Abstract
Xenotransplantation using pigs as donors offers the possibility of eliminating the chronic shortage of donor kidneys, but there are several obstacles to be overcome before this goal can be achieved. Preclinical studies have shown that while porcine renal xenografts are broadly compatible physiologically, they provoke a complex rejection process involving preformed and elicited antibodies, heightened innate immune cell reactivity, dysregulated coagulation, and a strong T cell-mediated adaptive response. Furthermore, the susceptibility of the xenograft to pro-inflammatory and pro-coagulant stimuli is probably increased by cross-species molecular defects in regulatory pathways. To balance these disadvantages, xenotransplantation has at its disposal a unique tool to address particular rejection mechanisms and incompatibilities: genetic modification of the donor. This review focuses on the pathophysiology of porcine renal xenograft rejection, and on the significant genetic, pharmacological and technical progress that has been made to prolong xenograft survival.
Keywords: immunosuppression; kidney; pig, genetically modified; pig-to-primate model; transgenesis; tolerance; xenotransplantation
Introduction
Kidney transplantation, the best treatment for end-stage renal disease, is limited by the shortage of human donors. Although the donor pool has been expanded by strategies such as paired donation and the use of blood group-incompatible and non-heart-beating donors, it remains unlikely to meet the increasing demand in the foreseeable future. This has driven a search for alternative sources of donor kidneys. Much recent activity has focused on the generation of transplantable tissue from autologous stem cells, but the complexity of the kidney makes this a long-term prospect at best (1). In contrast, xenotransplantation using pigs as donors has been studied for several decades (2, 3), and porcine cellular xenografts have already reached the stage of clinical trials (4). The pig is the animal donor of choice for a number of reasons including relatively similar organ size and physiology, high reproductive capacity, and the potential for genetic modification to prevent rejection and correct molecular incompatibilities. Preclinical studies indicate that pig kidney xenotransplantation is feasible, with renal xenografts supporting life for several weeks or months in non-human primate recipients (5-8). However, despite considerable progress in recent years, the immunological and pathophysiological barriers have not been completely overcome. The major challenge is to place renal xenografts on at least an equal footing with allografts i.e. with comparable survival rates under similar levels of immunosuppression. This is likely to require a combination of ‘humanized’ donors and clinically applicable immunosuppressive protocols. Herein we will review the mechanisms of porcine renal xenograft rejection and describe recent progress in moving kidney xenotransplantation to the clinic.
THE PIG-TO-PRIMATE PRECLINICAL MODEL
Current understanding of the function and immunobiology of pig renal xenografts is primarily drawn from studies using non-human primates (NHP) as recipients. The technical challenges of this model are significant, with a relatively high rate of failure from causes unrelated to rejection (7, 9-11), but it has provided invaluable information and insights.
Physiological compatibility
Although final proof must await clinical testing, extensive in vivo and ex vivo data indicate that pig kidneys will function adequately in humans (reviewed in (12)). The most comprehensive dataset on physiological compatibility comes from a study of 22 monkeys transplanted with human CD55-transgenic pig kidneys (survival: range 21-78 days, mean 41 days, median 38 days) (13). During the period of stable xenograft function, most serum electrolytes (urea, sodium, chloride, potassium and calcium) remained within the normal range, while creatinine was modestly elevated but steady. Of some concern, phosphate and haemoglobin levels progressively fell and serum albumin was consistently low after transplantation. The cause of hypophosphatemia was not established, while anemia was postulated to be due to molecular incompatibility of porcine erythropoietin with the primate Epo receptor, and was treated using recombinant human erythropoietin (13). Hypoalbuminemia and mild to severe proteinuria have also been reported in baboon recipients (5, 7), although whether this phenomenon is due to rejection-associated injury or to an inherent physiological difference remains to be determined. In either case, the solution may be provided by further genetic modification of the donor pig and/or pharmacological intervention.
Immunological considerations
Like humans, Old World primates (e.g. macaques and baboons) possess preformed antibodies to galactose-α1,3-galactose (αGal), a xenoantigen that is abundantly expressed on the surface of most pig cells (14, 15) (see details in following section). This makes these animals the preferred model recipients from an immunological perspective. However, two potential limitations should be noted. First, macaques appear to have a more ‘hypercoaguable’ phenotype than humans (16), suggesting that coagulation disturbances may be exaggerated in this model. Second, macaques and baboons lack at least some types of anti-pig antibodies that are naturally present in humans. For example, humans develop antibodies to the carbohydrate N-glycolylneuraminic acid (also known as Neu5Gc or Hanganutziu-Deicher antigen), which is expressed in both pigs and NHP (17, 18). Nevertheless, the macaque and baboon models have been critical to unravelling the complex immune response to renal xenografts and testing new genetic and immunosuppressive strategies.
THE IMMUNE RESPONSE TO PIG KIDNEY XENOGRAFTS
The evolutionary distance between pigs and primates has resulted in carbohydrate and protein differences that not only promote immune recognition of porcine xenografts but also affect the function of immunoregulatory pathways. The most striking example of the importance of differential glycosylation is the αGal xenoantigen. Most mammalian species including pigs express α1,3-galactosyltransferase (GalT), an enzyme that synthesises the terminal carbohydrate moiety galactose-α1,3-galactose (αGal) on glycoproteins and glycolipids (15). Humans and other higher primates have lost αGal expression due to mutations in the coding region of the GalT gene GGTA1 (19), possibly as an evolutionary immune defence against microbial pathogens (20), and develop anti-αGal antibodies in response to gut bacteria (21). In humans, anti-αGal comprises about 80% of preformed (‘natural’) anti-pig IgM (22) and is the most abundant natural IgG (23). This has profound consequences for kidney xenotransplantation, as outlined below.
The innate immune response and hyperacute rejection (HAR)
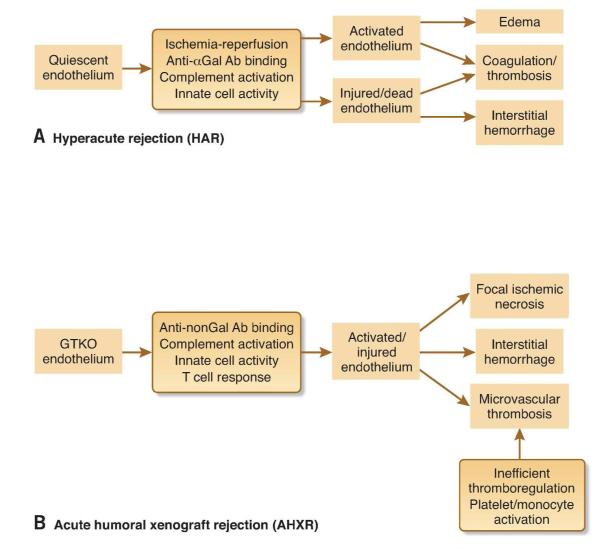
Unmodified pig kidneys provoke a rapid and powerful innate immune response in primates, characterized by binding of natural anti-pig antibodies to the xenograft vascular endothelium and activation of the classical complement pathway and the coagulation cascade. The resulting congestion, oedema and massive interstitial haemorrhage are hallmark features of this ‘hyperacute’ rejection (HAR) (24), which occurs within hours of reperfusion (25) (Figure 1A). The pivotal role of αGal is evident from the fact that specific depletion of anti-αGal antibodies prevented HAR of pig-to-macaque renal xenografts (26). Perhaps even more salient, elimination of αGal expression in the donor pig prevented HAR in the pig-to-baboon model in the absence of any other treatment (27). It is conceivable that natural human ‘non-Gal’ anti-pig antibodies, including those recognising other carbohydrate antigens such as Neu5Gc, may be present at sufficient levels in some individuals to precipitate HAR. Such antibodies have been detected in human serum (28), and at least some of them can mediate complement-dependent lysis and antibody-dependent cellular cytotoxicity to pig cells (29). However, the natural anti-non-Gal titer varies considerably between individuals, and it should be possible to minimize the impact of these antibodies by careful pre-screening of recipients (30).
Figure 1. Phases of kidney xenograft rejection.
A, Several factors contribute to hyperacute rejection of wild-type xenografts, but the key events are the binding of preformed anti-αGal antibodies (Ab) to xenograft vascular endothelial cells and subsequent activation of complement. HAR occurs within hours and can be prevented by deletion of αGal (GTKO) or transgenic expression of human complement-regulatory proteins (hCRPs). B, Acute humoral rejection of GTKO xenografts is also mediated by antibodies, in this case anti-non-Gal, but is a more prolonged process (days to weeks) which appears to involve the gradual development of a chronic pro-coagulant and pro-inflammatory vascular environment.
Differential glycosylation also contributes to direct recognition of pig cells by human NK cells and macrophages (31-33), leading to a response that may be further heightened by cross-species molecular incompatibilities affecting particular cellular interactions (34). Gene sequence analysis suggests that swine leukocyte antigen (SLA) class I, the porcine equivalent of HLA class I, will not transmit an inhibitory signal to receptors on human NK cells (35). Similarly, although human SIRPα has been shown to bind porcine CD47 in vitro (36), this interaction does not send a negative signal and thus does not prevent human macrophages from phagocytosing pig cells (37). These defects in the regulation of innate immune cell activity are likely to be most problematic for cellular xenografts, but may also contribute to renal xenograft rejection.
Dysregulated coagulation and inflammation
Dysregulated coagulation is a major barrier to the survival of pig kidney xenografts post-HAR (38). Thrombotic microangiopathy is observed in rejected renal xenografts (39, 40), albeit less often than in cardiac xenografts (41, 42). Furthermore, recipients frequently develop a consumptive coagulopathy characterized by thrombocytopenia, declining fibrinogen levels, increased D-dimer and thrombin-antithrombin levels, and prolonged clotting time (25, 43-46). This condition can be fatal once established (46), and only resolves upon removal of the xenograft (44). While the cause is yet to be formally determined, it is likely that several factors converge to promote excessive coagulation and inflammation. First, xenograft endothelial cells are activated by a range of mechanisms including ischemia-reperfusion, complement, antibody binding, and interaction with recipient immune cells (47-50), and consequently express tissue factor, the primary physiological initiator of coagulation. Second, it has been proposed that recipient tissue factor, expressed by platelets and monocytes activated by inflammation and contact with xenograft endothelium (51), also plays a role (46), although whether human platelets express tissue factor is controversial (52). Third, pig endothelial cells express an enzyme that converts human prothrombin to thrombin in a tissue factor-independent manner (53). Expression and activity of this direct prothrombinase (fgl2) is induced by pro-inflammatory cytokines (53, 54). Rejected pig-to-baboon renal xenografts showed fgl2 expression in close association with fibrin deposition in small vessels and glomerular capillaries (53).
