Abstract
The perception of fatigue is common in many disease states, however, the mechanisms of sensory muscle fatigue are not understood. In mice, rats and cats, muscle afferents signal metabolite production in skeletal muscle using a complex of ASIC, P2X and TRPV1 receptors. Endogenous muscle agonists for these receptors are combinations of protons, lactate, and ATP. Here we applied physiological concentrations of these agonists to muscle interstitium in human subjects to determine if this combination could activate sensations, and if so determined how these subjects described these sensations. Ten volunteers received infusions (0.2 ml over 30-s) containing protons, lactate and ATP under the fascia of a thumb muscle, abductor pollicis brevis (APB). Infusion of individual metabolites at maximum amounts evoked no fatigue or pain. Metabolite combinations found in resting muscles (pH 7.4+300nM ATP+1mM lactate) also evoked no sensation. The infusion of a metabolite-combination found in muscle during moderate endurance-exercise (pH 7.3+400nM ATP+5 mM lactate) produced significant fatigue sensations. Infusion of a metabolite-combination associated with vigorous exercise (pH 7.2+500nM ATP+10mM lactate) produced stronger sensations of fatigue and some ache. Higher levels of metabolites (as found with ischemic exercise) caused more ache but no additional fatigue-sensation. Thus, in a dose-dependent manner, intramuscular infusion of combinations of protons, lactate, and ATP leads to fatigue-sensation and eventually pain, probably through activation of ASIC, P2X, and TRPV1 receptors. This is the first demonstration in humans that metabolites normally produced by exercise act in combination to activate sensory neurons that signal sensations of fatigue and muscle pain.
Keywords: Fatigue, Muscle pain, Metabolites, Lactate, ATP, pH, exercise, skeletal muscle
Introduction
The sensation of fatigue as occurring during and after muscle contractions, or in certain pathological conditions (Kos et al., 2008), is the perception of tiredness or heaviness which can substantially reduce physical activity and performance (Amann & Dempsey, 2008; Amann et al., 2013). Muscle pain in undamaged skeletal muscle can also be evoked by ischemic exercise (Alam & Smirk, 1937). At present, the metabolites evoking and the molecular mechanisms detecting and leading to the sensations of fatigue and muscle pain remain largely unknown.
In skeletal muscle, nociceptive afferent neurons are mostly group III and IV neurons corresponding in conduction velocity to cutaneous Aδ and C afferents. Studies have shown that mechanical stimulation can activate at least some of these neurons (Mense & Schmidt, 1974; Mense, 1977; Kaufman et al., 1983; Kaufman et al., 1984; Kaufman & Rybicki, 1987; Bove & Light, 1995; Adreani et al., 1997; Graven-Nielsen, 2006). Additionally, intramuscular infusion of supra-physiological concentrations of various chemicals can activate these neurons (Mense, 1977; Kniffki et al., 1978; Hill et al., 1992; Decherchi et al., 1998; Khan & Sinoway, 2000).
More relevant to our understanding of the perception of fatigue is the fact that a variety of exercise-induced intramuscular metabolites, including lactate, adenosine 5′-triphosphate (ATP), and protons also stimulate a subset of these muscle afferents (Bangsbo et al., 1993; Hellsten et al., 1998; Bangsbo et al., 2001; Li et al., 2003, 2005; Mohr et al., 2007). Animal experiments have demonstrated that at least three different molecular receptors, namely a heteromere of ASIC1+3, or ASIC1+2 (Gautam & Benson, 2013) a P2X (P2X5 or P2X4, or P2X1/3 heteromere), and TRPV1, co-localized on cardiac and skeletal muscle afferent endings (Benson et al., 1999; Immke & McCleskey, 2001a, b; Sutherland et al., 2001; Connor et al., 2005; Molliver et al., 2005; Smith et al., 2005), cooperate to precisely detect physiological levels of a combination of these three metabolites (Naves & McCleskey, 2005; Yagi et al., 2006; Light et al., 2008; Birdsong et al., 2010; Jankowski et al., 2013). These metabolite receptors are reported to be densely localized near blood vessels just below the muscle fascia (Molliver et al., 2005), however, group III/IV afferent endings have also been localized to other parts of skeletal muscle including veins, and in the adventitia of lymphatic vessels (Andres et al., 1985).
We and others have recently shown that these three metabolites (lactate, ATP, and protons) act synergistically to activate at least two significant populations of dorsal root ganglion group III/IV afferent neurons innervating skeletal muscle of mice (Light et al., 2008; Jankowski et al., 2013). One population responds to the levels of metabolites produced by non-painful muscle contractions and may contribute significantly to the exercise pressor reflex [the increase in blood pressure that occurs in response to skeletal muscle contraction, (Alam & Smirk, 1937; Mitchell et al., 1968; Mitchell et al., 1983; Secher & Amann, 2012)] and, likely to the sensory experience of exercise-induced fatigue as was noted initially by Alam & Smirk (1937). The other population begins to respond at levels of metabolites that only occur during muscle ischemia, and could contribute to acute muscle pain as experienced during ischemic exercise or during muscle inflammation and injury. Additional mouse experiments verified that no individual or pair of these metabolites had the effectiveness of the proper combination of all three metabolites (Light et al., 2008).
Strong evidence in favour of a significant involvement of group III/IV muscle afferents in the perception of fatigue comes from recent human studies. When the inputs from these afferents were partially blocked by lumbar intrathecal injection of the μ-opioid agonist fentanyl, subjective ratings of perceived exertion and fatigue during lower limb exercise were significantly attenuated (Gandevia, 2001; Amann et al., 2009; Amann et al., 2010, 2011a; Amann et al., 2011b). These same studies also suggest that activation of group III/IV muscle afferents limits central motor drive (CMD) in exercising individuals which protects against excessive development of peripheral fatigue (fatigue caused by the reduction in ability of muscle fibers to contract) (Amann et al., 2011a). However, it is unclear whether human afferents utilize the same molecular receptors to detect the same metabolites as animal models. Therefore, the aims of this study were two-fold: 1. to create a method by which metabolites could be directly infused into muscle interstitium. 2. to determine if humans also utilize a unique combination of muscle contraction induced protons, lactate and ATP to activate group III and IV skeletal muscle afferents evoking sensations of fatigue and pain.
Methods
Subjects
Ten healthy volunteers (6 male, 4 female, ages 30–60) participated in these studies. Written informed consent was obtained from each participant. All procedures were approved by the University of Utah Institutional Review Board and conformed to the standards set by the Declaration of Helsinki.
Metabolite Solutions
Solvent free ATP was obtained from Sigma-Aldrich, all other metabolites and electrolytes were USP grade. All mixing procedures were conducted by an Anesthesiologistand supervised by a licensed pharmacist.
A series of metabolite solutions containing increasing concentrations of lactate, protons, and ATP (Table 1) were used in the present experiments. Details of the composition of the injected solutions are in the 1st Supplemental file. Lactate and pH levels in these solutions were derived from studies where blood samples, muscle biopsies, and direct measurements of muscle interstitial pH were obtained from healthy humans during exercise of varying intensities (Allsop et al., 1990; Bangsbo et al., 1993; Mohr et al., 2007). ATP levels were derived from studies in which interstitial muscle concentrations of ATP were measured during mild to intense exercise in humans, cats and rats (Hellsten et al., 1998; Li et al., 2003, 2005).
