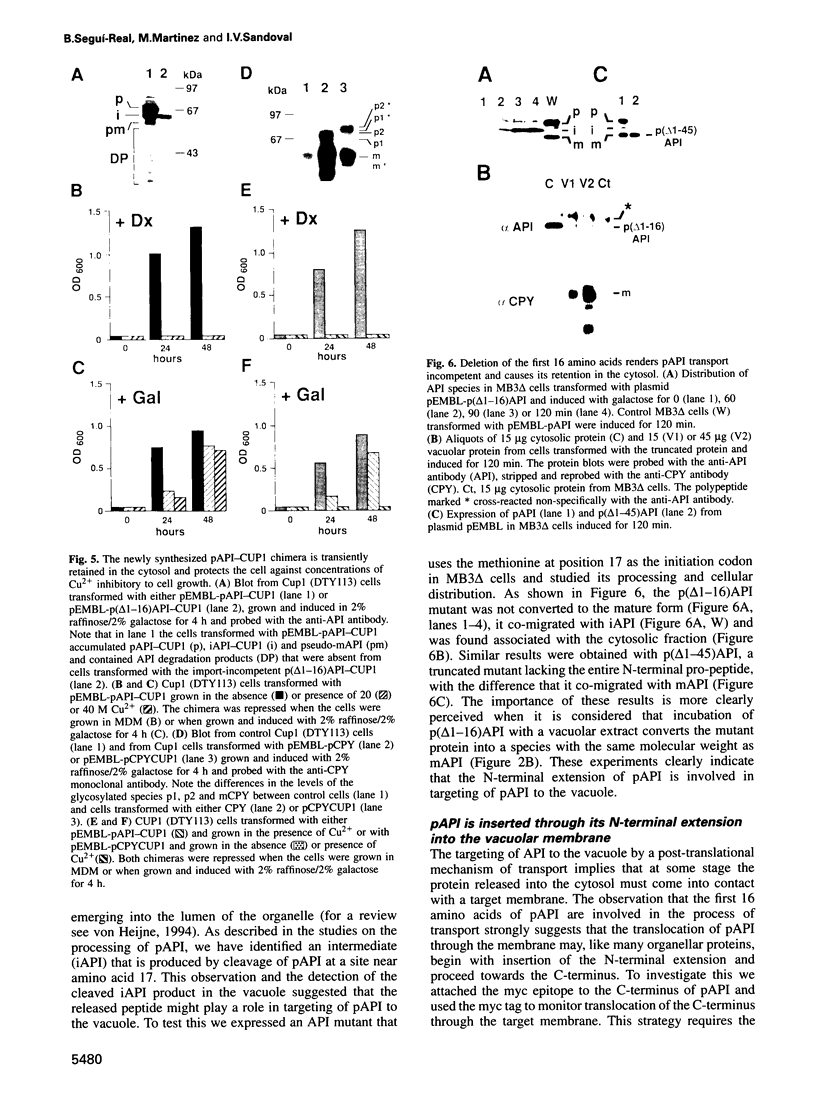

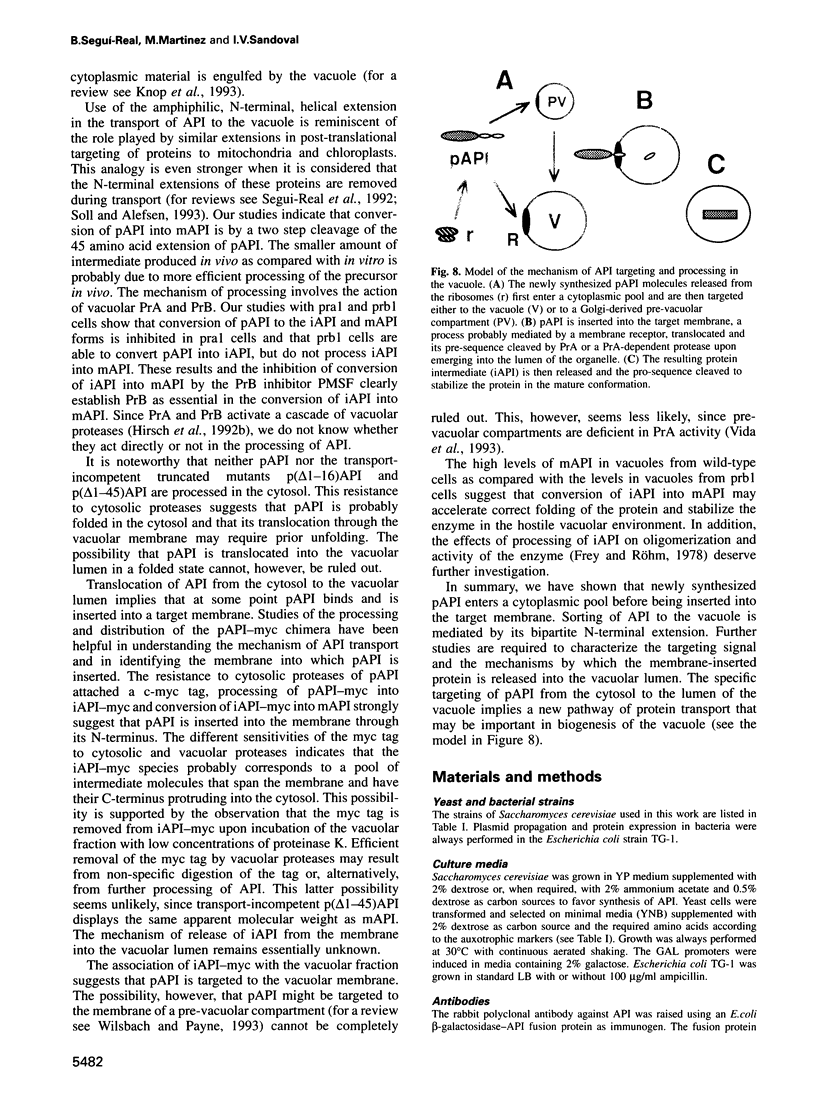


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- America T., Hageman J., Guéra A., Rook F., Archer K., Keegstra K., Weisbeek P. Methotrexate does not block import of a DHFR fusion protein into chloroplasts. Plant Mol Biol. 1994 Jan;24(2):283–294. doi: 10.1007/BF00020168. [DOI] [PubMed] [Google Scholar]
- Bankaitis V. A., Johnson L. M., Emr S. D. Isolation of yeast mutants defective in protein targeting to the vacuole. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9075–9079. doi: 10.1073/pnas.83.23.9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. H., Smith J. A. Molecular cloning and sequencing of genomic DNA encoding aminopeptidase I from Saccharomyces cerevisiae. J Biol Chem. 1989 Apr 25;264(12):6979–6983. [PubMed] [Google Scholar]
- Chapman R. E. Vacuolar sorting. Tracking down an elusive receptor. Curr Biol. 1994 Nov 1;4(11):1019–1022. doi: 10.1016/s0960-9822(00)00231-1. [DOI] [PubMed] [Google Scholar]
- Chen W. J., Douglas M. G. The role of protein structure in the mitochondrial import pathway. Unfolding of mitochondrially bound precursors is required for membrane translocation. J Biol Chem. 1987 Nov 15;262(32):15605–15609. [PubMed] [Google Scholar]
- Conradt B., Shaw J., Vida T., Emr S., Wickner W. In vitro reactions of vacuole inheritance in Saccharomyces cerevisiae. J Cell Biol. 1992 Dec;119(6):1469–1479. doi: 10.1083/jcb.119.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel B., Al E. J., Tabak H. F., Jones E. W. Synthesis and maturation of the yeast vacuolar enzymes carboxypeptidase Y and aminopeptidase I. Biochim Biophys Acta. 1983 Oct 13;741(1):128–135. doi: 10.1016/0167-4781(83)90019-2. [DOI] [PubMed] [Google Scholar]
- Frey J., Röhm K. H. Subcellular localization and levels of aminopeptidases and dipeptidase in Saccharomyces cerevisiae. Biochim Biophys Acta. 1978 Nov 10;527(1):31–41. doi: 10.1016/0005-2744(78)90253-x. [DOI] [PubMed] [Google Scholar]
- Gallusser A., Kirchhausen T. The beta 1 and beta 2 subunits of the AP complexes are the clathrin coat assembly components. EMBO J. 1993 Dec 15;12(13):5237–5244. doi: 10.1002/j.1460-2075.1993.tb06219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch H. H., Schiffer H. H., Müller H., Wolf D. H. Biogenesis of the yeast vacuole (lysosome). Mutation in the active site of the vacuolar serine proteinase yscB abolishes proteolytic maturation of its 73-kDa precursor to the 41.5-kDa pro-enzyme and a newly detected 41-kDa peptide. Eur J Biochem. 1992 Feb 1;203(3):641–653. doi: 10.1111/j.1432-1033.1992.tb16594.x. [DOI] [PubMed] [Google Scholar]