Finally, at least one key regulatory mechanism is compromised by molecular incompatibility. The thrombomodulin (TM) / protein C pathway is a critical regulator of coagulation and inflammation within the microvasculature (55). TM is an integral endothelial membrane protein that alters thrombin’s specificity from pro-coagulant and pro-inflammatory substrates to protein C, which in its activated form inhibits coagulation and inflammation (55, 56). Pig TM binds human thrombin but is a poor cofactor for activation of human protein C, with only 1-10% of the activity of human TM (57-59). Molecular incompatibility may also promote clotting; human platelets spontaneously aggregate in vitro upon contact with pig von Willebrand factor (vWF) due to an aberrant interaction between human platelet glycoprotein Ib and the O-glycosylated A1 domain of pig vWF (60-62).
The adaptive immune response and acute humoral xenograft rejection (AHXR)
T cells play a major role in xenograft rejection (reviewed in (63)). Both the direct and indirect presentation pathways are involved (64), and there is an extensive range of potential xenoantigens (65). If HAR is prevented and conventional immunosuppression is used to inhibit the T cell-mediated adaptive response, the survival of renal xenografts is prolonged for days to weeks before they are rejected by a second antibody-mediated process termed acute humoral xenograft rejection (AHXR), also known as acute vascular rejection or delayed xenograft rejection (66-68) (Figure 1B). AHXR is characterized by varying degrees of antibody and complement deposition, microvascular thrombosis, focal ischemic necrosis and interstitial haemorrhage, endothelial cell changes, and leukocytic infiltration (40, 69). In situations where anti-αGal has little or no role, AHXR is mediated by anti-non-Gal antibodies, either preformed (if present in sufficient titer) (11) or elicited (70, 71). Candidate non-Gal xenoantigens include carbohydrates (72-74) and proteins (75, 76) including important endothelial cell-protective regulators (77). It is reasonable to suggest that the susceptibility of renal xenografts to AHXR is increased by several of the factors described earlier, such as innate immune cell hyper-reactivity and defects in regulatory mechanisms; even low levels of anti-graft antibodies may trigger a vicious cycle of coagulation and inflammation.
Acute cellular and chronic xenograft rejection
Classical acute cellular rejection of organ xenografts is rarely observed, probably because it is usually preceded by AHXR. A histopathological analysis of pig-to-baboon renal biopsies showed increasing infiltration by T cells and macrophages, revealing cellular rejection when humoral rejection is avoided (40). However, most evidence suggests that the T cell response can be controlled by currently available immunosuppressive agents and may become less problematic as the innate immune response is better managed (see below). The frequency and characteristics of chronic xenograft rejection are unclear because few renal xenografts have survived long enough for this type of injury to develop, although one case of chronic glomerulopathy similar to that observed in allografts has been described (40).
PREVENTING KIDNEY XENOGRAFT REJECTION
It is evident from the preceding section that the rejection of pig renal xenografts is a complex and powerful process that will likely require a combination of approaches to prevent.
Genetic modification of the donor
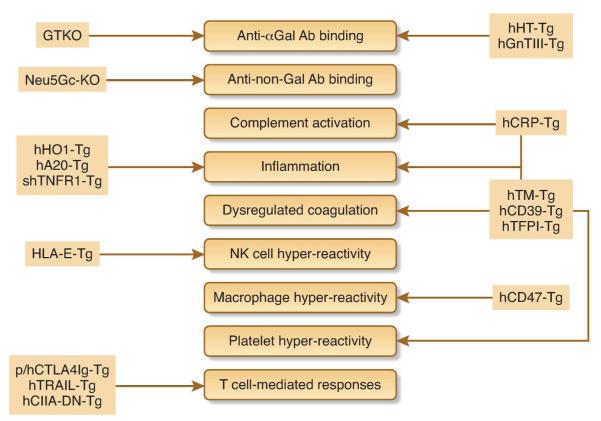
Perhaps the key advantage of xenotransplantation over allotransplantation is the ability to genetically modify the donor to protect grafts from the human immune system. The initial method of transgenesis by pronuclear microinjection (25, 78-80) has been superseded by somatic cell nuclear transfer (cloning), which can be used to delete porcine genes and / or add transgenes (reviewed in (81)). Recent technological advances allow precise engineering of the pig genome using zinc finger nucleases (ZFNs) (82) or transcription activator-like effector nucleases (TALENs) (83), and efficient co-expression of multiple transgenes using the 2A ‘ribosome skip’ signal (84). The main types of genetic modification that have been applied to pigs for the purpose of xenotransplantation are described below and summarized in Figure 2.
Figure 2. Donor genetic modification to prevent kidney xenograft rejection.
The targets of genetic modification are shown as filled boxes. Abbreviations: CIITA-DN, dominant-negative class II transactivator; CRP, complement regulatory protein; GnT-III, N-acetylglucosaminyltransferase III; GTKO, GalT knockout; h: human; HO-1, hemoxygenase-1; HT, H-transferase; Neu5Gc-KO, N-glycolylneuraminic acid knockout; HLA, human leukocyte antigen; p: pig; shTNFR1, soluble human TNF receptor 1; Tg: transgenic; TFPI, tissue factor pathway inhibitor; TM, thrombomodulin; TRAIL, TNF-related human apoptosis-inducing ligand.
Carbohydrate remodelling
The initial genetic approach to the αGal / α1,3-galactosyltransferase problem was to transgenically express alternative glycosyltransferases. The level of αGal in pigs was reduced by expressing either human α1,2-fucosyltransferase (H-transferase or HT), which adds the non-antigenic blood group O to the substrate of α1,3-galactosyltransferase (85), or N-acetylglucosaminyltransferase III (GnT-III), which downregulates both αGal and non-Gal xenoantigens by an unknown mechanism (86). However, the advent of cloning made it possible to eliminate αGal altogether (87-92). Renal xenografts from the resulting GalT knockout (Gal KO or GTKO) pigs were resistant to HAR (5, 10, 27), although one case of HAR of a GTKO heart in the pig-to-baboon model has been reported, presumably caused by non-Gal IgM (93). Deletion of αGal may also attenuate the innate cellular response, although this is yet to be examined in vivo.
With the αGal problem solved, attention has shifted to other potential carbohydrate targets, particularly Neu5Gc. Recently, ZFN technology was used in conjunction with cloning to simultaneously delete the porcine genes responsible for expression of αGal (GGTA1) and Neu5Gc (CMAH), in a process that took only 7 months (94). Peripheral blood mononuclear cells from the resulting ‘double-KO’ pigs showed significantly reduced binding of preformed antibodies in human sera compared to GTKO alone cells. This is very promising, although it will not be possible to test the impact of deleting Neu5Gc in the primate model because NHP express this carbohydrate and thus do not develop antibodies to it.
Regulation of complement activation
Complement activation within transplanted organs is controlled by the combined action of membrane-bound complement regulatory proteins (CRPs) on the donor organ and soluble factors of recipient origin. The former comprise CD46 (also known as membrane cofactor protein or MCP) and CD55 (decay accelerating factor or DAF), which regulate mid-pathway, and CD59, which inhibits formation of the membrane attack complex in the terminal pathway (95). Although it was once thought that pig CRPs may not efficiently inhibit primate complement, potentially contributing to the rejection of pig organ xenografts, this does not appear to be the case (96, 97). Nonetheless, transgenic expression of one or more human (h)CRPs is generally effective in preventing HAR of renal xenografts (13, 25, 98-100), presumably by providing supraphysiological regulatory capacity. Evidence from the cardiac model suggests that the combination of transgenic hCRP expression and deletion of αGal provides greater protection to organ xenografts than either alone (93).
Regulation of coagulation, inflammation and thrombosis
Inflammation and coagulation are tightly interlinked. The vascular lining of organ xenografts is the focus of antibody binding, complement activation and immune cell activity, leading to endothelial cell activation and downregulation or shedding of key protective molecules (101-103). Most genetic approaches have therefore targeted transgenic expression of anticoagulant / anti-inflammatory / anti-platelet molecules to the vascular endothelium. Mouse studies have demonstrated proof of concept for the benefits of expressing human TM and tissue factor pathway inhibitor (104, 105). Both molecules have been expressed in pigs (106-109) but efficacy in an in vivo transplant setting has not yet been reported. The ectonucleotidase CD39 has also been utilized because of its broad vasculoprotective functions (degradation of extracellular ATP and ADP, and promotion of adenosine generation) (110). Transgenic expression of human CD39 protected mouse hearts and kidneys from transplant-related and other vascular injury (111-114), and protected pig hearts from myocardial ischemia-reperfusion injury (115). However, renal expression of the CD39 transgene in this line of pigs appeared to be insufficient to prolong kidney xenograft survival in the pig-to-baboon model (116).
Hemoxygenase-1 (HO-1) has been widely studied in the transplant context because of its anti-inflammatory, antioxidant and cytoprotective properties (117). The potential benefits of human HO-1 expression in transgenic pigs have been demonstrated in vitro and ex vivo (118, 119), although in vivo efficacy has not been reported. Other promising candidate anti-inflammatory molecules that have reached a similar stage include human A20 (120) and soluble human TNF receptor 1 (shTNFR1) (121).
Regulation of innate immune cell activity
Genetic modification has been used to tackle the molecular incompatibilities affecting the heightened reactivity of human NK cells and macrophages to porcine targets. Endothelial cells from transgenic pigs expressing the non-classical human MHC class I molecule HLA-E were partially protected from human NK cell-mediated cytotoxicity in vitro (122). Expression of human CD47 on a pig lymphoblastoid cell line inhibited phagocytosis of the cells by human macrophages in vitro (37) and protected them from rejection in a mouse tumour model (123). Intuitively, these approaches would seem to be most applicable to cellular xenografts, and it remains to be seen whether they have any relevance to renal xenotransplantation.
Regulation of adaptive immunity
Several molecules have been expressed in transgenic pigs in attempts to block the T cell-mediated adaptive response. These include human TNF-related apoptosis-inducing ligand (TRAIL), an approach designed to kill xenograft-infiltrating T cells (124); pig dominant-negative class II transactivator (CIITA-DN), to inhibit upregulation of pig SLA class II (125); human CTLA4-Ig or its high-affinity variant LEA29Y (belatacept), to block costimulation of T cells associated with indirect antigen presentation (126, 127); and pig CTLA4-Ig, to block costimulation of T cells by direct antigen presentation (128). While in vitro analyses and limited cellular transplant studies in mice have produced promising results, no data are yet available from the pig-to-NHP model, and adverse health effects have been associated with one of the transgenes (128).
