Abstract
Background
Forty four percent of patients with pathologic node negative (pN0) non-small cell lung cancer (NSCLC) die within 5 years of curative-intent surgery. Heterogeneity in pathologic nodal examination practice raises concerns about the accuracy of nodal staging in these patients. We hypothesized a reciprocal relationship between the number of lymph nodes examined and probability of missed lymph node metastasis, and sought to identify the number of lymph nodes associated with the lowest mortality risk in pN0 NSCLC.
Methods
We analyzed resections for first primary, pN0 NSCLC in the United States Surveillance, Epidemiology, and End Results database from 1998 to 2009, with survival updated to December 31, 2009.
Results
In the 24,650 eligible patients, there was a significant, sequential reduction in mortality risk with examination of more lymph nodes. The lowest mortality risk occurred in those with 18 – 21 lymph nodes. Hazard ratio for all-cause mortality was 0.65, 95% confidence interval 0.57 – 0.73; for lung cancer specific mortality, 0.62, 0.53 – 0.73; p<.001 for both. The median number of lymph nodes examined was only 6.
Conclusions
Lymph node evaluation falls far short of optimal in patients with resected ‘pN0 NSCLC’, raising the odds of under-estimation of long-term mortality risk, and failure to identify candidates for post-operative adjuvant therapy. This represents a major quality gap, for which corrective intervention is warranted.
Keywords: Lung cancer, surgery, quality of care, outcomes
Introduction
With an annual death toll of 1.4 million lives worldwide and 160,000 in the US, lung cancer is the major oncologic public health burden of the present age [1,2]. Approximately 85% of lung cancer patients have non-small cell lung cancer (NSCLC). Although patients with early stage NSCLC may be cured by surgery, post-operative survival rates are relatively low [3]. Pathologic nodal stage is the strongest predictor of long-term post-operative survival in recipients of surgery, patients without lymph node metastasis have the best survival odds. However, 44% of patients with pathologic node-negative (pN0) NSCLC die within 5 years of resection [4]. The thoroughness with which pathologic staging procedures are applied influences the accuracy of staging, which may affect stage attribution and long-term outcomes [5,6].
There is evidence of a great deal of heterogeneity in the thoroughness with which lung resection specimens are examined [7–10]. At one extreme, non-examination of lymph nodes occurs in about 18% of all ‘node-negative’ resections in the US [11–14]. These so-called ‘pathologic NX’ cases have a significantly poorer survival than matched pN0 cases [13–15]. But pN0 is defined in these analyses as absence of lymph node metastasis in patients with at least 1 examined lymph node, and pN0 cohorts include patients with a wide range of lymph nodes. However, the probability of identifying lymph node metastasis may be directly proportional to the effort devoted to the search, a surrogate for which may be the number of nodes examined.
We examined the survival of patients with pN0 NSCLC in an effort to elucidate the relationship between the number of lymph nodes examined and survival. We hypothesized that a true correlation between thoroughness of examination and the probability of detecting lymph node metastasis should be manifest as serial improvement in long-term survival with increasing lymph node number, up to a certain point, beyond which there would be little further incremental survival benefit. We sought to determine the number of lymph nodes associated with the largest improvement in survival, which we proposed as the optimal number required to accurately determine the absence of nodal metastasis.
Patients and Methods
Study design
With the permission of the University of Tennessee Institutional Review Board, we conducted a retrospective analysis of the United States (US) Surveillance, Epidemiology, and End Results (SEER) database of patients treated for NSCLC from 1998 to 2009, with survival updated to December 31, 2009.
