Abstract
Management of coronary artery disease (CAD) has evolved over the past decade, but there are few prospective studies evaluating long-term outcomes in a real-world setting of evolving technical approaches and secondary prevention. The aim of this study was to determine how the mortality and morbidity of coronary artery disease has changed in patients who have undergone percutaneous coronary intervention (PCI), in the setting of co-morbidities and evolving management. The National Heart, Lung, and Blood Institute Dynamic Registry was a cohort study of patients undergoing PCI at various time points. Cohorts were enrolled in 1999 (cohort 2, n=2105), 2004 (cohort 4, n=2112), and 2006 (cohort 5, n= 2176), and each was followed out to 5 years. Primary outcomes were death, myocardial infarction (MI), coronary artery bypass grafting (CABG), repeat PCI, and repeat revascularization. Secondary outcomes were PCI for new obstructive lesions at 5 years, 5-year rate of death and MI stratified by the severity of coronary artery and co-morbid disease. Over time, patients were more likely to have multiple co-morbidities and more severe CAD. Despite greater disease severity, there was no significant difference in death (16.5% vs. 17.6%, adjusted hazard ratio (HR) 0.89 (0.74–1.08)), MI (11.0% vs. 10.6%, adjusted HR 0.87 (0.70–1.08)), or repeat PCI (20.4% vs. 22.2%, adjusted HR 0.98 (0.85–1.17)) at 5-year follow-up, but there was a significant decline inCABG (9.1% vs. 4.3%, adjusted HR 0.44 (0.32–0.59)). Patients with 5 co-morbidities had a 40–60% death rate at 5 years. There was a modestly high rate of repeat PCI for new lesions, indicating a potential failure of secondary prevention for this population in the face of increasing co-morbidity. Overall 5-year rates of death, MI, repeat PCI, and repeat PCI for new lesions did not change significantly in the context of increased co-morbidities and complex disease.
Keywords: coronary artery disease, percutaneous coronary intervention, outcomes
Introduction
Over the past 2 decades, there has been improved care of the cardiac patient through modification of cardiac risk factors, pharmacology, application of novel interventional approaches and education. The mortality rate of coronary artery disease (CAD) and the incidence of ST-elevation myocardial infarction have declined.1 However, there are data from survey-based studies indicating poor penetrance of best practice guidelines into clinical medicine. In this study, we sought to determine how long-term (5-year) mortality and morbidity from coronary artery disease in patients treated with percutaneous coronary intervention (PCI) changed over time, in the setting of evolving technology and medical management for patients. The National Heart, Lung, and Blood Institute Dynamic Registry, is unique in its long-term follow-up of unselected patients post-PCI, thereby allowing for evaluation of the impact of secondary prevention in patients with treated, obstructive CAD. In the Dynamic Registry, consecutive patients undergoing PCI were enrolled at various time intervals, reflecting periods of technological advancement plus changes in interventional and pharmacologic therapy.2
Methods
The Dynamic Registry was a prospective, multicenter study of patients undergoing PCI from 27 academic hospitals in the United States, Canada, and the Czech Republic.2 In this study, we analyzed results of 3 cohorts each followed out to 5-years (cohort 2: enrolled in 1999, n=2105 patients; cohort 4: enrolled in 2004, n=2112 patients; and cohort 5: enrolled in 2006, n=2176 patients). Each cohort was enriched with women and minorities, with race self-reported.
Demographic, angiographic, and procedural data were collected at baseline. Vital status and cardiac-related events post-discharge were collected annually via direct contact by trained study coordinators. Self-reported events were confirmed by reviewing hospital records. Patients provided written informed consent and the institutional review board of each participating site approved the data collection. Five-year follow-up rates were 70% for cohort 2, 85% for cohort 4, and 88% for cohort 5.
For cohorts 2 and 4, the Registry collected the Coronary Artery Surgery Study (CASS) segment number for repeat PCIs for all 5 years. After the first year of follow-up in cohort 5, the Registry stopped collecting segment numbers and instead asked for the treated vessels. PCI for new lesions was strictly defined as new obstructive stenoses requiring PCI, outside of the CASS segment stented at the time of enrollment in the Dynamic Registry or outside of the originally stented coronary artery/graft. This strict definition of additional lesions requiring PCI was applied to avoid any confounding by PCI for in-stent restenosis.
Primary outcomes of the study were deaths from any cause, myocardial infarction (MI), coronary artery bypass grafting (CABG), and any non-staged repeat PCI. Mortality data was gathered from the study coordinators at each site who performed a search of the National Death Index for patients who could not be located. MI was defined by evidence of at least 1 of the 2 following criteria: (1) Evolutionary ST-segment elevation, development of new Q-waves in 2 or more contiguous leads on an electrocardiogram, new or presumably new left bundle branch block pattern, (2) Biochemical evidence of myocardial necrosis manifested as: (a) creatine kinase (CK) CK-MB ≥ 3 times the upper limit of normal, (b) total CK ≥ 3 times the upper limit of normal, or (c) troponin level above the upper limit of normal. Repeat PCI was defined as any repeat PCI in the follow up period, either for in-stent restenosis or for additional obstructive lesions. Repeat revascularization was defined as repeat PCI and/or CABG during the follow up period.