Immunosuppression and other pharmacological treatments
Therapies directed at T and B cells
Most groups studying preclinical pig-to-NHP organ xenotransplantation have incorporated a T cell-depleting agent, most commonly anti-thymocyte globulin (ATG), in their induction regimens. An anti-human CD2 monoclonal antibody (mAb) and an anti-monkey CD3 recombinant immunotoxin (anti-CD3 rIT) have also been shown to effectively deplete T cells in the pig-to-baboon renal model (129). However, the anti-CD2 mAb did not prolong xenograft survival, and the anti-CD3 rIT-treated recipients died from complications before efficacy could be determined (129). Treatment with the specific B cell-depleting agent rituximab (anti-CD20 mAb) was beneficial in cardiac xenotransplantation (130, 131), but rituximab has not yet been tested in the renal model. Pan-lymphocyte depletion using the anti-human CD52 mAb alemtuzumab also remains untested in NHP models because CD52 is expressed on red blood cells in all NHP except cynomolgus monkeys of Indonesian origin (132). Interestingly, induction with cyclophosphamide has been more successful than T/B cell-specific therapies in prolonging renal xenograft survival (6), although this agent is rarely used today in solid organ transplantation.
While the combination of conventional maintenance immunosuppression (e.g. tacrolimus and rapamycin) with T and B cell depletion has produced encouraging results, there has been increasing interest in costimulation blockade using agents such as anti-CD154 mAb. Following induction with ATG ± rituximab, anti-CD154 therapy extended GTKO cardiac xenograft survival to up to 6-8 months in the pig-to-baboon model (131, 133). Anti-CD154 has been less successful in the renal xenograft model, but this was related to the relatively rapid onset of consumptive coagulopathy rather than failure to block T-cell mediated rejection or the elicited antibody response (46). With the co-transplantation of donor thymic tissue, anti-CD154 has contributed to significant prolongation of GTKO renal xenograft survival in baboons (5, 7) (see below). However, the anti-CD154 mAb employed in these studies is unlikely to be used clinically because of its thrombogenic properties (134-136). This has prompted an exploration of alternative costimulation blockade agents such as belatacept and anti-CD40 mAb, which have shown efficacy in the pig-to-macaque islet xenograft model (137).
Overall, the current experimental data suggest that costimulation blockade may be the preferred immunosuppressive therapy, but this would need to include blockade of both the CD40/CD154 and CD28/B7 pathways. It is conceivable, however, that conventional pharmacologic immunosuppressive therapy with agents such as tacrolimus and rapamycin may suffice, particularly if the pig has been genetically engineered to prevent or reduce activation of xenograft endothelial cells.
Anti-inflammatory / anti-coagulant treatments
The best prospect for control of inflammation and coagulation is likely to be genetic manipulation of the donor pig, as discussed earlier. However, there may still be a need for treatment with anti-inflammatory agents. In addition to corticosteroids, there is evidence that high-dose statin therapy not only reduces the inflammatory response and platelet activation (138), but also downregulates the primate cellular response to pig antigens (139).
Similarly, judicious treatment with anti-coagulant and/or anti-platelet agents may be beneficial even when genetically modified donor pigs are used. Some groups routinely administer continuous heparin to baboon recipients of pig renal (42) and cardiac (140, 141) xenografts, although it is difficult to assess the efficacy of this treatment. Administration of recombinant human antithrombin was clearly protective in the first week after pig-to-baboon renal xenotransplantation (98), but had no apparent long-term benefit in the pig-to-macaque renal model (142), even when combined with human activated protein C (143). Other reagents have also produced mixed results, and it would be reasonable to conclude that both genetic modification and pharmacotherapy will be necessary to fully control inflammation and coagulation in renal xenotransplantation (144).
Induction of tolerance
As in allotransplantation, the induction of tolerance is the ultimate goal of those involved in xenotransplantation research, although it should be noted that tolerance is a long-term prospect at best and is not a prerequisite for clinical renal xenotransplantation. Prolonged kidney xenograft survival under clinically acceptable chronic immunosuppression remains the initial goal. The hurdles for tolerance in xenotransplantation are in some respects greater than those in allotransplantation, but there are also some advantages. The prior identification of the donor pig will allow pre-transplant preparation of the recipient, which is not possible with transplantation using organs from deceased human donors. Furthermore, the donor pig may be able to be genetically modified to facilitate the induction of tolerance. Efforts to induce tolerance to a xenograft have largely been confined to three major approaches: mixed chimerism, co-transplantation of donor thymic tissue, and cellular therapy with regulatory T cells (Tregs) or mesenchymal stem cells (MSCs).
Mixed chimerism
Based on a successful approach in human renal allotransplantation (145), great efforts have been made to induce mixed chimerism in NHP by the infusion of pig hematopoietic stem cells (146). However, phagocytosis of pig cells by human macrophages, probably as a result of the failure of pig CD47 to transmit a negative signal to human SIRPα, may be a major barrier to the development of mixed chimerism (147). In vitro (37) and small animal models (148) suggest that transgenic expression of human CD47 in pigs may be the solution to this problem.
Co-transplantation of donor thymic tissue
This concept is based on the depletion of mature T cells before the combined transplantation of the organ xenograft and donor thymic tissue, thus allowing new T cells to recognize pig antigens as self, and T cells directed to pig antigenic specificities to be deleted. After recipient pre-treatment, transplantation of donor-specific pig thymus tissue with a kidney graft, either in the form of a previously-prepared ‘thymokidney’ or as a thymic lobe transplant, extended graft survival to almost three months (5, 7). Antibody-mediated rejection remained problematic, though most baboons died from the effects of the intensive immunosuppressive therapy rather than from graft failure. Whether T cell tolerance will be precluded by the additional barriers to xenotransplantation remains uncertain. It may be necessary to overcome the innate immune response and coagulation dysregulation through other means before T cell tolerance can be achieved.
Cellular therapy
Although no studies of expanded Tregs or MSCs have been carried out in pig-to-NHP organ transplantation models, there have been several in vitro studies (149-152), and some experience of MSC therapy in pig-to-baboon islet transplantation (153). Tregs have effects on both the direct and indirect T cell response, and inhibition of the CD40/CD154 pathway seems to be particularly beneficial in enhancing Treg activity (154, 155). MSCs have received considerable attention in recent years for their immunomodulatory, anti-inflammatory and regenerative effects. MSCs function across species barriers (156), and pig MSCs suppress the human T cell response to pig tissues (157, 158). Pig MSCs have the advantage that they can be obtained in very large numbers from adipose tissue or bone marrow, and therefore require less expansion in vitro before infusion into the xenograft recipient. In addition, the same donor can be used for the MSCs and the xenograft, avoiding allogeneic differences.
MANAGING INFECTIOUS RISK
Transplantation carries the possibility of transmission of infectious agents from the donor organ to the immunocompromised recipient. In xenotransplantation, this potential risk is compounded by fears of a wider risk to the community posed by human-to-human transmission of novel pig-derived pathogens, such as porcine endogenous retrovirus (PERV). While the degree of risk associated with renal xenotransplantation remains unknown in the absence of clinical trials, preclinical data and the limited data from human trials indicate that infectious transmission will occur rarely, if at all (159). Nevertheless, clinical trial guidelines have been established to manage infectious risk (160), with basic principles including national and global oversight, routine screening and maintenance of source pigs in specific or defined pathogen-free facilities, pre-and post-transplant screening of recipients, and long-term archiving of animal and patient samples. Those readers interested in learning more about this topic are directed to a recent review (161). Another cross-species aspect that has received less attention is the possibility of ‘reverse’ infection: viral transmission from the recipient to the xenograft. Several human viruses are capable of productively infecting pig cells (162). However, it is likely that this potential problem will be manageable by careful monitoring and management.
One of the difficulties in extrapolating from the NHP model to predict infectious risk in the clinical setting is the markedly different environment of NHP and human recipients, both pre-and post-transplant. The recent availability of specific pathogen-free primates may help in this regard, although this option would add significantly to the cost of this already expensive model.
CONCLUSIONS AND PERSPECTIVE
Advancing kidney xenotransplantation to the clinic is a daunting challenge. The glycosylation and protein differences between pigs and humans provoke a powerful humoral and cellular immune response to porcine xenografts that cannot be controlled by conventional immunosuppression. Progress in the difficult pig-to-NHP preclinical model has been painstaking and slow, with maximum graft survival currently limited to about 3 months. However, few would disagree that the reward for success – elimination of the donor kidney shortage – warrants continued effort. The key goal is to overcome the humoral, coagulation and inflammatory responses by genetic modification of the donor pig. While clinical application is probably still several years away, the increasingly sophisticated tools for precise manipulation of the pig genome, along with the development of novel immunosuppressive agents and tolerance-inducing protocols, bode well for the future.
Several questions remain. First, what level of preclinical success would justify moving to the next stage? We suggest that the consistent demonstration of life-supporting renal function for at least 5-6 months in the pig-to-NHP model, with clinically applicable immunosuppression and no evidence of infection, would be sufficient. Second, which potential recipient group would be most appropriate for the first clinical trials? Highly allosensitized patients with broadly reactive preformed alloantibodies are the obvious choice; they are less unlikely than other patients on the waiting list to receive a human kidney graft, and in vitro analyses indicate that they are at no greater risk of xenoreactivity to porcine tissue (163). Third, will renal xenotransplantation result in sensitization to alloantigens, and thus potentially jeopardize subsequent allotransplantation? The answer to this question is less clear, although the demonstration that baboons sensitized to pig antigens did not develop increased humoral or cellular alloreactivity (164) is encouraging. Finally, what is the best way to move the field forward and efficiently address the many issues still facing the pig-to-NHP model? Ideas and technologies are not lacking, but stable funding support is difficult to obtain and the intellectual property landscape is far from clear. Real progress is likely to require expanded collaboration between groups working on different organs and tissues, including the sharing of new genetically modified pigs.
