Abstract
Purpose
OLC1 was recently identified to be a potential oncogene. However, the role of OLC1 in human esophageal cell carcinoma (ESCC) is unknown. The aim of this study was therefore to evaluate the expression of OLC1 in human ESCC from normal, premalignant, and malignant lesions, and to clarify the mechanisms by which OLC1 contributes to the progression of ESCC.
Experimental Design
Two hundred and fourteen paired ESCC specimens, and an independent set from 28 ESCC patients, were used to analyze the correlation between OLC1 expression and the pathological characteristics of tumors using immunohistochemistry. Stable OLC1-overexpressing and OLC1-interfering esophageal cancer cells were established and a series of experimental methods were used to investigate the biological functions and mechanisms of action of OLC1.
Results
We showed that OLC1 was overexpressed in 145 of 214 (67.8%) of human ESCC specimens, compared with in only 59 of 214 (27.57%) paired adjacent normal tissues (P<0.001). OLC1 overexpression occurred at a rate of 35% (10/28) at the stage of mild/moderate dysplasia, but was significantly upregulated to 66% (22/33) at the stages of severe dysplasia and in situ carcinoma, while 71% positive staining (22/28) was observed in invasive carcinoma tissues compared with normal tissues (P<0.05). We also provided evidence that OLC1 abnormalities significantly altered the cell proliferation and apoptosis induced by cytotoxic agents. OLC1 overexpression suppressed apoptosis, and was associated with attenuated caspase-3 activation and increased Bcl-2 stability.
Conclusion
Our study provides strong evidence suggesting OLC1 abnormalities may contribute to the development of human ESCC and have some important clinical significance.
Introduction
Human esophageal carcinoma is one of the most common malignant tumors worldwide, and 90% of cases are esophageal squamous cell carcinomas (ESCC). Compared with Western countries, cases of ESCC in the People’s Republic of China are characterized by a higher incidence and mortality rate. Several studies investigating ESCC pathophysiology have focused on changes in protein expression, which can contribute to the discovery of new biomarkers and help to develop novel strategies for the early diagnosis and individualized treatment of ESCC [1]. It was previously reported that p53 [2]–[4], Cyclin D1 [5]–[7], E-cadherin [8], K-ras [9], and VEGF [10]–[13] have altered expression in ESCC, suggesting that abnormalities in the expression of oncogenes and tumor suppressor genes contributes to esophageal carcinogenesis and malignant development. These abnormally expressed proteins may therefore serve as potential biomarkers for ESCC. We have recently demonstrated that Aurora-A and cyclin B1, which are key regulators of cell cycle progression, are overexpressed in patients with ESCC, and are associated with increased tumor invasion or metastasis. These findings suggest that Aurora-A and cyclin B1 may therefore serve as useful biomarkers for evaluating advanced ESCC and determining disease prognosis [14]–[18].
OLC1 was recently identified to be a candidate oncogene. It was originally identified by Yuan et al. [19] as one of fifty uncharacterized genes that were differentially expressed in squamous cell lung tumors compared with normal bronchial epithelial cells, and they named it OLC1. Recent studies from Yuan et al. suggest that OLC1 is overexpressed in human lung cancer, which may be linked to activation of the NF-κB pathway. In addition, a large-scale screening study of human genes has revealed that OLC1 can activate the MAPK signaling pathway [20].
The OLC1 gene is also known as KIAA0174, and its accession number is AK057902. OLC1 gene spans 33.47 kb on human chromosome 16q22.2, encoding the OLC1 protein that consists of 364 amino acids. This gene was first identified in non-lethal yeast mutants, and was named the Increased Sodium Tolerance 1 (IST1) gene [21]. The gene product of OLC1/IST1 is highly conserved from humans to yeast. In yeast, IST1 regulates the disassembly of endosomal sorting complexes, based on subcellular localization and the identification of specific protein-binding partners [22]–[25]. To our knowledge, few studies reporting cancer-related activities of OLC1 were made until recently. Because it is highly conserved, studying the roles of OLC1 could provide more insights into elucidating its biological role in human tumorigenesis.
The role of OLC1 in human esophageal carcinoma remains unknown. In this study, we evaluated the expression of OLC1 in human ESCC at different stages of tumorigenesis, from normal, premalignant, and malignant lesions. We also established stable OLC1-overexpressing and OLC1-interfering cell lines, based on human esophageal cancer cell lines, to investigate the biological functions of OLC1. We identified relationships between the dysregulation of OLC1 and the effects of cytotoxic agents on cell proliferation and apoptosis. Our study provides strong evidence suggesting that OLC1 may contribute to the development of esophageal cancer.
Materials and Methods
Cell Lines and Cell Culture
Human ESCC Cell lines were grown in RPMI 1640 medium (Invitrogen) supplemented with 10% fetal bovine serum, and maintained at 37°C in a humidified 5% CO2 incubator. The human EC9706, KYSE510, KYSE180, KYSE450 and KYSE150 were the origin of gifted cell lines indicated in our previous published references [15]–[18].
Plasmids, Cell Transfections, and the Establishment of Stable Cell Lines
The plasmids pEGFP-N1, pEGFP-N1-OLC1, PGCsi-U6/neo/GFP-control and PGCsi-U6/neo/GFP-OLC1 were all kind gifts from Prof. Shujun Cheng (Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China). Plasmid transfection was carried out using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions, and transfected cells were selected with G418 at 400 µg/mL (Geneticin sulfate, Gibco). For each transfection, colonies were trypsinized and collected to produce stable cell pools. The OLC1 stable cell lines were validated by western blot analysis, RT-PCR, and GFP fluorescence using fluorescence microscopy (Olympus).
Patients and Tissue Samples
All specimens used for the study were obtained from the paraffin-embedded tissue archives of our Department of Pathology, as indicated in our previous published references [14], [15]. This study was approved by the ethics committee of Cancer Institute and Hospital, Chinese Academy of Medical Sciences (approval number: 12-71/605. issued date: July 26, 2012). All cancer patients in our Hospital were signed the clinical informed consent of surgical treatment. The tumor specimens resected were allowed for further pathological diagnosis and research program approved by the ethics committee of Cancer Institute and Hospital, Chinese Academy of Medical Sciences.
