Abstract
Objective
Fluctuations in ovarian hormones during the menstrual cycle and psychosocial stress contribute to eating disorder (ED) behavior.
Methods
Using ecological momentary assessment techniques, this study examined relationships between stress and binge eating, self-induced vomiting, and dietary restriction based on menstrual cycle status in anorexia nervosa (AN). 109 females with full and subthreshold AN (17–45 years old) recorded ED behavior and stress ratings over two weeks. Using hierarchical linear modeling, individuals with eumenorrhea and those with amenorrhea or oligomenorrhea were compared.
Results
Following episodes of meal skipping, momentary stress decreased in individuals with normal menstrual cycles and increased in those with irregular menstrual cycles.
Discussion
Results suggest that changes in stress severity in response to food restriction may differ based on ovarian hormonal status and may be a mechanism by which AN is maintained in individuals without menstrual disturbance.
Anorexia nervosa (AN) is a severe psychiatric illness associated with high rates of morbidity and mortality1,2. Characterized by an intense drive for thinness and desire to lose weight, individuals with AN fear fatness, experience body image distortion and exaggerate the importance of body shape and size in self-evaluation. Biological factors are thought to influence AN symptomatology; however, mechanisms underlying development and maintenance of the disorder remain incompletely understood3.
Several lines of research have investigated reproductive hormonal function in the context of eating disorder (ED) psychopathology. In a longitudinal study of female twins, Klump, Burt, McGue, and Iacono4 examined changes in genetic and environmental influence on disordered eating (DE) throughout adolescence. While environmental contributions remained stable or decreased over time, variance in DE accounted for by genetic factors increased between early (6%) and late adolescence (45%). Changes during pubertal development are thought to account for differing heritability estimates5. More specifically, estradiol, an ovarian hormone (measured via saliva) that increases during puberty, has been shown to moderate genetic influence on DE, such that correlations between measures of DE are greater in monozygotic twins than dizygotic twins who have higher concentrations of estradiol compared to those with lower concentrations of the hormone6. It is possible that rising levels of ovarian hormones during adolescence increase the risk for ED development in individuals with a genetic vulnerability.
In addition to etiological influences, research in rodent and human populations has indicated that ovarian hormones are associated with normal and aberrant patterns of eating behavior. For instance, increased food intake in ovariectomized rats has been shown to reverse with administration of exogenous estrogen7, where progesterone seems to attenuate estradiol’s anorexigenic effect, increasing food intake8. Studies in non-clinical populations have shown that food craving and intake are associated with hormonal changes across the normal menstrual cycle. Women have reported higher levels of craving and consumption in late luteal menstrual phases, when progesterone and estrogen are high but decreasing, compared to the follicular phase when estrogen is elevated9. Prospective studies of ovarian hormones in community samples and in individuals diagnosed with bulimia nervosa who report current, regular menses have shown that decreasing levels of estradiol and increasing levels of progesterone are linked to higher rates of clinically significant binge eating10,11 whereas interactions between estrogen and progesterone during the mid-luteal phase are associated with increases in emotional eating12, or eating in response to negative emotions, not physiological hunger cues.
Research has yet to examine ovarian hormone function and ED pathology in AN. AN-like symptomatology is seen across a wide spectrum of menstrual cycle regularity, where individuals with AN symptoms report menstruation frequency ranging from absent or infrequent to more regular and consistent13. Although the DSM-IV-TR required amenorrhea (cessation of three or more consecutive menstrual cycles14), it has been removed from the DSM-5 on account of evidence that suggests amenorrhea reflects nutritional status, not differences in core psychological or behavioral symptoms15,16. Individuals with amenorrhea or oligomenorrhea (present but irregularly or infrequently occurring menstrual cycles; A/O) possess decreased mean levels of ovarian hormones compared to eumenorrhic (EU), or normal menstruating, females17. Comparing EU to A/O can, therefore be a proxy for studying differences between normal versus aberrant ovarian hormonal function. Doing so in AN could improve our understanding of endocrine factors that contribute to maintenance of ED behavior in this population.
Stress has also been widely studied in EDs and has been shown to be associated with AN symptomatology. For instance, severe life stress, assessed retrospectively, differs between AN and control samples, predating AN onset in 67% of cases18. In epidemiological samples, chronic stress has been frequently reported within the year prior to ED onset19, and individuals with acute AN have reported higher levels of total life stress and more difficulty coping with stress than controls20.