The SEER database
SEER is designed to be representative of the US population, with patient-level data abstracted from 18 specific geographically diverse populations representing rural, urban and regional populations. During the time span included in this study, the SEER data collection sites included up to 28% of the US population [16].
Patient selection
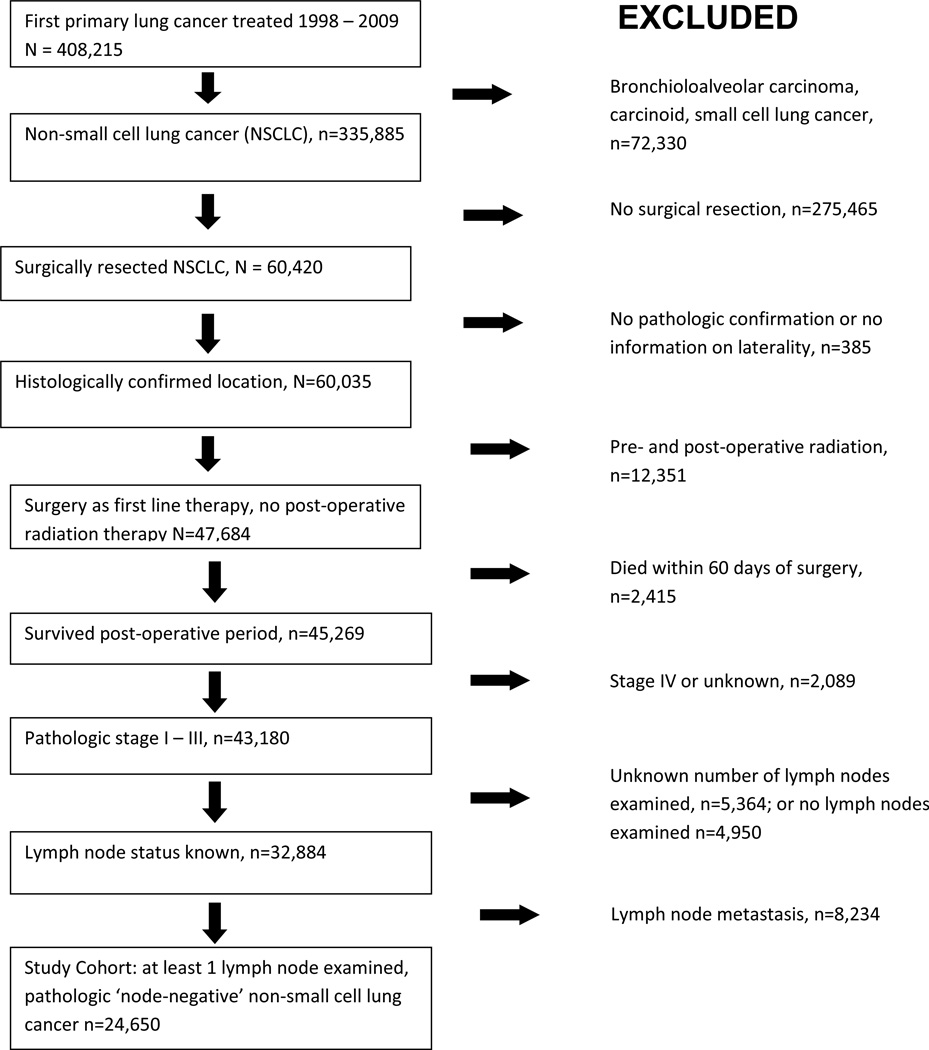
Eligible patients had initial treatment for a first primary NSCLC in the SEER database from 1998 to 2009. We eliminated patients with bronchioloalveolar carcinoma, small cell lung cancer and benign neuro-endocrine tumors because nodal status is not as impactful on the prognosis and treatment of these patients. We also eliminated those who did not undergo surgical resection, recipients of radiation therapy, patients with lymph node or distant metastasis, those without nodal examination, and all who died within 60 days of surgery (Figure 1).
Figure 1.
Selection of study cohort.
Outcomes and data
Our primary objective was to determine the number of examined lymph nodes associated with the lowest risk of death within 5 years after curative surgery for pN0 NSCLC. We also examined the distribution of the number of lymph nodes examined per patient, and the characteristics associated with higher numbers of examined lymph nodes.
Statistical analysis
We used the Chi Square test to compare differences between categorical variables, the t test and trend test for continuous variables, unadjusted Kaplan-Meier method for visualization of survival curves, and the Log Rank test to compare survival curves. The Cox Proportional Hazards model was used to determine the effect of the number of lymph nodes on overall survival, adjusted for patient socio-demographic factors, tumor characteristics, and treatment, including the extent of surgical resection. We used the Fine & Gray competing risk model to examine the lung cancer-specific survival [17]. The threshold of the number of lymph nodes was determined by examining the trend of hazard ratios in the multivariate analysis. The critical turning point in the hazard ratio curve was the optimal number of lymph nodes. We set p <0.001 in the threshold analysis of the number of lymph nodes examined and survival, based on the Bonferroni adjustment for multiple comparisons. The p value for significance in all other analyses, not involving multiple testing, was <0.01.
We performed sensitivity analyses in the threshold analysis by grouping lymph nodes using various lymph node number brackets, and by directly modeling the number of lymph nodes with both linear and quadratic terms. We also performed additional analyses to evaluate the consistency of our findings, including analysis with and without patients who received post-operative adjuvant radiation therapy, given its potentially deleterious implications for survival in resected early stage NSCLC; the impact of a post-operative mortality exclusion window of 30-versus 60-days; examination of the lobectomy sub-cohort; separate analyses for each T-category; and finally, the trend in number of lymph nodes examined per pN0 patient over the12-year time span of resections included in this study.
Results
Cohort characteristics and the distribution of lymph node counts
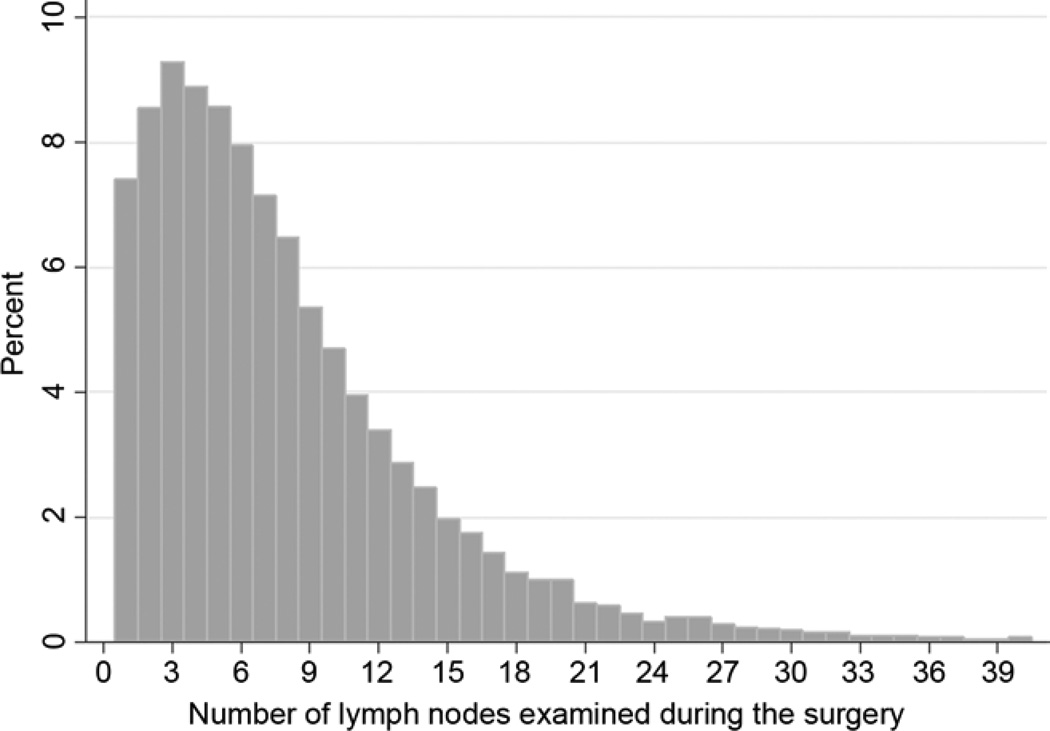
From 1998 to 2009, the SEER database had 24,650 patients eligible for inclusion in our analysis cohort (Figure 1). The distribution of the number of lymph nodes examined reveals a tendency to examine relatively few lymph nodes (Figure 2). The median number of lymph nodes examined was 6. There were significant differences in demographic and clinical characteristics between patients with ≥6, and those with <6 lymph nodes examined (Table 1). The extent of resection was strongly associated with lymph node counts. Whereas only 28% of patients who had a wedge or segmental resection had 6 or more lymph nodes, 60% of lobectomy and 79% of pneumonectomy recipients did.
