Abstract
Translocation of proteins across the endoplasmic reticulum membrane is initiated by the signal recognition particle (SRP), a cytoplasmic ribonucleoprotein complex consisting of a 7S RNA and six polypeptides. To investigate the functions of the SRP components, we have tested the activities of several SRP subparticles. We show that the SRP GTPase (SRP54) alone binds a signal sequence and discriminates it from a non-signal sequence. Although SRP54 alone is unable to promote translocation, SRP54 in a complex with SRP RNA is both necessary and sufficient to promote translocation of an elongation-arrested nascent protein in a GTP-regulated manner. For co-translational translocation, additional SRP components are required. We discuss the implications of our results for the function of the Escherichia coli SRP which is homologous to the SRP54/SRP-RNA complex.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein H. D., Poritz M. A., Strub K., Hoben P. J., Brenner S., Walter P. Model for signal sequence recognition from amino-acid sequence of 54K subunit of signal recognition particle. Nature. 1989 Aug 10;340(6233):482–486. doi: 10.1038/340482a0. [DOI] [PubMed] [Google Scholar]
- Bernstein H. D., Zopf D., Freymann D. M., Walter P. Functional substitution of the signal recognition particle 54-kDa subunit by its Escherichia coli homolog. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5229–5233. doi: 10.1073/pnas.90.11.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos N., Palau J., Torrent M., Ludevid D. Signal recognition-like particles are present in maize. J Biol Chem. 1988 Jul 15;263(20):9646–9650. [PubMed] [Google Scholar]
- Connolly T., Gilmore R. Formation of a functional ribosome-membrane junction during translocation requires the participation of a GTP-binding protein. J Cell Biol. 1986 Dec;103(6 Pt 1):2253–2261. doi: 10.1083/jcb.103.6.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly T., Gilmore R. The signal recognition particle receptor mediates the GTP-dependent displacement of SRP from the signal sequence of the nascent polypeptide. Cell. 1989 May 19;57(4):599–610. doi: 10.1016/0092-8674(89)90129-3. [DOI] [PubMed] [Google Scholar]
- Connolly T., Rapiejko P. J., Gilmore R. Requirement of GTP hydrolysis for dissociation of the signal recognition particle from its receptor. Science. 1991 May 24;252(5009):1171–1173. doi: 10.1126/science.252.5009.1171. [DOI] [PubMed] [Google Scholar]
- Gilmore R., Collins P., Johnson J., Kellaris K., Rapiejko P. Transcription of full-length and truncated mRNA transcripts to study protein translocation across the endoplasmic reticulum. Methods Cell Biol. 1991;34:223–239. doi: 10.1016/s0091-679x(08)61683-0. [DOI] [PubMed] [Google Scholar]
- Gundelfinger E. D., Krause E., Melli M., Dobberstein B. The organization of the 7SL RNA in the signal recognition particle. Nucleic Acids Res. 1983 Nov 11;11(21):7363–7374. doi: 10.1093/nar/11.21.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlich D., Hartmann E., Prehn S., Rapoport T. A. A protein of the endoplasmic reticulum involved early in polypeptide translocation. Nature. 1992 May 7;357(6373):47–52. doi: 10.1038/357047a0. [DOI] [PubMed] [Google Scholar]
- Görlich D., Prehn S., Hartmann E., Kalies K. U., Rapoport T. A. A mammalian homolog of SEC61p and SECYp is associated with ribosomes and nascent polypeptides during translocation. Cell. 1992 Oct 30;71(3):489–503. doi: 10.1016/0092-8674(92)90517-g. [DOI] [PubMed] [Google Scholar]