Reactivity of the stress system appears to fluctuate with changes in ovarian hormones. In animal studies, administration of estrogen alpha and beta receptor agonists have been shown to increase and decrease stress-related behavior respectively21. In healthy women, physiological responses to physical and psychological stressors, indicated by increased heart rate, noradrenaline and cortisol secretion has been shown to increase during the luteal phase of the menstrual cycle22,23. Similarly, an fMRI study examining the effects of stress on neural responsivity to emotional stimuli concluded that stress sensitivity, measured by amygdala and medial prefrontal cortex activity following exposure to a psychological stressor, was greater during the luteal phase of the cycle compared to the follicular phase24. Action of ovarian hormones in brain regions that modulate activity of the hypothalamic-pituitary-adrenal axis are thought to contribute to observed changes in stress sensitivity across the menstrual cycle25. Although evidence suggests that ovarian hormones and stress affect ED behavior, little is known about the relationship between these variables and how they influence momentary food restriction, binge eating, and self-induced vomiting in AN. One means of teasing apart the temporal order of these variables is with a methodology known as ecological momentary assessment (EMA).
EMA has received increasing attention in ED research26–28 and offers many advantages over other types of assessments conducted in circumscribed laboratory visits. It measures variables in real-world environments that are more ecologically valid29. EMA reduces retrospective recall bias and memory errors inherent in standard self-report. It also facilitates repeated assessment that, with computerized technology, can date and time-stamp recordings, allowing for investigation of temporal relationships30.
Research has yet to examine how differences in menstrual cycle regularity, an indirect measure of ovarian hormone function, interacts with changes in stress to affect eating in individuals with AN. To begin to explore this question, this study is the first to examine the momentary relationship between stress and ED behavior in AN as a function of menstrual cycle status using EMA. We hypothesized that ratings of stress would increase prior to and decrease following discrete episodes of meal skipping, binge eating, and self-induced vomiting in individuals with both A/O and EU but that the rate of change in stress ratings would be greater in those reporting normal menstrual cycle function.
METHODS
Participants
Data from 109 females, 17–45 years old, were analyzed from part of a larger 3-site study. A total of 121 participants met full eligibility criteria, agreed to participate and were enrolled. Of those enrolled as part of the larger study, three participants with EMA compliance rates less than 50% and nine who endorsed use of hormonal contraceptives were excluded from analyses in the present study. Additionally, participants had to meet DSM-IV-TR criteria for restricting or binge-purge type AN or subclinical AN. Subclinical AN was defined as meeting criteria for full AN with any single one of the following exceptions: 1) BMI between 17.5 and 18.5, or, 2) absence of amenorrhea, or 3) denial of fearing fatness or body image disturbance. Individuals without fear of fatness or body image disturbance but who were significantly low weight and endorsed amenorrhea were included in this study based on research suggesting that these individuals are highly similar to those with full threshold AN in regards to eating disorder symptoms and general personality and psychopathology variables31–33. Adopting more lenient AN inclusion criteria allowed us to examine differences in stress and eating disorder variables based on menstrual cycle status among a more inclusive spectrum of AN pathology. Non-English speakers and those who endorsed psychosis, substance dependence, gastrointestinal surgery or who were medically unstable, pregnant, breastfeeding, or had been hospitalized within six weeks prior to study onset were excluded. The study did not enroll individuals recently initiating psychotherapy or pharmacotherapy; however, those on stable treatment regimens (at least 6 weeks) who continued to show a consistent pattern of ED behavior were included. Participants were divided into groups based on self-reported menstrual cycle frequency assessed during the Eating Disorder Examination34. An EU group (n=47) was comprised of individuals reporting 5 to 7 menstrual cycles over the previous 6 months (equivalent to 26–36 day cycles). The A/O group (n=62) consisted of individuals reporting 4 or fewer cycles over the same time period. Participants were asked to report how many total menstrual cycles they had over the previous six months; however, information regarding which months menstruation occurred was not provided. This prevented the separation between amenorrhea and oligomenorrhea; therefore, the two were combined for analysis.
Assessments
Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-I/P35)
This study employed the SCID-I/P, a semi-structured clinical interview used to assess AN and other current and lifetime DSM-IV-TR Axis 1 disorders.
Eating Disorder Examination (EDE34)
The EDE is a clinician-administered interview that served as the primary measure of ED pathology. This instrument assesses restraint, eating concerns, shape concerns and weight concerns as well as frequency of ED behavior. Both the SCID-I/P and EDE have well documented reliability and validity34–36 and are commonly used in psychiatric research. Twenty-five percent of interviews were randomly selected and rated by an independent assessor. Kappa coefficients for current AN diagnoses on the SCID-I/P (n=30) were .929. Intraclass correlation coefficients for the EDE scales (n=31) ranged from .894 (Shape Concerns) to .997 (Restraint).
