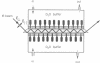
Abstract
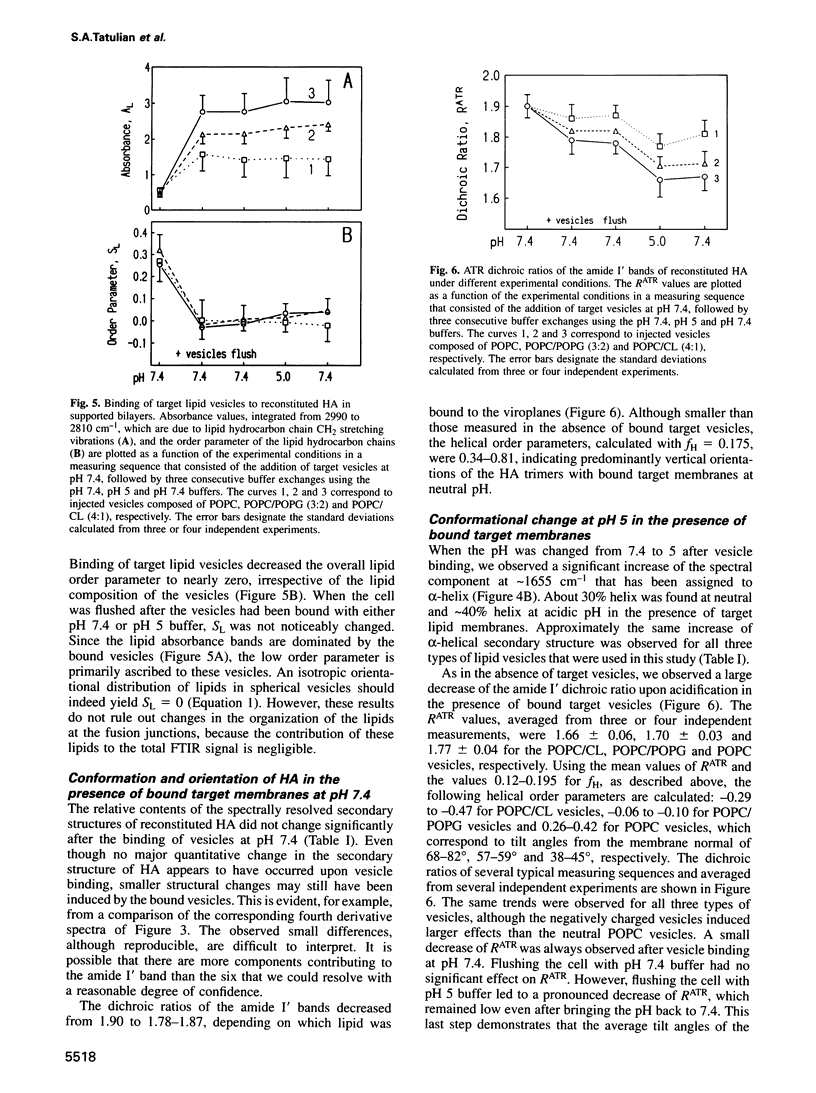
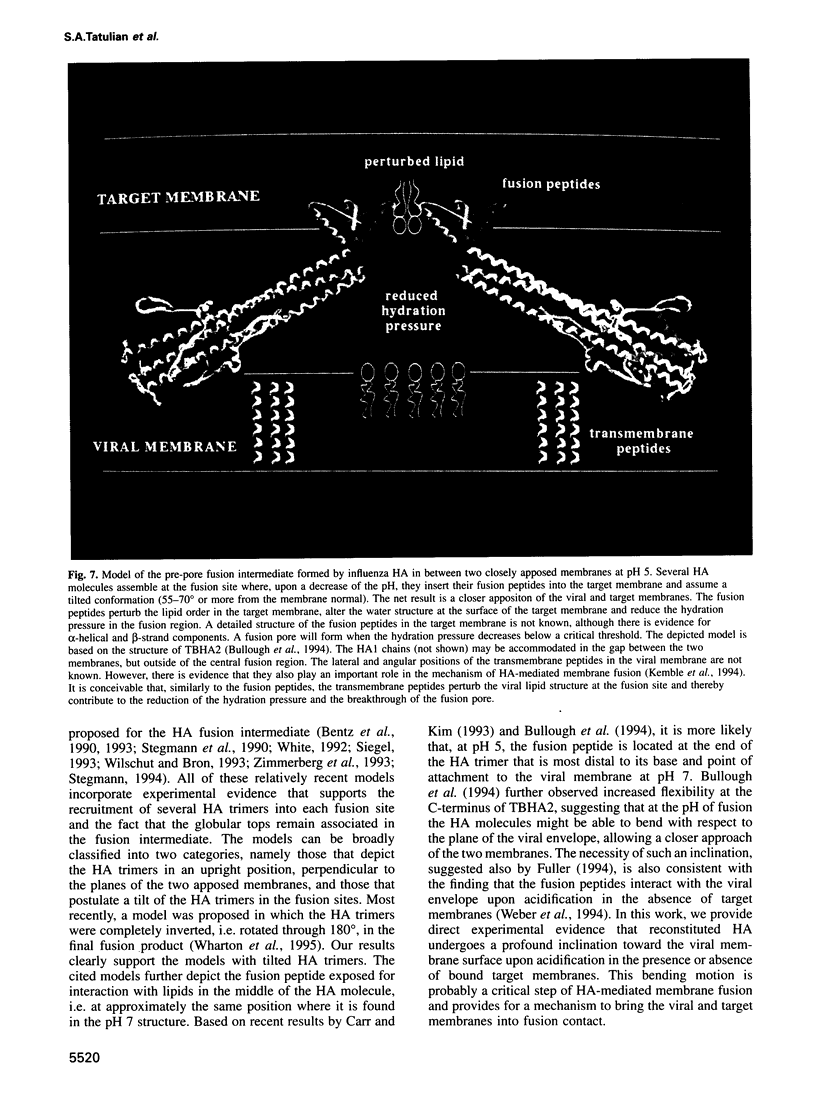
Fusion of influenza virus with target membranes is mediated by an acid-induced conformational change of the viral fusion protein hemagglutinin (HA) involving an extensive reorganization of the alpha-helices. A 'spring-loaded' displacement over at least 100 A provides a mechanism for the insertion of the fusion peptide into the target membrane, but does not explain how the two membranes are brought into fusion contact. Here we examine, by attenuated total reflection Fourier transform infrared spectroscopy, the secondary structure and orientation of HA reconstituted in planar membranes. At neutral pH, the orientation of the HA trimers in planar membranes is approximately perpendicular to the membrane. However, at the pH of fusion, the HA trimers are tilted 55-70 degrees from the membrane normal in the presence or absence of bound target membranes. In the absence of target membranes, the overall secondary structure of HA at the fusion pH is similar to that at neutral pH, but approximately 50-60 additional residues become alpha-helical upon the conformational change in the presence of bound target membranes. These results are discussed in terms of a structural model for the fusion intermediate of influenza HA.