Acknowledgments
Sources of support: PJC, AJFd’A: National Health and Medical Research Council of Australia; Juvenile Diabetes Research Foundation
DKCC: US National Institutes of Health; Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Inc., Blacksburg, VA
Abbreviations
- APC
activated protein C
- AHXR
acute humoral xenograft rejection
- CIITA-DN
dominant-negative class II transactivator
- CRP
complement regulatory protein
- EPCR
endothelial protein C receptor
- GnT-III
N-acetylglucosaminyltransferase III
- GTKO
GalT knockout
- HAR
hyperacute rejection
- HO-1
hemoxygenase-1
- HT
α1,2-fucosyltransferase or H-transferase
- mAb
monoclonal antibody
- MSCs
mesenchymal stem cells
- Neu5Gc
N-glycolylneuraminic acid
- NHP
non-human primate
- PERV
porcine endogenous retrovirus
- SLA
swine leukocyte antigen
- shTNFR1
soluble human TNF receptor 1
- TFPI
tissue factor pathway inhibitor
- TM
thrombomodulin
- TRAIL
TNF-related human apoptosis-inducing ligand
- Tregs
regulatory T cells
- vWF
von Willebrand factor
Footnotes
Disclosure There are no financial disclosures or conflicts of interest to report for any of the authors
REFERENCES
- 1.Pondrom S. The AJT Report: news and issues that affect organ and tissue transplantation. Am J Transplant. 2012 Oct;12(10):2565–6. doi: 10.1111/j.1600-6143.2012.04296.x. [DOI] [PubMed] [Google Scholar]
- 2.Cooper DKC. A brief history of cross-species organ transplantation. Proc (Bayl Univ Med Cent) 2012;25(1):49–57. doi: 10.1080/08998280.2012.11928783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bengtsson A, Svalander CT, Molne J, Rydberg L, Breimer ME. Extracorporeal (“ex vivo”) connection of pig kidneys to humans. III. Studies of plasma complement activation and complement deposition in the kidney tissue. Xenotransplantation. 1998 Aug;5(3):176–83. doi: 10.1111/j.1399-3089.1998.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 4.Ekser B, Ezzelarab M, Hara H, van der Windt DJ, Wijkstrom M, Bottino R, et al. Clinical xenotransplantation: the next medical revolution? Lancet. 2012 Feb 18;379(9816):672–83. doi: 10.1016/S0140-6736(11)61091-X. [DOI] [PubMed] [Google Scholar]
- 5.Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005 Jan;11(1):32–4. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 6.Cozzi E, Bhatti F, Schmoeckel M, Chavez G, Smith KG, Zaidi A, et al. Long-term survival of nonhuman primates receiving life-supporting transgenic porcine kidney xenografts. Transplantation. 2000 Jul 15;70(1):15–21. [PubMed] [Google Scholar]
- 7.Griesemer AD, Hirakata A, Shimizu A, Moran S, Tena A, Iwaki H, et al. Results of gal-knockout porcine thymokidney xenografts. Am J Transplant. 2009 Dec;9(12):2669–78. doi: 10.1111/j.1600-6143.2009.02849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldan N, Rigotti P, Calabrese F, Cadrobbi R, Dedja A, Iacopetti I, et al. Ureteral stenosis in HDAF pig-to-primate renal xenotransplantation: a phenomenon related to immunological events? Am J Transplant. 2004 Apr;4(4):475–81. doi: 10.1111/j.1600-6143.2004.00407.x. [DOI] [PubMed] [Google Scholar]
- 9.Gollackner B, Knosalla C, Houser S, Mauiyyedi S, Buhler L, Kawai T, et al. Pig kidney transplantation in baboons treated intravenously with a bovine serum albumin-Galalpha1-3Gal conjugate. Xenotransplantation. 2003 Nov;10(6):606–14. doi: 10.1034/j.1399-3089.2003.00065.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen G, Qian H, Starzl T, Sun H, Garcia B, Wang X, et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat Med. 2005 Dec;11(12):1295–8. doi: 10.1038/nm1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen G, Sun H, Yang H, Kubelik D, Garcia B, Luo Y, et al. The role of anti-non-Gal antibodies in the development of acute humoral xenograft rejection of hDAF transgenic porcine kidneys in baboons receiving anti-Gal antibody neutralization therapy. Transplantation. 2006 Jan 27;81(2):273–83. doi: 10.1097/01.tp.0000188138.53502.de. [DOI] [PubMed] [Google Scholar]
- 12.Ibrahim Z, Busch J, Awwad M, Wagner R, Wells K, Cooper DK. Selected physiologic compatibilities and incompatibilities between human and porcine organ systems. Xenotransplantation. 2006 Nov;13(6):488–99. doi: 10.1111/j.1399-3089.2006.00346.x. [DOI] [PubMed] [Google Scholar]
- 13.Soin B, Smith KG, Zaidi A, Cozzi E, Bradley JR, Ostlie DJ, et al. Physiological aspects of pig-to-primate renal xenotransplantation. Kidney Int. 2001 Oct;60(4):1592–7. doi: 10.1046/j.1523-1755.2001.00973.x. [DOI] [PubMed] [Google Scholar]
- 14.Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem. 1988 Nov 25;263(33):17755–62. [PubMed] [Google Scholar]
- 15.Macher BA, Galili U. The Galalpha1,3Galbeta1,4GlcNAc-R (alpha-Gal) epitope: a carbohydrate of unique evolution and clinical relevance. Biochim Biophys Acta. 2008 Feb;1780(2):75–88. doi: 10.1016/j.bbagen.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spiezia L, Bertini D, Boldrin M, Radu C, Bulato C, Gavasso S, et al. Reference values for thromboelastometry (ROTEM(R)) in cynomolgus monkeys (Macaca fascicularis) Thromb Res. 2010 Oct;126(4):e294–7. doi: 10.1016/j.thromres.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 17.Miwa Y, Kobayashi T, Nagasaka T, Liu D, Yu M, Yokoyama I, et al. Are N-glycolylneuraminic acid (Hanganutziu-Deicher) antigens important in pig-to-human xenotransplantation? Xenotransplantation. 2004 May;11(3):247–53. doi: 10.1111/j.1399-3089.2004.00126.x. [DOI] [PubMed] [Google Scholar]
- 18.Taylor RE, Gregg CJ, Padler-Karavani V, Ghaderi D, Yu H, Huang S, et al. Novel mechanism for the generation of human xeno-autoantibodies against the nonhuman sialic acid N-glycolylneuraminic acid. J Exp Med. 2010 Aug 2;207(8):1637–46. doi: 10.1084/jem.20100575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larsen RD, Rivera-Marrero CA, Ernst LK, Cummings RD, Lowe JB. Frameshift and nonsense mutations in a human genomic sequence homologous to a murine UDP-Gal:beta-D-Gal(1,4)-D-GlcNAc alpha(1,3)-galactosyltransferase cDNA. J Biol Chem. 1990 Apr 25;265(12):7055–61. [PubMed] [Google Scholar]
- 20.Varki A, Gagneux P. Multifarious roles of sialic acids in immunity. Ann N Y Acad Sci. 2012 Apr;1253:16–36. doi: 10.1111/j.1749-6632.2012.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galili U, Mandrell RE, Hamadeh RM, Shohet SB, Griffiss JM. Interaction between human natural anti-alpha-galactosyl immunoglobulin G and bacteria of the human flora. Infect Immun. 1988 Jul;56(7):1730–7. doi: 10.1128/iai.56.7.1730-1737.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parker W, Bruno D, Holzknecht ZE, Platt JL. Characterization and affinity isolation of xenoreactive human natural antibodies. J Immunol. 1994 Oct 15;153(8):3791–803. [PubMed] [Google Scholar]
- 23.Galili U, Rachmilewitz EA, Peleg A, Flechner I. A unique natural human IgG antibody with anti-alpha-galactosyl specificity. J Exp Med. 1984 Nov 1;160(5):1519–31. doi: 10.1084/jem.160.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimizu A, Yamada K. Pathology of renal xenograft rejection in pig to non-human primate transplantation. Clin Transplant. 2006;20(Suppl 15):46–52. doi: 10.1111/j.1399-0012.2006.00550.x. [DOI] [PubMed] [Google Scholar]
- 25.Cowan PJ, Aminian A, Barlow H, Brown AA, Chen CG, Fisicaro N, et al. Renal xenografts from triple-transgenic pigs are not hyperacutely rejected but cause coagulopathy in non-immunosuppressed baboons. Transplantation. 2000 Jun 27;69(12):2504–15. doi: 10.1097/00007890-200006270-00008. [DOI] [PubMed] [Google Scholar]
- 26.Xu Y, Lorf T, Sablinski T, Gianello P, Bailin M, Monroy R, et al. Removal of anti-porcine natural antibodies from human and nonhuman primate plasma in vitro and in vivo by a Galalpha1-3Galbeta1-4betaGlc-X immunoaffinity column. Transplantation. 1998 Jan 27;65(2):172–9. doi: 10.1097/00007890-199801270-00005. [DOI] [PubMed] [Google Scholar]
- 27.Cowan PJ, Salvaris EJ, Crikis S, Barlow H, Aminian A, Hawthorne WJ, et al. Gal KO pig renal xenografts are not hyperacutely rejected by non-immunosuppressed baboons. Am J Transplant. 2006;6(s2):1050. [Google Scholar]
- 28.Saethre M, Baumann BC, Fung M, Seebach JD, Mollnes TE. Characterization of natural human anti-non-gal antibodies and their effect on activation of porcine gal-deficient endothelial cells. Transplantation. 2007 Jul 27;84(2):244–50. doi: 10.1097/01.tp.0000268815.90675.d5. [DOI] [PubMed] [Google Scholar]
- 29.Baumann BC, Stussi G, Huggel K, Rieben R, Seebach JD. Reactivity of human natural antibodies to endothelial cells from Galalpha(1,3)Gal-deficient pigs. Transplantation. 2007 Jan 27;83(2):193–201. doi: 10.1097/01.tp.0000250478.00567.e5. [DOI] [PubMed] [Google Scholar]
- 30.Cowan PJ, Roussel JC, d’Apice AJ. The vascular and coagulation issues in xenotransplantation. Curr Opin Organ Transplant. 2009 Apr;14(2):161–7. doi: 10.1097/mot.0b013e3283279591. [DOI] [PubMed] [Google Scholar]
- 31.Artrip JH, Kwiatkowski P, Michler RE, Wang SF, Tugulea S, Ankersmit J, et al. Target cell susceptibility to lysis by human natural killer cells is augmented by alpha(1,3)-galactosyltransferase and reduced by alpha(1, 2)-fucosyltransferase. J Biol Chem. 1999 Apr 16;274(16):10717–22. doi: 10.1074/jbc.274.16.10717. [DOI] [PubMed] [Google Scholar]