EMA Measures
Daily Stress
Twenty-three self-report items assessed daily stress. Fifteen were drawn from the Daily Stress Inventory37 to measure interpersonal stressors (e.g., argued with family). This questionnaire has shown convergent validity with endocrine stress measures38. Eight additional items measured body image (e.g., saw body), eating (e.g., eating high risk food), and ED treatment-related stressors (e.g., saw therapist). These situations were selected to assess potentially stressful situations specific to patients with AN. Items were included to reflect clinical and empirical significance in this population while reducing the overall burden of assessment. Participants reported which stressful events occurred and how stressful each event was on a 5-point scale (1=not at all, 5=very much), yielding a stress severity score.
ED Behaviors
Participants were asked to report all eating episodes and to specify whether they ate an unusually large amount of food (that which “would be considered an excessive quantity by most people”) and/or felt a loss of control (“an inability to stop eating”) or drive to eat (“an inability to prevent the episode”)3,4. Frequency of objective bulimic episodes (OBE; eating a large amount of food accompanied by a sense of loss of control) and subjective bulimic episodes (SBE; eating a small/modest quantity of food associated with a sense of loss of control) for each participant was obtained with this measure. Participants also recorded self-induced vomiting and meal skipping episodes (MSEs).
Procedures
Participants were recruited at the three sites from ED treatment facilities, provider mailings, and flyers throughout the community. Institutional review board approval was obtained at each site. Interested participants underwent an initial phone screening to assess basic inclusion criteria. Those eligible were scheduled for a visit where they signed informed consent. Enrolled participants completed two subsequent visits consisting of 1) a physical examination with a research physician to ensure medical stability, and 2) diagnostic interviews administered by trained research staff. The EMA assessment schedule used in this study has been described previously27. To maximize data quality and optimize the strengths of different recording methods, this study combined the use of interval, random, and event-contingent recordings. Participants were instructed to complete EMA ratings following six semi-random daily beeps delivered to a Palm Pilot computer between 8:30a.m. and 9:50p.m. (random recordings). They also completed ratings at the end of each day (interval recording) and whenever they engaged in target behaviors (event-contingent recording). If individuals felt unable to reply (e.g., during class) or if responding posed a safety risk (e.g., while driving), individuals were instructed to delay responding until the environment permitted. Participants received extensive training in how to define behaviors of interest and operate Palm Pilot equipment. They were asked to carry the device for two practice days before meeting with research staff to receive feedback. Practice data were excluded from analysis. Participants were then asked to carry the Palm Pilot and complete recordings for 2 weeks. They were compensated $100 per week and given a $50 bonus for complying with at least 80% of all recordings.
Statistics
Results were analyzed using SPSS (Version 19.0). Demographic data, behavioral frequency, and EDE subscale scores were examined using independent sample t-tests, Pearson chi-square tests, and analysis of variance (ANOVA) respectively. A mixed-effects, hierarchical linear model (days nested within participant) was employed to test differences in stress ratings between groups on days when ED events occurred compared to non-ED behavior days. Momentary analyses were conducted using hierarchical linear modeling described previously27.
RESULTS
EMA Assessments
Compliance rates to random signals averaged 86% (range = 58–100%) and 87% (range = 69–99%) for the A/O and EU groups respectfully; 77% (range = 31–99%) of all signals were responded to within 45 minutes. End-of-day rating compliance averaged 90% for the A/O group (range = 28–100%) and 89% for individuals with EU (range = 24–100%).
Demographic, Behavioral and Clinical Data
See Table 1 for demographic and clinical variables. Groups did not differ in age or ethnic background. 69.4% of the A/O group met diagnostic criteria for the restricting subtype, compared to 53.7% of the EU group. Similarly, individuals with A/O were more likely to meet diagnostic criteria for full AN (χ2(1, N=109)=9.23, p<0.05), by definition. As expected, there was a trend towards higher average BMI in the EU group compared to the A/O group. To account for this trend, BMI was added as a covariate in subsequent analyses.
Table 1.
Demographic and Clinical Variables.