- Haeuptle M. T., Frank R., Dobberstein B. Translation arrest by oligodeoxynucleotides complementary to mRNA coding sequences yields polypeptides of predetermined length. Nucleic Acids Res. 1986 Feb 11;14(3):1427–1448. doi: 10.1093/nar/14.3.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High S., Flint N., Dobberstein B. Requirements for the membrane insertion of signal-anchor type proteins. J Cell Biol. 1991 Apr;113(1):25–34. doi: 10.1083/jcb.113.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High S., Görlich D., Wiedmann M., Rapoport T. A., Dobberstein B. The identification of proteins in the proximity of signal-anchor sequences during their targeting to and insertion into the membrane of the ER. J Cell Biol. 1991 Apr;113(1):35–44. doi: 10.1083/jcb.113.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High S., Martoglio B., Görlich D., Andersen S. S., Ashford A. J., Giner A., Hartmann E., Prehn S., Rapoport T. A., Dobberstein B. Site-specific photocross-linking reveals that Sec61p and TRAM contact different regions of a membrane-inserted signal sequence. J Biol Chem. 1993 Dec 15;268(35):26745–26751. [PubMed] [Google Scholar]
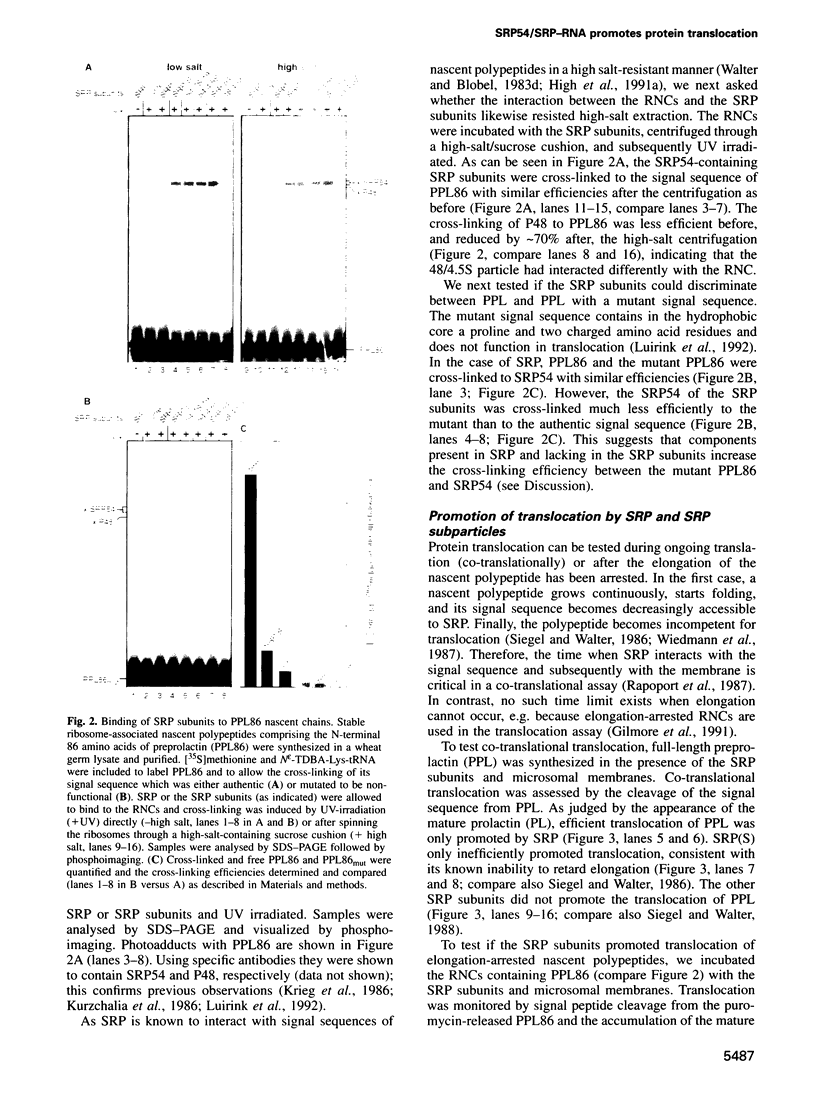
- Krieg U. C., Walter P., Johnson A. E. Photocrosslinking of the signal sequence of nascent preprolactin to the 54-kilodalton polypeptide of the signal recognition particle. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8604–8608. doi: 10.1073/pnas.83.22.8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzchalia T. V., Wiedmann M., Girshovich A. S., Bochkareva E. S., Bielka H., Rapoport T. A. The signal sequence of nascent preprolactin interacts with the 54K polypeptide of the signal recognition particle. Nature. 1986 Apr 17;320(6063):634–636. doi: 10.1038/320634a0. [DOI] [PubMed] [Google Scholar]
- Lauring B., Sakai H., Kreibich G., Wiedmann M. Nascent polypeptide-associated complex protein prevents mistargeting of nascent chains to the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5411–5415. doi: 10.1073/pnas.92.12.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentzen G., Dobberstein B., Wintermeyer W. Formation of SRP-like particle induces a conformational change in E. coli 4.5S RNA. FEBS Lett. 1994 Jul 18;348(3):233–238. doi: 10.1016/0014-5793(94)00599-0. [DOI] [PubMed] [Google Scholar]
- Lingelbach K., Zwieb C., Webb J. R., Marshallsay C., Hoben P. J., Walter P., Dobberstein B. Isolation and characterization of a cDNA clone encoding the 19 kDa protein of signal recognition particle (SRP): expression and binding to 7SL RNA. Nucleic Acids Res. 1988 Oct 25;16(20):9431–9442. doi: 10.1093/nar/16.20.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luirink J., High S., Wood H., Giner A., Tollervey D., Dobberstein B. Signal-sequence recognition by an Escherichia coli ribonucleoprotein complex. Nature. 1992 Oct 22;359(6397):741–743. doi: 10.1038/359741a0. [DOI] [PubMed] [Google Scholar]
- Luirink J., ten Hagen-Jongman C. M., van der Weijden C. C., Oudega B., High S., Dobberstein B., Kusters R. An alternative protein targeting pathway in Escherichia coli: studies on the role of FtsY. EMBO J. 1994 May 15;13(10):2289–2296. doi: 10.1002/j.1460-2075.1994.tb06511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütcke H., High S., Römisch K., Ashford A. J., Dobberstein B. The methionine-rich domain of the 54 kDa subunit of signal recognition particle is sufficient for the interaction with signal sequences. EMBO J. 1992 Apr;11(4):1543–1551. doi: 10.1002/j.1460-2075.1992.tb05199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütcke H. Signal recognition particle (SRP), a ubiquitous initiator of protein translocation. Eur J Biochem. 1995 Mar 15;228(3):531–550. doi: 10.1111/j.1432-1033.1995.tb20293.x. [DOI] [PubMed] [Google Scholar]