Secondary outcomes included the number of co-morbidities, angiographic severity of CAD, and presence of new stenoses requiring PCI in segments and/or vessels not stented at the time of enrollment PCI. The number of co-morbidities was defined as one or any combination of the following; type II diabetes, hypertension, hypercholesterolemia, renal insufficiency (history or presence of renal failure diagnosed and treated with medication, low protein diet or dialysis), or peripheral arterial disease (history of claudication, rest pain, amputation, vascular reconstruction, bypass surgery or angioplasty to the extremities, or aortic aneurysm). Severe CAD was defined as one or any combination of the following; type C lesion, calcified lesion, total occlusion, left main stenosis ≥50%, or triple vessel disease.
Temporal trends in patient, procedural and lesion characteristics were assessed across the cohorts using the Cochran-Armitage test for dichotomous variables and the Jonckeheere-Terpsta test for continuous and nominal/ordinal variables. Clinical event rates at 5 years for death, combined death/MI, repeat PCI, CABG, and repeat revascularization with PCI or CABG were calculated by the Kaplan-Meier approach and compared by means of the log-rank test for trend. Patients who did not experience the outcome of interest were censored at the last known date of contact or at 5 years, if contact extended slightly beyond their anniversary date. Cox proportional hazards regression methods were used to estimate 5-year hazard ratios of clinical events in relation to study cohort, with the time to event beginning at the time of procedure. Demographic and clinical variables were initially screened for univariate association with clinical outcomes of interest at P ≤ 0.20. Individual variables identified were then assessed in a forward stepwise manner with the use of a P-value criterion of ≤ 0.05. In instances when the variables for recruitment cohort did not “step” into a model, they were included in the model after entry of all other significant variables. The list of co-variates is listed in Appendix I. The proportionality assumption was assessed for all Cox proportional-hazards models graphically and statistically and the hazard ratio represents the average risk during the 5 years after the index procedure. All analyses were performed with SAS version 9.2 (SAS Institute, Inc.) and STATA version 7.0 (StataCorp LP).
Results
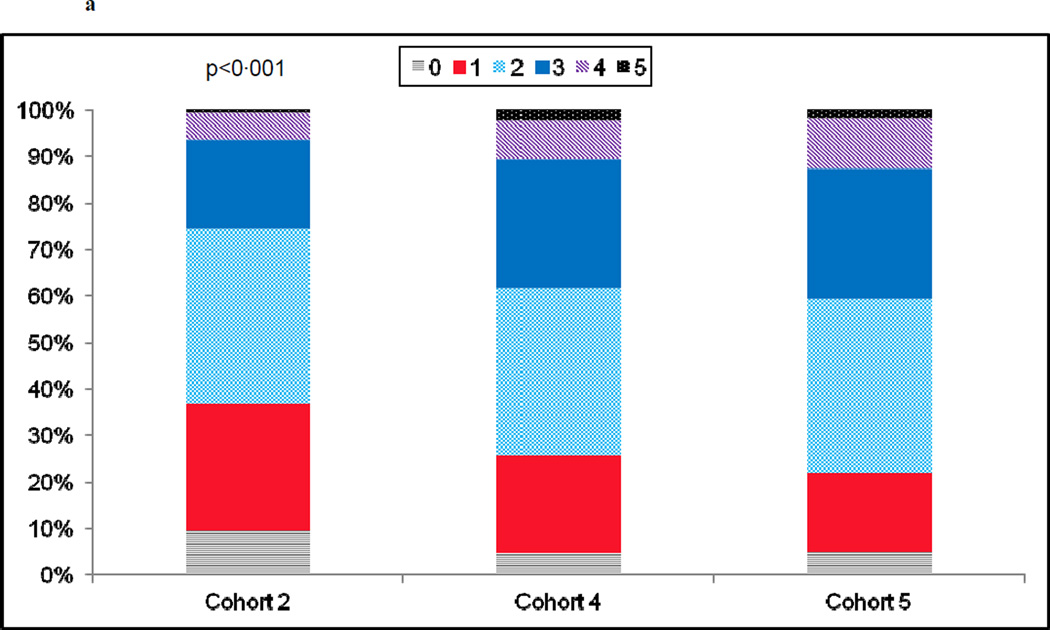
Patients enrolled in the 3 cohorts were of a similar age (mean of 63–64 years old), and by design, approximately one third female, and at least 20% non-white (Table I). Cohorts 4 and 5 presented with a higher prevalence of co-morbid conditions than their cohort 2 counterpart with higher body mass indices, worsening renal insufficiency, higher rates of type II diabetes mellitus, hypertension, hypercholesterolemia, and a high prevalence of current and former smoking (Table 1). The number of patients presenting for PCI with multiple co-morbidities increased over time, while the percentage presenting with zero or a single co-morbidity decreased (Fig. 1a). Patients in cohort 5 were over twice as likely as those in cohort 2 to rely on public assistance for their insurance coverage (Table 1).
Table 1.