- 32.Jin R, Greenwald A, Peterson MD, Waddell TK. Human monocytes recognize porcine endothelium via the interaction of galectin 3 and alpha GAL. J Immunol. 2006 Jul 15;177(2):1289–95. doi: 10.4049/jimmunol.177.2.1289. [DOI] [PubMed] [Google Scholar]
- 33.Matter-Reissmann UB, Forte P, Schneider MK, Filgueira L, Groscurth P, Seebach JD. Xenogeneic human NK cytotoxicity against porcine endothelial cells is perforin/granzyme B dependent and not inhibited by Bcl-2 overexpression. Xenotransplantation. 2002 Sep;9(5):325–37. doi: 10.1034/j.1399-3089.2002.01074.x. [DOI] [PubMed] [Google Scholar]
- 34.Li S, Waer M, Billiau AD. Xenotransplantation: role of natural immunity. Transpl Immunol. 2009 Jun;21(2):70–4. doi: 10.1016/j.trim.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan JA, Oettinger HF, Sachs DH, Edge AS. Analysis of polymorphism in porcine MHC class I genes: alterations in signals recognized by human cytotoxic lymphocytes. J Immunol. 1997 Sep 1;159(5):2318–26. [PubMed] [Google Scholar]
- 36.Subramanian S, Parthasarathy R, Sen S, Boder ET, Discher DE. Species- and cell type-specific interactions between CD47 and human SIRPalpha. Blood. 2006 Mar 15;107(6):2548–56. doi: 10.1182/blood-2005-04-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ide K, Wang H, Tahara H, Liu J, Wang X, Asahara T, et al. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc Natl Acad Sci U S A. 2007 Mar 20;104(12):5062–6. doi: 10.1073/pnas.0609661104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cowan PJ, Robson SC, d’Apice AJ. Controlling coagulation dysregulation in xenotransplantation. Curr Opin Organ Transplant. 2011 Apr;16(2):214–21. doi: 10.1097/MOT.0b013e3283446c65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimizu A, Yamada K, Yamamoto S, Lavelle JM, Barth RN, Robson SC, et al. Thrombotic microangiopathic glomerulopathy in human decay accelerating factor-transgenic swine-to-baboon kidney xenografts. J Am Soc Nephrol. 2005 Sep;16(9):2732–45. doi: 10.1681/ASN.2004121148. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu A, Yamada K, Robson SC, Sachs DH, Colvin RB. Pathologic characteristics of transplanted kidney xenografts. J Am Soc Nephrol. 2012 Feb;23(2):225–35. doi: 10.1681/ASN.2011040429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Houser SL, Kuwaki K, Knosalla C, Dor FJ, Gollackner B, Cheng J, et al. Thrombotic microangiopathy and graft arteriopathy in pig hearts following transplantation into baboons. Xenotransplantation. 2004 Sep;11(5):416–25. doi: 10.1111/j.1399-3089.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- 42.Ezzelarab M, Garcia B, Azimzadeh A, Sun H, Lin CC, Hara H, et al. The innate immune response and activation of coagulation in alpha1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 2009 Mar 27;87(6):805–12. doi: 10.1097/TP.0b013e318199c34f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ierino FL, Kozlowski T, Siegel JB, Shimizu A, Colvin RB, Banerjee PT, et al. Disseminated intravascular coagulation in association with the delayed rejection of pig-to-baboon renal xenografts. Transplantation. 1998 Dec 15;66(11):1439–50. doi: 10.1097/00007890-199812150-00006. [DOI] [PubMed] [Google Scholar]
- 44.Buhler L, Basker M, Alwayn IP, Goepfert C, Kitamura H, Kawai T, et al. Coagulation and thrombotic disorders associated with pig organ and hematopoietic cell transplantation in nonhuman primates. Transplantation. 2000 Nov 15;70(9):1323–31. doi: 10.1097/00007890-200011150-00010. [DOI] [PubMed] [Google Scholar]
- 45.Cozzi E, Simioni P, Boldrin M, Seveso M, Calabrese F, Baldan N, et al. Alterations in the coagulation profile in renal pig-to-monkey xenotransplantation. Am J Transplant. 2004 Mar;4(3):335–45. doi: 10.1046/j.1600-6143.2003.00349.x. [DOI] [PubMed] [Google Scholar]
- 46.Lin CC, Ezzelarab M, Shapiro R, Ekser B, Long C, Hara H, et al. Recipient tissue factor expression is associated with consumptive coagulopathy in pig-to-primate kidney xenotransplantation. Am J Transplant. 2010 Jul;10(7):1556–68. doi: 10.1111/j.1600-6143.2010.03147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saadi S, Platt JL. Transient perturbation of endothelial integrity induced by natural antibodies and complement. J Exp Med. 1995 Jan 1;181(1):21–31. doi: 10.1084/jem.181.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palmetshofer A, Robson SC, Bach FH. Tyrosine phosphorylation following lectin mediated endothelial cell stimulation. Xenotransplantation. 1998 Feb;5(1):61–6. doi: 10.1111/j.1399-3089.1998.tb00010.x. [DOI] [PubMed] [Google Scholar]
- 49.Palmetshofer A, Galili U, Dalmasso AP, Robson SC, Bach FH. Alpha-galactosyl epitope-mediated activation of porcine aortic endothelial cells: type II activation. Transplantation. 1998 Apr 15;65(7):971–8. doi: 10.1097/00007890-199804150-00018. [DOI] [PubMed] [Google Scholar]
- 50.Gollackner B, Goh SK, Qawi I, Buhler L, Knosalla C, Daniel S, et al. Acute vascular rejection of xenografts: roles of natural and elicited xenoreactive antibodies in activation of vascular endothelial cells and induction of procoagulant activity. Transplantation. 2004 Jun 15;77(11):1735–41. doi: 10.1097/01.tp.0000131167.21930.b8. [DOI] [PubMed] [Google Scholar]
- 51.Lin CC, Chen D, McVey JH, Cooper DK, Dorling A. Expression of tissue factor and initiation of clotting by human platelets and monocytes after incubation with porcine endothelial cells. Transplantation. 2008 Sep 15;86(5):702–9. doi: 10.1097/TP.0b013e31818410a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Osterud B, Olsen JO. Human platelets do not express tissue factor. Thromb Res. 2013 Apr 25; doi: 10.1016/j.thromres.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 53.Ghanekar A, Mendicino M, Liu H, He W, Liu M, Zhong R, et al. Endothelial induction of fgl2 contributes to thrombosis during acute vascular xenograft rejection. J Immunol. 2004 May 1;172(9):5693–701. doi: 10.4049/jimmunol.172.9.5693. [DOI] [PubMed] [Google Scholar]
- 54.Miwa Y, Yamamoto K, Onishi A, Iwamoto M, Yazaki S, Haneda M, et al. Potential value of human thrombomodulin and DAF expression for coagulation control in pig-to-human xenotransplantation. Xenotransplantation. 2010 Jan-Feb;17(1):26–37. doi: 10.1111/j.1399-3089.2009.00555.x. [DOI] [PubMed] [Google Scholar]
- 55.Esmon CT, Esmon NL. The link between vascular features and thrombosis. Annu Rev Physiol. 2011 Mar 17;73:503–14. doi: 10.1146/annurev-physiol-012110-142300. [DOI] [PubMed] [Google Scholar]
- 56.Bae JS, Yang L, Rezaie AR. Receptors of the protein C activation and activated protein C signaling pathways are colocalized in lipid rafts of endothelial cells. Proc Natl Acad Sci U S A. 2007 Feb 20;104(8):2867–72. doi: 10.1073/pnas.0611493104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roussel JC, Moran CJ, Salvaris EJ, Nandurkar HH, d’Apice AJ, Cowan PJ. Pig thrombomodulin binds human thrombin but is a poor cofactor for activation of human protein C and TAFI. Am J Transplant. 2008 Jun;8(6):1101–12. doi: 10.1111/j.1600-6143.2008.02210.x. [DOI] [PubMed] [Google Scholar]
- 58.Siegel JB, Grey ST, Lesnikoski BA, Kopp CW, Soares M, Schulte am Esch J, 2nd, et al. Xenogeneic endothelial cells activate human prothrombin. Transplantation. 1997 Sep 27;64(6):888–96. doi: 10.1097/00007890-199709270-00017. [DOI] [PubMed] [Google Scholar]
- 59.Lawson JH, Daniels LJ, Platt JL. The evaluation of thrombomodulin activity in porcine to human xenotransplantation. Transplant Proc. 1997 Feb-Mar;29(1-2):884–5. doi: 10.1016/s0041-1345(96)00192-3. [DOI] [PubMed] [Google Scholar]
- 60.Mazzucato M, De Marco L, Pradella P, Masotti A, Pareti FI. Porcine von Willebrand factor binding to human platelet GPIb induces transmembrane calcium influx. Thromb Haemost. 1996 Apr;75(4):655–60. [PubMed] [Google Scholar]
- 61.Schulte am Esch J, 2nd, Cruz MA, Siegel JB, Anrather J, Robson SC. Activation of human platelets by the membrane-expressed A1 domain of von Willebrand factor. Blood. 1997 Dec 1;90(11):4425–37. [PubMed] [Google Scholar]
- 62.Schulte Am Esch J, 2nd, Robson SC, Knoefel WT, Hosch SB, Rogiers X. O-linked glycosylation and functional incompatibility of porcine von Willebrand factor for human platelet GPIb receptors. Xenotransplantation. 2005 Jan;12(1):30–7. doi: 10.1111/j.1399-3089.2004.00187.x. [DOI] [PubMed] [Google Scholar]
- 63.Scalea J, Hanecamp I, Robson SC, Yamada K. T-cell-mediated immunological barriers to xenotransplantation. Xenotransplantation. 2012 Jan-Feb;19(1):23–30. doi: 10.1111/j.1399-3089.2011.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamada K, Sachs DH, DerSimonian H. Direct and indirect recognition of pig class II antigens by human T cells. Transplant Proc. 1995 Feb;27(1):258–9. [PubMed] [Google Scholar]
- 65.Galili U. Induced anti-non gal antibodies in human xenograft recipients. Transplantation. 2012 Jan 15;93(1):11–6. doi: 10.1097/TP.0b013e31823be870. [DOI] [PubMed] [Google Scholar]