Full text
PDF
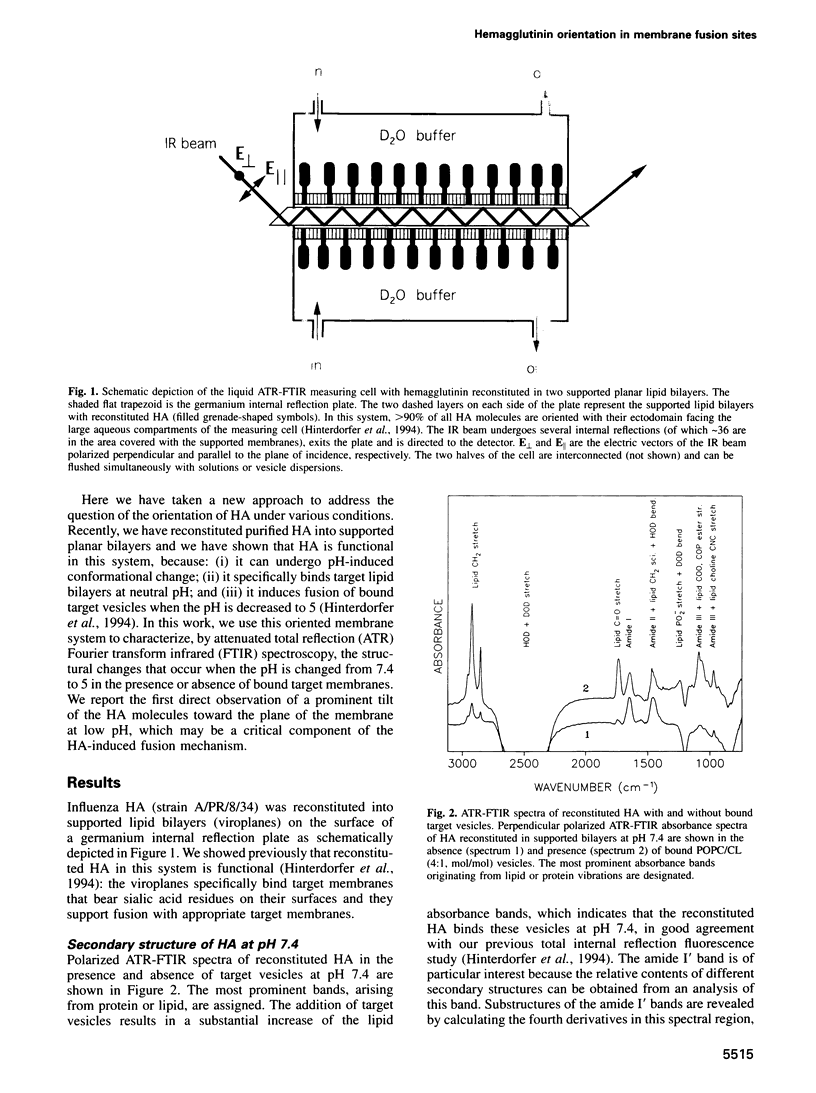
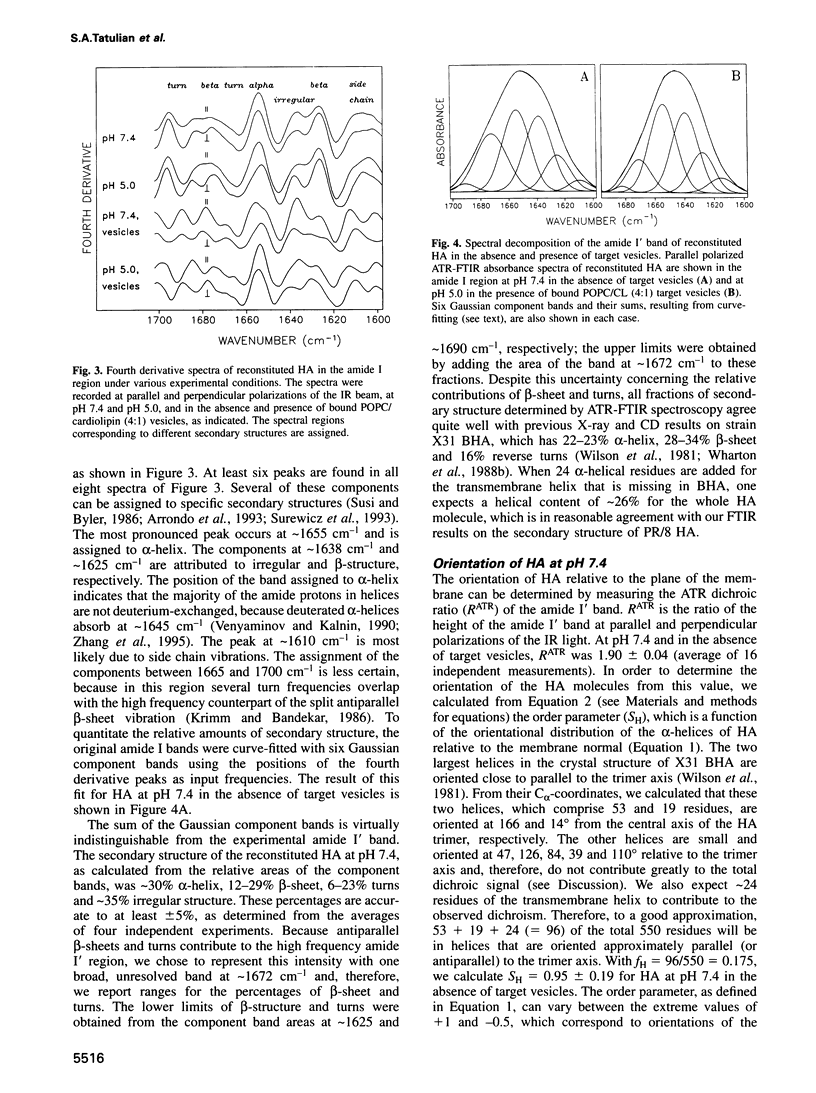
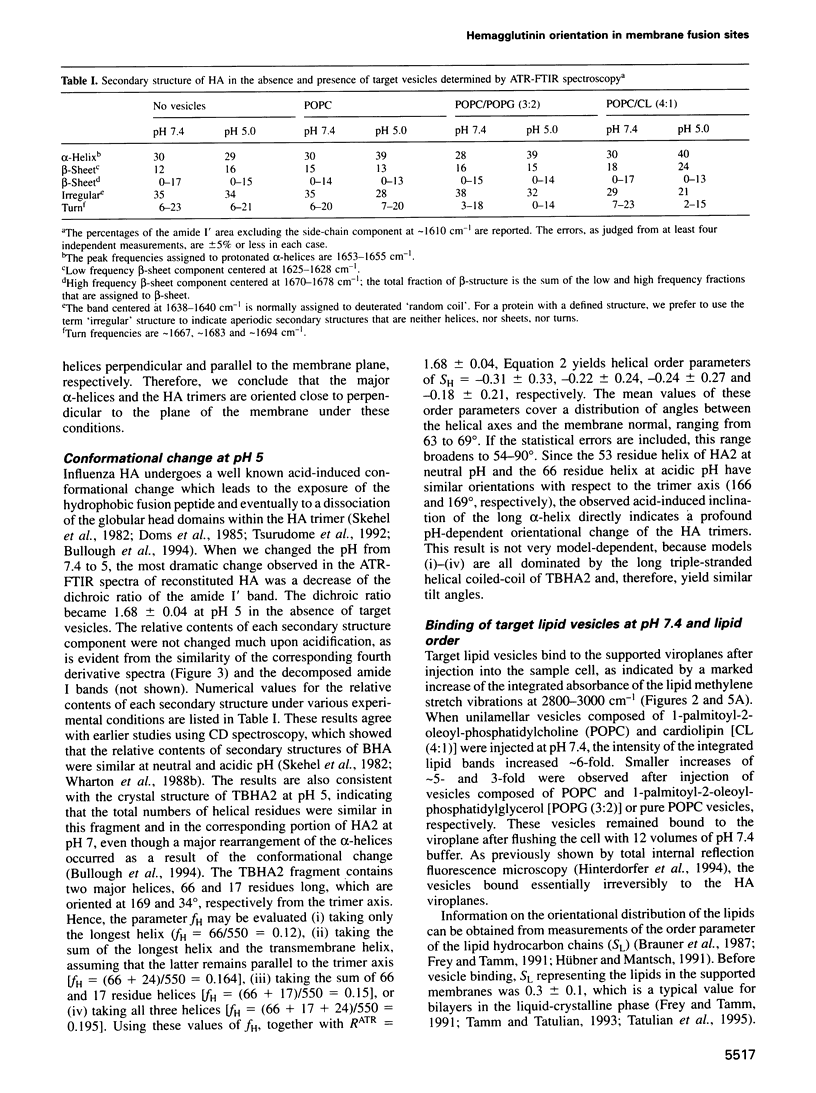




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrondo J. L., Muga A., Castresana J., Goñi F. M. Quantitative studies of the structure of proteins in solution by Fourier-transform infrared spectroscopy. Prog Biophys Mol Biol. 1993;59(1):23–56. doi: 10.1016/0079-6107(93)90006-6. [DOI] [PubMed] [Google Scholar]
- BRADBURY E. M., BROWN L., DOWNIE A. R., ELLIOTT A., FRASER R. D., HANBY W. E. The structure of the omegaform of poly-Beta-benzyl-L-aspartate. J Mol Biol. 1962 Aug;5:230–247. doi: 10.1016/s0022-2836(62)80086-2. [DOI] [PubMed] [Google Scholar]
- Bentz J., Ellens H., Alford D. An architecture for the fusion site of influenza hemagglutinin. FEBS Lett. 1990 Dec 10;276(1-2):1–5. doi: 10.1016/0014-5793(90)80492-2. [DOI] [PubMed] [Google Scholar]
- Brauner J. W., Mendelsohn R., Prendergast F. G. Attenuated total reflectance Fourier transform infrared studies of the interaction of melittin, two fragments of melittin, and delta-hemolysin with phosphatidylcholines. Biochemistry. 1987 Dec 15;26(25):8151–8158. doi: 10.1021/bi00399a020. [DOI] [PubMed] [Google Scholar]
- Brunner J. Testing topological models for the membrane penetration of the fusion peptide of influenza virus hemagglutinin. FEBS Lett. 1989 Nov 6;257(2):369–372. doi: 10.1016/0014-5793(89)81574-1. [DOI] [PubMed] [Google Scholar]
- Bullough P. A., Hughson F. M., Skehel J. J., Wiley D. C. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature. 1994 Sep 1;371(6492):37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- Carr C. M., Kim P. S. A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell. 1993 May 21;73(4):823–832. doi: 10.1016/0092-8674(93)90260-w. [DOI] [PubMed] [Google Scholar]