All 214 formalin-fixed, paraffin-embedded specimens were obtained from patients who were diagnosed with ESCC between 1999 and 2002. None of the patients had received radiotherapy or chemotherapy before surgery. All the cases were T3 stage with invasion to adventitia, and none had shown evidence of distance metastases (M0). Among these patients, 110 were T3N1M0 with lymph-node metastases, and 104 were T3N0M0 without lymph-node metastases, with a gender distribution of 172 male to 42 female. The age of patients ranged from 31 to 81 years, with a median of 58.87. The 214-paired ESCC tissues were used to prepare tissue microarrays, which were then used to analyze the correlation of OLC1 expression with the malignant features of the tumors using immunohistochemistry (IHC).
An independent set of paraffin-embedded tissue sections from 28 ESCC patients were used to verify the correlation between OLC1 protein expression and the stages of tumorigenesis of human ESCC. Twenty-three of these samples were from males, while five were from females, and the age of the patients ranged from 33 to 69 years-of-age. These samples included 25 normal adjacent, 28 mild to moderate dysplasia, 33 severe dysplasia/carcinoma in situ (CIS), and 28 invasive carcinoma tissues.
Two pathologists independently diagnosed all samples. One section was stained with hematoxylin and eosin (H&E) for histological evaluation, and the others were used for IHC analysis.
Immunohistochemical Staining for Evaluating OLC1 Protein Expression in ESCC
The tissue sections were deparaffinized in xylene, and rehydrated in graded ethanol. After antigen retrieval with sodium citrate, sections were blocked with 1.5% normal blocking serum in PBS for 1-hr at room temperature. They were then incubated with a 1∶100 dilution of the primary anti-OLC1 antibody (provided by Prof. Shujun Cheng) at 4°C overnight. Sections were then washed 3 times with PBS, and incubated with streptavidin-horseradish peroxidase-conjugated anti-rabbit or -mouse immunoglobulin G for 30 min at room temperature. Finally, sections were incubated with H2O2-diaminobenzidine at room temperature until the desired intensity of stain had developed. All sections were counterstained with hematoxylin, dehydrated, and mounted to slides.
Semi-quantitative Evaluation of Immunohistochemical Staining
In the analysis of IHC-stained slides, visible brown granules in the cytoplasm were identified as positive OLC1 staining. The staining intensity and extent of each specimen was reviewed. The staining intensity was rated as negative (0), bordering (1), weak (2), or strong (3), while the staining extent was rated based on the percentage of positive cells in the field. Samples with no stained cells were rated as 0, those with <25% were rated 1, those with 25%–50% were rated 2, and those with >50% of cells stained were rated as 3. The results of the staining intensity and extent gave an overall staining score. The samples in which the overall score was 0 were marked as (−), those that scored 1 to 2 marked as (±), 3 to 4 labelled (+), and 5 to 6 marked as (++). All slides were blindly labeled and scored by two independent pathologists.
Statistical Analysis
All data represented at least three independent experiments and were statistically evaluated by Pearson chi-square test or Student’s t test with the statistical analysis software SPSS version 19.0 (IBM). Results are expressed as mean ± SEM, and P values less than 0.05 were considered to be statistically significant.
Cell Extraction and Western Blot
Cell extraction and western blotting analyses were performed as previously described [26]. Caspase-3 (sc-7148), Bcl-2 (sc-509), and ß-actin (sc-8432) antibodies were purchased from Santa Cruz Biotechnology. All experiments were repeated three times.
RT-PCR
Total RNA was isolated from cells using Trizol reagent (Invitrogen) following the manufacturer’s instructions, and total RNA was reverse-transcribed as described (Invitrogen). The sequences of the RT-PCR primers for OLC1 were: 5′-ACAGTGGGAGAGAGCACGTT-3′ (forward primer); 5′-GCACCTTGTCCTTTCTCTGC-3′ (reverse primer). These primers resulted in a PCR product that was 435 bp in length. For GAPDH, the primers were as follows: 5′-GCTGAGAACGGGAAGCTTGT-3′ (forward primer); 5′-GCCAGGGGTGCTAAGCAG-3′ (reverse primer), and they resulted in a PCR product of 299 bp.
Cell Growth
Cancer cells in the exponential growth phase were digested with trypsin, suspended in culture medium containing 10% fetal bovine serum, and then seeded (2×104 cells per 35-mm plates) in triplicate. For each plate, the cells were counted on days 1, 2, 3, 4, and 5, and the growth curves were plotted. All experiments were repeated three times.
Colony Formation Assay
Cells were plated at a density of 1×103 cells per well, in triplicate, on 6-well plates. After 14 days in a humidified 5% CO2 incubator at 37°C, the plates were washed with PBS, and the cells were fixed in cold methanol and stained with 0.5% crystal violet. Colonies with >50 cells were counted, and all experiments were repeated three times.
DAPI Staining
Cells were trypsinized while in their exponential growth phase, suspended in culture with 10% fetal bovine serum, plated on to 30 mm plates, and incubated for 24 hr maintained at 37°C in a humidified 5% CO2 incubator. Cells were then treated with different doses of CDDP (cis-dichlorodiamine platinum, Haosen Pharmaceutical, Inc, Jiangsu, China). Cells were fixed with methanol, and nuclei were stained with 0.1 µg/mL DAPI (4′,6′-diamidino-2-phenylindole hydrochloride, Sigma). Cells with condensed nuclei when DAPI staining was visualized under a fluorescent microscope were deemed to be apoptotic.