Patient and lesion characteristics
| Characteristics Years of Follow-up Number at Follow-up/Enrolled |
Cohort 2 1999–2004 (n=1666/2105) |
Cohort 4 2004–09 (n=1965/2112) |
Cohort 5 2006–11 (n=2077/2176) |
p Value (test for trend) |
|---|---|---|---|---|
| Mean Age (Years) | 63.2 | 63.7 | 64.0 | 0.05 |
| Age over 65 | 45.8% | 46.4% | 46.8% | 0.58 |
| Women | 36.9% | 32.8% | 32.8% | 0.01 |
| Non-white | 20.6% | 22.9% | 24.7% | 0.003 |
| Mean Body Mass Index (kg/m2) | 28.8 | 29.2 | 29.6 | <0.001 |
| Prior Percutaneous Procedure | 25.3% | 29.7% | 34.3% | <0.001 |
| Reason for Revascularization | ||||
| Unstable Angina Pectoris | 44.9% | 34.7% | ||
| Acute Myocardial Infarction (MI) | 27.6% | 28.4% | ||
| Prior Coronary Artery Bypass Grafting (CABG) | 16.2% | 19.3% | ||
| Prior Myocardial Infarction | 30.6% | 25.0% | ||
| Renal disease | 4.6% | 8.6% | ||
| Hypertension | 63.6% | 77.2% | ||
| Hypercholesterolemia | 62.5% | 75.0% | ||
| Diabetes Mellitus | 27.0% | 33.5% | ||
| Current Treatment for Diabetes | ||||
| Diet | 15.0% | 13.3% | ||
| Oral Medication | 50.0% | 54.6% | ||
| Insulin | 35.0% | 32.2% | ||
| Congestive Heart Failure | 9.1% | 8.9% | ||
| Ejection Fraction, mean, median | 53.1, 55% | 51.7, 55% | ||
| Smoker | ||||
| Never | 32.3% | 37.4% | ||
| Current | 27.4% | 22.6% | ||
| Former | 40.3% | 40.0% | ||
| Discharge Medications | ||||
| Aspirin | 93.9% | 96.6% | 98.2% | <0.001 |
| Beta blocker | 69.9% | 80.8% | 82.3% | <0.001 |
| Cholesterol modifying agent | 61.2% | 86.9% | 88.2% | <0.001 |
| Angiotensin converting enzyme inhibitor | 36.5% | 55.2% | 50.9% | <0.001 |
| Thienopyridine | 92.1% | 97.9% | 98.3% | <0.001 |
| Method of Payment | ||||
| Medicare | 44.2% | 34.4% | 38.2% | |
| Public | 7.8% | 15.5% | 17.9% | 0.62 |
| Private | 42.7% | 45.9% | 41.0% | |
| Self | 5.4% | 4.2% | 2% | |
| Coronary Artery Disease Location | ||||
| Left Anterior Descending, Left Circumflex and Left Main | 2.2% | 4.2% | 4.8% | <0.001 |
| Triple vessel disease | 24.1% | 30.6% | 28.6% | 0.05 |
| Left Main with a stenosis ≥50 percent | 3.6% | 6.0% | 6.9% | <0.001 |
| Any total occlusion | 34.2% | 38.4% | 37.5% | 0.05 |
| Mean number significant lesions | 2.8 | 3.1 | 3.1 | <0.001 |
| Drug-eluting stent implanted | 0% | 74.3% | 90.0% | <0.001 |
| Technically amenable to complete revascularization with CABG | 86.5% | 77.0% | 72.7% | <0.001 |
| Technically amenable to complete revascularization with Percutaneous | 82.3% | 87.4% | 90.8% | <0.001 |
| Coronary Intervention (PCI) | ||||
| Procedural success | 98.0% | 98.5% | 99.1% | 0.004 |
| Attempted Lesions | (n=2417) | (n=2627) | (n=2863) | p Value |
| Calcified | 24.4% | 25.2% | 32.0% | <0.001 |
| ACC/AHA Classification16 | ||||
| A | 12.9% | 10.8% | 11.7% | |
| B1 | 32.2% | 36.3% | 29.8% | <0.001 |
| B2 | 38.3% | 32.8% | 29.5% | |
| C | 16.5% | 20.2% | 28.9% | |
| Drug type | ||||
| Paclitaxel | 0% | 23.9% | 31.9% | <0.001 |
| Limus drugs (everolimus, zotarolimus, sirolimus) | 0% | 45.4% | 54.6% | <0.001 |
| PCI success | 96.4% | 97.9% | 98.5% | <0.001 |
Fig. 1.