Table 1.
Metabolite concentrations used in study
| Metabolite Chart | ||||
|---|---|---|---|---|
| pH | ATP | Lactate | Exercise Level | Order of Presentation |
| 7.4 | 300 nM | 1 mM | Resting (neutral solution) | 1 |
| 7.3 | 400 nM | 4 mM | Mild | 2 |
| 7.2 | 500 nM | 10 mM | Moderate | 3 |
| 7.0 | 1000 nM | 15 mM | High | 4 |
| 6.8 | 2000 nM | 20 mM | Very High | 5 |
| 6.6 | 5000 nM | 50 mM | Ischemic (painful) | 6 |
The values of metabolites in the solution at pH 7.4 approximate those found in interstitial muscle fluid when muscles are at rest. Generally, the low metabolite values associated with pH > 7.0 are likely to be non-noxious, whereas higher metabolite values at pH < 7.0 may be associated with pain. The metabolite concentrations used here (pH 7.4, lactate 1mM, ATP 300nM to pH 6.6, lactate 50 mM, ATP 5000nM) bracket the physiological range of metabolites found in skeletal muscle interstitium at rest and during exercise and ischemic contractions (Steinhagen et al., 1976; Graham et al., 1993; Bangsbo et al., 2001; Reeh & Kress, 2001; Li et al., 2003, 2005).
The solutions were mixtures of sodium, calcium, magnesium, potassium, and glucose buffered with 30mM sodium phosphate and adjusted to normal interstitial levels to balance the osmolarity at between 280 and 300 mOsmol/L. Because ATP degrades quickly when in solution at and above room temperature, ATP was thawed just before use, and added by an anesthesiologist in a sterile hood to obtain the values in Table 1 at the time of the experiment. Once mixed solutions containing ATP were used within 20 minutes. Once combined, all solutions were kept at muscle temperature (35°C).
Injection Placement
The desired metabolite solution was drawn into a 5cc syringe and placed in a clinical grade syringe pump attached to sterile catheter tubing mating with a sterile 33-gauge needle.
An anesthesiologist, using ultrasound guidance, inserted 33-gauge needles bilaterally just under the fascia of the abductor pollicis brevis (APB) muscle of the thumb (see Figure 1). This permitted intramuscular infusion of the series of six sterile metabolite solutions of increasing concentrations in the APB of one hand (e.g., the left hand), and in 5 of the subjects a series of six solutions corresponding to only the resting neutral (pH 7.4) metabolite levels (see Table 1) in the APB in the other hand (e.g., the right hand). This served as a control for volume effects of the injections. Four other subjects (see below) received injections of single metabolites. The dead space in the needle was filled with warmed (35°C) neutral (pH 7.4) metabolite solution prior to placement in the muscle, and the catheter tubing was purged of air before mating with the cannula. A sensation of aching pain often (but not always) was perceived when the needle penetrated the superficial muscle fascia. This sensation was allowed to diminish before solutions were injected. To ensure that positioning would not itself produce muscle fatigue, the forearms of volunteers were rested on pillows to maintain a neutral position of the APB muscles.
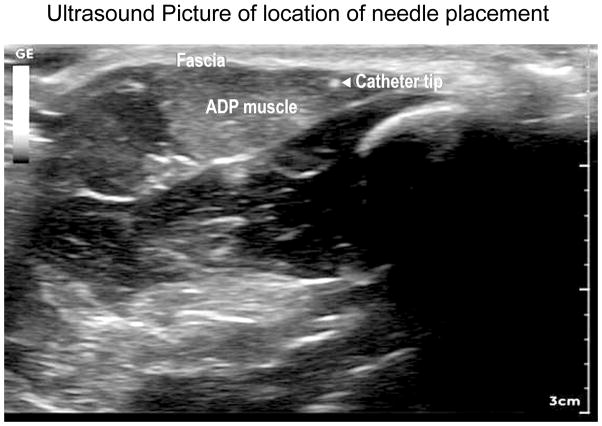
Figure 1.
Ultrasound Picture of location of needle placement.
Ultrasound of needle placement under muscle fascia of abductor pollicis brevis (ADP) muscle of the thumb. Fascia layer indicated, as are the muscle fibers of ADP. The tip of the needle is indicated by the bright spot with the arrow pointing to it.
Metabolite Application
200 μL of each solution in Table 1 was infused over 30 seconds in an ascending series in one thumb. At least 5 minutes was allowed between all infusions. For later infusions (pH 7.0 - pH 6.6) the time between infusions averaged 10 minutes. Between each infusion in the series, a neutral solution (pH 7.4) of the same volume was infused over 30 seconds in the opposite thumb. This allowed for the control of any sensations related to volume effects. These volumes and rates have been used with hypotonic solutions to evoke pain in the same muscle in past experiments (Coppieters et al., 2006; Graven-Nielsen, 2006).
Pain and Fatigue reporting
Subjects were asked to report and describe non-pain and pain sensations every 30 seconds during and after each metabolite infusion until the subjects could not detect any evoked sensations (an average of 3.4 minutes, range 0.2–4.8 minutes). Subjects were not cued, or briefed on possible descriptions of these sensations. They were simply asked if they felt anything, and whether it was painful or non-painful, and for their description of what it felt like. Duration of sensations was estimated by subtracting the time of the last report from the time of the first report.
Subjects were also asked to quantify the magnitude of non-pain and pain sensations using a Verbal Response Scale (VRS) ranging from 0 to 100, with 0 being no sensation and 100 being the strongest non-pain, or pain sensation they could imagine. These scales have been used for human experiments to assess noxious sensation and have been proven valid (Ferreira-Valente et al., 2011). A two-minute rest period was given upon cessation of all subject-reported sensations before administering the next solution in the series.
At the conclusion of the series of infusions, ultrasound was again applied to the APB. Needle position was examined to look for possible migration. In addition, the muscle and subcutaneous tissues were closely examined to look for evidence of fluid build-up observable as a “pocket” of fluid, or leakage of injected fluid out of the muscle, into the overlying tissues.
Individual Metabolites
To test for the effects of the maximal amounts of individual metabolites, and also potential residual effects of injected metabolites that might be affecting subsequent metabolite injections we conducted the following experiment.
Four healthy volunteers received infusions over 30 seconds of 200 μL of each of the 3 individual metabolites (protons, lactate, or ATP) at maximal levels (see Table 2), each in the same phosphate buffered solution with normal interstitial levels of sodium, calcium, magnesium, and potassium. (See Supplemental file #2 for details of the composition of these solutions).
Table 2.