| Eumenorrhea (n=47) | Amenorrhea/Oligomenorrhea (n=62) | |||
|---|---|---|---|---|
|
| ||||
| Demographics | Mean (SD) | Mean (SD) | t-statistic | p-value |
|
| ||||
| Age | 23.91 (6.26) | 24.81 (7.50) | 0.66 | .51 |
| BMIa | 17.36 (0.97) | 17.00 (1.09) | −1.80 | .07 |
|
| ||||
| Ethnicity | % of Participants (n) | % of Participants (n) | χ2 | p-value |
|
| ||||
| Caucasian | 93.60 (44) | 98.40 (61) | 2.74 | .25 |
| African American | 2.10 (1) | 1.60 (1) | ||
| Other | 4.30 (2) | 0.00 (0) | ||
|
| ||||
| AN Subtype Diagnosis | ||||
|
| ||||
| Full ANb | 31.91 (15) | 61.29 (38) | 9.23 | .00 |
| Subthreshold AN | 68.08 (32) | 38.70 (24) | ||
| Restricting Subtype | 53.20 (25) | 69.40 (43) | 2.98 | .08 |
| Binge/Purge Subtype | 46.80 (22) | 30.60 (19) | ||
|
| ||||
| Comorbidity | ||||
|
| ||||
| Mood Disorder | 21.30 (10) | 24.20 (15) | 0.13 | .72 |
| Anxiety Disorder | 38.30 (18) | 45.20 (28) | 0.52 | .47 |
|
| ||||
| Clinical Variables | Mean(SD) | Mean(SD) | F statistic | p-value |
|
| ||||
| EDEc | ||||
|
| ||||
| Eating Concern | 1.95 (1.54) | 2.24 (1.20) | 1.39 | .24 |
| Weight Concern | 2.89 (1.67) | 3.10 (1.58) | 1.11 | .29 |
| Shape Concern | 2.93 (1.74) | 3.08 (1.46) | 0.96 | .32 |
| Dietary Restraint | 2.63 (1.73) | 2.74 (1.48) | 0.46 | .49 |
|
| ||||
| ED Behavior Frequency | % of Daysf | % of Days | X2 statistic | p-value |
|
| ||||
| Meal Skipping | 32.00 | 31.60 | 0.03 | .86 |
| OBEd | 13.30 | 9.10 | 7.45 | .01 |
| SBEe | 3.90 | 2.80 | 1.57 | .21 |
| Self-induced Vomiting | 19.40 | 18.40 | 0.25 | .61 |
body mass index.
participants that met full DSM-IV-TR criteria for AN compared to those with subthreshold AN.
Eating Disorder Examination.
objective bulimic episode.
subjective bulimic episode.
percent of days when at least one ED behavior was reported.
To examine differences in ED pathology, EDE subscales and behavioral frequencies were compared. There were no differences in rates of MSEs, vomiting, or SBEs between A/O or EU. The EU group reported a higher percentage of days with OBE episodes compared to the A/O group. Groups did not differ in their degree of Dietary Restraint, Eating Concern, Weight Concern, or Shape Concern as assessed by the EDE. Similar rates of comorbid anxiety and mood disorders were observed for both groups. It is important to note that there were no differences in variables of interest (i.e., ED behavior frequency, stress ratings) between those meeting full versus subthreshold AN.
EMA Between and Within Day (Momentary) Results
Similar to methods used in previous EMA studies27, repeated stress ratings within days were combined to produce a daily measure of overall stress severity. When aggregated over the 2-week study period, individuals with A/O reported higher total stress severity (M=18.89, SE=1.46) compared to individuals with EU (M=14.27, SE=1.67, F(1, 106)=4.27, p<.05). Trends suggest that this difference may be due to the A/O group reporting a greater number of stressors (M=7.70, SE=0.52) over the two weeks (EU: M=6.29, SE=0.60, F(1, 106)=3.09, p=.08) which were rated as more stressful on average (M=6.34, SE=0.29) compared to those with EU (M=2.81, SE=0.34, F(1, 106)=3.38, p=.07). For the entire sample, stress severity scores on days when OBEs (M=19.20, SE=1.41), SBEs (M=21.78, SE=1.82), and self-induced vomiting (M=19.55, SE=1.35) occurred were greater compared to symptom-free days (OBE M=16.21, SE=1.10; SBE M=16.46, SE=1.10; Vomiting M=15.90, SE=1.11, all p’s<0.01). This pattern was not observed for meal skipping.
There were no differences in stress ratings between groups on days when binge eating or vomiting occurred; however, there was a significant interaction between menstrual cycle status and meal skipping (F(1,1488)=4.309, p<.05). The A/O group reported increased stress severity when MSEs occurred (M=19.68, SE=1.58) compared to non-MSE days (M=18.52, SE = 1.47). The opposite pattern was observed in the EU group (MSE days M=12.93, SE=1.82; non-MSE days M=15.02, SE = 1.69).
Hierarchical linear modeling was used to examine changes in stress severity ratings preceding and following binge eating, vomiting, and MSEs. Because ED behavior itself can affect subsequent levels of subjective stress, only the first reported behavioral episode of each day was included in the model in order to avoid using ratings confounded by previous events, an approach employed previously27. Main effects and interactions included in the multilevel model are presented in Table 2. Stress severity prior to MSEs did not differ; however, stress following meal skipping appeared to differentiate A/O from EU. Specifically, following MSEs, stress decreased in individuals with EU but increased in those with A/O (F(1,1243)=4.12, p<.05). No differences were observed in momentary stress ratings surrounding binge eating and vomiting between the two groups. Momentary results are presented in Figure 1.