- Doms R. W., Helenius A., White J. Membrane fusion activity of the influenza virus hemagglutinin. The low pH-induced conformational change. J Biol Chem. 1985 Mar 10;260(5):2973–2981. [PubMed] [Google Scholar]
- Frey S., Tamm L. K. Orientation of melittin in phospholipid bilayers. A polarized attenuated total reflection infrared study. Biophys J. 1991 Oct;60(4):922–930. doi: 10.1016/S0006-3495(91)82126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S. Influenza haemagglutinin: illuminating fusion. Structure. 1994 Oct 15;2(10):903–906. doi: 10.1016/s0969-2126(94)00090-5. [DOI] [PubMed] [Google Scholar]
- Gallaher W. R., Segrest J. P., Hunter E. Are fusion peptides really "sided" insertional helices? Cell. 1992 Aug 21;70(4):531–532. doi: 10.1016/0092-8674(92)90423-a. [DOI] [PubMed] [Google Scholar]
- Harter C., James P., Bächi T., Semenza G., Brunner J. Hydrophobic binding of the ectodomain of influenza hemagglutinin to membranes occurs through the "fusion peptide". J Biol Chem. 1989 Apr 15;264(11):6459–6464. [PubMed] [Google Scholar]
- Hinterdorfer P., Baber G., Tamm L. K. Reconstitution of membrane fusion sites. A total internal reflection fluorescence microscopy study of influenza hemagglutinin-mediated membrane fusion. J Biol Chem. 1994 Aug 12;269(32):20360–20368. [PubMed] [Google Scholar]
- Hübner W., Mantsch H. H. Orientation of specifically 13C=O labeled phosphatidylcholine multilayers from polarized attenuated total reflection FT-IR spectroscopy. Biophys J. 1991 Jun;59(6):1261–1272. doi: 10.1016/S0006-3495(91)82341-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemble G. W., Danieli T., White J. M. Lipid-anchored influenza hemagglutinin promotes hemifusion, not complete fusion. Cell. 1994 Jan 28;76(2):383–391. doi: 10.1016/0092-8674(94)90344-1. [DOI] [PubMed] [Google Scholar]
- Krimm S., Bandekar J. Vibrational spectroscopy and conformation of peptides, polypeptides, and proteins. Adv Protein Chem. 1986;38:181–364. doi: 10.1016/s0065-3233(08)60528-8. [DOI] [PubMed] [Google Scholar]