Control metabolite solutions
| pH | ATP | Lactate | |
|---|---|---|---|
| Maximal Lactate | 7.4 | 0 | 50mM |
| Maximal protons | 6.6 | 0 | 0 |
| Maximal ATP | 7.4 | 5000 nM | 0 |
For this procedure, the 4 subjects were first infused with 200 μL of “neutral solution” (pH 7.4+300nM ATP+1 mM lactate). Next, subjects were infused with each of the 3 individual metabolites (protons, lactate, or ATP) 10 minutes apart at maximal levels. Solutions administered were counter balanced across subjects to control for order effects. As a control for volume effects after the individual metabolite administrations, three of the four subjects received two infusions (10 minutes apart) of the “neutral solution (pH 7.4+ATP 300nM+lactate 1mM). After each infusion, non-pain and pain sensations were again recorded, and the same VRS was used to quantify sensation magnitude.
Analysis
Data from each patient was recorded by a researcher during the metabolite infusions. Data containing number of subjects reporting sensations, onset times, sensation duration, sensation descriptors, and VRS score for both non-pain and pain sensations for each metabolite series was later transferred to an Excel spreadsheet for further analysis. Mean values and Standard Error of the Mean (SEMs) for each of the interval values collected were calculated, and comparisons were made between values for non-pain and pain sensations for each metabolite series. VRS scores were analyzed first using 1-way repeated measures ANOVAs (df =5,45). For the non-pain data, F = 3.915, p< 0.005. For the pain data, F = 15.13, p< 0.00001. Individual contrasts between baseline and other solutions were analyzed using 1 tailed, paired T-tests with the a priori hypotheses being that the first three non-baseline solutions (pH 7.3, pH 7.2, pH 7.0) would evoke increased non-pain sensations while pain sensations would not be evoked until pH 7.2, and would increase in intensity as the metabolites were increased (pH 7.0, pH 6.8, pH 6.6). Similarly, VRS scores for maximum combination metabolite infusions were compared with VRS scores for the maximum of each individual metabolite using paired T-tests with the a priori hypothesis being that the combination of metabolites would have a higher VRS score than the mean of each of VRS scores evoked by individual maximum values of metabolites. Because of the floor effect created by the many zero VRS scores reported, VRS scores were not normally distributed. To create normality for parametric statistics, a constant (1) was added to each measurement, and this value was log transformed. No corrections were made for multiple comparisons, as only a priori hypotheses were tested. Statistical tests were not done on the duration data as no a priori hypotheses were created, and the variance in these values was high, thus, only descriptive values are shown.
Results
Of note, the adjectives, “sharp,” “stinging,” “prickling,” “pins and needles,” and “soreness,” referred to the skin above the muscle were reported by three individuals. Ultrasound examination at the completion of the infusion series in these three individuals revealed extravasation of metabolite solutions into subcutaneous tissues superficial to the muscle in these individuals. In all other subjects infusions were entirely restricted to the muscle, and the only descriptions concomitant with pain were “ache” and “hot” (See Table 3). These descriptions are reported in table 3, but for clarity, only data with the descriptors “ache” and “hot” were used in graphs and statistical analyses of pain.
Table 3.
Descriptors (in order of how many times described)
| Non-pain descriptors | Pain Descriptors | ||
|
Pressure related (total = 19) |
pressure (8) full (4) heavy (3) strip (2) puffy (1) swollen (1) |
ache (27) (modifier-dull (4)) (modifier throbbing (1)) |
|
|
Movement related (total = 17) |
shaking (9) twitching (2) effervescent (1) pulsing (1) flowing (1) shooshing (1) vibration (1) tingly (1) |
||
| Thermal (total =14) |
warm (9) cool (5) |
Hot (3) | |
|
Fatigue related (total = 9) |
fatigue (4) tired (2) exhausted (1) exercised (1) well used (1) |
(For pain sensations, only ache and hot were NOT associated with back leakage of the metabolites into the skin !) |
Sharp (3) stinging (3) soreness (4) pins and needles (1) (total = 11) |
| Other (total = 3) | pins and needles (2) raw (1) |
||
Non-pain sensations
As illustrated in figures 2 – 4, the infusion of metabolites at levels found in muscles at rest (pH 7.4+300nM ATP+1mM lactate) evoked no sensations in 9 subjects and very mild (VRS score =1) non-pain sensations in one of the 10 subjects. This lasted only 6 seconds.
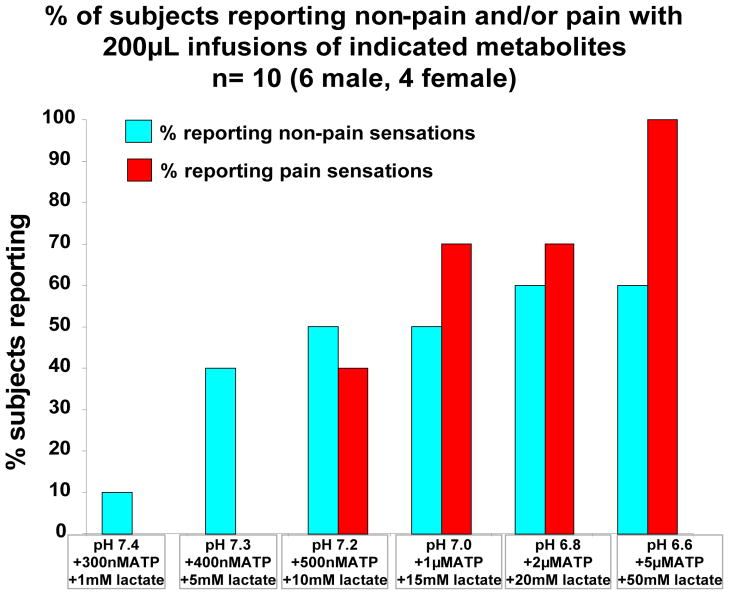
Figure 2. % of subjects reporting non-pain and/or pain with 200μL infusions of indicated metabolites n= 10 (6 male, 4 female).
% of the 10 subjects reporting non-pain and pain sensations during and/or after infusion of indicated levels of metabolite combinations. 200 μL of metabolites was infused over 30 seconds.
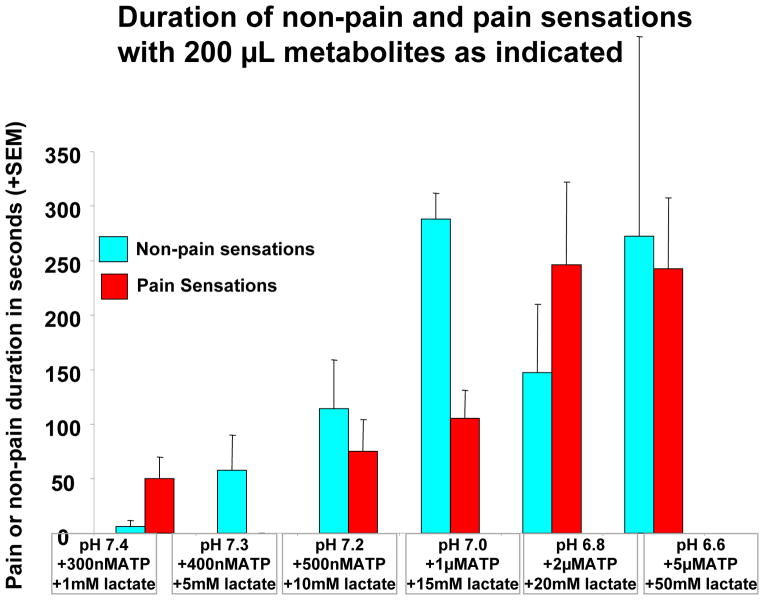
Figure 4. Duration of non-pain and pain sensations with 200 μL metabolites as indicated.