- Hirsch H. H., Schiffer H. H., Wolf D. H. Biogenesis of the yeast vacuole (lysosome). Proteinase yscB contributes molecularly and kinetically to vacuolar hydrolase-precursor maturation. Eur J Biochem. 1992 Aug 1;207(3):867–876. doi: 10.1111/j.1432-1033.1992.tb17118.x. [DOI] [PubMed] [Google Scholar]
- Höhfeld J., Hartl F. U. Post-translational protein import and folding. Curr Opin Cell Biol. 1994 Aug;6(4):499–509. doi: 10.1016/0955-0674(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Johnsson N., Varshavsky A. Ubiquitin-assisted dissection of protein transport across membranes. EMBO J. 1994 Jun 1;13(11):2686–2698. doi: 10.1002/j.1460-2075.1994.tb06559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D. J., Cueva R., Yaver D. S. Aminopeptidase I of Saccharomyces cerevisiae is localized to the vacuole independent of the secretory pathway. J Cell Biol. 1992 Oct;119(2):287–299. doi: 10.1083/jcb.119.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop M., Schiffer H. H., Rupp S., Wolf D. H. Vacuolar/lysosomal proteolysis: proteases, substrates, mechanisms. Curr Opin Cell Biol. 1993 Dec;5(6):990–996. doi: 10.1016/0955-0674(93)90082-2. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNew J. A., Goodman J. M. An oligomeric protein is imported into peroxisomes in vivo. J Cell Biol. 1994 Dec;127(5):1245–1257. doi: 10.1083/jcb.127.5.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr S. F., Stevens T. H. Sorting of membrane proteins in the yeast secretory pathway. J Biol Chem. 1994 Apr 8;269(14):10185–10188. [PubMed] [Google Scholar]
- Reisdorf P., Maarse A. C., Daignan-Fornier B. Epitope-tagging vectors designed for yeast. Curr Genet. 1993 Feb;23(2):181–183. doi: 10.1007/BF00352019. [DOI] [PubMed] [Google Scholar]
- Segui-Real B., Stuart R. A., Neupert W. Transport of proteins into the various subcompartments of mitochondria. FEBS Lett. 1992 Nov 16;313(1):2–7. doi: 10.1016/0014-5793(92)81171-h. [DOI] [PubMed] [Google Scholar]
- Silver P. A. How proteins enter the nucleus. Cell. 1991 Feb 8;64(3):489–497. doi: 10.1016/0092-8674(91)90233-o. [DOI] [PubMed] [Google Scholar]