Table 2.
Multilevel results for within day stress severity ratings before and after the first reported meal skipping episode (MSE) of the day.
| Variable | Estimate | Standard Error | T-statistic |
|---|---|---|---|
|
| |||
| Intercept | 5.06 | 0.46 | 10.96** |
| Time prior to MSE | −0.01 | 0.31 | −0.02 |
| Time prior to MSE2 | −0.02 | 0.07 | −0.33 |
| Time prior to MSE3 | 0.00 | 0.00 | −0.48 |
| Time prior to MSE* First MSE | 0.48 | 0.46 | 1.05 |
| Time prior to MSE2* First MSE | −0.05 | 0.08 | −0.64 |
| Time prior to MSE3* First MSE | 0.00 | 0.00 | 1.07 |
| Group | 0.03 | 0.71 | 0.04 |
| Time prior to MSE* Group | 0.59 | 0.48 | 1.22 |
| Time prior to MSE2* Group | 0.12 | 0.10 | 1.12 |
| Time prior to MSE3* Group | 0.00 | 0.00 | 1.00 |
| Time prior to MSE* First MSE* Group | −1.52 | 0.71 | −2.12* |
| Time prior to MSE3* First MSE* Group | −0.01 | 0.00 | −1.66 |
Note: The intercept reflects stress severity ratings at the time that the first MSE of the day was reported. Time prior to meal skipping, time prior to meal skipping2, and time prior to meal skipping3 reflect the linear change in stress severity leading up to reported MSE, the acceleration in the slope of stress severity ratings, and changes in the direction of the slope, respectively. Interactions between time and MSE reflect trajectories of stress severity ratings following meal skipping episodes. Three-way interactions (time x First MSE x group) represent differences in the trajectories of stress severity ratings following meal skipping episodes between the two groups.
p<0.05.
p<0.01.
Figure 1.
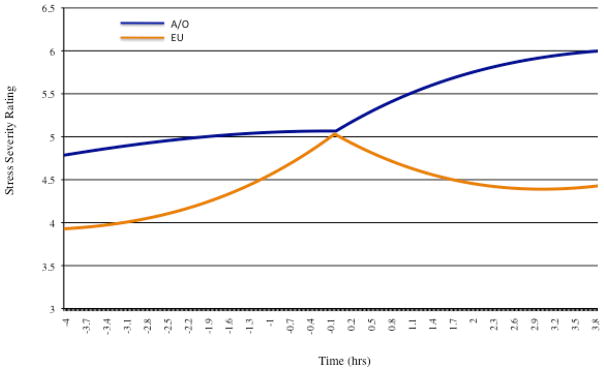
Momentary stress severity ratings captured within 4 hours before and after meal skipping (time=0). Trajectories were significant (p<.05) post meal skipping.
DISCUSSION
Previous research suggests that ovarian hormones and exposure to stressors contribute to ED symptomatology. EMA provides a novel approach for investigating how these variables are related. To our knowledge, this is the first study to specifically examine momentary relationships between stress and ED behavior in AN based on menstrual cycle status. The main finding of this study is that trajectories of self-reported stress differ between individuals with A/O and EU following discrete meal skipping episodes. Results suggest that in AN, normal menstrual cycles are associated with changes in stress severity surrounding distinct episodes of dietary restriction and that restrictive behavior has a fundamental relationship to such stress (i.e., reduces stress). Conversely, those with aberrant menstrual function have persistently higher levels of stress that are less affected by the same behavior. Trends suggest that the A/O group was more likely to meet criteria for the restrictive AN subtype and to have a lower BMI. However, because meal skipping frequency and EDE scores did not differ between the groups and because BMI was covaried statistically, it is unlikely that the findings reflect any underlying differences in illness severity.
These results suggest that dietary restriction in AN may be maintained differently based on hormonal status, where individuals with eumenorrhea are more sensitive to negative reinforcing effects of stress reduction following restriction. With chronically lower levels of estrogen and progesterone, restriction may be reinforced by other mechanisms in individuals with A/O or, if learned early in the illness, it may be slow to extinguish even after reinforcing effects are no longer present. Low levels of estradiol have been previously shown to impair extinction learning in healthy adult women39 and in women diagnosed with posttraumatic stress disorder40, so it is possible that continued restriction in A/O in the absence of reinforcing effects may be due, in part, to decreased secretion of ovarian hormones. As hormonal levels increase and normalize with weight gain during recovery, it is possible that negative reinforcing effects re-emerge and further strengthen the pairing of stress reduction and restrictive eating behaviors. This is consistent with both animal41 and human studies42 that suggest behavioral conditioning and learning is facilitated by higher estrogen concentrations through widespread effects on neural functioning in the brain43. This pattern may partially contribute to the intractability of symptoms frequently observed in AN, especially in the context of weight restoration. Additional research that more directly examines within person changes in estrogen and progesterone in AN is needed to clarify the mechanism by which ovarian hormones influence conditioning and extinction learning in this population.