Results
The Expression of OLC1 Protein was Progressively Increased in the Different Stages of ESCC
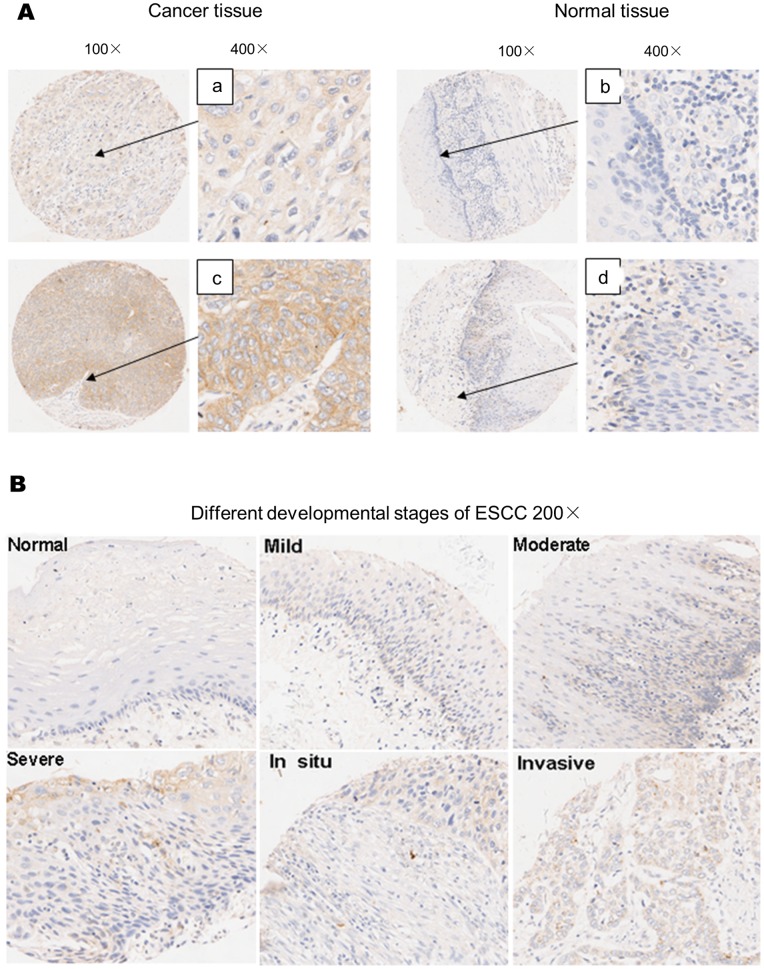
To detect the expression of OLC1 expression in human ESCC, 214 paired ESCC specimens were assessed by IHC staining followed by chi-squared analysis. The tumor samples all exhibited cytoplasmic staining of OLC1 (Figure 1A–a and 1A–c), but the paired adjacent normal tissues showed no or faint cytoplasmic staining (Figure 1A–b and 1A–d). Immunohistochemical analysis showed that OLC1 was overexpressed in 145 out of 214 (67.8%) human ESCC, compared with only 59 of 214 (27.57%) paired adjacent normal tissues (P<0.001). However, we found no significant differences in OLC1 expression between T3N0M0 (63.46%, 66/104) and T3N1M0 (70.91%, 78/110) in human ESCC tissues (P = 0.394) (Table 1).
Figure 1. Expression of OLC1 in normal esophageal epithelium, dysplasia, and ESCC, as assessed by immunohistochemistry.
(A) Images of representative IHC staining of OLC1 in human ESCC and paired adjacent normal tissue, magnified 100- and 400-fold, as indicated. OLC1 staining in the cytoplasm of ESCC tissue was positive (A–a) and strongly positive (A–c), but the paired adjacent normal tissues showed no or faint OLC1 staining (A–b and A–d). (B) The correlation of OLC1 protein expression with the developmental stages of human ESCC. Representative images of OLC1 staining in normal adjacent, mild dysplasia, moderate dysplasia, severe dysplasia, carcinoma in situ, and invasive carcinoma tissues (x200 magnification).
Table 1. The relationship between OLC1 expression and patient clinicopathological characteristics.
| Expression of OLC1 protein | Total Patients | Percent positive (%) | P value* | |||
| (−)(±)# | (+)# | (++)# | ||||
| General | ||||||
| Normal Adjacent tissue | 155 | 51 | 8 | 214 | 27.57 | |
| Tumor tissue | 69 | 115 | 30 | 214 | 67.80 | <0.001 |
| Gender | ||||||
| Male | 54 | 93 | 25 | 172 | ||
| Female | 16 | 21 | 5 | 42 | >0.05 | |
| Age | ||||||
| <60 | 41 | 68 | 14 | 123 | ||
| ≥60 | 29 | 46 | 16 | 91 | >0.05 | |
| TNM Stage | ||||||
| T3N0M0 | 38 | 54 | 12 | 104 | 63.46 | |
| T3N1M0 | 32 | 60 | 18 | 110 | 70.91 | >0.05 |
(−) = no staining; (±) = background or faint staining; (+) = positive staining; (++) = intense staining. Samples marked (+) or (++) were considered positive for OLC1 protein expression.
*Significance was determined by Pearson chi-square test.
An independent set of tissue sections taken from 28 ESCC patients were used to verify the correlation between OLC1 expression and the different stages of tumorigenesis of human ESCC using IHC. As shown in Figure 1B, mild/moderate dysplasia (Mi-DYs/Mo-DYs) samples showed weak OLC1 staining, while severe dysplasia(S-DYs)/carcinoma in situ (CIS), and invasive carcinoma tissues showed strong cytoplasmic staining. In contrast, normal tissues were negative for OLC1 cytoplasmic staining. OLC1 overexpression was detected in 35% (10/28) of mild/moderate dysplasia specimens, but was significantly upregulated in 66% (22/33) of severe dysplasia and carcinoma in situ tissues (P<0.05). The OLC1expression in invasive carcinoma samples was 71% (22/28), while 16% (4/25) positive staining was observed in normal tissues (P<0.05) (Table 2).
Table 2. OLC1 expression in esophageal samples with different lesions.
| Expression of OLC1 protein | Total cases | Percent Positive (%) | |||
| Developmental stages | (−)(±)# | (+)# | (++)# | ||
| Normal adjacent tissues | 21 | 4 | 0 | 25 | 16 |
| Mi-DYS/Mo-DYS | 18 | 7 | 3 | 28 | 35 |
| S-DYs/CIS | 11 | 15 | 7 | 33 | 66 |
| Invasive Carcinoma | 6 | 12 | 10 | 28 | 71 |
(−) = no staining; (±) = background or faint staining; (+) = positive staining; (++) = intense staining. Samples marked (+) or (++) were considered positive for OLC1 protein expression.
Significance was determined by Pearson chi-square test. S-DYs/CIS vs. Normal adjacent tissues, P<0.05; Invasive carcinoma vs. Normal adjacent tissues, P<0.05; Mi-DYS/Mo-DYS vs. Normal adjacent tissues, P>0.05; S-DYs/CIS vs. Mi-DYs/Mo-DYs, P<0.05; Invasive carcinoma vs. Mi-DYs/Mo-DYs, P<0.05; Invasive carcinoma vs. S-DYs/CIS, P>0.05.