- Miller J. D., Bernstein H. D., Walter P. Interaction of E. coli Ffh/4.5S ribonucleoprotein and FtsY mimics that of mammalian signal recognition particle and its receptor. Nature. 1994 Feb 17;367(6464):657–659. doi: 10.1038/367657a0. [DOI] [PubMed] [Google Scholar]
- Miller J. D., Wilhelm H., Gierasch L., Gilmore R., Walter P. GTP binding and hydrolysis by the signal recognition particle during initiation of protein translocation. Nature. 1993 Nov 25;366(6453):351–354. doi: 10.1038/366351a0. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Imai Y., Nakamura A., Yamane K. Small cytoplasmic RNA of Bacillus subtilis: functional relationship with human signal recognition particle 7S RNA and Escherichia coli 4.5S RNA. J Bacteriol. 1992 Apr;174(7):2185–2192. doi: 10.1128/jb.174.7.2185-2192.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips G. J., Silhavy T. J. The E. coli ffh gene is necessary for viability and efficient protein export. Nature. 1992 Oct 22;359(6397):744–746. doi: 10.1038/359744a0. [DOI] [PubMed] [Google Scholar]
- Poritz M. A., Bernstein H. D., Strub K., Zopf D., Wilhelm H., Walter P. An E. coli ribonucleoprotein containing 4.5S RNA resembles mammalian signal recognition particle. Science. 1990 Nov 23;250(4984):1111–1117. doi: 10.1126/science.1701272. [DOI] [PubMed] [Google Scholar]
- Prehn S., Wiedmann M., Rapoport T. A., Zwieb C. Protein translocation across wheat germ microsomal membranes requires an SRP-like component. EMBO J. 1987 Jul;6(7):2093–2097. doi: 10.1002/j.1460-2075.1987.tb02475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapiejko P. J., Gilmore R. Protein translocation across the ER requires a functional GTP binding site in the alpha subunit of the signal recognition particle receptor. J Cell Biol. 1992 May;117(3):493–503. doi: 10.1083/jcb.117.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport T. A., Heinrich R., Walter P., Schulmeister T. Mathematical modeling of the effects of the signal recognition particle on translation and translocation of proteins across the endoplasmic reticulum membrane. J Mol Biol. 1987 Jun 5;195(3):621–636. doi: 10.1016/0022-2836(87)90186-0. [DOI] [PubMed] [Google Scholar]
- Ribes V., Römisch K., Giner A., Dobberstein B., Tollervey D. E. coli 4.5S RNA is part of a ribonucleoprotein particle that has properties related to signal recognition particle. Cell. 1990 Nov 2;63(3):591–600. doi: 10.1016/0092-8674(90)90454-m. [DOI] [PubMed] [Google Scholar]
- Römisch K., Webb J., Herz J., Prehn S., Frank R., Vingron M., Dobberstein B. Homology of 54K protein of signal-recognition particle, docking protein and two E. coli proteins with putative GTP-binding domains. Nature. 1989 Aug 10;340(6233):478–482. doi: 10.1038/340478a0. [DOI] [PubMed] [Google Scholar]
- Römisch K., Webb J., Lingelbach K., Gausepohl H., Dobberstein B. The 54-kD protein of signal recognition particle contains a methionine-rich RNA binding domain. J Cell Biol. 1990 Nov;111(5 Pt 1):1793–1802. doi: 10.1083/jcb.111.5.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel V., Walter P. Each of the activities of signal recognition particle (SRP) is contained within a distinct domain: analysis of biochemical mutants of SRP. Cell. 1988 Jan 15;52(1):39–49. doi: 10.1016/0092-8674(88)90529-6. [DOI] [PubMed] [Google Scholar]
- Siegel V., Walter P. Removal of the Alu structural domain from signal recognition particle leaves its protein translocation activity intact. Nature. 1986 Mar 6;320(6057):81–84. doi: 10.1038/320081a0. [DOI] [PubMed] [Google Scholar]