Duration of non-pain and pain sensations evoked by 200 μL of metabolites as indicated above (same subjects as in previous figures). For this graph, all sensations (including “sharp,” “stinging,” “prickling,” “pins and needles,” and “soreness,”) were included. Removal of these sensations would eliminate the pain sensations at baseline, and would very slightly reduce the variance in pain duration at other metabolite levels.
Metabolites at pH 7.3+400nM ATP + 5mM lactate (found with minimal muscle contraction) evoked fatigue related descriptions (fatigue, warming, flowing, pulsation, see also Table 3) in 4 subjects (average VRS =1.33 ± 0.6, P<0.049 when compared to baseline) (Figures 2 and 3) and evoked tremor in the APB muscle in 2 subjects.
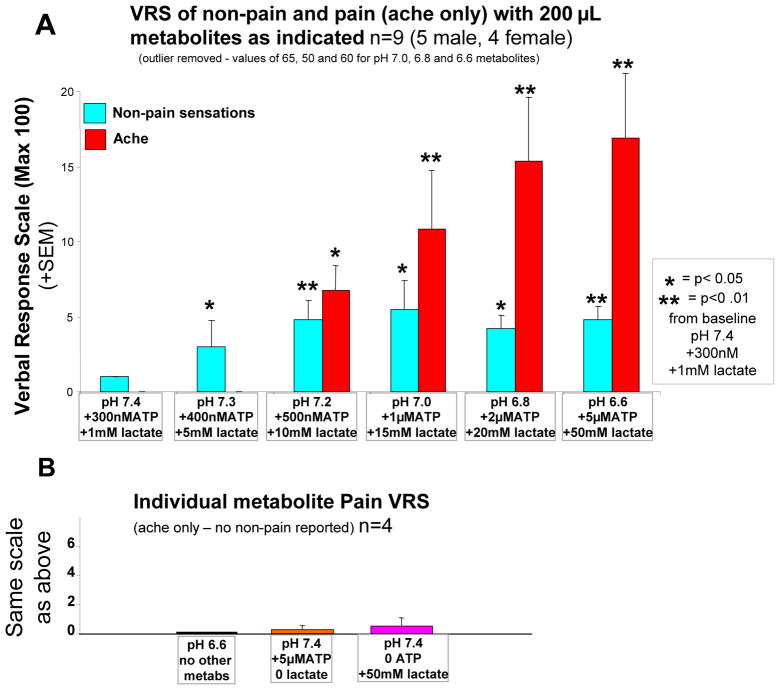
Figure 3.
A, average of verbal reports of amount of non-pain and pain sensations with combinations of metabolites as indicated (n=9, 5 male 4 female). One subject (male) was removed because of VRS scores for non-pain sensations 10 times greater than any other subjects for the highest 3 metabolite infusions. B, average verbal reports of amount of pain sensations caused by 200 μL infusions of individual metabolites indicated below each bar (n=4, 2 male, 2 female).
* P<.05, ** P<.01 increase from baseline -- paired T-test.
Metabolites at pH 7.2+500nM ATP+10mM lactate evoked non-pain sensations in 5 subjects, with “pressure” being the most common descriptor. Average VRS was 2.90 ± 1.08 (P< 0.01, when compared to baseline).
At pH 7.0+1μM ATP+15mM lactate (found with moderate exercise) metabolites evoked non-pain sensations in 5 subjects with an average VRS of 2.44 (± 1.25) P<0.03, (0.04) and lasting 276 (± 195) seconds (for this VRS score, a single outlier was removed). This male outlier reported a non-pain VRS score of 65 (the highest other score was 10) for this metabolite administration. If this outlier remained in the analysis, the mean and SEM would have been 8.70 and 6.69 respectively).
“Warm” and “tired” were the most common descriptors (others included “full”, “fatigued”, “strip-like”, “shaking”, and “vibration”). In most cases, the non-pain sensations were referred to large regions of the thumb, sometimes including the entire region from the origin of the muscle to the insertion (this resulted in the description of “strip-like”). The sensations waxed and waned in a pulse like fashion. However, the increases and decreases in sensation were not related to either the heart rate or respiratory rate as determined by manual palpation of pulse and observation of respiratory movements by the anesthesiologist.
Higher levels of metabolites evoked no greater non-pain sensations (VRS score 2.33 ± 0.9 and 2.67 ± 1.0 for pH 6.8 +2μMATP + 20mM lactate and pH 6.6 + 5 μMATP + 50mM lactate respectively) although VRS scores at both of these levels were different from the baseline, P<0.02 and 0.01 respectively. The most common specific non-pain sensations described by participants for these last two metabolite levels were “shaking”, “pressure” and “warm”, see also Table 3).
Figure 4 indicates that the duration of non-pain and pain sensations increased in asimilar pattern to the pain VRS scores as metabolite concentrations were increased.
It should be noted that none of the “pressure” related descriptions were accompanied by actual swelling of the thumb. In addition, ultrasound examination of the thumb indicated that no “pocket” of metabolites was formed with the injection that could have been perceived as pressure or swelling when these descriptors were used. Similarly, when warm or cooling were described, the thumb was not actually warmer or cooler. Movement descriptors were also not accompanied by actual movement of the solutions in the muscle. In all cases, the solution administration had ended long before the subjects reported any movement related sensations. “Shaking” and tremor related descriptions, however, were accompanied by actual tremor of the ADP muscle of the thumb.
Pain sensations
Figures 2 – 4 also show that metabolites at levels found in muscles at rest (pH 7.4+300nM ATP+1mM lactate) evoked no sensations of ache or hot. Higher metabolites at pH 7.3+400nM ATP + 5mM lactate (found with minimal muscle contraction) also evoked no pain sensations. pH 7.2+500nM ATP+10mM lactate evoked some pain sensations in 4 subjects (ache, VRS 6.8, P<0.05 when compared to baseline). However, pH 7.0+1μM ATP+15mM lactate (found with moderate exercise) evoked stronger pain sensations in 7 of the 10 subjects (VRS =10.9, P<0.01 when compared to either baseline or pH 7.2 metabolites). Higher levels of metabolites (corresponding to extreme exercise levels) evoked more and stronger pain sensations in all 10 subjects (VRS = 15.4 and 16.9 for pH 6.8 and 6. 6 respectively, max VRS = 50, P< 0.001 for both). While VRS at 6.6 and 6.8 were not different from each other, 6.6 was different from VRS at pH 7.0 (P<.00003).
Consistent adjectives corresponding to pain sensations used by subjects were the words “ache” and “hot”, see also Table 3. Sensations of ache and hot were most often referred to the base of the thumb, a site remote from the location of the insertion or the tip of the infusion needle. In some cases, referral extended to the back of the thumb, and to the adjacent index finger. As with non-pain sensations, pain sensations waxed and waned in a manner that was described as “throbbing” by several subjects. Again, the throbbing frequency was not related to either the heart rate or respiratory rate.