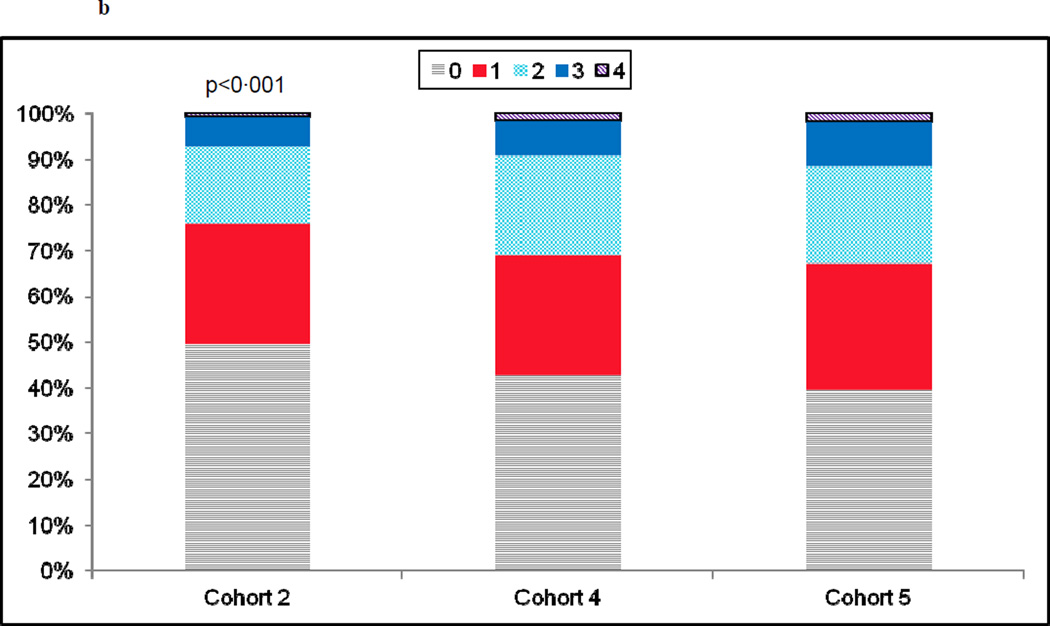
a) Prevalence in the number of co-morbid conditions by recruitment cohort. Co-morbidities were defined as: smoking, diabetes, renal insufficiency, peripheral arterial disease, hypertension, and/or hypercholesterolemia. b)Prevalence in the number of severe coronary artery disease characteristics by recruitment cohort. Severe coronary artery disease was defined as: calcified stenoses, total occlusions, left main lesions ≥ 50%, type C lesions and/or triple vessel disease.
Over time there was a rise in lesion complexity, with treated lesions in cohorts 4 and 5 more likely to be calcified, totally occluded, located in the left main with at least a 50% stenosis, and classified as type C (Table 1). Approximately two-thirds of each cohort had 1 and 2 vessel disease. Triple vessel disease, however, was more common in cohorts 4 and 5 as compared to cohort 2. The indication for PCI was acute coronary syndrome in approximately two-thirds of each cohort (Table I). There was a significant increase in the percentage of patients having 1 or more of the angiographic characteristics described above (Fig. 1b).
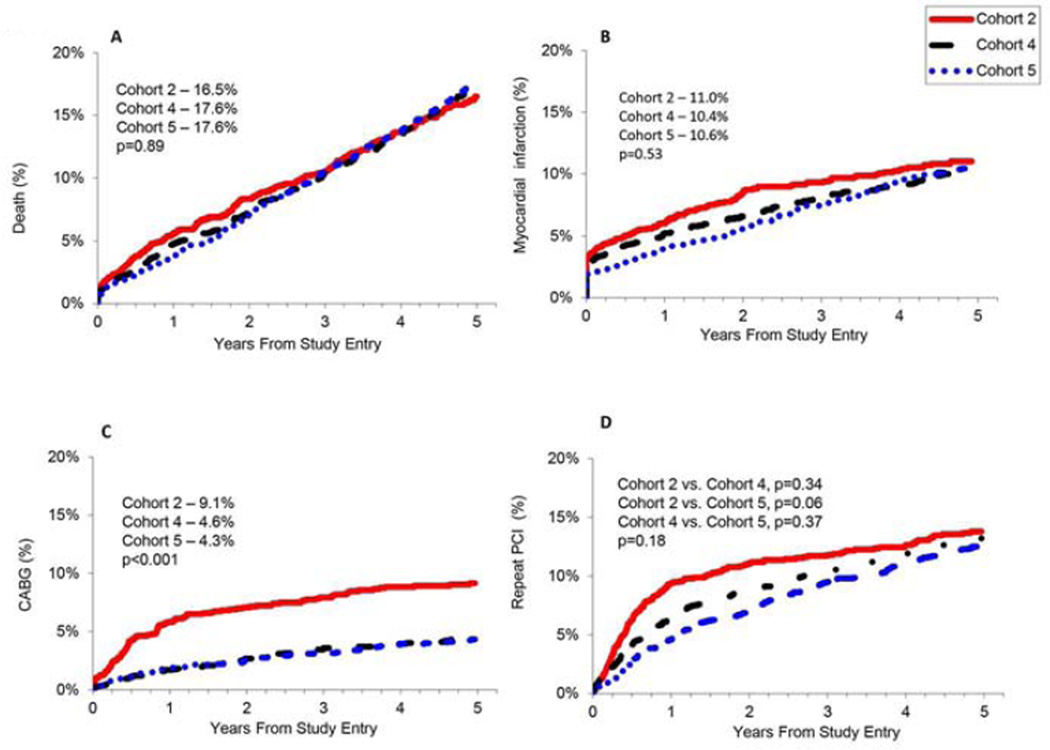
Despite increasing patient and lesion complexity, the 5-year death rate was unchanged between 2004 and 2011, even when adjusted for age, body mass index, race/ethnicity, co-morbid conditions, reason and acuity for initial revascularization including cardiogenic shock, and lesion characteristics (Table 2). The incidence of death, MI and repeat PCI were similar at 5 years across the 3 recruitment cohorts (Table 2, Fig. 2). For cohort 2, a large proportion of repeat PCI were performed in the vessel stented at the time of enrollment and likely due to in-stent restenosis (Fig. 2d) For cohorts 4 and 5, more repeat PCI was performed for stenoses outside of the index vessel, likely due to disease progression (Fig. 3). The incidence of CABG declined significantly over time (Table 2, Fig. 2c) and the overall need for repeat revascularization decreased with time, driven by the decline in CABG. Both emergency and planned bypass grafting decreased over time as operators reported a higher rate of successful percutaneous revascularization (Table 1).