Irrespective of menstrual cycle status, between day analyses indicated that greater stress was reported on days with binge eating or self-induced vomiting, which is consistent with previous findings in BN27. In addition, patterns of daily stress associated with meal skipping differentiated A/O and EU. Greater stress accompanied days when meal skipping occurred in individuals with A/O whereas reductions in daily stress were associated with meal skipping in participants with EU.
Several limitations are important to note. First, menstrual cycle status was determined via retrospective self-report, not direct hormonal assay (e.g., saliva, blood samples). Similarly, because this study did not assess prospective changes in hormonal concentrations, current menstrual phase in those with EU was not accounted for. Ovarian hormones differ throughout each phase of the menstrual cycle, and thus this should be addressed and controlled for in future research. In addition, the assessment procedures prevented the separation of individuals with current/past amenorrhea from those with oligomenorrhea. Ovarian hormone function undoubtedly differs between these two conditions, and future research using more precise biological measures should clarify how the relationship between stress and dietary restriction differs as a result. Stress severity was similarly measured by self-report and only examined within eight hours of a MSE. Stress experienced outside of this time period likely affects the frequency of restrictive behavior at later time points. Future studies should expand upon these findings, examining patterns of stress and AN behavior over longer time periods. Lastly, these results should be replicated using more direct biological measures of stress reactivity and ovarian hormones (e.g. salivary estradiol and cortisol) and used to examine how within person changes in circulating estrogen and progesterone concentrations and momentary changes in stress directly influence ED behavior in AN.
Overall, findings from this study suggest that momentary changes in stress following discrete episodes of food restriction in AN differ based on self-reported menstrual cycle status. Individuals with normal ovarian hormonal function may be more sensitive to behavior-induced reductions in subjective stress. If true, this suggests that optimal treatments for AN may be personalized based on individual hormonal function. For example, brief modifications of estrogen through pharmacological interventions could potentially reduce the likelihood that negative reinforcement will maintain restrictive eating in individuals who present with normal menses (e.g., early during the illness as individuals are losing weight or after menses have resumed following weight restoration). Additional research is needed to replicate these findings, further investigating how hormonal factors directly affect maintenance of AN symptoms. Such research has the potential to clarify the viability of ovarian hormones as potential treatment targets for AN during certain phases of the illness.
Acknowledgments
This study was supported by funding from NIH RO1 MH 59674 and P30 DK 50456.
Contributor Information
Leah M. Jappe, Department of Psychology, University of Minnesota.
Li Cao, Neuropsychiatric Research Institute, Department of Psychiatry, University of North Dakota.
Ross D. Crosby, Neuropsychiatric Research Institute, Department of Psychiatry, University of North Dakota.
Scott J. Crow, Department of Psychiatry, University of Minnesota.
Carol B. Peterson, Department of Psychiatry, University of Minnesota.
Daniel Le Grange, Department of Psychiatry and Behavioral Neuroscience, University of Chicago.
Scott G. Engel, Neuropsychiatric Research Institute Department of Psychiatry, University of North Dakota.
Stephen A. Wonderlich, Neuropsychiatric Research Institute Department of Psychiatry, University of North Dakota.