Overexpression of OLC1 Promotes the Growth and Colony Formation of ESCC Cells
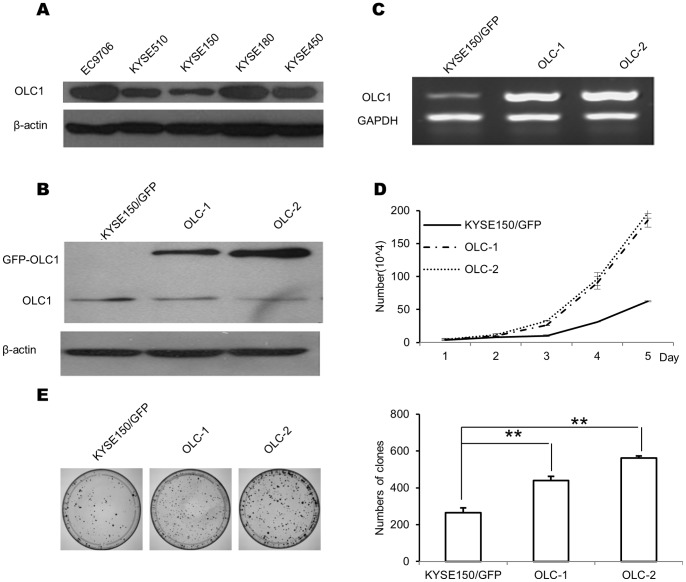
To evaluate the role of OLC1 expression in ESCC, the cell line KYSE150, which expresses a relatively low level of endogenous OLC1 protein (Figure 2A), was used. KYSE150 cells were transfected with pEGFP-N1-OLC1 or pEGFP-N1, and the stable OLC1-overexpressing cell line was named KYSE150/GFP-OLC1, while the null control clone was named KYSE150/GFP. Two OLC1 overexpressing clones (OLC-1 and OLC-2) and one null control clone were used for further analyses. As expected, the GFP-OLC1 fusion protein (68 kDa) was detected in OLC-1 and OLC-2, but not in control cells (Figure 2B). Consistent with these data, RT-PCR analysis showed a strong band in OLC-1 and OLC-2 cells, but only a weak band in KYSE150/GFP cells (Figure 2C). We then used the 3 cell lines to assess the effects of OLC1 overexpression on the characteristics of KYSE150 cells using cell proliferation and colony formation assays. OLC-1 and OLC-2 grew significantly faster than KYSE150/GFP (P<0.05; Figure 2D). In addition, the number of colonies formed by OLC-1 and OLC-2 cells were 440.33±22.12 and 562±11.53, which were significantly higher than that those formed by control cells (264.67±26.27; P<0.001; Figure 2E).
Figure 2. Construction of stable OLC1-overexpressing cell lines, and the effects of OLC1 overexpression on KYSE150 cells.
(A) To investigate OLC1 expressions among ESCC cell lines (EC9706, KYSE 510, KYSE 150, KYSE450 and KYSE180), the relative protein levels of OLC1 from these cell lines were examined by western blotting analysis. (B) KYSE150 cells were transfected with pEGFP-N1-OLC1 and pEGFP-N1, and stable, positive clones were selected using with G418 (400 µg/mL) from candidate clones. Two positive clones were designated as OLC-1 and OLC-2, the null control colony was designated KYSE150/GFP. Confirmed by western blotting, the expressed GFP-OLC1 fusion protein (68 kDa) was detected in OLC-1 and OLC-2, but not in KYSE150/GFP cells (C) mRNA levels of OLC1 in OLC-1, OLC-2 and KYSE150/GFP cells, assessed by RT-PCR. (D) Cell proliferation curves were calculated after counting the numbers of OLC-1, OLC-2 and KYSE150/GFP cells for 5 days. (E) The numbers of colonies of each cell line were counted, and then compared. All experiments were repeated three times. Significance was determined by the Student’s t test. * = P<0.05; ** = P<0.001.
Apoptosis Inhibited by OLC1 Overexpression is Associated with Attenuated Caspase-3 Activation and Increased Bcl-2 Stability
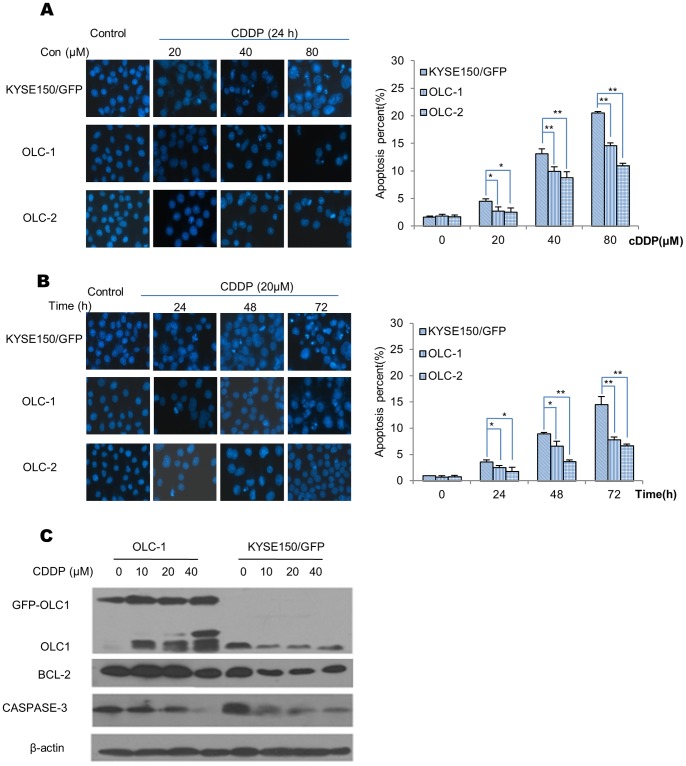
We further investigated the role of OLC1 overexpression on CDDP-induced apoptosis in OLC-1 and OLC-2 and KYSE150/GFP. CDDP induced apoptosis in a dose-dependent manner in the 3 cells. However, the increases were lower in OLC-1 and OLC-2 compared with KYSE150/GFP (Figure 3A). When the cells were treated with 20 µM CDDP, apoptosis increased over time in the 3 cell lines. The percentages of apoptotic OLC-1 and OLC-2 cells were also lower than their control cells (Figure 3B). Taken together, these results suggest that the overexpression of OLC1 inhibited CDDP-induced apoptosis in a time- and dose-dependent manner.