Figure 2.
Distribution of number of lymph nodes examined in patients with resected pathologic node-negative non-small cell lung cancer: US Surveillance, Epidemiology and End Results database, 1998 to 2009.
Table 1.
Cohort characteristics in relation to lymph node counts
| Characteristics | Total | Lymph node groups | |||
|---|---|---|---|---|---|
| N=24,650 (100%) | <6 N=10,471 (42.5%) |
>=6 N=14,179 (57.5%) |
P-value | ||
| Demographic | |||||
| Age: Mean (SD) | 66.9 (10.1) | 67.2 (10.2) | 66.6 (10.1) | <0.01 | |
| <50 | 1,338 (5.4) | 563 (5.4) | 775 (5.5) | <0.01 | |
| 50 – 64 | 8,008 (32.5) | 3,254 (31.1) | 4,754 (33.5) | ||
| 65 – 74 | 9,208 (37.4) | 3,920 (37.4) | 5,288 (37.3) | ||
| >74 | 6,096 (24.7) | 2,734 (26.1) | 3,362 (23.7) | ||
| Sex | |||||
| Male | 12,736 (51.7) | 5,258 (50.2) | 7,478 (52.7) | <0.01 | |
| Female | 11,914 (48.3) | 5,213 (49.8) | 6,701 (47.3) | ||
| Race | |||||
| White | 21,138 (85.8) | 8,888 (84.9) | 12,250 (86.4) | 0.002 | |
| Black | 2,041 (8.3) | 937 (8.9) | 1,104 (7.8) | ||
| Other | 1,471 (6.0) | 646 (6.2) | 825 (5.8) | ||
| Marriage status | |||||
| married | 14,356 (58.2) | 5,940 (56.7) | 8,416 (59.4) | <0.01 | |
| live alone | 10,294 (41.8) | 4,531 (43.3) | 5,763 (40.6) | ||
| Metropolitan status | |||||
| No | 3,543 (14.4) | 1,519 (14.5) | 12,155 (85.7) | 0.61 | |
| Yes | 21,107 (85.6) | 8,952 (85.5) | 12,155 (85.7) | ||
| Tumor | |||||
| Histology | |||||
| Adenocarcinoma | 12,419 (50.4) | 5,418 (51.7) | 7,001 (49.4) | <0.01 | |
| Squamous | 7,891 (32.0) | 3,177 (30.3) | 4,714 (33.3) | ||
| Large cell | 1,266 (5.1) | 551 (5.3) | 715 (5.0) | ||
| Other | 3,074 (12.5) | 1,325 (12.7) | 1,749 (12.3) | ||
| Grade | |||||
| 1 | 2,414 (9.8) | 1,089 (10.4) | 1,325 (9.3) | <0.01 | |
| 2 | 10,144 (41.2) | 4,285 (40.9) | 5,859 (41.3) | ||
| 3 | 9,775 (39.7) | 4,063 (38.8) | 5,712 (40.3) | ||
| 4 | 881 (3.6) | 369 (3.5) | 512 (3.6) | ||
| Unknown | 1,436 (5.8) | 665 (6.4) | 771 (5.4) | ||
| Tumor size | |||||