As with the non-pain descriptors, sensations of ache were not accompanied by actual swelling of the ADP, or by pockets of metabolites building up in the muscle. Sensations of “hot” were also not related to actual heating of the ADP muscle by the administered solutions.
Individual Metabolites
Four healthy volunteers received infusions of 200μL over 30 seconds of individual metabolites at concentrations corresponding to levels that accumulate in muscle interstitium during extreme exercise (pH 6.6, or ATP 5uM, or lactate 50mM, see Table 2). As shown in figure 3B, infusions of pH6.6 with no ATP or lactate (protons only) evoked no non-pain or pain sensations, as measured by VRS scores and was significantly less than VRS evoked by the combination of all three metabolites at pH 6.6 in the opposite thumb of these same 4 subjects (P<0.02, paired T-test). Similarly, infusions of 5μM ATP at resting values of pH and no lactate (ATP only) produced a mean VRS score of 0.25, significantly less than this same concentration of ATP when combined with protons and lactate in these same 4 subjects (P< 0.02). Lactate at 50mM at resting values of pH with no added ATP (lactate only) evoked an average VRS score of 0.5, again significantly less that the same concentration of lactate when combined with the other two metabolites (P<0.01). This compares with the average VRS score of 16 from all 10 subjects when all three of these same metabolites were administered together.
Control Side injections
Controls for volume effects on evoked sensations included injections in the thumb on the side opposite to the active metabolite injections in 5 subjects. This consisted of a series of six infusions of neutral resting solution (pH 7.4+300nM ATP+1mM lactate-all solutions buffered with 30mM phosphate), each infusion being 200 μL over 30 seconds. One subject reported the non-pain sensation of warmth (VRS=2) after the 6th presentation. None of the other 3 subjects reported any non-pain sensations. Three subjects reported no pain sensations during or after the first three presentations of the neutral solutions. One subject reported ache and sharp around the needle site during the first three injections. This subject and two others (total 3) reported a mild ache or soreness (average VRS 6.25) after the 4th presentation. However, two of these 3 subjects reported no sensations with the 5th presentation. The other subject continued to report “sharp” localized to the needle tip with the 5th presentation. With the 6th and final presentation, two subjects reported no sensations, while one reported a mild “twinge” VRS=2 around the needle while the other reported mild ache, VRS=2, referred to the thumb. Overall, minimal pain sensations were induced with these infusions (mean VRS = 3.30, average duration = 86.22 seconds).
Discussion
Our results suggest at least two populations of sensory neurons in humans that are activated by intramuscular metabolites: one mainly responding to lower metabolite concentrations causing the perception of fatigue, the other mainly responding to higher metabolite concentrations and causing the sensation of pain. As shown in figures 2 – 4, fatigue-related descriptions and duration of sensations increased linearly as the concentrations of the infused metabolites increased from resting values to levels corresponding to moderate to high intensity exercise. Higher levels of infused metabolites produced no further increases in fatigue sensations. The results for ache and hot pain sensations differed substantially from this pattern. Low levels of metabolites evoked no pain sensations; pH 7.2, 1μM ATP, 10mM lactate elicited mild pain sensations in 4 subjects, and as metabolite levels increased beyond this, pain sensations continued to show a linear increase to the maximum levels applied, pH 6.6, 5 μM ATP, 50mM lactate (see figures 2–4).
Agreement with Previous Animal Studies
The current findings are consistent with calcium responses previously seen in isolated mouse dorsal root ganglion neurons (DRG) and in responses of identified group III and IV fibers innervating the mouse foot muscles (Light et al., 2008; Jankowski et al., 2013). These previous experiments also showed two distinct populations of skeletal muscle afferents that respond to metabolites. One population responded to non-painful levels of metabolites, the other population only responded to levels of metabolites that are associated with ischemic and painful muscle contractions. In addition, our mouse experiments also documented that no individual metabolite in the physiological range, activated calcium responses in DRG.
Two subsets of metabosensitive muscle afferents in humans?
Subjects’ sensation descriptors showed two sets of adjectives consistently used. One set was elicited by metabolites at low concentrations, the other by higher concentrations of metabolites. The adjectives used at lower concentrations were non-pain descriptors. These included 8 traditional post-exercise fatigue adjectives, “tired, fatigued, tremor, twitching, shaking, pressure, heaviness, and exhausted” (Blanchard et al., 2001; Semmler et al., 2007), as well as other descriptors consistent with non-painful muscle activity, “pulsing, puffy, swollen, flowing, effervescent, cool, and warm.” We believe all of these adjectives were related to sensations of muscle fatigue similar to that experienced during and immediately after moderate to heavy exercise.
We also found a distinct set of adjectives used by subjects during infusion of higher concentrations of metabolites. In this case, adjectives were synonymous with pain and included “ache” and “hot.” Interestingly, unlike previous muscle infusion experiments that used hypertonic saline (Graven-Nielsen et al., 2003), or very low pH using phosphate buffered saline (Frey Law et al., 2008), “cramp” was not used by any of the subjects in the present study. Similarly, microneurographical stimulation of group III mechanoreceptors evoked the sensation of “cramp,” not ache or hot (Marchettini et al., 1996). Whether this is due to quantitative differences in the amount of pain evoked in the present study, or if this represents qualitative differences in the types of sensory afferents activated with the combination of metabolites used hereis unknown. Taken together, these findings suggest that low levels of metabolites activate muscle sensory neurons that contribute to the perception of fatigue, while high levels of muscle metabolites activate muscle sensory neurons that contribute to the perception of pain.
Combinations of Metabolites are necessary to evoke sensations of fatigue and ache
A critical finding of our experiments is that individual metabolites are ineffective at evoking the perception of fatigue or pain when applied at concentrations occurring during normal and even ischemic exercise (Figure 3). This is consistent with previous findings in animal models that showed that individually applied ATP, lactate, or protons were ineffective at low concentrations and only evoked responses when applied at very high concentrations corresponding to vascular and muscle injury (Rybicki et al., 1985; Graham et al., 1986; Kaufman & Rybicki, 1987; Thimm & Baum, 1987; Rotto & Kaufman, 1988; Sinoway et al., 1993; Reeh & Kress, 2001; Li & Sinoway, 2002; Reinohl et al., 2003; Hanna & Kaufman, 2004; Hoheisel et al., 2004; Kindig et al., 2006; Light et al., 2008).
Conversely, the combination of at least 3 of the metabolites actually produced by muscle contraction (protons, lactate and ATP) activate a large proportion of the sensory neurons innervating skeletal muscle, and evoke sensations of fatigue and pain in humans. This synergy of metabolites activating muscle innervating sensory neurons was suggested in previous animal studies (Immke & McCleskey, 2001b, 2003; Naves & McCleskey, 2005; Yagi et al., 2006; Birdsong et al., 2010). These investigations also suggested that ATP, via a P2X receptor, enhanced ASIC3 responses to protons. TRPV1 receptors may also play a role in this synergy (Light et al., 2008). Since the present study showed that these same metabolites evoke sensations of fatigue and pain when injected into human skeletal muscle, it is possible that ASICs, P2X, and TRPV1 receptors also mediate the response of muscle afferents to intramuscular concentrations of contraction induced metabolites in humans as well.