Table 2.
Unadjusted event rates, adjusted hazard ratios and 95% Confidence Intervals for death, myocardial infarction (MI), coronary artery bypass grafting (CABG), repeat percutaneous coronary intervention (PCI), and repeat revascularization (variables used to adjust the model appear in Appendix I)
| Event Rates | Cohort 4 vs. Cohort 2 | Cohort 5 vs. Cohort 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cohort 2 |
Cohort 4 |
Cohort 5 |
p- Value |
HR | 95% CI |
p- Value |
HR | 95% CI | p-Value | |
| Death | 16.5% | 17.6% | 17.6% | 0.66 | 0.97 | 0.80–1.17 | 0.74 | 0.89 | 0.74–1.08 | 0.25 |
| MI | 11.0% | 10.4% | 10.6% | 0.23 | 0.89 | 0.71–1.10 | 0.28 | 0.87 | 0.70–1.08 | 0.21 |
| Death or MI | 24.1% | 25.2% | 24.4% | 0.52 | 0.91 | 0.79–1.06 | 0.23 | 0.83 | 0.72–0.96 | 0.01 |
| CABG | 9.1% | 4.6% | 4.3% | <0.001 | 0.47 | 0.35–0.64 | <0.001 | 0.44 | 0.32–0.59 | <0.001 |
| Repeat PCI | 20.4% | 21.9% | 22.2% | 0.76 | 1.00 | 0.85–1.17 | 0.99 | 0.98 | 0.85–1.17 | 0.79 |
| Repeat Revascularization | 26.8% | 24.9% | 25.0% | 0.02 | 0.85 | 0.73–1.00 | 0.045 | 0.83 | 0.71–0.97 | 0.02 |
Fig. 2. Five-year unadjusted rates of a) death, b) myocardial infarction (MI), c) coronary artery bypass grafting (CABG), and d) repeat percutaneous coronary intervention (PCI).
Fig. 2d showsin-stent restenosis and repeat PCI to additional segments within the same coronary artery/graft stented at the time of enrollment into the Dynamic Registry.
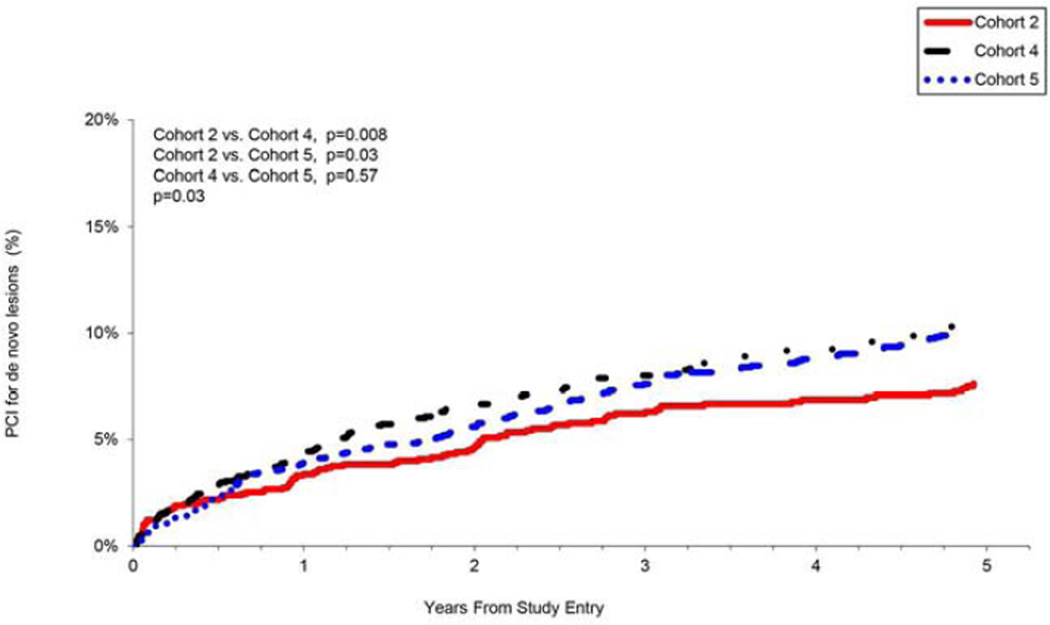
Fig 3. Repeat percutaneous coronary intervention (PCI) for de novo lesions.