References
- 1.Agras WS, Brandt HA, Bulik CM, Dolan-Sewell R, Fairburn CG, Halmi KA, et al. Report of the National Institutes of Health workshop on overcoming barriers to treatment research in anorexia nervosa. Int J Eat Disord. 2004;35:509–521. doi: 10.1002/eat.10261. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan PF. Mortality in anorexia nervosa. Am J Psychiatry. 1995;152:1073–1074. doi: 10.1176/ajp.152.7.1073. [DOI] [PubMed] [Google Scholar]
- 3.Striegel-Moore RH, Bulik CM. Risk factors for eating disorders. Am Psychol. 2007;62:181–198. doi: 10.1037/0003-066X.62.3.181. [DOI] [PubMed] [Google Scholar]
- 4.Klump KL, Burt SA, McGue M, Iacono WG. Changes in genetic and environmental influences on disordered eating across adolescence: a longitudinal twin study. Arch Gen Psychiatry. 2007;64:1409–1415. doi: 10.1001/archpsyc.64.12.1409. [DOI] [PubMed] [Google Scholar]
- 5.Klump KL, Perkins PS, Alexandra Burt S, McGue M, Iacono WG. Puberty moderates genetic influences on disordered eating. Psychol Med. 2007;37:627–634. doi: 10.1017/S0033291707000189. [DOI] [PubMed] [Google Scholar]
- 6.Klump KL, Keel PK, Sisk C, Burt SA. Preliminary evidence that estradiol moderates genetic influences on disordered eating attitudes and behaviors during puberty. Psychol Med. 2010;40:1745–1753. doi: 10.1017/S0033291709992236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Biol Sci. 2006;361:1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butera PC. Estradiol and the control of food intake. Physiol Behav. 2010;99:175–180. doi: 10.1016/j.physbeh.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McVay MA, Copeland AL, Geiselman PJ. Eating disorder pathology and menstrual cycle fluctuations in eating variables in oral contraceptive users and non-users. Eat Behav. 2011;12:49–55. doi: 10.1016/j.eatbeh.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Edler C, Lipson SF, Keel PK. Ovarian hormones and binge eating in bulimia nervosa. Psychol Med. 2007;37:131–141. doi: 10.1017/S0033291706008956. [DOI] [PubMed] [Google Scholar]
- 11.Klump KL, Keel PK, Culbert KM, Edler C. Ovarian hormones and binge eating: exploring associations in community samples. Psychol Med. 2008;38:1749–1757. doi: 10.1017/S0033291708002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klump KL, Keel PK, Racine SE, Burt SA, Neale M, Sisk CL, et al. The interactive effects of estrogen and progesterone on changes in emotional eating across the menstrual cycle. J Abnorm Psychol. 2013;122:131–137. doi: 10.1037/a0029524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poyastro Pinheiro A, Thornton LM, Plotonicov KH, Tozzi F, Klump KL, Berrettini WH, et al. Patterns of menstrual disturbance in eating disorders. Int J Eat Disord. 2007;40:424–434. doi: 10.1002/eat.20388. [DOI] [PubMed] [Google Scholar]
- 14.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Arlington, VA: 2000. Text Revision (DSM-IV-TR) ed. [Google Scholar]
- 15.Attia E, Roberto CA. Should amenorrhea be a diagnostic criterion for anorexia nervosa? Int J Eat Disord. 2009;42:581–589. doi: 10.1002/eat.20720. [DOI] [PubMed] [Google Scholar]
- 16.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. American Psychiatric Publishing; 2013. (DSM-5) ed. [Google Scholar]
- 17.Siegberg R, Nilsson CG, Stenman UH, Widholm O. Endocrinologic features of oligomenorrheic adolescent girls. Fertil Steril. 1986;46:852–857. [PubMed] [Google Scholar]
- 18.Schmidt U, Oldershaw A, Jichi F, Sternheim L, Startup H, McIntosh V, et al. Out-patient psychological therapies for adults with anorexia nervosa: randomised controlled trial. Br J Psychiatry. 2012;201:392–399. doi: 10.1192/bjp.bp.112.112078. [DOI] [PubMed] [Google Scholar]
- 19.Rojo L, Conesa L, Bermudez O, Livianos L. Influence of stress in the onset of eating disorders: data from a two-stage epidemiologic controlled study. Psychosom Med. 2006;68:628–635. doi: 10.1097/01.psy.0000227749.58726.41. [DOI] [PubMed] [Google Scholar]
- 20.Soukup VM, Beiler ME, Terrell F. Stress, coping style, and problem solving ability among eating-disordered inpatients. J Clin Psychol. 1990;46:592–599. doi: 10.1002/1097-4679(199009)46:5<592::aid-jclp2270460508>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 21.Weiser MJ, Foradori CD, Handa RJ. Estrogen receptor beta activation prevents glucocorticoid receptor-dependent effects of the central nucleus of the amygdala on behavior and neuroendocrine function. Brain Res. 2010;1336:78–88. doi: 10.1016/j.brainres.2010.03.