Several other metabolites increase with muscle contraction and may also enhance the sensory responses to muscle contraction in humans. These include bradykinin, potassium, and arachidonic acid metabolites such as prostaglandin E2 and various cytokines (Mense & Schmidt, 1974; Mense, 1977; Kaufman et al., 1983; Rotto & Kaufman, 1988; Hoheisel et al., 2005; Cui et al., 2007). It is also possible that these metabolites either directly stimulate, or enhance the sensitivity of metaboreceptors under a variety of conditions such as metabolic derangements and disease states.
Implications of Findings
Some of the many interesting implications of the current findings are listed below.
Because the same combination of metabolites that evokes sensations of fatigue at low concentrations evokes pain at higher concentrations, disease or stress might change the relative ratio of these metabolites to differentially affect muscle pain vs. fatigue. Further, this implies that disorders altering sensory fatigue could also alter muscle pain.
The synergistic action of metabolites explains why most conditions that cause large increases in the individual metabolites (for example metabolic acidosis) do not cause sensations of fatigue and muscle pain.
The temporal and spatial differences reported for ache vs. hot imply that different sensory neurons may signal hot vs. ache. Previous studies showed that only 48°C evoked sensations of pain in human muscle (Graven-Nielsen et al., 2002). We found that combinations of metabolites in muscle evoked the non-pain sensation of “warmth”, and higher levels evoked “hot”. This suggests that metabolites may be a better stimulus for sensations of warm and hot in muscle than actual increases in muscle temperature.
The “throbbing” nature of aching pain may not be related to muscle movement, heart rate or ventilation. Instead this phenomenon may be related to the mechanisms of sensory receptor activation or brain processing as per (Mo et al., 2013).
Metabolites evoked sensations of mechanical movement, including pressure, heavy, flowing and vibration in the absence of any mechanical stimulus. This suggest that metabolites activate at least some mechanoreceptive neurons in skeletal muscle.
Experimental Considerations
We recognize several potential limitations of our experiments. Metabolite infusions were not blinded or randomized, and subjects knew when an infusion was occurring. Because we were unsure of the potential intensity of evoked sensations we administered metabolite solutions in an ascending series from resting levels to levels associated with ischemia. Although this could have led to a progressive accumulation of metabolites within the muscle, this potential scenario is unlikely since we waited, before administering the next dose, for at least 5 min after the sensations of the previous infusion has ceased. Furthermore, because we administered all components of the interstitial fluid with the metabolites, the resulting final concentration of metabolites should not exceed the concentration injected, and likely will be less. We also ruled out volume effects in these experiments using 6 infusions of resting metabolites in the contralateral APB, which evoked minimal sensations.
The average VRS values were low (average 16 out of 100 maximum for pain). However two subjects’ values were 30 and 40. It is unclear if low pain VRS values resulted from the small amount of solution, and therefore area of the muscle affected, or from the dilution of the metabolites by interstitial fluid.
One problem was the often long delay between the infusion and sensations of fatigue and pain. This delay implied that the sensory neuron receptors were not near the administration site. Thus, it is possible that our assumption that receptors for the metabolites were on the superficial fascia of the muscle was incorrect.
The APB muscle is quite specialized. It is highly vascular and highly innervated. For these reasons, it is likely that at least some of the activation characteristics for sensations in other skeletal muscles will differ from those reported here.
We used an on demand method to determine subject’s descriptions of fatigue and pain sensations. This was necessary because of the short time between changes in pain and fatigue sensations we observed in pilot studies, and because of the lack of data on previous descriptions of fatigue related sensations. Using data generated from this study, a short list of descriptions from which subjects may choose may be viable in the future.
Finally, we eliminated the VRS scores resulting from reports of “sharp,” “stinging,” “prickling,” “pins and needles,” and “soreness,” from the statistical analyses and Figures 2 and 3 because they were associated with extravasation of metabolites into the skin and because these sensations were referred to the skin overlying the muscle. We inferred that these sensations were being generated by skin receptor neurons, not those found in muscle. These sensations occurred in three of the subjects, and in two of these, began with the resting levels of metabolites, and reoccurred with most of the other metabolite injections, with the VRS scores diminishing as the metabolite concentrations increased. Removing these sensations from the analyses had no effect on significant differences reported here but did cause a small decrease in the variance, and removed the appearance of pain sensations at rest in Figures 2 and 3, which is shown in the uncorrected duration graph in figure 4.
Conclusion
Our findings suggest that physiological levels of metabolites produced by contracting muscles - specifically protons, lactate, and ATP - activate human muscle afferent neurons via metabolite receptors similar to those found in animal skeletal muscle. These receptors likely include ASICs, at least one P2X, and TRPV1. Furthermore, all three metabolites are required for the sensations of fatigue and pain, though it is possible that other metabolites and receptors are also involved.
In addition, the muscle afferents in humans responding to these metabolites appear to consist of at least two separate populations. One population responds to lower levels of metabolites and is associated with the perception of exercise-induced fatigue. The other population responds to higher levels of metabolites that are seen with ischemic exercise, or during post-exercise muscle ischemia, and is associated with the perception of pain. This is consistent with prior animal experiments.
Supplementary Material
What is the central question of this study?
Can physiological concentrations of metabolite combinations evoke sensations of fatigue and pain when injected into skeletal muscle? If so, what sensations are evoked?
What is the main finding and its importance?
Low concentrations of protons, lactate and ATP evoked sensations related to fatigue. Higher concentrations of these metabolites evoked pain. Single metabolites evoked no sensations. This suggests that an ASIC receptor combined with a P2X receptor are required for signaling fatigue and pain. They also suggest 2 types of sensory neurons encode metabolites. One detects low concentrations of metabolites and signals sensations of fatigue. The other detects higher levels of metabolites and signals ache and hot.
Acknowledgments
Funding:
These studies were funded by a grant from the University of Utah Department of Anesthesiology and by NIAMS AR060336 to KCL. Markus Amann and A. R. Light were supported by a grants from NHLBI (HL-103786) and (HL107529) respectively.
Footnotes
Competing interests
None of the investigators have financial or other obligations that constitute a conflict of interest related to the outcome of these studies.
- Dr. Kelly A. Pollak, MD. Dr. Pollak was the lead author on this manuscript. She also was involved in the final design for the experiments reported here. She participated in all studies, mixed metabolite solutions, and was responsible for collecting behavioral data from each subject. She helped in the analysis of this data.
- Dr. Jeffrey D. Swenson, MD. Dr. Swenson designed the method for obtaining ultrasound recordings from all subjects. He determined the best method for determining the position of the tip of the needle in the APB muscle. He also helped design the administration protocol. He participated in many of the studies reported here.
- Dr. Timothy A. Vanhaitsma, Ph.D. Dr. Vanhaitsma helped design the composition of the metabolite solutions, and helped prepare and deliver solutions to all subjects. He helped in the preparation of this manuscript. He also helped conduct preliminary experiments essential for the experiments reported here.