Repeat PCI for new obstructive lesions outside of the segment stented at the time of enrollment in either the same vessel or other vessels, by recruitment cohort.
Within the first 2 years following the index-PCI, the rate of repeat PCI procedures was lower in cohorts 4 and 5 as compared to cohort 2 (Fig. 2d), but by year 5, the rates of repeat PCI across the cohorts were similar, possibly driven by the development of additional obstructive lesions among patients with more severe co-morbid disease (Fig. 3). More patients underwent additional PCI for de novo obstructive plaque and/or progression of atherosclerosis by 5years in cohorts 4 and 5 (Fig. 3).
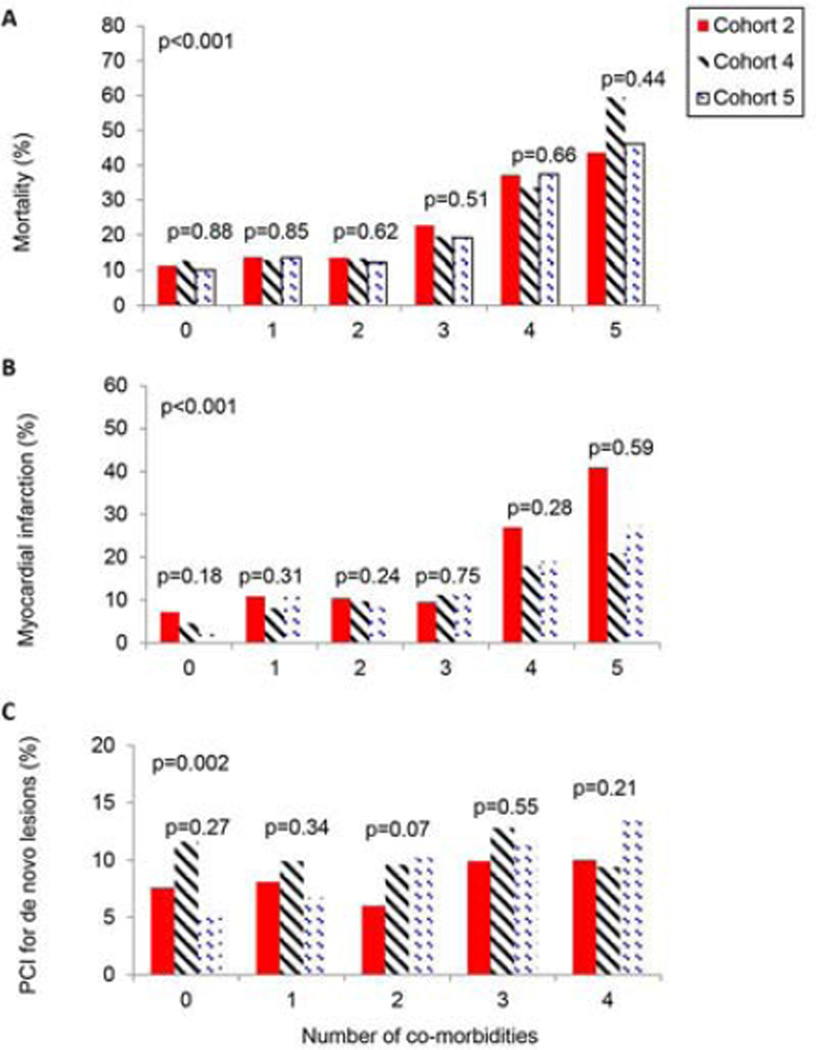
Patients presenting with more than 3 co-morbidities had an exponential increase in their unadjusted 5-year death rates (Fig. 4a). Furthermore, there was no improvement in mortality over time. PCI for additional obstructive lesions was associated with increasing number of co-morbidities over time (Fig. 4c).
Fig 4. Cumulative five-year incidence of a) death, b) myocardial infarction (MI), and c) percutaneous coronary intervention (PCI) of de novo lesions by number of co-morbidities and recruitment cohort.
The number of co-morbidities was defined as any combination of one or more of the following; smoking, diabetes mellitus type II, renal insufficiency, peripheral arterial disease, hypertension, and/or hypercholesterolemia.
Discussion
This study evaluated the effect of current interventional and pharmacologic therapies in unselected patients treated for obstructive CAD, in a real world setting. Despite the increased use of proven secondary prevention measures over the past decade,3 death and myocardial infarction rates were not reduced, indicating that such secondary prevention measures appear to be limited by the increase of co-morbidities during this period. Over time, a greater percentage of patients were, in fact, discharged on aspirin, statins, beta-blockers, angiotensin converting enzyme (ACE) inhibitors, and dual antiplatelet therapy in accordance with guidelines. Procedural outcomes improved, resulting in less in-stent restenosis and CABG, while overall 5-year mortality and MI rates did not change.