Figure 3. The effects of OLC1 overexpression on CDDP-induced apoptosis.
(A) Cell lines were treated with CDDP at 0, 20, 40, or 80 µM for 24 h, followed by DAPI staining and analysis by fluorescent microscopy and photography. The total number of the apoptotic cells was counted, and the percentage of apoptotic cells was calculated. (B) Cells were treated with 20 µM CDDP for different time periods (including 0, 24, 48, 72 h) as indicated, followed by DAPI staining. (C) Cells were treated with different doses of CDDP (including 0, 10, 20, 40 µM) for 24 h, and the effects of OLC1 on the expression of the apoptosis-related proteins caspase-3 and Bcl-2 was assessed by western blotting. Significance was determined by the Student’s t test. * = P<0.05; ** = P<0.001.
Next, western blotting was used to assess the expression of OLC1, Bcl-2, and caspase-3 after cells had been treated with CDDP. As the concentration of CDDP increased (Figure 3C), a concurrent fall in caspase-3 pro-band was seen in both cell lines. However, less pro-band was seen in KYSE150/GFP compared with OLC-1 cells. In addition, Bcl-2 was more stable in OLC-1 cells compared with KYSE150/GFP after treatment with CDDP. These data suggest that OLC1 overexpression inhibits apoptosis, by stabilizing Bcl-2.
Stable Knockdown of Endogenous OLC1 Inhibits Cell Growth and Increases CDDP-induced Apoptosis
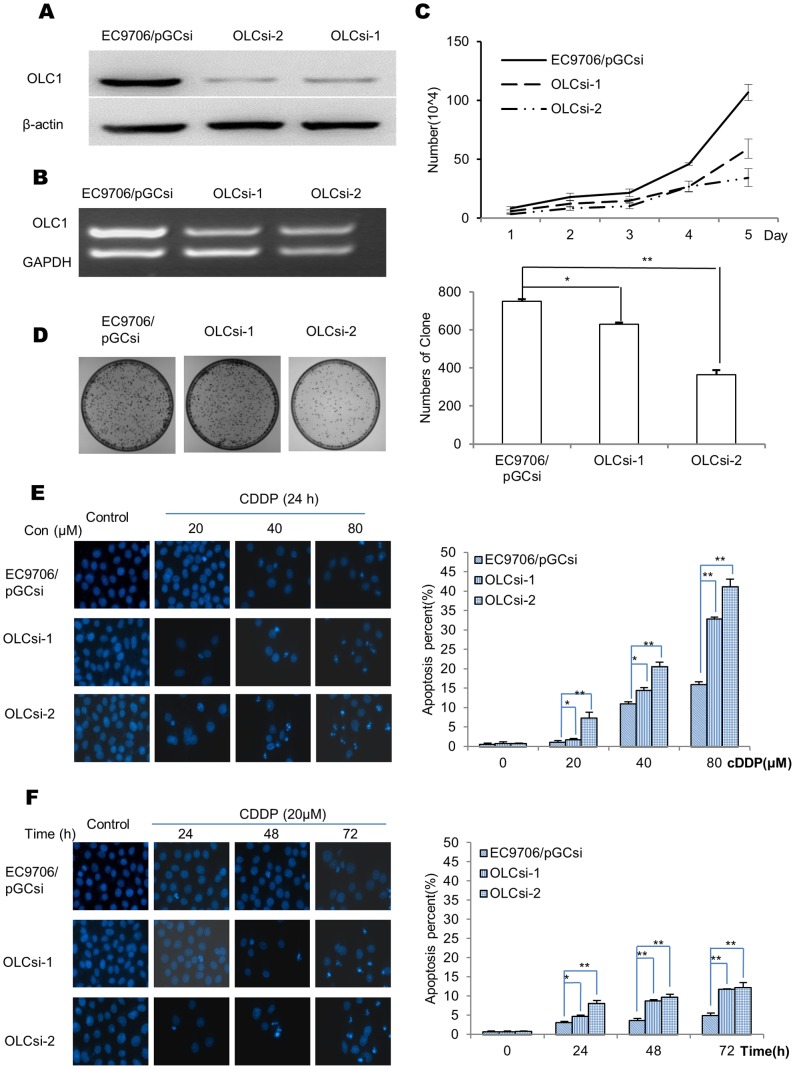
EC9706 cells, which express relatively higher levels of endogenous OLC1 (Figure 2A), were transfected with PGCsi-U6/neo/GFP-control or PGCsi-U6/neo/GFP-OLC1. We designated the 2 stable OLC1 knockdown clones as OLCsi-1 and OLCsi-2, and the negative control as EC9706/pGCsi. As expected, the mRNA and protein levels of OLC1 were reduced in OLCsi-1 and OLCsi-2 cells compared with EC9706/pGCsi, as assessed by western blotting and RT-PCR, respectively (Figure 4A and 4B). OLCsi-1 and OLCsi-2 cells grew slower (P<0.05) and formed fewer colonies (P<0.001) than EC9706/pGCsi cells (Figure 4C and 4D).
Figure 4. OLC1-interfering cell lines and their differential effects on cell growth and apoptosis in response to CDDP treatment.
(A) EC9706 cells were transfected with PGCsi-U6/neo/GFP-control or PGCsi-U6/neo/GFP-OLC1. The 2 stable OLC1-interferencing cell lines and paired control were verified by western blotting, and named OLCsi-1, OLCsi-2 and EC9706/pGCsi, respectively. (B) Evaluation of the mRNA levels of OLC1 in OLCsi-1, OLCsi-2 and EC9706/pGCsi cells, assessed by RT-PCR. (C) The cell proliferation curve was calculated from cell counting data over 5 days. (D) Graph showing the total numbers of colonies of OLCsi-1, OLCsi-2 and EC9706/pGCsi cells. (E) OLCsi-1, OLCsi-2 and EC9706/pGCsi cells were treated with CDDP at concentrations of 0, 20, 40, 80 µM for 24 h, and apoptosis was assessed by DAPI staining. The graph shows the percent of apoptotic cells from each cell line. Significance was determined by the Student’s t test. * = P<0.05; ** = P<0.001.