| <3 | 19,655 (79.7) | 8,329 (79.5) | 11,326 (79.9) | <0.01 | |
| 3–5 | 3,091 (12.5) | 1,386 (13.24) | 1,705 (12.0) | ||
| >5 | 1,727 (7.0) | 672 (6.4) | 1,055 (7.4) | ||
| Unknown | 177 (0.7) | 84 (0.8) | 93 (0.7) | ||
| Tumor location | |||||
| Upper | 15,157 (61.5) | 6,150 (58.7) | 9,007 (63.5) | <0.01 | |
| Middle | 1,199 (4.9) | 701 (6.7) | 498 (3.5) | ||
| Lower | 7,505 (30.5) | 3,350 (32.0) | 4,155 (29.3) | ||
| NOS | 789 (3.2) | 270 (2.6) | 519 (3.7) | ||
| T-category | |||||
| 1 | 15,382 (62.4) | 6,765 (64.6) | 8,617 (60.8) | <0.01 | |
| 2 | 7,229 (29.3) | 2,933 (28.0) | 4,296 (30.3) | ||
| 3 | 1,044 (4.2) | 380 (3.6) | 664 (4.7) | ||
| 4 | 951 (3.9) | 375 (3.6) | 576 (4.1) | ||
| Unknown | 44 (0.2) | 18 (0.2) | 26 (0.2) | ||
| Extent of resection | |||||
| Lobectomy | 21,140 (85.8) | 8,503 (81.2) | 12,637 (89.1) | <0.01 | |
| Pneumonectomy | 1,038 (4.2) | 217 (2.1) | 821 (5.8) | ||
| Wedge/segment | 2,385 (9.7) | 1,717 (16.4) | 668 (4.7) | ||
| Other | 87 (0.4) | 34 (0.3) | 53 (0.4) | ||
Impact of lymph node counts on survival
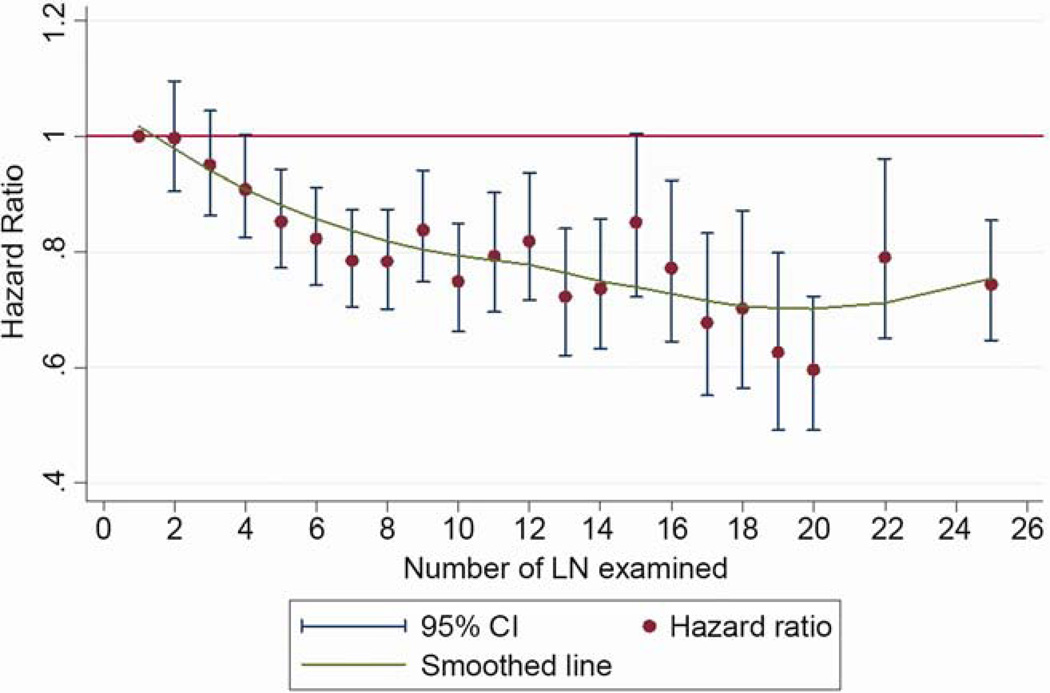
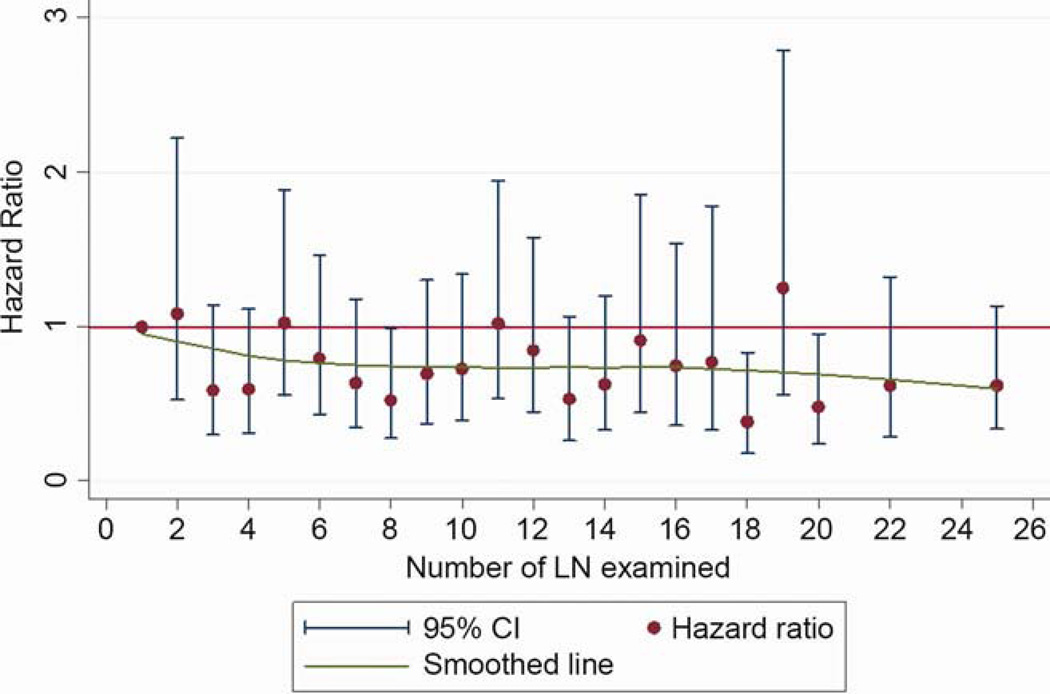
The overall 5-year survival rate of the cohort was 58.6% (95% confidence interval [CI]: 57.8 – 59.3%). For patients with <6 lymph nodes, the 5-year survival rate was 55% (95% CI: 53.9 – 56.2%), and for those with ≥6 lymph nodes, it was 61.4% (95% CI: 60.4 – 62.3%). The hazard ratio for mortality decreased sequentially with increasing number of lymph nodes, until a maximal benefit was achieved with examination of 20 nodes (Figure 3). After examination of 20 lymph nodes, the sequential improvement in the hazard ratio for death was no longer evident. This pattern was consistent for all-cause, and lung cancer-specific, mortality (Figures 3a and 3b). In the threshold analysis, a minimum of 6 lymph nodes was required to show a significant reduction in mortality risk over examination of only 1 lymph node for both all-cause, and lung cancer specific, mortality (p<.001).