There are several explanations for the lack of significant declines in mortality, MI, or repeat PCI across the cohorts. One is that secondary prevention strategies may have merely obviated the increased risk of co-morbidities observed in this and other studies.4–6 A second explanation is that patients in this study were not maintained on guideline-recommended therapy or did not achieve targeted goals. We only had data for medications at discharge, but not at 5-year follow-up and it is possible that some patients or providers were non-adherent to instructions and therapy. Patients often need 4 medications to adhere to evidence based recommendations, but might not be prescribed all due to co-morbidities or perceived intolerance.7–9 Patients may lack understanding of their illnesses if not given information appropriate to their level of health literacy, which would reduce adherence to medications.10 Adherence can be challenging, particularly as the number of daily doses increase.11 A significant percentage of patients prematurely discontinue aspirin and statins even after an MI, despite obtaining free medications,12 and some patients have side effects from statins and ACE inhibitors that preclude their use. In addition, few eligible patients are referred for cardiac rehabilitation which could impact recurrent events.13 This study did not assess whether or not patients attended rehabilitation.
While there appeared to be a decline in revascularization in the first 1 to 2 years of follow-up, predominantly in-stent restenosis this difference was no longer present by 5 years, denoting an increase in revascularization for obstructive lesion progression. This would appear to be related to the increase in co-morbities (Table I), which supports previous studies showing that insulin-requiring diabetes and previous PCI were predictive of future ischemic events in PROSPECT, while multi-vessel disease was strongly predictive in the Dynamic Registry study.14,15 CABG rates declined significantly over time reflecting a steep decline in emergent CABG for acute vessel closure. Operators may have also been more confident in treating challenging anatomy with drug-eluting stents. Lastly, there may have been increased referrals of patients for intervention rather than surgery due to a rise in their operative risk as the number of co-morbidities increased.
The strengths of this study include its prospective, all-comers design, large sample size, geographic diversity of sites, inclusion of a significant number of women and minorities, high retention and reflection of routine clinical practice over a decade. There are, however, limitations. First, patient enrollment was limited to select academic hospital centers, which might limit generalizability. Second, there may have been some referral bias in the patients referred for cardiac catheterization over time since they may have been more or less complex than those who were referred for surgery or medical management alone. Thirdly, a lack of core-lab analysis and independent adjudication might have affected event reporting. Lastly, PCI for additional lesions was an outcome that we defined post-hoc. We used the strictest definition in order to minimize confounding, but may have underreported the true incidence of intervention for new lesions. This study adds new important information about the long-term outcomes of patients with coronary artery disease who present for PCI and suggests a potential failure to prevent additional obstructive lesions in an era of evolving medical therapy for CAD.
Acknowledgements
Grant support: This work was support by the National Institutes of Health.
Appendix 1
List of variables adjusted for in the final model
Death rates were adjusted for: age, body mass index (BMI), race/ethnicity, congestive heart failure (CHF), diabetes mellitus type II (DM-II), hypertension (HTN), hypercholesterolemia (HL), reason for index revascularization, cardiogenic shock, number of significant coronary lesions, total occlusion found at index, urgency of index procedure, thienopyridine use, chronic kidney disease (CKD), peripheral arterial disease (PAD), chronic obstructive pulmonary disease (COPD) and cancer.
MI rates were adjusted for: age, sex, BMI, prior MI, prior PCI, DM-II, reason for revascularization, number of significant coronary lesions, number of lesions attempted during index procedure, class C lesion at index procedure, graft treated at index procedure, CKD, PAD, and cerebrovascular disease (CVD), defined as loss of neurological function caused by an ischemic event with residual symptoms at least 72 hours after onset, loss of neurological function caused by ischemia with symptoms at least 24 hours after onset but with complete return of function within 72 hours, loss of neurological function caused by ischemia that was abrupt in onset but with complete return of function within 24 hours or complete mental unresponsiveness and no evidence of psychological or physiologically appropriate responses to stimulation).
The rate of death or MI was adjusted for: age, BMI, race, CHF, prior MI, DM-II, HTN, HL, reason for index revascularization, cardiogenic shock, treated calcified lesion at index procedure, number of significant coronary lesions, number of lesions attempted during index procedure, total occlusion found at index, CVD, CKD and PAD. CABG rates were adjusted for: age, prior CABG, DM-II, HL, vessel disease, reason for index revascularization, cardiogenic shock, attempted lesion that receives collaterals at index procedure, ulcerated lesion at index procedure, class-C lesion at index procedure, number of significant lesions and thienopyridine use.
Repeat PCI rates were adjusted for: age, prior PCI, prior CABG, DM-II, HTN, vessel disease, reason for index revascularization, found total occlusion at index procedure, attempted lesion that receives collaterals at index procedure, number of significant lesions, number of lesions treated during index procedure, and graft lesion treated during index procedure.
Rates of repeat revascularization were adjusted for: age, prior PCI, prior MI, DM-II, HTN, vessel disease, reason for index revascularization, found total occlusion at index procedure, attempted lesion that receives collaterals at index procedure, number of significant lesions, number of lesions treated during index procedure, graft lesion treated during index procedure and thienopyridine use.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data presented, in part, at the American College of Cardiology 2012 Scientific Sessions in Chicago, Illinois, March 24, 2012.
Conflict of interest: The authors have no conflicts of interest to report.