We further evaluated the effects of OLC1-interference on apoptosis with DAPI staining. CDDP-induced apoptosis in EC9706/pGCsi cells was markedly lower than in OLCsi-1 and OLCsi-2 cells, and they were all in a dose- and time-dependent manner (Figure 4E and 4F).
Discussion
ESCC is usually diagnosed when the tumor is at an advanced stage, and so frequently has poor prognosis. It is therefore important to identify new candidate markers to facilitate the early diagnosis and treatment of ESCC. The abnormal expression of several proteins, including p63 [27]–[29], SMAD6 [30], SMAD7 [30], [31], FHIT [3], [32], [33], and Annexin I [34], [35], has been reported in the early stages of ESCC.
In this study, several experiments revealed that the expression of OLC1 is progressively increased during the development of esophageal dysplasia and ESCC. This suggests that elevated expression of OLC1 occurs before tumor metastasis, and that high OLC1 levels are probably maintained until ESCC metastasizes. In addition, immunohistochemical analysis of 214 ESCC specimens at the T3 stage, which show no significant differences in OLC1 expression between the non-lymph node (T3N0M0) and lymph node metastasis groups (T3N1M0) (Table 1), support this conclusion. Alteration of OLC1 expression could therefore be a critical event during the developmental of human esophageal carcinoma. Yuan et al. provided evidence that OLC1 overexpression may be involved in human lung carcinogenesis, especially during the early stages, which is consistent with our IHC data from ESCC patients. This suggests that the dysregulation of OLC1 may contribute to the development of human ESCC.
ESCC is one of the most common malignant neoplasms, and apoptotic dysfunction plays an important role in its development. Accordingly, a better understanding of the molecular mechanisms by which OLC1-induces the malignant cellular phenotypes of ESCC is required. Here, we provided novel evidence that dysregulation of OLC1 expression significantly alter the cell proliferation and apoptosis induced by cytotoxic agents. We observed that OLC1 overexpressing cells had increased proliferation and resistance to cisplatin-induced apoptosis compared with control cells. Conversely, we constructed OLC1-knockdown cell lines in EC9706 cells using siRNA, and stable knockdown of endogenous OLC1 inhibited cell growth and sensitized cells to CDDP-induced apoptosis. In addition, OLC1 overexpression led to reduced caspase-3 activation, and enhanced stability of Bcl-2. Caspase-3 is an important executioner caspase whose cleavage and activation leads to apoptosis executed by both death receptor-mediated or mitochondrial-dependent pathways [26], [36]–[40]. Bcl-2, an anti-apoptotic protein, suppresses apoptosis in several ways, including inhibiting cytochrome c release from the mitochondria [41]–[45]. Our findings suggest that OLC1 overexpression suppresses cellular apoptosis, possibly by interfering with caspase-3 activation and enhancing Bcl-2 stability. Taken together, these results suggest that the dysregulation of OLC1 disrupts the delicate balance between cell growth and apoptosis. Our results therefore help to explain why abnormalities in OLC1 expression contribute to a malignant cellular phenotype in ESCC.
Yuan et al. transformed NIH3T3 cells with OLC1 and injected them into nude mice to assess the tumorigenicity of OLC1 [19]. Fibrosarcomas were detected in all animals that were inoculated with OLC1-expressing NIH3T3 cells, but not in the control groups injected with the parental or empty-vector transfected cells. As a novel potential oncogene, our results reveal that OLC1 is a cell cycle-dependent protein that may be involved with ubiquitin-dependent degradation [46]. OLC1 also plays a role in cytokinesis [47]–[51]. Taken together, these data suggest that OLC1 may play an important role in regulating the cell cycle, and ultimately cellular growth and apoptosis. However, more studies are needed to explore the underlying mechanisms of OLC1 dysregulation in esophageal tumorigenesis.
In conclusion, we report that OLC1 is overexpressed in human ESCC; OLC1 abnormalities may contribute to the development of human ESCC and have some important clinical significance.
Acknowledgments
We thank Dr. Shimada at Kyoto University, Japan, for providing us with KYSE150, KYSE510, KYSE180, KYSE450 and Prof. Mingrong Wang at Chinese Academy of Medical Sciences & Peking Union Medical College, China, for providing us with EC9706 cells. We also are grateful to Prof. Shujun Cheng at Chinese Academy of Medical Sciences & Peking Union Medical College, China, for generously providing us with the pEGFP-N1, pEGFP-N1-OLC1, PGCsi-U6/neo/GFP-control and PGCsi-U6/neo/GFP-OLC1 plasmids.