- Lear J. D., DeGrado W. F. Membrane binding and conformational properties of peptides representing the NH2 terminus of influenza HA-2. J Biol Chem. 1987 May 15;262(14):6500–6505. [PubMed] [Google Scholar]
- Rafalski M., Ortiz A., Rockwell A., van Ginkel L. C., Lear J. D., DeGrado W. F., Wilschut J. Membrane fusion activity of the influenza virus hemagglutinin: interaction of HA2 N-terminal peptides with phospholipid vesicles. Biochemistry. 1991 Oct 22;30(42):10211–10220. doi: 10.1021/bi00106a020. [DOI] [PubMed] [Google Scholar]
- Ruigrok R. W., Martin S. R., Wharton S. A., Skehel J. J., Bayley P. M., Wiley D. C. Conformational changes in the hemagglutinin of influenza virus which accompany heat-induced fusion of virus with liposomes. Virology. 1986 Dec;155(2):484–497. doi: 10.1016/0042-6822(86)90210-2. [DOI] [PubMed] [Google Scholar]
- Skehel J. J., Bayley P. M., Brown E. B., Martin S. R., Waterfield M. D., White J. M., Wilson I. A., Wiley D. C. Changes in the conformation of influenza virus hemagglutinin at the pH optimum of virus-mediated membrane fusion. Proc Natl Acad Sci U S A. 1982 Feb;79(4):968–972. doi: 10.1073/pnas.79.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmann T., Delfino J. M., Richards F. M., Helenius A. The HA2 subunit of influenza hemagglutinin inserts into the target membrane prior to fusion. J Biol Chem. 1991 Sep 25;266(27):18404–18410. [PubMed] [Google Scholar]
- Stegmann T., Hoekstra D., Scherphof G., Wilschut J. Kinetics of pH-dependent fusion between influenza virus and liposomes. Biochemistry. 1985 Jun 18;24(13):3107–3113. doi: 10.1021/bi00334a006. [DOI] [PubMed] [Google Scholar]
- Stegmann T. Membrane fusion. Anchors aweigh. Curr Biol. 1994 Jun 1;4(6):551–554. doi: 10.1016/s0960-9822(00)00123-8. [DOI] [PubMed] [Google Scholar]