Figure 3.
Evolution of hazard ratio for mortality with the number of lymph nodes examined. A) All-cause mortality. B) Lung cancer-specific mortality.
In the multivariate model, which adjusted for all relevant demographic and clinical factors (including the extent of resection), the number of lymph nodes examined was a strong independent predictor of mortality risk, which sequentially diminished with increasing number of lymph nodes examined until the 18–21 lymph node subset, after which the hazard ratio rose slightly (Table 2). Other factors, including sex, marital status, residence in a metropolitan area, tumor grade, tumor size, tumor location, T-category and extent of resection were also independently associated with mortality.
Table 2.
Multivariate analysis of survival determinants in resected pN0 non-small cell lung cancer
| All-cause mortality HR (95%CI) |
Lung cancer specific mortality HR (95%CI) |
||
|---|---|---|---|
| Lymph node exam | |||
| 1–3 | ref | ref | |
| 4–5 | 0.90 (0.85–0.96) | 0.89 (0.82–0.96) | |
| 6–8 | 0.82 (0.77–0.87) | 0.78 (0.72–0.84) | |
| 9–11 | 0.81 (0.76–0.87) | 0.77 (0.70–0.84) | |
| 12–17 | 0.79 (0.73–0.84) | 0.75 (0.68–0.82) | |
| 18–21 | 0.65 (0.57–0.73) | 0.62 (0.53–0.73) | |
| 22+ | 0.77 (0.69–0.86) | 0.79 (0.69–0.91) | |
| Age | 1.04 (1.03–1.04) | 1.02 (1.02–1.02) | |
| Sex | |||
| Male | ref | ref | |
| Female | 0.70 (0.67–0.73) | 0.81 (0.76–0.85) | |
| Race | |||
| White | ref | ref | |
| Black | 1.12 (1.04–1.20) | 1.08 (0.98–1.18) | |
| Other | 0.86 (0.79–0.95) | 0.95 (0.85–1.07) | |
| Marital status | |||
| married | ref | ref | |
| live alone | 1.23 (1.18–1.28) | 1.12 (1.06–1.19) | |
| Metropolitan status | |||
| No | ref | ref | |
| Yes | 0.87 (0.82–0.92) | 0.89 (0.82–0.95) | |
| Tumor | |||
| Histology | |||
| Adenocarcinoma | ref | ref | |
| Squamous | 1.12 (1.07–1.18) | 0.97 (0.91–1.03) | |
| Large cell | 1.13 (1.02–1.26) | 1.14 (1.0–1.30) | |
| Other | 1.04 (0.98–1.12) | 1.0 (0.91–1.09) | |
| Grade | |||
| 1 | ref | ref | |
| 2 | 1.42 (1.30–1.55) | 1.47 (1.31–1.65) | |
| 3 | 1.62 (1.49–1.77) | 1.74 (1.55–1.96) | |
| 4 | 1.60 (1.39–1.84) | 1.72 (1.43–2.07) | |
| Unknown | 1.51 (1.35–1.70) | 1.61 (1.39–1.87) | |
| Tumor size | |||
| <3 | ref | ref | |
| 3–5 | 1.24 (1.17–1.30) | 1.35 (1.26–1.45) | |
| >5 | 1.39 (1.30–1.49) | 1.68 (1.54–1.83) | |
| Unknown | 1.18 (0.97–1.44) | 1.12 (0.86–1.47) | |
| Tumor location | |||
| Upper | ref | ref | |