Funding Statement
This work was supported by the grants from the National Natural Science Foundation of China (31250001), and the 973 National Key Fundamental Research Program of China (2009CB521801). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lin DC, Du XL, Wang MR (2009) Protein alterations in ESCC and clinical implications: a review. Dis Esophagus 22: 9–20. [DOI] [PubMed] [Google Scholar]
- 2. Mathew R, Arora S, Khanna R, Mathur M, Shukla NK, et al. (2002) Alterations in p53 and pRb pathways and their prognostic significance in oesophageal cancer. Eur J Cancer 38: 832–841. [DOI] [PubMed] [Google Scholar]
- 3. Silveira AP, Da Silva Manoel-Caetano F, Aoki S, Yamasaki LH, Rahal P, et al. (2011) Gene mutations and polymorphisms of TP53 and FHIT in chronic esophagitis and esophageal carcinoma. Anticancer Res 31: 1685–1690. [PubMed] [Google Scholar]
- 4. Matsumoto M, Furihata M, Kurabayashi A, Sasaguri S, Araki K, et al. (2004) Prognostic significance of serine 392 phosphorylation in overexpressed p53 protein in human esophageal squamous cell carcinoma. Oncology 67: 143–150. [DOI] [PubMed] [Google Scholar]
- 5. Hussain S, M Y, Thakur N, Salam I, Singh N, et al. (2011) Association of cyclin D1 gene polymorphisms with risk of esophageal squamous cell carcinoma in Kashmir Valley: a high risk area. Mol Carcinog 50: 487–498. [DOI] [PubMed] [Google Scholar]
- 6. Mega S, Miyamoto M, Ebihara Y, Takahashi R, Hase R, et al. (2005) Cyclin D1, E2F1 expression levels are associated with characteristics and prognosis of esophageal squamous cell carcinoma. Dis Esophagus 18: 109–113. [DOI] [PubMed] [Google Scholar]
- 7. Itami A, Shimada Y, Watanabe G, Imamura M (1999) Prognostic value of p27(Kip1) and CyclinD1 expression in esophageal cancer. Oncology 57: 311–317. [DOI] [PubMed] [Google Scholar]
- 8. Uchikado Y, Natsugoe S, Okumura H, Setoyama T, Matsumoto M, et al. (2005) Slug Expression in the E-cadherin preserved tumors is related to prognosis in patients with esophageal squamous cell carcinoma. Clin Cancer Res 11: 1174–1180. [PubMed] [Google Scholar]
- 9. Lyronis ID, Baritaki S, Bizakis I, Krambovitis E, Spandidos DA (2008) K-ras mutation, HPV infection and smoking or alcohol abuse positively correlate with esophageal squamous carcinoma. Pathol Oncol Res 14: 267–273. [DOI] [PubMed] [Google Scholar]
- 10. Shimada H, Takeda A, Nabeya Y, Okazumi SI, Matsubara H, et al. (2001) Clinical significance of serum vascular endothelial growth factor in esophageal squamous cell carcinoma. Cancer 92: 663–669. [DOI] [PubMed] [Google Scholar]
- 11. Kimura Y, Watanabe M, Ohga T, Saeki H, Kakeji Y, et al. (2003) Vascular endothelial growth factor C expression correlates with lymphatic involvement and poor prognosis in patients with esophageal squamous cell carcinoma. Oncol Rep 10: 1747–1751. [DOI] [PubMed] [Google Scholar]
- 12. Shih CH, Ozawa S, Ando N, Ueda M, Kitajima M (2000) Vascular endothelial growth factor expression predicts outcome and lymph node metastasis in squamous cell carcinoma of the esophagus. Clin Cancer Res 6: 1161–1168. [PubMed] [Google Scholar]
- 13. Luo KJ, Hu Y, Wen J, Fu JH (2011) CyclinD1, p53, E-cadherin, and VEGF discordant expression in paired regional metastatic lymph nodes of esophageal squamous cell carcinoma: a tissue array analysis. J Surg Oncol 104: 236–243. [DOI] [PubMed] [Google Scholar]
- 14. Shi J, Xue LY, Yin N, Lu J, Fu M, et al. (2010) Clinical significance of abnormal expression of Aurora-A in human esophageal squamous cell carcinoma with or without lymph node metastasis. Zhonghua Zhong Liu Za Zhi 32: 748–751. [PubMed] [Google Scholar]
- 15. Tong T, Zhong Y, Kong J, Dong L, Song Y, et al. (2004) Overexpression of Aurora-A contributes to malignant development of human esophageal squamous cell carcinoma. Clin Cancer Res 10: 7304–7310. [DOI] [PubMed] [Google Scholar]
- 16. Wang XX, Liu R, Jin SQ, Fan FY, Zhan QM (2006) Overexpression of Aurora-A kinase promotes tumor cell proliferation and inhibits apoptosis in esophageal squamous cell carcinoma cell line. Cell Res 16: 356–366. [DOI] [PubMed] [Google Scholar]
- 17. Song Y, Zhao C, Dong L, Fu M, Xue L, et al. (2008) Overexpression of cyclin B1 in human esophageal squamous cell carcinoma cells induces tumor cell invasive growth and metastasis. Carcinogenesis 29: 307–315. [DOI] [PubMed] [Google Scholar]
- 18. Wang X, Dong L, Xie J, Tong T, Zhan Q (2009) Stable knockdown of Aurora-A by vector-based RNA interference in human esophageal squamous cell carcinoma cell line inhibits tumor cell proliferation, invasion and enhances apoptosis. Cancer Biol Ther 8: 1852–1859. [DOI] [PubMed] [Google Scholar]
- 19. Yuan J, Ma J, Zheng H, Shi T, Sun W, et al. (2008) Overexpression of OLC1, cigarette smoke, and human lung tumorigenesis. J Natl Cancer Inst 100: 1592–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matsuda A, Suzuki Y, Honda G, Muramatsu S, Matsuzaki O, et al. (2003) Large-scale identification and characterization of human genes that activate NF-kappaB and MAPK signaling pathways. Oncogene 22: 3307–3318. [DOI] [PubMed] [Google Scholar]
- 21. Entian KD, Schuster T, Hegemann JH, Becher D, Feldmann H, et al. (1999) Functional analysis of 150 deletion mutants in Saccharomyces cerevisiae by a systematic approach. Mol Gen Genet 262: 683–702. [DOI] [PubMed] [Google Scholar]
- 22. Krogan NJ, Cagney G, Yu H, Zhong G, Guo X, et al. (2006) Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature 440: 637–643. [DOI] [PubMed] [Google Scholar]
- 23. Tarassov K, Messier V, Landry CR, Radinovic S, Serna Molina MM, et al. (2008) An in vivo map of the yeast protein interactome. Science 320: 1465–1470. [DOI] [PubMed] [Google Scholar]
- 24. Dimaano C, Jones CB, Hanono A, Curtiss M, Babst M (2008) Ist1 regulates Vps4 localization and assembly. Mol Biol Cell 19: 465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rue SM, Mattei S, Saksena S, Emr SD (2008) Novel Ist1-Did2 complex functions at a late step in multivesicular body sorting. Mol Biol Cell 19: 475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tong T, Ji J, Jin S, Li X, Fan W, et al. (2005) Gadd45a expression induces Bim dissociation from the cytoskeleton and translocation to mitochondria. Mol Cell Biol 25: 4488–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hu H, Xia SH, Li AD, Xu X, Cai Y, et al. (2002) Elevated expression of p63 protein in human esophageal squamous cell carcinomas. Int J Cancer 102: 580–583. [DOI] [PubMed] [Google Scholar]