- 66.Platt JL, Lin SS, McGregor CG. Acute vascular rejection. Xenotransplantation. 1998 Aug;5(3):169–75. doi: 10.1111/j.1399-3089.1998.tb00024.x. [DOI] [PubMed] [Google Scholar]
- 67.Dorling A. Are anti-endothelial cell antibodies a pre-requisite for the acute vascular rejection of xenografts? Xenotransplantation. 2003 Jan;10(1):16–23. doi: 10.1034/j.1399-3089.2003.01134.x. [DOI] [PubMed] [Google Scholar]
- 68.Bach FH, Winkler H, Ferran C, Hancock WW, Robson SC. Delayed xenograft rejection. Immunol Today. 1996 Aug;17(8):379–84. doi: 10.1016/0167-5699(96)10024-4. [DOI] [PubMed] [Google Scholar]
- 69.Shimizu A, Yamada K. Histopathology of xenografts in pig to non-human primate discordant xenotransplantation. Clin Transplant. 2010 Jul;24(Suppl 22):11–5. doi: 10.1111/j.1399-0012.2010.01270.x. [DOI] [PubMed] [Google Scholar]
- 70.Tseng YL, Moran K, Dor FJ, Sanderson TM, Li W, Lancos CJ, et al. Elicited antibodies in baboons exposed to tissues from alpha1,3-galactosyltransferase gene-knockout pigs. Transplantation. 2006 Apr 15;81(7):1058–62. doi: 10.1097/01.tp.0000197555.16093.98. [DOI] [PubMed] [Google Scholar]
- 71.Lam TT, Paniagua R, Shivaram G, Schuurman HJ, Borie DC, Morris RE. Anti-non-Gal porcine endothelial cell antibodies in acute humoral xenograft rejection of hDAF-transgenic porcine hearts in cynomolgus monkeys. Xenotransplantation. 2004 Nov;11(6):531–5. doi: 10.1111/j.1399-3089.2004.00175.x. [DOI] [PubMed] [Google Scholar]
- 72.Breimer ME. Gal/non-Gal antigens in pig tissues and human non-Gal antibodies in the GalT-KO era. Xenotransplantation. 2011 Jul-Aug;18(4):215–28. doi: 10.1111/j.1399-3089.2011.00644.x. [DOI] [PubMed] [Google Scholar]
- 73.Miyagawa S, Ueno T, Nagashima H, Takama Y, Fukuzawa M. Carbohydrate antigens. Curr Opin Organ Transplant. 2012 Apr;17(2):174–9. doi: 10.1097/MOT.0b013e3283508189. [DOI] [PubMed] [Google Scholar]
- 74.Yeh P, Ezzelarab M, Bovin N, Hara H, Long C, Tomiyama K, et al. Investigation of potential carbohydrate antigen targets for human and baboon antibodies. Xenotransplantation. 2010 May-Jun;17(3):197–206. doi: 10.1111/j.1399-3089.2010.00579.x. [DOI] [PubMed] [Google Scholar]
- 75.Byrne GW, Stalboerger PG, Davila E, Heppelmann CJ, Gazi MH, McGregor HC, et al. Proteomic identification of non-Gal antibody targets after pig-to-primate cardiac xenotransplantation. Xenotransplantation. 2008 Jul;15(4):268–76. doi: 10.1111/j.1399-3089.2008.00480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Burlak C, Wang ZY, Chihara RK, Lutz AJ, Wang Y, Estrada JL, et al. Identification of human preformed antibody targets in GTKO pigs. Xenotransplantation. 2012 Mar-Apr;19(2):92–101. doi: 10.1111/j.1399-3089.2012.00695.x. [DOI] [PubMed] [Google Scholar]
- 77.Byrne GW, Stalboerger PG, Du Z, Davis TR, McGregor CG. Identification of new carbohydrate and membrane protein antigens in cardiac xenotransplantation. Transplantation. 2011 Feb 15;91(3):287–92. doi: 10.1097/TP.0b013e318203c27d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fodor WL, Williams BL, Matis LA, Madri JA, Rollins SA, Knight JW, et al. Expression of a functional human complement inhibitor in a transgenic pig as a model for the prevention of xenogeneic hyperacute organ rejection. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):11153–7. doi: 10.1073/pnas.91.23.11153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cozzi E, Langford GA, Richards A, Elsome K, Lancaster R, Chen P, et al. Expression of human decay accelerating factor in transgenic pigs. Transplant Proc. 1994 Jun;26(3):1402–3. [PubMed] [Google Scholar]
- 80.McCurry KR, Kooyman DL, Alvarado CG, Cotterell AH, Martin MJ, Logan JS, et al. Human complement regulatory proteins protect swine-to-primate cardiac xenografts from humoral injury. Nat Med. 1995 May;1(5):423–7. doi: 10.1038/nm0595-423. [DOI] [PubMed] [Google Scholar]
- 81.Sachs DH, Galli C. Genetic manipulation in pigs. Curr Opin Organ Transplant. 2009 Apr;14(2):148–53. doi: 10.1097/mot.0b013e3283292549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hauschild J, Petersen B, Santiago Y, Queisser AL, Carnwath JW, Lucas-Hahn A, et al. Efficient generation of a biallelic knockout in pigs using zinc-finger nucleases. Proc Natl Acad Sci U S A. 2011 Jul 19;108(29):12013–7. doi: 10.1073/pnas.1106422108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carlson DF, Tan W, Lillico SG, Stverakova D, Proudfoot C, Christian M, et al. Efficient TALEN-mediated gene knockout in livestock. Proc Natl Acad Sci U S A. 2012 Oct 23;109(43):17382–7. doi: 10.1073/pnas.1211446109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fisicaro N, Londrigan SL, Brady JL, Salvaris E, Nottle MB, O’Connell PJ, et al. Versatile co-expression of graft-protective proteins using 2A-linked cassettes. Xenotransplantation. 2011 Mar-Apr;18(2):121–30. doi: 10.1111/j.1399-3089.2011.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sharma A, Okabe J, Birch P, McClellan SB, Martin MJ, Platt JL, et al. Reduction in the level of Gal(alpha1,3)Gal in transgenic mice and pigs by the expression of an alpha(1,2)fucosyltransferase. Proc Natl Acad Sci U S A. 1996 Jul 9;93(14):7190–5. doi: 10.1073/pnas.93.14.7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miyagawa S, Murakami H, Takahagi Y, Nakai R, Yamada M, Murase A, et al. Remodeling of the major pig xenoantigen by N-acetylglucosaminyltransferase III in transgenic pig. J Biol Chem. 2001 Oct 19;276(42):39310–9. doi: 10.1074/jbc.M104359200. [DOI] [PubMed] [Google Scholar]
- 87.Dai Y, Vaught TD, Boone J, Chen SH, Phelps CJ, Ball S, et al. Targeted disruption of the alpha1,3-galactosyltransferase gene in cloned pigs. Nat Biotechnol. 2002 Mar;20(3):251–5. doi: 10.1038/nbt0302-251. [DOI] [PubMed] [Google Scholar]
- 88.Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, Im GS, et al. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002 Feb 8;295(5557):1089–92. doi: 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- 89.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003 Jan 17;299(5605):411–4. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kolber-Simonds D, Lai L, Watt SR, Denaro M, Arn S, Augenstein ML, et al. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci U S A. 2004 May 11;101(19):7335–40. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nottle MB, Beebe LF, Harrison SJ, McIlfatrick SM, Ashman RJ, O’Connell PJ, et al. Production of homozygous alpha-1,3-galactosyltransferase knockout pigs by breeding and somatic cell nuclear transfer. Xenotransplantation. 2007 Jul;14(4):339–44. doi: 10.1111/j.1399-3089.2007.00417.x. [DOI] [PubMed] [Google Scholar]
- 92.Fujimura T, Takahagi Y, Shigehisa T, Nagashima H, Miyagawa S, Shirakura R, et al. Production of alpha 1,3-galactosyltransferase gene-deficient pigs by somatic cell nuclear transfer: a novel selection method for gal alpha 1,3-Gal antigen-deficient cells. Mol Reprod Dev. 2008 Sep;75(9):1372–8. doi: 10.1002/mrd.20890. [DOI] [PubMed] [Google Scholar]
- 93.McGregor CG, Ricci D, Miyagi N, Stalboerger PG, Du Z, Oehler EA, et al. Human CD55 expression blocks hyperacute rejection and restricts complement activation in Gal knockout cardiac xenografts. Transplantation. 2012 Apr 15;93(7):686–92. doi: 10.1097/TP.0b013e3182472850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lutz AJ, Li P, Estrada JL, Sidner RA, Chihara RK, Downey SM, et al. Double knockout pigs deficient in N-glycolylneuraminic acid and Galactose alpha-1,3-Galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation. 2013 Jan-Feb;20(1):27–35. doi: 10.1111/xen.12019. [DOI] [PubMed] [Google Scholar]
- 95.Cowan PJ, d’Apice AJ. Complement activation and coagulation in xenotransplantation. Immunol Cell Biol. 2009 Mar-Apr;87(3):203–8. doi: 10.1038/icb.2008.107. [DOI] [PubMed] [Google Scholar]
- 96.Morgan BP, Berg CW, Harris CL. “Homologous restriction” in complement lysis: roles of membrane complement regulators. Xenotransplantation. 2005 Jul;12(4):258–65. doi: 10.1111/j.1399-3089.2005.00237.x. [DOI] [PubMed] [Google Scholar]
- 97.Fisicaro N, Aminian A, Hinchliffe SJ, Morgan BP, Pearse MJ, D’Apice AJ, et al. The pig analogue of CD59 protects transgenic mouse hearts from injury by human complement. Transplantation. 2000 Sep 27;70(6):963–8. doi: 10.1097/00007890-200009270-00014. [DOI] [PubMed] [Google Scholar]
- 98.Cowan PJ, Aminian A, Barlow H, Brown AA, Dwyer K, Filshie RJ, et al. Protective effects of recombinant human antithrombin III in pig-to-primate renal xenotransplantation. Am J Transplant. 2002 Jul;2(6):520–5. doi: 10.1034/j.1600-6143.2002.20605.x. [DOI] [PubMed] [Google Scholar]
- 99.Loveland BE, Milland J, Kyriakou P, Thorley BR, Christiansen D, Lanteri MB, et al. Characterization of a CD46 transgenic pig and protection of transgenic kidneys against hyperacute rejection in non-immunosuppressed baboons. Xenotransplantation. 2004 Mar;11(2):171–83. doi: 10.1046/j.1399-3089.2003.00103.x. [DOI] [PubMed] [Google Scholar]
- 100.Menoret S, Plat M, Blancho G, Martinat-Botte F, Bernard P, Karam G, et al. Characterization of human CD55 and CD59 transgenic pigs and kidney xenotransplantation in the pig-to-baboon combination. Transplantation. 2004 May 15;77(9):1468–71. doi: 10.1097/01.tp.0000111758.35048.ea. [DOI] [PubMed] [Google Scholar]