References
- 1.Fang J, Alderman MH, Keenan NL, Ayala C. Acute myocardial infarction hospitalization in the United States, 1979 to 2005. Am J Med. 2010;123:259–266. doi: 10.1016/j.amjmed.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 2.Venkitachalam L, Kip KE, Mulukutla SR, Selzer F, Laskey W, Slater J, Cohen HA, Wilensky RL, Williams DO, Marroquin OC, Sutton-Tyrrell K, Bunker CH, Kelsey SF. Temporal trends in patient-reported angina at 1 year after percutaneous coronary revascularization in the stent era: a report from the National Heart, Lung, and Blood Institute-sponsored 1997–2006 dynamic registry. Circ Cardiovasc Qual Outcomes. 2009;2:607–615. doi: 10.1161/CIRCOUTCOMES.109.869131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith SC, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW, Lloyd-Jones DM, Minissian M, Mosca L, Peterson ED, Sacco RL, Spertus J, Stein JH, Taubert KA. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients With Coronary and Other Atherosclerotic Vascular Disease: 2011 Update: A Guideline From the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458–2473. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 4.Shay CM, Ning H, Allen NB, Carnethon MR, Chiuve SE, Greenlund KJ, Daviglus ML, Lloyd-Jones DM. Status of cardiovascular health in US adults: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2003–2008. Circulation. 2012;125:45–56. doi: 10.1161/CIRCULATIONAHA.111.035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, Gillespie C, Merritt R, Hu FB. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA. 2012;307:1273–1283. doi: 10.1001/jama.2012.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenlund KJ, Zheng ZJ, Keenan NL, Giles WH, Casper ML, Mensah GA, Croft JB. Trends in self-reported multiple cardiovascular disease risk factors among adults in the United States, 1991–1999. Arch Intern Med. 2004;164:181–188. doi: 10.1001/archinte.164.2.181. [DOI] [PubMed] [Google Scholar]
- 7.Lahoud R, Howe M, Krishnan SM, Zacharias S, Jackson EA. Effect of use of combination evidence-based medical therapy after acute coronary syndromes on long-term outcomes. Am J Cardiol. 2012;109:159–164. doi: 10.1016/j.amjcard.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 8.Kumar A, Fonarow GC, Eagle KA, Hirsch AT, Califf RM, Alberts MJ, Boden WE, Steg PG, Shao M, Bhatt DL, Cannon CP. Regional and Practice Variation in Adherence to Guideline Recommendations for Secondary and Primary Prevention Among Outpatients With Atherothrombosis or Risk Factors in the United States. Critical Pathways in Cardiology: A Journal of Evidence-Based Medicine. 2009;8:104–111. doi: 10.1097/HPC.0b013e3181b8395d. [DOI] [PubMed] [Google Scholar]
- 9.Newby LK. Long-Term Adherence to Evidence-Based Secondary Prevention Therapies in Coronary Artery Disease. Circulation. 2006;113:203–212. doi: 10.1161/CIRCULATIONAHA.105.505636. [DOI] [PubMed] [Google Scholar]
- 10.Gazmararian JA, Baker DW, Williams MV, Parker RM, Scott TL, Green DC, Fehrenbach SN, Ren J, Koplan JP. Health literacy among Medicare enrollees in a managed care organization. JAMA. 1999;281:545–551. doi: 10.1001/jama.281.6.545. [DOI] [PubMed] [Google Scholar]
- 11.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clinical therapeutics. 2001;23:1296–1310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 12.Choudhry NK, Avorn J, Glynn RJ, Antman EM, Schneeweiss S, Toscano M, Reisman L, Fernandes J, Spettell C, Lee JL, Levin R, Brennan T, Shrank WH. Full coverage for preventive medications after myocardial infarction. N Engl J Med. 2011;365:2088–2097. doi: 10.1056/NEJMsa1107913. [DOI] [PubMed] [Google Scholar]
- 13.Brown TM, Hernandez AF, Bittner V, Cannon CP, Ellrodt G, Liang L, Peterson ED, Piña IL, Safford MM, Fonarow GC. Predictors of cardiac rehabilitation referral in coronary artery disease patients. J Am Coll Cardiol. 2009;54:515–521. doi: 10.1016/j.jacc.2009.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, Templin B, White R, Zhang Z, Serruys PW PROSPECT Investigators. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 15.Glaser R, Selzer F, Faxon DP, Laskey WK, Cohen HA, Slater J, Detre KM, Wilensky RL. Clinical progression of incidental, asymptomatic lesions discovered during culprit vessel coronary intervention. Circulation. 2005;111:143–149. doi: 10.1161/01.CIR.0000150335.01285.12. [DOI] [PubMed] [Google Scholar]
- 16.Ryan TJ, Faxon DP, Gunnar RM, Kennedy JW, King SB, 3rd, Loop FD, Peterson KL, Reeves TJ, Williams DO, Winters WL, Jr, Fisch C, DeSanctis RW, Dodge HT, Reeves TJ, Weinberg SL. Guidelines for percutaneous transluminal coronary angioplasty. A report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Subcommittee on Percutaneous Transluminal Coronary Angioplasty) Circulation. 1988;78:486–502. doi: 10.1161/01.cir.78.2.486. [DOI] [PubMed] [Google Scholar]