- Strub K., Moss J., Walter P. Binding sites of the 9- and 14-kilodalton heterodimeric protein subunit of the signal recognition particle (SRP) are contained exclusively in the Alu domain of SRP RNA and contain a sequence motif that is conserved in evolution. Mol Cell Biol. 1991 Aug;11(8):3949–3959. doi: 10.1128/mcb.11.8.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Stueber D., Ibrahimi I., Cutler D., Dobberstein B., Bujard H. A novel in vitro transcription-translation system: accurate and efficient synthesis of single proteins from cloned DNA sequences. EMBO J. 1984 Dec 20;3(13):3143–3148. doi: 10.1002/j.1460-2075.1984.tb02271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Disassembly and reconstitution of signal recognition particle. Cell. 1983 Sep;34(2):525–533. doi: 10.1016/0092-8674(83)90385-9. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Preparation of microsomal membranes for cotranslational protein translocation. Methods Enzymol. 1983;96:84–93. doi: 10.1016/s0076-6879(83)96010-x. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Purification of a membrane-associated protein complex required for protein translocation across the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7112–7116. doi: 10.1073/pnas.77.12.7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature. 1982 Oct 21;299(5885):691–698. doi: 10.1038/299691a0. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Signal recognition particle: a ribonucleoprotein required for cotranslational translocation of proteins, isolation and properties. Methods Enzymol. 1983;96:682–691. doi: 10.1016/s0076-6879(83)96057-3. [DOI] [PubMed] [Google Scholar]
- Walter P., Ibrahimi I., Blobel G. Translocation of proteins across the endoplasmic reticulum. I. Signal recognition protein (SRP) binds to in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):545–550. doi: 10.1083/jcb.91.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Johnson A. E. Signal sequence recognition and protein targeting to the endoplasmic reticulum membrane. Annu Rev Cell Biol. 1994;10:87–119. doi: 10.1146/annurev.cb.10.110194.000511. [DOI] [PubMed] [Google Scholar]
- Wiedmann B., Sakai H., Davis T. A., Wiedmann M. A protein complex required for signal-sequence-specific sorting and translocation. Nature. 1994 Aug 11;370(6489):434–440. doi: 10.1038/370434a0. [DOI] [PubMed] [Google Scholar]
- Wiedmann M., Kurzchalia T. V., Bielka H., Rapoport T. A. Direct probing of the interaction between the signal sequence of nascent preprolactin and the signal recognition particle by specific cross-linking. J Cell Biol. 1987 Feb;104(2):201–208. doi: 10.1083/jcb.104.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin S. L., Walter P. Signal recognition particle mediates a transient elongation arrest of preprolactin in reticulocyte lysate. J Cell Biol. 1989 Dec;109(6 Pt 1):2617–2622. doi: 10.1083/jcb.109.6.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood H., Luirink J., Tollervey D. Evolutionary conserved nucleotides within the E.coli 4.5S RNA are required for association with P48 in vitro and for optimal function in vivo. Nucleic Acids Res. 1992 Nov 25;20(22):5919–5925. doi: 10.1093/nar/20.22.5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zopf D., Bernstein H. D., Johnson A. E., Walter P. The methionine-rich domain of the 54 kd protein subunit of the signal recognition particle contains an RNA binding site and can be crosslinked to a signal sequence. EMBO J. 1990 Dec;9(13):4511–4517. doi: 10.1002/j.1460-2075.1990.tb07902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zopf D., Bernstein H. D., Walter P. GTPase domain of the 54-kD subunit of the mammalian signal recognition particle is required for protein translocation but not for signal sequence binding. J Cell Biol. 1993 Mar;120(5):1113–1121. doi: 10.1083/jcb.120.5.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]