- Stegmann T., Nir S., Wilschut J. Membrane fusion activity of influenza virus. Effects of gangliosides and negatively charged phospholipids in target liposomes. Biochemistry. 1989 Feb 21;28(4):1698–1704. doi: 10.1021/bi00430a041. [DOI] [PubMed] [Google Scholar]
- Stegmann T., White J. M., Helenius A. Intermediates in influenza induced membrane fusion. EMBO J. 1990 Dec;9(13):4231–4241. doi: 10.1002/j.1460-2075.1990.tb07871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surewicz W. K., Mantsch H. H., Chapman D. Determination of protein secondary structure by Fourier transform infrared spectroscopy: a critical assessment. Biochemistry. 1993 Jan 19;32(2):389–394. doi: 10.1021/bi00053a001. [DOI] [PubMed] [Google Scholar]
- Susi H., Byler D. M. Resolution-enhanced Fourier transform infrared spectroscopy of enzymes. Methods Enzymol. 1986;130:290–311. doi: 10.1016/0076-6879(86)30015-6. [DOI] [PubMed] [Google Scholar]
- Tamm L. K., Tatulian S. A. Orientation of functional and nonfunctional PTS permease signal sequences in lipid bilayers. A polarized attenuated total reflection infrared study. Biochemistry. 1993 Aug 3;32(30):7720–7726. doi: 10.1021/bi00081a017. [DOI] [PubMed] [Google Scholar]
- Tatulian S. A., Jones L. R., Reddy L. G., Stokes D. L., Tamm L. K. Secondary structure and orientation of phospholamban reconstituted in supported bilayers from polarized attenuated total reflection FTIR spectroscopy. Biochemistry. 1995 Apr 4;34(13):4448–4456. doi: 10.1021/bi00013a038. [DOI] [PubMed] [Google Scholar]
- Tsurudome M., Glück R., Graf R., Falchetto R., Schaller U., Brunner J. Lipid interactions of the hemagglutinin HA2 NH2-terminal segment during influenza virus-induced membrane fusion. J Biol Chem. 1992 Oct 5;267(28):20225–20232. [PubMed] [Google Scholar]
- Venyaminov SYu, Kalnin N. N. Quantitative IR spectrophotometry of peptide compounds in water (H2O) solutions. II. Amide absorption bands of polypeptides and fibrous proteins in alpha-, beta-, and random coil conformations. Biopolymers. 1990;30(13-14):1259–1271. doi: 10.1002/bip.360301310. [DOI] [PubMed] [Google Scholar]