- 28. Cao LY, Yin Y, Li H, Jiang Y, Zhang HF (2009) Expression and clinical significance of S100A2 and p63 in esophageal carcinoma. World J Gastroenterol 15: 4183–4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thepot A, Hautefeuille A, Cros MP, Abedi-Ardekani B, Petre A, et al. (2010) Intraepithelial p63-dependent expression of distinct components of cell adhesion complexes in normal esophageal mucosa and squamous cell carcinoma. Int J Cancer 127: 2051–2062. [DOI] [PubMed] [Google Scholar]
- 30. Osawa H, Nakajima M, Kato H, Fukuchi M, Kuwano H (2004) Prognostic value of the expression of Smad6 and Smad7, as inhibitory Smads of the TGF-beta superfamily, in esophageal squamous cell carcinoma. Anticancer Res 24: 3703–3709. [PubMed] [Google Scholar]
- 31. Puhringer-Oppermann F, Sarbia M, Ott N, Brucher BL (2010) The predictive value of genes of the TGF-beta1 pathway in multimodally treated squamous cell carcinoma of the esophagus. Int J Colorectal Dis 25: 515–521. [DOI] [PubMed] [Google Scholar]
- 32. Chava S, Mohan V, Shetty PJ, Manolla ML, Vaidya S, et al. (2012) Immunohistochemical evaluation of p53, FHIT, and IGF2 gene expression in esophageal cancer. Dis Esophagus 25: 81–87. [DOI] [PubMed] [Google Scholar]
- 33. Kitamura A, Yashima K, Okamoto E, Andachi H, Hosoda A, et al. (2001) Reduced Fhit expression occurs in the early stage of esophageal tumorigenesis: no correlation with p53 expression and apoptosis. Oncology 61: 205–211. [DOI] [PubMed] [Google Scholar]
- 34. Chung JY, Braunschweig T, Hu N, Roth M, Traicoff JL, et al. (2006) A multiplex tissue immunoblotting assay for proteomic profiling: a pilot study of the normal to tumor transition of esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 15: 1403–1408. [DOI] [PubMed] [Google Scholar]
- 35. Zhang J, Wang K, Zhang J, Liu SS, Dai L, et al. (2011) Using proteomic approach to identify tumor-associated proteins as biomarkers in human esophageal squamous cell carcinoma. J Proteome Res 10: 2863–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu PP, Chung HW, Liu KC, Wu RS, Yang JS, et al. (2011) Diallyl sulfide induces cell cycle arrest and apoptosis in HeLa human cervical cancer cells through the p53, caspase- and mitochondria-dependent pathways. Int J Oncol 38: 1605–1613. [DOI] [PubMed] [Google Scholar]
- 37. Cryns V, Yuan J (1998) Proteases to die for. Genes Dev 12: 1551–1570. [DOI] [PubMed] [Google Scholar]
- 38. Tyagi A, Gu M, Takahata T, Frederick B, Agarwal C, et al. (2011) Resveratrol selectively induces DNA Damage, independent of Smad4 expression, in its efficacy against human head and neck squamous cell carcinoma. Clin Cancer Res 17: 5402–5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tung EW, Winn LM (2011) Valproic acid-induced DNA damage increases embryonic p27(KIP1) and caspase-3 expression: a mechanism for valproic-acid induced neural tube defects. Reprod Toxicol 32: 255–260. [DOI] [PubMed] [Google Scholar]
- 40. Li Y, Qian H, Li X, Wang H, Yu J, et al. (2009) Adenoviral-mediated gene transfer of Gadd45a results in suppression by inducing apoptosis and cell cycle arrest in pancreatic cancer cell. J Gene Med 11: 3–13. [DOI] [PubMed] [Google Scholar]
- 41. Cory S, Huang DC, Adams JM (2003) The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene 22: 8590–8607. [DOI] [PubMed] [Google Scholar]
- 42. Wagner AK, Amin KB, Niyonkuru C, Postal BA, McCullough EH, et al. (2011) CSF Bcl-2 and cytochrome C temporal profiles in outcome prediction for adults with severe TBI. J Cereb Blood Flow Metab 31: 1886–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huang P, Yu C, Liu XQ, Ding YB, Wang YX, et al. (2011) Cytotoxicity of tubeimoside I in human choriocarcinoma JEG-3 cells by induction of cytochrome c release and apoptosis via the mitochondrial-related signaling pathway. Int J Mol Med 28: 579–587. [DOI] [PubMed] [Google Scholar]
- 44. Lan CH, Sheng JQ, Fang DC, Meng QZ, Fan LL, et al. (2010) Involvement of VDAC1 and Bcl-2 family of proteins in VacA-induced cytochrome c release and apoptosis of gastric epithelial carcinoma cells. J Dig Dis 11: 43–49. [DOI] [PubMed] [Google Scholar]
- 45. Ling Q, Xu X, Wei X, Wang W, Zhou B, et al. (2011) Oxymatrine induces human pancreatic cancer PANC-1 cells apoptosis via regulating expression of Bcl-2 and IAP families, and releasing of cytochrome c. J Exp Clin Cancer Res 30: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang X, Xiao T, Cheng S, Tong T, Gao Y (2011) Cigarette smoke suppresses the ubiquitin-dependent degradation of OLC1. Biochem Biophys Res Commun 407: 753–757. [DOI] [PubMed] [Google Scholar]
- 47. Bajorek M, Morita E, Skalicky JJ, Morham SG, Babst M, et al. (2009) Biochemical analyses of human IST1 and its function in cytokinesis. Mol Biol Cell 20: 1360–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Agromayor M, Carlton JG, Phelan JP, Matthews DR, Carlin LM, et al. (2009) Essential role of hIST1 in cytokinesis. Mol Biol Cell 20: 1374–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee S, Chang J, Renvoise B, Tipirneni A, Yang S, et al. (2012) MITD1 is recruited to midbodies by ESCRT-III and participates in cytokinesis. Mol Biol Cell 23: 4347–4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Renvoise B, Parker RL, Yang D, Bakowska JC, Hurley JH, et al. (2010) SPG20 protein spartin is recruited to midbodies by ESCRT-III protein Ist1 and participates in cytokinesis. Mol Biol Cell 21: 3293–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Renvoise B, Stadler J, Singh R, Bakowska JC, Blackstone C (2012) Spg20−/− mice reveal multimodal functions for Troyer syndrome protein spartin in lipid droplet maintenance, cytokinesis and BMP signaling. Hum Mol Genet 21: 3604–3618. [DOI] [PMC free article] [PubMed] [Google Scholar]