- Stack J. H., Emr S. D. Genetic and biochemical studies of protein sorting to the yeast vacuole. Curr Opin Cell Biol. 1993 Aug;5(4):641–646. doi: 10.1016/0955-0674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- Subramani S. Protein import into peroxisomes and biogenesis of the organelle. Annu Rev Cell Biol. 1993;9:445–478. doi: 10.1146/annurev.cb.09.110193.002305. [DOI] [PubMed] [Google Scholar]
- Söllner T., Rassow J., Pfanner N. Analysis of mitochondrial protein import using translocation intermediates and specific antibodies. Methods Cell Biol. 1991;34:345–358. doi: 10.1016/s0091-679x(08)61689-1. [DOI] [PubMed] [Google Scholar]
- Takeshige K., Baba M., Tsuboi S., Noda T., Ohsumi Y. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J Cell Biol. 1992 Oct;119(2):301–311. doi: 10.1083/jcb.119.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumbly R. J., Bradley G. Isolation and characterization of aminopeptidase mutants of Saccharomyces cerevisiae. J Bacteriol. 1983 Oct;156(1):36–48. doi: 10.1128/jb.156.1.36-48.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida T. A., Huyer G., Emr S. D. Yeast vacuolar proenzymes are sorted in the late Golgi complex and transported to the vacuole via a prevacuolar endosome-like compartment. J Cell Biol. 1993 Jun;121(6):1245–1256. doi: 10.1083/jcb.121.6.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland M., Subramani S. Cytosol-dependent peroxisomal protein import in a permeabilized cell system. J Cell Biol. 1993 Feb;120(3):675–685. doi: 10.1083/jcb.120.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsbach K., Payne G. S. Dynamic retention of TGN membrane proteins in Saccharomyces cerevisiae. Trends Cell Biol. 1993 Dec;3(12):426–432. doi: 10.1016/0962-8924(93)90031-u. [DOI] [PubMed] [Google Scholar]
- Yaffe M. P., Schatz G. Two nuclear mutations that block mitochondrial protein import in yeast. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4819–4823. doi: 10.1073/pnas.81.15.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihisa T., Anraku Y. A novel pathway of import of alpha-mannosidase, a marker enzyme of vacuolar membrane, in Saccharomyces cerevisiae. J Biol Chem. 1990 Dec 25;265(36):22418–22425. [PubMed] [Google Scholar]
- von Heijne G. Signals for protein targeting into and across membranes. Subcell Biochem. 1994;22:1–19. doi: 10.1007/978-1-4615-2401-4_1. [DOI] [PubMed] [Google Scholar]