- 101.Platt JL, Vercellotti GM, Lindman BJ, Oegema TR, Jr., Bach FH, Dalmasso AP. Release of heparan sulfate from endothelial cells. Implications for pathogenesis of hyperacute rejection. J Exp Med. 1990 Apr 1;171(4):1363–8. doi: 10.1084/jem.171.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Menschikowski M, Hagelgans A, Eisenhofer G, Siegert G. Regulation of endothelial protein C receptor shedding by cytokines is mediated through differential activation of MAP kinase signaling pathways. Exp Cell Res. 2009 Sep 10;315(15):2673–82. doi: 10.1016/j.yexcr.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 103.Robson SC, Kaczmarek E, Siegel JB, Candinas D, Koziak K, Millan M, et al. Loss of ATP diphosphohydrolase activity with endothelial cell activation. J Exp Med. 1997 Jan 6;185(1):153–63. doi: 10.1084/jem.185.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen D, Weber M, McVey JH, Kemball-Cook G, Tuddenham EG, Lechler RI, et al. Complete inhibition of acute humoral rejection using regulated expression of membrane-tethered anticoagulants on xenograft endothelium. Am J Transplant. 2004 Dec;4(12):1958–63. doi: 10.1111/j.1600-6143.2004.00625.x. [DOI] [PubMed] [Google Scholar]
- 105.Crikis S, Zhang XM, Dezfouli S, Dwyer KM, Murray-Segal LM, Salvaris E, et al. Anti-inflammatory and anticoagulant effects of transgenic expression of human thrombomodulin in mice. Am J Transplant. 2010 Feb;10(2):242–50. doi: 10.1111/j.1600-6143.2009.02939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Petersen B, Ramackers W, Tiede A, Lucas-Hahn A, Herrmann D, Barg-Kues B, et al. Pigs transgenic for human thrombomodulin have elevated production of activated protein C. Xenotransplantation. 2009 Nov-Dec;16(6):486–95. doi: 10.1111/j.1399-3089.2009.00537.x. [DOI] [PubMed] [Google Scholar]
- 107.Yazaki S, Iwamoto M, Onishi A, Miwa Y, Hashimoto M, Oishi T, et al. Production of cloned pigs expressing human thrombomodulin in endothelial cells. Xenotransplantation. 2012 Mar-Apr;19(2):82–91. doi: 10.1111/j.1399-3089.2012.00696.x. [DOI] [PubMed] [Google Scholar]
- 108.Lee HJ, Lee BC, Kim YH, Paik NW, Rho HM. Characterization of transgenic pigs that express human decay accelerating factor and cell membrane-tethered human tissue factor pathway inhibitor. Reprod Domest Anim. 2011 Apr;46(2):325–32. doi: 10.1111/j.1439-0531.2010.01670.x. [DOI] [PubMed] [Google Scholar]
- 109.Lin CC, Ezzelarab M, Hara H, Long C, Lin CW, Dorling A, et al. Atorvastatin or transgenic expression of TFPI inhibits coagulation initiated by anti-nonGal IgG binding to porcine aortic endothelial cells. J Thromb Haemost. 2010 Sep;8(9):2001–10. doi: 10.1111/j.1538-7836.2010.03950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kaczmarek E, Koziak K, Sevigny J, Siegel JB, Anrather J, Beaudoin AR, et al. Identification and characterization of CD39/vascular ATP diphosphohydrolase. J Biol Chem. 1996 Dec 20;271(51):33116–22. doi: 10.1074/jbc.271.51.33116. [DOI] [PubMed] [Google Scholar]
- 111.Dwyer KM, Robson SC, Nandurkar HH, Campbell DJ, Gock H, Murray-Segal LJ, et al. Thromboregulatory manifestations in human CD39 transgenic mice and the implications for thrombotic disease and transplantation. J Clin Invest. 2004 May;113(10):1440–6. doi: 10.1172/JCI19560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Crikis S, Lu B, Murray-Segal LM, Selan C, Robson SC, D’Apice AJ, et al. Transgenic overexpression of CD39 protects against renal ischemia-reperfusion and transplant vascular injury. Am J Transplant. 2010 Dec;10(12):2586–95. doi: 10.1111/j.1600-6143.2010.03257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huttinger ZM, Milks MW, Nickoli MS, Aurand WL, Long LC, Wheeler DG, et al. Ectonucleotide triphosphate diphosphohydrolase-1 (CD39) mediates resistance to occlusive arterial thrombus formation after vascular injury in mice. Am J Pathol. 2012 Jul;181(1):322–33. doi: 10.1016/j.ajpath.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cai M, Huttinger ZM, He H, Zhang W, Li F, Goodman LA, et al. Transgenic over expression of ectonucleotide triphosphate diphosphohydrolase-1 protects against murine myocardial ischemic injury. J Mol Cell Cardiol. 2011 Dec;51(6):927–35. doi: 10.1016/j.yjmcc.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wheeler DG, Joseph ME, Mahamud SD, Aurand WL, Mohler PJ, Pompili VJ, et al. Transgenic swine: expression of human CD39 protects against myocardial injury. J Mol Cell Cardiol. 2012 May;52(5):958–61. doi: 10.1016/j.yjmcc.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Le Bas-Bernardet S, Tillou X, Poirier N, Dilek N, Chatelais M, Devalliere J, et al. Xenotransplantation of Galactosyl-Transferase Knockout, CD55, CD59, CD39, and Fucosyl-Transferase Transgenic Pig Kidneys Into Baboons. Transplant Proc. 2011 Nov;43(9):3426–30. doi: 10.1016/j.transproceed.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 117.Ollinger R, Pratschke J. Role of heme oxygenase-1 in transplantation. Transpl Int. 2010 Nov;23(11):1071–81. doi: 10.1111/j.1432-2277.2010.01158.x. [DOI] [PubMed] [Google Scholar]
- 118.Yeom HJ, Koo OJ, Yang J, Cho B, Hwang JI, Park SJ, et al. Generation and characterization of human heme oxygenase-1 transgenic pigs. PLoS One. 2012;7(10):e46646. doi: 10.1371/journal.pone.0046646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Petersen B, Ramackers W, Lucas-Hahn A, Lemme E, Hassel P, Queisser AL, et al. Transgenic expression of human heme oxygenase-1 in pigs confers resistance against xenograft rejection during ex vivo perfusion of porcine kidneys. Xenotransplantation. 2011 Nov;18(6):355–68. doi: 10.1111/j.1399-3089.2011.00674.x. [DOI] [PubMed] [Google Scholar]
- 120.Oropeza M, Petersen B, Carnwath JW, Lucas-Hahn A, Lemme E, Hassel P, et al. Transgenic expression of the human A20 gene in cloned pigs provides protection against apoptotic and inflammatory stimuli. Xenotransplantation. 2009 Nov-Dec;16(6):522–34. doi: 10.1111/j.1399-3089.2009.00556.x. [DOI] [PubMed] [Google Scholar]
- 121.Cho B, Koo OJ, Hwang JI, Kim H, Lee EM, Hurh S, et al. Generation of soluble human tumor necrosis factor-alpha receptor 1-Fc transgenic pig. Transplantation. 2011 Jul 27;92(2):139–47. doi: 10.1097/TP.0b013e3182215e7e. [DOI] [PubMed] [Google Scholar]
- 122.Weiss EH, Lilienfeld BG, Muller S, Muller E, Herbach N, Kessler B, et al. HLA-E/human beta2-microglobulin transgenic pigs: protection against xenogeneic human anti-pig natural killer cell cytotoxicity. Transplantation. 2009 Jan 15;87(1):35–43. doi: 10.1097/TP.0b013e318191c784. [DOI] [PubMed] [Google Scholar]
- 123.Wang C, Wang H, Ide K, Wang Y, Van Rooijen N, Ohdan H, et al. Human CD47 expression permits survival of porcine cells in immunodeficient mice that express SIRPalpha capable of binding to human CD47. Cell Transplant. 2011;20(11-12):1915–20. doi: 10.3727/096368911X566253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kemter E, Lieke T, Kessler B, Kurome M, Wuensch A, Summerfield A, et al. Human TNF-related apoptosis-inducing ligand-expressing dendritic cells from transgenic pigs attenuate human xenogeneic T cell responses. Xenotransplantation. 2012 Jan-Feb;19(1):40–51. doi: 10.1111/j.1399-3089.2011.00688.x. [DOI] [PubMed] [Google Scholar]
- 125.Cooper DK, Ayares D. The immense potential of xenotransplantation in surgery. Int J Surg. 2011;9(2):122–9. doi: 10.1016/j.ijsu.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 126.Martin C, Plat M, Nerriere-Daguin V, Coulon F, Uzbekova S, Venturi E, et al. Transgenic expression of CTLA4-Ig by fetal pig neurons for xenotransplantation. Transgenic Res. 2005 Aug;14(4):373–84. doi: 10.1007/s11248-004-7268-4. [DOI] [PubMed] [Google Scholar]
- 127.Klymiuk N, van Buerck L, Bahr A, Offers M, Kessler B, Wuensch A, et al. Xenografted islet cell clusters from INSLEA29Y transgenic pigs rescue diabetes and prevent immune rejection in humanized mice. Diabetes. 2012 Jun;61(6):1527–32. doi: 10.2337/db11-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Phelps CJ, Ball SF, Vaught TD, Vance AM, Mendicino M, Monahan JA, et al. Production and characterization of transgenic pigs expressing porcine CTLA4-Ig. Xenotransplantation. 2009 Nov-Dec;16(6):477–85. doi: 10.1111/j.1399-3089.2009.00533.x. [DOI] [PubMed] [Google Scholar]
- 129.Nishimura H, Scalea J, Wang Z, Shimizu A, Moran S, Gillon B, et al. First experience with the use of a recombinant CD3 immunotoxin as induction therapy in pig-to-primate xenotransplantation: the effect of T-cell depletion on outcome. Transplantation. 2011 Sep 27;92(6):641–7. doi: 10.1097/TP.0b013e31822b92a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McGregor CG, Davies WR, Oi K, Teotia SS, Schirmer JM, Risdahl JM, et al. Cardiac xenotransplantation: recent preclinical progress with 3-month median survival. J Thorac Cardiovasc Surg. 2005 Sep;130(3):844–51. doi: 10.1016/j.jtcvs.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 131.Mohiuddin MM, Corcoran PC, Singh AK, Azimzadeh A, Hoyt RF, Jr., Thomas ML, et al. B-cell depletion extends the survival of GTKO.hCD46Tg pig heart xenografts in baboons for up to 8 months. Am J Transplant. 2012 Mar;12(3):763–71. doi: 10.1111/j.1600-6143.2011.03846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.van der Windt DJ, Smetanka C, Macedo C, He J, Lakomy R, Bottino R, et al. Investigation of lymphocyte depletion and repopulation using alemtuzumab (Campath-1H) in cynomolgus monkeys. Am J Transplant. 2010 Apr;10(4):773–83. doi: 10.1111/j.1600-6143.2010.03050.x. [DOI] [PubMed] [Google Scholar]