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tersman Z, Collins A, Eneroth P. Cardiovascular responses to psychological and physiological stressors during the menstrual cycle. Psychosom Med. 1991;53:185–197. doi: 10.1097/00006842-199103000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Lustyk MK, Olson KC, Gerrish WG, Holder A, Widman L. Psychophysiological and neuroendocrine responses to laboratory stressors in women: implications of menstrual cycle phase and stressor type. Biol Psychol. 2010;83:84–92. doi: 10.1016/j.biopsycho.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Ossewaarde L, Hermans EJ, van Wingen GA, Kooijman SC, Johansson IM, Backstrom T, et al. Neural mechanisms underlying changes in stress-sensitivity across the menstrual cycle. Psychoneuroendocrinology. 2010;35:47–55. doi: 10.1016/j.psyneuen.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience. 2009;159:883–895. doi: 10.1016/j.neuroscience.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wonderlich SA, Crosby RD, Engel SG, Mitchell JE, Smyth J, Miltenberger R. Personality-based clusters in bulimia nervosa: differences in clinical variables and ecological momentary assessment. J Pers Disord. 2007;21:340–357. doi: 10.1521/pedi.2007.21.3.340. [DOI] [PubMed] [Google Scholar]
- 27.Smyth JM, Wonderlich SA, Heron KE, Sliwinski MJ, Crosby RD, Mitchell JE, et al. Daily and momentary mood and stress are associated with binge eating and vomiting in bulimia nervosa patients in the natural environment. J Consult Clin Psychol. 2007;75:629–638. doi: 10.1037/0022-006X.75.4.629. [DOI] [PubMed] [Google Scholar]
- 28.Burd C, Mitchell JE, Crosby RD, Engel SG, Wonderlich SA, Lystad C, et al. An assessment of daily food intake in participants with anorexia nervosa in the natural environment. Int J Eat Disord. 2009;42:371–374. doi: 10.1002/eat.20628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- 30.Smyth J, Wonderlich S, Crosby R, Miltenberger R, Mitchell J, Rorty M. The use of ecological momentary assessment approaches in eating disorder research. Int J Eat Disord. 2001;30:83–95. doi: 10.1002/eat.1057. [DOI] [PubMed] [Google Scholar]
- 31.Le Grange D, Crosby RD, Engel SG, Cao L, Ndungu A, Crow SJ, et al. DSM-IV-defined anorexia nervosa versus subthreshold anorexia nervosa (EDNOS-AN) Eur Eat Disord Rev. 2013;21:1–7. doi: 10.1002/erv.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crow SJ, Stewart Agras W, Halmi K, Mitchell JE, Kraemer HC. Full syndromal versus subthreshold anorexia nervosa, bulimia nervosa, and binge eating disorder: a multicenter study. Int J Eat Disord. 2002;32:309–318. doi: 10.1002/eat.10088. [DOI] [PubMed] [Google Scholar]
- 33.McIntosh VV, Jordan J, Carter FA. Strict versus lenient weight criterion in anorexia nervosa. European Eating Disorders Review. 2004;12:51–60. [Google Scholar]
- 34.Fairburn CG, Wilson GT. Binge eating: nature, assessment, and treatment. New York: Guilford Press; 1993. [Google Scholar]
- 35.First MB, Spitzer R, Gibbon M, Williams JBW, editors. Structured Clinical Interview for DSM-IV Axis I Disorders. New York, NY: Biometrics; 1995. Patient Edition (SCID-I/P) [Google Scholar]
- 36.Fairburn CG. Cognitive Behavior Therapy in Eating Disorders. New York: Guilford Press; 2008. [Google Scholar]
- 37.Brantley PJ, Waggoner CD, Jones GN, Rappaport NB. A Daily Stress Inventory: development, reliability, and validity. J Behav Med. 1987;10:61–74. doi: 10.1007/BF00845128. [DOI] [PubMed] [Google Scholar]
- 38.Brantley PJ, Dietz LS, McKnight GT, Jones GN, Tulley R. Convergence between the Daily Stress Inventory and endocrine measures of stress. J Consult Clin Psychol. 1988;56:549–551. doi: 10.1037//0022-006x.56.4.549. [DOI] [PubMed] [Google Scholar]
- 39.Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, et al. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168:652–658. doi: 10.1016/j.neuroscience.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glover EM, Jovanovic T, Mercer KB, Kerley K, Bradley B, Ressler KJ, et al. Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biol Psychiatry. 2012;72:19–24. doi: 10.1016/j.biopsych.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.ter Horst GJ. Estrogen in the limbic system. Vitam Horm. 2010;82:319–338. doi: 10.1016/S0083-6729(10)82017-5. [DOI] [PubMed] [Google Scholar]
- 42.Gasbarri A, Tavares MC, Rodrigues RC, Tomaz C, Pompili A. Estrogen, cognitive functions and emotion: an overview on humans, non-human primates and rodents in reproductive years. Rev Neurosci. 2012;23:587–606. doi: 10.1515/revneuro-2012-0051. [DOI] [PubMed] [Google Scholar]
- 43.McEwen BS, Akama KT, Spencer-Segal JL, Milner TA, Waters EM. Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms. Behav Neurosci. 2012;126:4–16. doi: 10.1037/a0026708. [DOI] [PMC free article] [PubMed] [Google Scholar]