- Weber T., Paesold G., Galli C., Mischler R., Semenza G., Brunner J. Evidence for H(+)-induced insertion of influenza hemagglutinin HA2 N-terminal segment into viral membrane. J Biol Chem. 1994 Jul 15;269(28):18353–18358. [PubMed] [Google Scholar]
- Wharton S. A., Calder L. J., Ruigrok R. W., Skehel J. J., Steinhauer D. A., Wiley D. C. Electron microscopy of antibody complexes of influenza virus haemagglutinin in the fusion pH conformation. EMBO J. 1995 Jan 16;14(2):240–246. doi: 10.1002/j.1460-2075.1995.tb06997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton S. A., Martin S. R., Ruigrok R. W., Skehel J. J., Wiley D. C. Membrane fusion by peptide analogues of influenza virus haemagglutinin. J Gen Virol. 1988 Aug;69(Pt 8):1847–1857. doi: 10.1099/0022-1317-69-8-1847. [DOI] [PubMed] [Google Scholar]
- Wharton S. A., Ruigrok R. W., Martin S. R., Skehel J. J., Bayley P. M., Weis W., Wiley D. C. Conformational aspects of the acid-induced fusion mechanism of influenza virus hemagglutinin. Circular dichroism and fluorescence studies. J Biol Chem. 1988 Mar 25;263(9):4474–4480. [PubMed] [Google Scholar]
- White J. M. Membrane fusion. Science. 1992 Nov 6;258(5084):917–924. doi: 10.1126/science.1439803. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- Zhang Y. P., Lewis R. N., Henry G. D., Sykes B. D., Hodges R. S., McElhaney R. N. Peptide models of helical hydrophobic transmembrane segments of membrane proteins. 1. Studies of the conformation, intrabilayer orientation, and amide hydrogen exchangeability of Ac-K2-(LA)12-K2-amide. Biochemistry. 1995 Feb 21;34(7):2348–2361. doi: 10.1021/bi00007a031. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J., Vogel S. S., Chernomordik L. V. Mechanisms of membrane fusion. Annu Rev Biophys Biomol Struct. 1993;22:433–466. doi: 10.1146/annurev.bb.22.060193.002245. [DOI] [PubMed] [Google Scholar]