- 133.Kuwaki K, Tseng YL, Dor FJ, Shimizu A, Houser SL, Sanderson TM, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005 Jan;11(1):29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 134.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000 Feb;6(2):114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 135.Knosalla C, Gollackner B, Cooper DK. Anti-CD154 monoclonal antibody and thromboembolism revisted. Transplantation. 2002 Aug 15;74(3):416–7. doi: 10.1097/00007890-200208150-00024. [DOI] [PubMed] [Google Scholar]
- 136.Ezzelarab MB, Ekser B, Echeverri G, Hara H, Ezzelarab C, Long C, et al. Costimulation blockade in pig artery patch xenotransplantation - a simple model to monitor the adaptive immune response in nonhuman primates. Xenotransplantation. 2012 Jul-Aug;19(4):221–32. doi: 10.1111/j.1399-3089.2012.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Thompson P, Cardona K, Russell M, Badell IR, Shaffer V, Korbutt G, et al. CD40-specific costimulation blockade enhances neonatal porcine islet survival in nonhuman primates. Am J Transplant. 2011 May;11(5):947–57. doi: 10.1111/j.1600-6143.2011.03509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li Q, Deng SB, Xia S, Du JL, She Q. Impact of intensive statin use on the level of inflammation and platelet activation in stable angina after percutaneous coronary intervention: A clinical study. Med Clin (Barc) 2012 Nov 20; doi: 10.1016/j.medcli.2012.05.042. [DOI] [PubMed] [Google Scholar]
- 139.Ezzelarab M, Welchons D, Torres C, Hara H, Long C, Yeh P, et al. Atorvastatin down-regulates the primate cellular response to porcine aortic endothelial cells in vitro. Transplantation. 2008 Sep 15;86(5):733–7. doi: 10.1097/TP.0b013e3181821cad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kuwaki K, Knosalla C, Dor FJ, Gollackner B, Tseng YL, Houser S, et al. Suppression of natural and elicited antibodies in pig-to-baboon heart transplantation using a human anti-human CD154 mAb-based regimen. Am J Transplant. 2004 Mar;4(3):363–72. doi: 10.1111/j.1600-6143.2004.00353.x. [DOI] [PubMed] [Google Scholar]
- 141.Tseng YL, Kuwaki K, Dor FJ, Shimizu A, Houser S, Hisashi Y, et al. alpha1,3-Galactosyltransferase gene-knockout pig heart transplantation in baboons with survival approaching 6 months. Transplantation. 2005 Nov 27;80(10):1493–500. doi: 10.1097/01.tp.0000181397.41143.fa. [DOI] [PubMed] [Google Scholar]
- 142.Cozzi E, Simioni P, Boldrin M, Seveso M, Calabrese F, Baldan N, et al. Effects of long-term administration of high-dose recombinant human antithrombin in immunosuppressed primate recipients of porcine xenografts. Transplantation. 2005 Nov 27;80(10):1501–10. doi: 10.1097/01.tp.0000178377.55615.8b. [DOI] [PubMed] [Google Scholar]
- 143.Simioni P, Boldrin M, Gavasso S, Seveso M, Radu C, Bulato C, et al. Effects of long-term administration of recombinant human protein C in xenografted primates. Transplantation. 2011 Jan 27;91(2):161–8. doi: 10.1097/TP.0b013e318200ba0e. [DOI] [PubMed] [Google Scholar]
- 144.Schmelzle M, Cowan PJ, Robson SC. Which anti-platelet therapies might be beneficial in xenotransplantation? Xenotransplantation. 2011 Mar-Apr;18(2):79–87. doi: 10.1111/j.1399-3089.2011.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008 Jan 24;358(4):353–61. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tseng YL, Dor FJ, Kuwaki K, Ryan D, Wood J, Denaro M, et al. Bone marrow transplantation from alpha1,3-galactosyltransferase gene-knockout pigs in baboons. Xenotransplantation. 2004 Jul;11(4):361–70. doi: 10.1111/j.1399-3089.2004.00151.x. [DOI] [PubMed] [Google Scholar]
- 147.Yang YG. CD47 in xenograft rejection and tolerance induction. Xenotransplantation. 2010 Jul-Aug;17(4):267–73. doi: 10.1111/j.1399-3089.2010.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang C, Wang H, Ide K, Wang Y, Van Rooijen N, Ohdan H, et al. Human CD47 expression permits survival of porcine cells in immunodeficient mice that express SIRPalpha capable of binding to human CD47. Cell Transplant. 2011 Apr 29; doi: 10.3727/096368911X566253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fu Y, Yi S, Wu J, Jimenez E, Simond D, Hawthorne WJ, et al. In vitro suppression of xenoimmune-mediated macrophage activation by human CD4+CD25+ regulatory T cells. Transplantation. 2008 Sep 27;86(6):865–74. doi: 10.1097/TP.0b013e31818530fd. [DOI] [PubMed] [Google Scholar]
- 150.Porter CM, Horvath-Arcidiacono JA, Singh AK, Horvath KA, Bloom ET, Mohiuddin MM. Characterization and expansion of baboon CD4+CD25+ Treg cells for potential use in a non-human primate xenotransplantation model. Xenotransplantation. 2007 Jul;14(4):298–308. doi: 10.1111/j.1399-3089.2007.00416.x. [DOI] [PubMed] [Google Scholar]
- 151.Wu J, Yi S, Ouyang L, Jimenez E, Simond D, Wang W, et al. In vitro expanded human CD4+CD25+ regulatory T cells are potent suppressors of T-cell-mediated xenogeneic responses. Transplantation. 2008 Jun 27;85(12):1841–8. doi: 10.1097/TP.0b013e3181734793. [DOI] [PubMed] [Google Scholar]
- 152.Sun L, Yi S, O’Connell PJ. Foxp3 regulates human natural CD4+CD25+ regulatory T-cell-mediated suppression of xenogeneic response. Xenotransplantation. 2010 Mar-Apr;17(2):121–30. doi: 10.1111/j.1399-3089.2010.00571.x. [DOI] [PubMed] [Google Scholar]
- 153.Veriter S, Gianello P, Igarashi Y, Beaurin G, Ghyselinck A, Aouassar N, et al. Improvement of Subcutaneous Bioartificial Pancreas Vascularization and Function by Co-Encapsulation of Pig Islets and Mesenchymal Stem Cells in Primates. Cell Transplant. 2013 Feb 4; doi: 10.3727/096368913X663550. [DOI] [PubMed] [Google Scholar]
- 154.Muller YD, Golshayan D, Ehirchiou D, Wekerle T, Seebach JD, Buhler LH. T regulatory cells in xenotransplantation. Xenotransplantation. 2009 May-Jun;16(3):121–8. doi: 10.1111/j.1399-3089.2009.00531.x. [DOI] [PubMed] [Google Scholar]
- 155.Muller YD, Ehirchiou D, Golshayan D, Buhler LH, Seebach JD. Potential of T-regulatory cells to protect xenografts. Curr Opin Organ Transplant. 2012 Apr;17(2):155–61. doi: 10.1097/MOT.0b013e3283508e17. [DOI] [PubMed] [Google Scholar]
- 156.Li J, Ezzelarab MB, Cooper DK. Do mesenchymal stem cells function across species barriers? Relevance for xenotransplantation. Xenotransplantation. 2012 Sep-Oct;19(5):273–85. doi: 10.1111/xen.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ezzelarab M, Ezzelarab C, Wilhite T, Kumar G, Hara H, Ayares D, et al. Genetically-modified pig mesenchymal stromal cells: xenoantigenicity and effect on human T-cell xenoresponses. Xenotransplantation. 2011 May-Jun;18(3):183–95. doi: 10.1111/j.1399-3089.2011.00635.x. [DOI] [PubMed] [Google Scholar]
- 158.Kumar G, Hara H, Long C, Shaikh H, Ayares D, Cooper DK, et al. Adipose-derived mesenchymal stromal cells from genetically modified pigs: immunogenicity and immune modulatory properties. Cytotherapy. 2012 Apr;14(4):494–504. doi: 10.3109/14653249.2011.651529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Onions D, Cooper DK, Alexander TJ, Brown C, Claassen E, Foweraker JE, et al. An approach to the control of disease transmission in pig-to-human xenotransplantation. Xenotransplantation. 2000 May;7(2):143–55. doi: 10.1034/j.1399-3089.2000.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.First WHO Global Consultation on Regulatory Requirements for Xenotransplantation Clinical Trials: Changsha, China, 19-21 November 2008. The Changsha Communique. Xenotransplantation. 2009 Mar-Apr;16(2):61–3. doi: 10.1111/j.1399-3089.2009.00520.x. [DOI] [PubMed] [Google Scholar]
- 161.Fishman JA, Scobie L, Takeuchi Y. Xenotransplantation-associated infectious risk: a WHO consultation. Xenotransplantation. 2012 Mar-Apr;19(2):72–81. doi: 10.1111/j.1399-3089.2012.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Millard AL, Mueller NJ. Can human viruses infect porcine xenografts? Xenotransplantation. 2010 Jan-Feb;17(1):6–10. doi: 10.1111/j.1399-3089.2009.00566.x. [DOI] [PubMed] [Google Scholar]
- 163.Wong BS, Yamada K, Okumi M, Weiner J, O’Malley PE, Tseng YL, et al. Allosensitization does not increase the risk of xenoreactivity to alpha1,3-galactosyltransferase gene-knockout miniature swine in patients on transplantation waiting lists. Transplantation. 2006 Aug 15;82(3):314–9. doi: 10.1097/01.tp.0000228907.12073.0b. [DOI] [PubMed] [Google Scholar]
- 164.Baertschiger RM, Dor FJ, Prabharasuth D, Kuwaki K, Cooper DK. Absence of humoral and cellular alloreactivity in baboons sensitized to pig antigens. Xenotransplantation. 2004 Jan;11(1):27–32. doi: 10.1111/j.1399-3089.2004.00075.x. [DOI] [PubMed] [Google Scholar]