| Middle | 1.06 (0.96–1.17) | 1.11 (0.98–1.26) | |
| Lower | 1.10 (1.05–1.15) | 1.18 (1.11–1.25) | |
| NOS | 1.14 (1.02–1.27) | 1.26 (1.10–1.45) | |
| T-category | |||
| 1 | ref | ref | |
| 2 | 1.14 (1.09–1.20) | 1.25 (1.18–1.33) | |
| 3 | 1.89 (1.73–2.05) | 2.06 (1.84–2.30) | |
| 4 | 1.76 (1.60–1.94) | 2.00 (1.77–2.26) | |
| Unknown | 1.40 (0.94–2.07) | 1.29 (0.78–2.14) | |
| Extent of resection | |||
| Lobectomy | ref | ref | |
| Pneumonectomy | 1.45 (1.32–1.59) | 1.52 (1.35–1.72) | |
| Wedge/segment | 1.18 (1.10–1.26) | 1.18 (1.08–1.29) | |
| Other | 1.28 (0.94–1.75) | 1.44 (0.99–2.10) | |
Sensitivity analyses of the relationship between lymph node count and survival
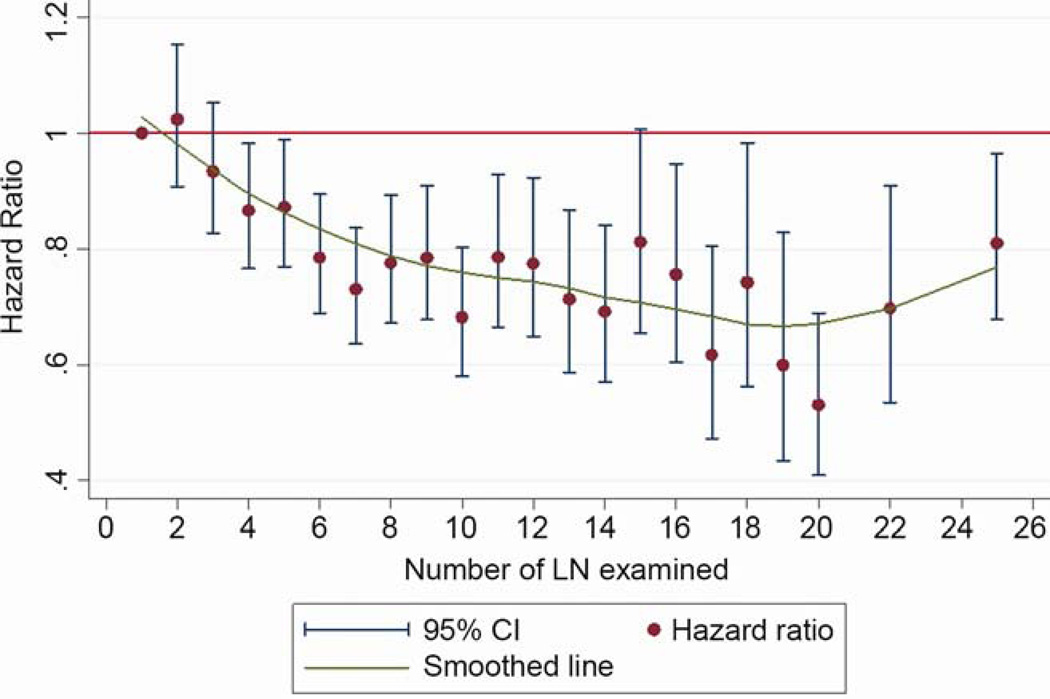
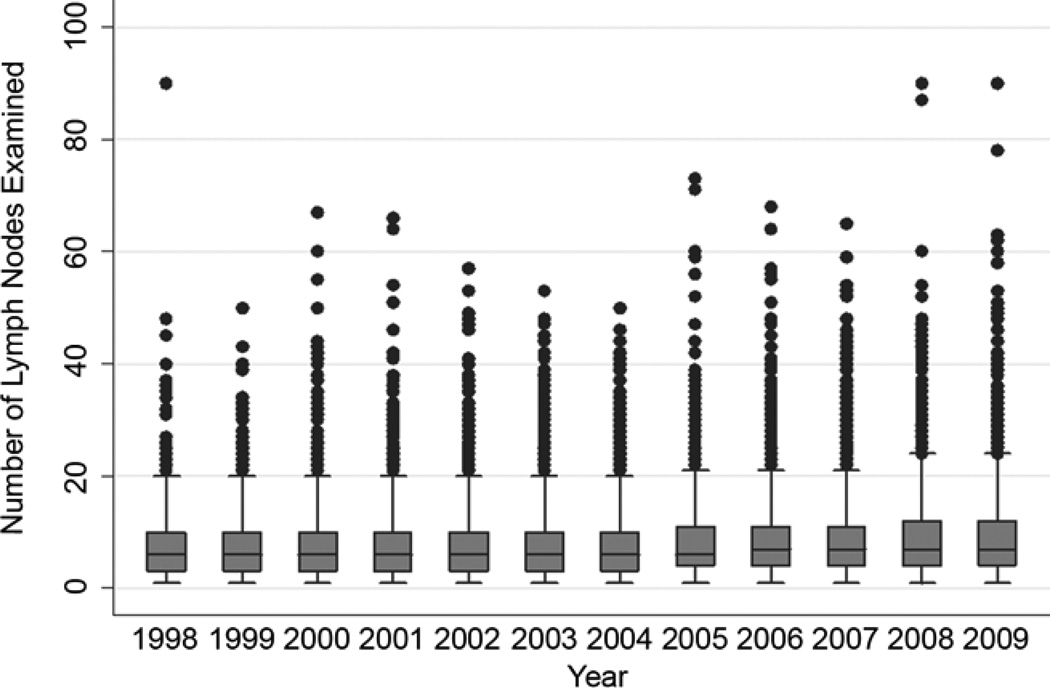
The pattern of relationship between the lymph node count and survival was affected neither by inclusion or exclusion of patients who received post-operative adjuvant radiation therapy, nor inclusion or exclusion of patients who died within 30 or 60 days of surgery (data not shown). The pattern was also consistent within each pathologic T-category (data not shown). In the lobectomy sub-cohort, the pattern of improvement in survival up to the 18–20 lymph node count remained, although with wider confidence intervals due to a smaller sample size (Figure 4). Over the span of 12 years from 1998 to 2009, there was a modest trend towards increasing number of examined lymph nodes, with an average increase of 0.15 lymph nodes per year (p<.001; Figure 5). The median number of lymph nodes examined increased from 6 in the early period to 7 in the late period.
Figure 4.
Evolution of hazard ratio for mortality with the number of lymph nodes examined in lobectomy cases.
Figure 5.
Trend analysis of the number of lymph nodes examined in resected pathologic node-negative non-small cell lung cancer: US Surveillance, Epidemiology and End Results database, 1998 to 2009.
Comment
In the quest to improve lung cancer survival, every effort must be made to distinguish between high and low risk patients, in order to optimize the benefit of treatment for all. Patients who undergo surgical resection are the most likely to survive, but those with lymph node metastasis remain at considerably higher risk for death after surgery alone. It is important to accurately identify these patients because they benefit from post-operative adjuvant therapy and are eligible for adjuvant therapy trials [18–21].
Logic suggests that the thoroughness of examination will determine the likelihood of finding lymph node metastasis. The number of examined lymph nodes is a simple surrogate for the thoroughness of examination, although it may be confounded by fragmentation of lymph nodes [22]. The number of stations examined is another such surrogate, without the disadvantage of confounding by fragmentation. However, the number of lymph nodes examined is more reliably identified in pathology reports. These two measures depend on the actions of surgeons during the operation (the collection of hilar and mediastinal lymph nodes) and pathologists after the operation (the retrieval of intrapulmonary nodes, and thorough examination of all provided specimens). Despite the above caveats, determining the optimal number of lymph nodes required to reliably assert node negativity is a useful quality benchmark.
Three prior population-based studies have addressed the association between lymph node examination and survival in pN0 NSCLC. Ludwig et al examined postoperative survival among 16,800 recipients of surgical resection for stage IA or IB NSCLC from 1990 – 2000 in the SEER database [23]. They found that survival peaked at approximately 13 to 16 lymph nodes, with no incremental improvement from evaluating more than 16 nodes. Varlotto, et al, examined 24,273 patients with stage I NSCLC in the SEER database from 1992 to 2002, and found improved outcomes with increasing lymph node number, with a plateau at ‘11 or more lymph nodes’ [11]. In a sub-cohort from 1998 to 2002 with general information on the location of examined lymph nodes, they compared the outcomes of 1414 patients with no nodal dissection, to 2683 patients with only N1, and 1019 patients with only N2 nodal dissection.
In patients with only N1 lymph nodes examined, they reported a maximal survival difference after 11–16 lymph nodes, and trends for worse survival in 61 patients with 17 or more nodes in comparison to 158 patients with 11 to 16 nodes. The N2-nodal dissection only group had a maximal survival difference at 7 to 10 lymph nodes, and those with 11 or more N2 nodes had significantly worse survival than those with 7 to 10 nodes. They speculated about ‘a survival detriment in having a very large number of recovered lymph nodes in patients having only N1 or only N2 dissections.’ Finally, Ou and Zell compared the survival impact of ranges of lymph node examination in 2545 patients who underwent lobectomy for stage IA NSCLC from 1999 – 2003 and found that removal of 11 to 15 nodes conferred the lowest hazard ratio for death in comparison to those with no nodes [12].
Our findings, though generally similar to the three prior studies cited above, are different in specifics. The most striking differences are that we identified 20 as the optimal lymph node number, and found no survival impairment with the higher lymph node counts. A minimum of 6 lymph nodes needed to be examined in order to reach the sequentially beneficial part of the survival curve. This improvement in survival with higher lymph node counts is most likely attributable to reduction in the probability of missed lymph node metastasis.
Several factors may explain the difference in our findings. We included patients with T3 and T4 tumors, in whom the probability of lymph node metastasis is significantly higher [24], and for whom N1 metastasis would translate to stage IIIA disease (compared to IIA for those with T1, and IIB for those with T2 lesions), thereby expanding the risk of missed lymph node metastasis in our study population. Secondly, our examination cohort is larger than each of the prior studies, giving us greater statistical power to examine the lymph node number subsets, especially the higher numbers which are proportionally much smaller (Figure 2). The reported survival detriment in the study by Varlotto et al was based on analysis of only 219 patients out of a total cohort of 24,273. The safety analysis from the American College of Surgery Oncology Group Z0030 trial (ACOSOG Z0030) refutes any suggestion of increased danger from thorough mediastinal lymph node sampling [25,26], and improving lymph node retrieval from intrapulmonary stations certainly poses no additional risk to patients [10]. Thirdly, we excluded patients with bronchioloalveolar cell carcinoma and carcinoid tumors, in whom the incidence and survival impact of lymph node metastasis is lower.
Examination of the lobectomy population suggests that the main survival impact occurs in patients with sub-lobar resection and pneumonectomy, in whom other competing risks beyond lymph node metastasis may account for the heightened survival impact. However, the shape of the cumulative survival hazard ratio comparison curve is similar, although not as steep as in the unselected population (Figure 4). The need for a sufficient number of lymph nodes to ensure accurate pathologic nodal staging must not be conflated with debates about the optimal mediastinal lymph node staging procedure. Although Z0030 revealed no survival difference between patients with early stage NSCLC who received an elaborate systematic sampling procedure versus those who had mediastinal nodal dissection [27], the vast majority of patients who undergo lung cancer resection in the US do not meet minimum systematic nodal sampling criteria [9,26–28]. Besides, poor pathologic nodal staging reflects a dual failure of surgical retrieval [9,28] and pathology examination [10].
Examination of approximately 18 to 20 lymph nodes is optimally associated with reduced mortality risk in patients with resected ‘node-negative’ NSCLC. The rate of attainment of this level of thoroughness in nodal staging should be adopted as an institutional quality parameter. Prevailing practice falls considerably short of this target, necessitating rigorous testing of corrective interventions to improve the pathologic nodal staging of resected NSCLC in the US.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Raymond U. Osarogiagbon, Thoracic Oncology Research Group, Baptist Cancer Center, Memphis, TN
Obiageli Ogbata, Thoracic Oncology Research Group, Baptist Cancer Center, Memphis, TN.
Xinhua Yu, Department of Epidemiology and Biostatistics, School of Public Health, University of Memphis, Memphis, TN.
References
- 1.Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Goldstraw P, Crowley J, Chansky K, et al. The IASLC lung cancer staging project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumors. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 4.Rusch VW, Crowley J, Giroux DJ, et al. The IASLC lung cancer staging project: Proposals for the revision of the N descriptors in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2007;2:603–612. doi: 10.1097/JTO.0b013e31807ec803. [DOI] [PubMed] [Google Scholar]
- 5.Gajra A, Newman N, Gamble GP, et al. Effect of number of lymph nodes sampled on outcome of patients with stage I non-small-cell lung cancer. J Clin Oncol. 2003;21:1029–1034. doi: 10.1200/JCO.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 6.Detterbeck FC, Puchelski J, Rubinowitz A, et al. Classification of the thoroughness of mediastinal staging of lung cancer. CHEST. 2010;137(2):436–442. doi: 10.1378/chest.09-1378. [DOI] [PubMed] [Google Scholar]
- 7.Gephardt GN, Baker PB. Lung carcinoma surgical pathology report adequacy. Arch Pathol Lab Med. 1996;120:922–927. [PubMed] [Google Scholar]
- 8.Farooq A, Osarogiagbon RU, Allen JW, et al. Accuracy and comprehensiveness of pathology reportage after lung cancer resection. J Clin Oncol. 2009;27(suppl):15s. abstr 6523. [Google Scholar]
- 9.Osarogiagbon RU, Yu X. Mediastinal lymph node examination and survival in resected early stage non-small cell lung cancer in the surveillance, epidemiology and end results database. J Thorac Oncol. 2012;7:1798–1806. doi: 10.1097/JTO.0b013e31827457db. [DOI] [PubMed] [Google Scholar]
- 10.Ramirez RA, Wang CG, Miller LE, et al. Incomplete intrapulmonary lymph node retrieval after routine pathologic examination of resected lung cancer. J Clin Oncol. 2012;30:2823–2828. doi: 10.1200/JCO.2011.39.2589. [DOI] [PubMed] [Google Scholar]
- 11.Varlotto JM, Recht A, Nikolov M, et al. Extent of lymphadenectomy and outcome for patients with stage I nonsmall cell lung cancer. Cancer. 2009;115:851–858. doi: 10.1002/cncr.23985. [DOI] [PubMed] [Google Scholar]
- 12.Ou SI, Zell JA. Prognostic Significance of the Number of Lymph Nodes Removed at Lobectomy in Stage IA Non-small Cell Lung Cancer. J Thorac Oncol. 2008;3:880–886. doi: 10.1097/JTO.0b013e31817dfced. [DOI] [PubMed] [Google Scholar]
- 13.Osarogiagbon RU, Allen JW, Farooq A, et al. Outcome of surgical resection for pathologic N0 and Nx non-small cell lung cancer. J Thorac Oncol. 2010;5:191–196. doi: 10.1097/JTO.0b013e3181c8cc32. [DOI] [PubMed] [Google Scholar]
- 14.Osarogiagbon RU, Yu X. Nonexamination of lymph nodes and survival after resection of non-small cell lung cancer. Ann Thorac Surg 2013. 2013 Jul 30; doi: 10.1016/j.athoracsur.2013.05.021. [Epub ahead of print] PMID: 23910633. [DOI] [PubMed] [Google Scholar]
- 15.Osarogiagbon RU, Ramirez RA, Wang C, et al. Survival analysis of patients with/without lymph node examination after lung cancer resection. J Clin Oncol. 2011;29(suppl) abstr 7054. [Google Scholar]
- 16.Overview of the SEER program. [Accessed November 6, 2012]; at http://seer.cancer.gov/about/overview.html.
- 17.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 18.The International Adjuvant Lung Cancer Trial Collaborative Group. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 19.Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 20.Pignon J, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE collaborative group. J Clin Oncol. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 21.Lally BE, Zelterman D, Colasanto JM, et al. Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the surveillance, epidemiology, and end results database. J Clin Oncol. 2006;24:2998–3006. doi: 10.1200/JCO.2005.04.6110. [DOI] [PubMed] [Google Scholar]
- 22.Wu Y, Lin CJ, Hsu W, et al. Long-term results of pathological stage I non-small cell lung cancer: validation of using the number of totally removed lymph nodes as a staging control. Eur J Cardiothorac Surg. 2003;24:994–1001. doi: 10.1016/s1010-7940(03)00567-0. [DOI] [PubMed] [Google Scholar]
- 23.Ludwig MS, Goodman M, Miller DL, et al. Postoperative Survival and the Number of Lymph Nodes Sampled During Resection of Node-Negative Non-Small Cell Lung Cancer. CHEST. 2005;128:1545–1550. doi: 10.1378/chest.128.3.1545. [DOI] [PubMed] [Google Scholar]
- 24.Graham ANJ, Chan KJM, Pastorino U, Goldstraw P. Systematic nodal dissection in the intrathoracic staging of patients with non-small cell lung cancer. J Thorac Cardiovasc Surg. 1999;117:246–251. doi: 10.1016/S0022-5223(99)70419-8. [DOI] [PubMed] [Google Scholar]
- 25.Allen MS, Darling GE, Pechet TTV, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg. 2006;81:1013–1020. doi: 10.1016/j.athoracsur.2005.06.066. [DOI] [PubMed] [Google Scholar]
- 26.Darling GE, Allen MS, Decker PA, et al. Number of lymph nodes harvested from a mediastinal lymphadenectomy. Chest. 2011;139:1124–1129. doi: 10.1378/chest.10-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma : results of the American College of Surgery Oncology Group Z0030 trial. J Thorac Cardiovasc Surg. 2011;141:661–670. doi: 10.1016/j.jtcvs.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osarogiagbon RU, Allen JW, Farooq A, et al. Objective review of mediastinal lymph node examination in a lung cancer resection cohort. J Thorac Oncol. 2012;7:390–396. doi: 10.1097/JTO.0b013e31823e5e2d. [DOI] [PubMed] [Google Scholar]