- Ronald W. Hughen. Ronald Hughen designed the composition for the metabolite solutions, tested these metabolites on mouse models prior to human studies, helped anesthesiologists mix the solutions used with subjects, helped with all methods reported here, and helped with the preparation of this manuscript.
- Dr. Daehyun Jo, MD. Daehyun Jo helped with placing needles and catheters using ultrasound for placements, did ultrasound checks on location of metabolite administration, checked for leakage, interpreted ultrasound images. He also helped with the design of administration equipment, and helped in the preparation of this manuscript.
- Dr. Kathleen C. Light, Ph.D. Dr. Light helped conceptualize these experiments. She helped translate the mouse data on which these experiments are based to make the appropriate changes in protocol that could be used with human subjects. She helped design the first experiments described here, and worked out the method for collecting the behavioral data reported here. She wrote the IRB protocol for the studies reported here. She helped with the analysis behavioral data reported here, and helped with manuscript preparation.
- Dr. Petra Schweinhardt, M.D. Dr. Schweinhardt participated in the pilot studies that led to the data reported here. She was instrumental in designing the infusion device, and in helping to design the behavioral measurements, and making the first behavioral measurements in these experiments. She was the driving force behind the first experiments, and contributed intellectually as well as physically in the preparation of this manuscript.
- Dr. Markus Amann, Ph.D. Dr. Amann is an expert in fatigue research. He helped design the fatigue component of the experiments reported here. He helped collect the behavioral and ultrasound data from the subjects reported here.
- Dr. Alan R. Light, Ph.D. Dr. Light was the senior investigator for this project. He conceived the idea that synergistic metabolites could encode fatigue in addition to muscle pain. He conceived of, and designed and conducted the mouse experiments on which these human experiments are based. He participated in all pilot and full experiments. He helped design the metabolite solutions used here, and helped with the collection of behavioral data. He also helped analyze the data, and created all figures and tables in this manuscript. He also helped with the completion of this manuscript.
References
- Adreani CM, Hill JM, Kaufman MP. Responses of group III and IV muscle afferents to dynamic exercise. J Appl Physiol. 1997;82:1811–1817. doi: 10.1152/jappl.1997.82.6.1811. [DOI] [PubMed] [Google Scholar]
- Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol. 1937;89:372–383. doi: 10.1113/jphysiol.1937.sp003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsop P, Cheetham M, Brooks S, Hall GM, Williams C. Continuous intramuscular pH measurement during the recovery from brief, maximal exercise in man. Eur J Appl Physiol Occup Physiol. 1990;59:465–470. doi: 10.1007/BF02388630. [DOI] [PubMed] [Google Scholar]
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol. 2010;109:966–976. doi: 10.1152/japplphysiol.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Implications of group III and IV muscle afferents for high-intensity endurance exercise performance in humans. J Physiol. 2011a;589:5299–5309. doi: 10.1113/jphysiol.2011.213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Dempsey JA. Locomotor muscle fatigue modifies central motor drive in healthy humans and imposes a limitation to exercise performance. J Physiol. 2008;586:161–173. doi: 10.1113/jphysiol.2007.141838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol. 2009;587:271–283. doi: 10.1113/jphysiol.2008.163303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR, Richardson RS. On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol. 2011b;589:3855–3866. doi: 10.1113/jphysiol.2011.209353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Venturelli M, Ives SJ, McDaniel J, Layec G, Rossman MJ, Richardson RS. Peripheral fatigue limits endurance exercise via a sensory feedback-mediated reduction in spinal motoneuronal output. J Appl Physiol. 2013;115:355–364. doi: 10.1152/japplphysiol.00049.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres KH, von During M, Schmidt RF. Sensory innervation of the Achilles tendon by group III and IV afferent fibers. Anat Embryol. 1985;172:145–156. doi: 10.1007/BF00319597. [DOI] [PubMed] [Google Scholar]
- Bangsbo J, Johansen L, Graham T, Saltin B. Lactate and H+ effluxes from human skeletal muscles during intense, dynamic exercise. J Physiol. 1993;462:115–133. doi: 10.1113/jphysiol.1993.sp019546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsbo J, Krustrup P, Gonzalez-Alonso J, Saltin B. ATP production and efficiency of human skeletal muscle during intense exercise: effect of previous exercise. Am J Physiol Endocrinol Metab. 2001;280:E956–964. doi: 10.1152/ajpendo.2001.280.6.E956. [DOI] [PubMed] [Google Scholar]
- Benson CJ, Eckert SP, McCleskey EW. Acid-evoked currents in cardiac sensory neurons: A possible mediator of myocardial ischemic sensation. Circ Res. 1999;84:921–928. doi: 10.1161/01.res.84.8.921. [DOI] [PubMed] [Google Scholar]
- Birdsong WT, Fierro L, Williams FG, Spelta V, Naves LA, Knowles M, Marsh-Haffner J, Adelman JP, Almers W, Elde RP, McCleskey EW. Sensing muscle ischemia: coincident detection of acid and ATP via interplay of two ion channels. Neuron. 2010;68:739–749. doi: 10.1016/j.neuron.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard CM, Rodgers WM, Spence JC, Courneya KS. Feeling state responses to acute exercise of high and low intensity. J Sci Med Sport. 2001;4:30–38. doi: 10.1016/s1440-2440(01)80005-0. [DOI] [PubMed] [Google Scholar]
- Bove GM, Light AR. Unmyelinated nociceptors of rat paraspinal tissues. J Neurophysiol. 1995;73:1752–1762. doi: 10.1152/jn.1995.73.5.1752. [DOI] [PubMed] [Google Scholar]
- Connor M, Naves LA, McCleskey EW. Contrasting phenotypes of putative proprioceptive and nociceptive trigeminal neurons innervating jaw muscle in rat. Mol Pain. 2005;1:31. doi: 10.1186/1744-8069-1-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppieters MW, Alshami AM, Hodges PW. An experimental pain model to investigate the specificity of the neurodynamic test for the median nerve in the differential diagnosis of hand symptoms. Arch Phys Med Rehabil. 2006;87:1412–1417. doi: 10.1016/j.apmr.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Cui J, McQuillan P, Momen A, Blaha C, Moradkhan R, Mascarenhas V, Hogeman C, Krishnan A, Sinoway LI. The role of the cyclooxygenase products in evoking sympathetic activation in exercise. Am J Physiol Heart Circ Physiol. 2007;293:H1861–1868. doi: 10.1152/ajpheart.00258.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decherchi P, Darques JL, Jammes Y. Modifications of afferent activities from Tibialis anterior muscle in rat by tendon vibrations, increase of interstitial potassium or lactate concentration and electrically-induced fatigue. J Peripher Nerv Syst. 1998;3:267–276. [PubMed] [Google Scholar]
- Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain. 2011;152:2399–2404. doi: 10.1016/j.pain.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Frey Law LA, Sluka KA, McMullen T, Lee J, Arendt-Nielsen L, Graven-Nielsen T. Acidic buffer induced muscle pain evokes referred pain and mechanical hyperalgesia in humans. Pain. 2008;140:254–264. doi: 10.1016/j.pain.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Gautam M, Benson CJ. Acid-sensing ion channels (ASICs) in mouse skeletal muscle afferents are heteromers composed of ASIC1a, ASIC2, and ASIC3 subunits. FASEB J. 2013;27:793–802. doi: 10.1096/fj.12-220400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R, Jammes Y, Delpierre S, Grimaud C, Roussos C. The effects of ischemia, lactic acid and hypertonic sodium chloride on phrenic afferent discharge during spontaneous diaphragmatic contraction. Neurosci Lett. 1986;67:257–262. doi: 10.1016/0304-3940(86)90318-6. [DOI] [PubMed] [Google Scholar]
- Graham T, Bangsbo J, Saltin B. Skeletal muscle ammonia production and repeated, intense exercise in humans. Can J Physiol Pharmacol. 1993;71:484–490. doi: 10.1139/y93-070. [DOI] [PubMed] [Google Scholar]
- Graven-Nielsen T. Fundamentals of muscle pain, referred pain, and deep tissue hyperalgesia. Scand J Rheumatol Suppl. 2006;122:1–43. doi: 10.1080/03009740600865980. [DOI] [PubMed] [Google Scholar]
- Graven-Nielsen T, Arendt-Nielsen L, Mense S. Thermosensitivity of muscle: high-intensity thermal stimulation of muscle tissue induces muscle pain in humans. J Physiol. 2002;540:647–656. doi: 10.1113/jphysiol.2001.013336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graven-Nielsen T, Jansson Y, Segerdahl M, Kristensen JD, Mense S, Arendt-Nielsen L, Sollevi A. Experimental pain by ischaemic contractions compared with pain by intramuscular infusions of adenosine and hypertonic saline. Eur J Pain. 2003;7:93–102. doi: 10.1016/s1090-3801(02)00069-1. [DOI] [PubMed] [Google Scholar]
- Hanna RL, Kaufman MP. Activation of thin-fiber muscle afferents by a P2X agonist in cats. J Appl Physiol. 2004;96:1166–1169. doi: 10.1152/japplphysiol.01020.2003. [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Maclean D, Radegran G, Saltin B, Bangsbo J. Adenosine concentrations in the interstitium of resting and contracting human skeletal muscle. Circulation. 1998;98:6–8. doi: 10.1161/01.cir.98.1.6. [DOI] [PubMed] [Google Scholar]
- Hill JM, Pickar JG, Parrish MD, Kaufman MP. Effects of hypoxia on the discharge of group III and IV muscle afferents in cats. J Appl Physiol. 1992;73:2524–2529. doi: 10.1152/jappl.1992.73.6.2524. [DOI] [PubMed] [Google Scholar]
- Hoheisel U, Reinohl J, Unger T, Mense S. Acidic pH and capsaicin activate mechanosensitive group IV muscle receptors in the rat. Pain. 2004;110:149–157. doi: 10.1016/j.pain.2004.03.043. [DOI] [PubMed] [Google Scholar]
- Hoheisel U, Unger T, Mense S. Excitatory and modulatory effects of inflammatory cytokines and neurotrophins on mechanosensitive group IV muscle afferents in the rat. Pain. 2005;114:168–176. doi: 10.1016/j.pain.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Immke DC, McCleskey EW. ASIC3: a lactic acid sensor for cardiac pain. TheScientificWorldJournal. 2001a;1:510–512. doi: 10.1100/tsw.2001.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci. 2001b;4:869–870. doi: 10.1038/nn0901-869. [DOI] [PubMed] [Google Scholar]
- Immke DC, McCleskey EW. Protons open acid-sensing ion channels by catalyzing relief of Ca2+ blockade. Neuron. 2003;37:75–84. doi: 10.1016/s0896-6273(02)01130-3. [DOI] [PubMed] [Google Scholar]
- Jankowski MP, Rau KK, Ekmann KM, Anderson CE, Koerber HR. Comprehensive phenotyping of group III and IV muscle afferents in mouse. J Neurophysiol. 2013;109:2374–2381. doi: 10.1152/jn.01067.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol. 1983;55:105–112. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Rybicki KJ. Discharge properties of group III and IV muscle afferents: their responses to mechanical and metabolic stimuli. Circ Res. 1987;61:I60–65. [PubMed] [Google Scholar]
- Kaufman MP, Waldrop TG, Rybicki KJ, Ordway GA, Mitchell JH. Effects of static and rhythmic twitch contractions on the discharge of group III and IV muscle afferents. Cardiovasc Res. 1984;18:663–668. doi: 10.1093/cvr/18.11.663. [DOI] [PubMed] [Google Scholar]
- Khan MH, Sinoway LI. Muscle reflex control of sympathetic nerve activity in heart failure: the role of exercise conditioning. Heart Fail Rev. 2000;5:87–100. doi: 10.1023/A:1009802308872. [DOI] [PubMed] [Google Scholar]
- Kindig AE, Hayes SG, Hanna RL, Kaufman MP. P2 antagonist PPADS attenuates responses of thin fiber afferents to static contraction and tendon stretch. Am J Physiol Heart Circ Physiol. 2006;290:H1214–H1219. doi: 10.1152/ajpheart.01051.2005. [DOI] [PubMed] [Google Scholar]
- Kniffki KD, Mense S, Schmidt RF. Responses of group IV afferent units from skeletal muscle to stretch, contraction and chemical stimulation. Exp Brain Res. 1978;31:511–522. doi: 10.1007/BF00239809. [DOI] [PubMed] [Google Scholar]
- Kos D, Kerckhofs E, Nagels G, D’Hooghe MB, Ilsbroukx S. Origin of fatigue in multiple sclerosis: review of the literature. Neurorehabil Neural Repair. 2008;22:91–100. doi: 10.1177/1545968306298934. [DOI] [PubMed] [Google Scholar]
- Li J, King NC, Sinoway LI. ATP concentrations and muscle tension increase linearly with muscle contraction. J Appl Physiol. 2003;95:577–583. doi: 10.1152/japplphysiol.00185.2003. [DOI] [PubMed] [Google Scholar]
- Li J, King NC, Sinoway LI. Interstitial ATP and norepinephrine concentrations in active muscle. Circulation. 2005;111:2748–2751. doi: 10.1161/CIRCULATIONAHA.104.510669. [DOI] [PubMed] [Google Scholar]
- Li J, Sinoway LI. ATP stimulates chemically sensitive and sensitizes mechanically sensitive afferents. Am J Physiol Heart Circ Physiol. 2002;283:H2636–H2643. doi: 10.1152/ajpheart.00395.2002. [DOI] [PubMed] [Google Scholar]
- Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol. 2008;100:1184–1201. doi: 10.1152/jn.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchettini P, Simone DA, Caputi G, Ochoa JL. Pain from excitation of identified muscle nociceptors in humans. Brain Res. 1996;740:109–116. doi: 10.1016/s0006-8993(96)00851-7. [DOI] [PubMed] [Google Scholar]
- Mense S. Nervous outflow from skeletal muscle following chemical noxious stimulation. J Physiol. 1977;267:75–88. doi: 10.1113/jphysiol.1977.sp011802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S, Schmidt RF. Activation of group IV afferent units from muscle by algesic agents. Brain Res. 1974;72:305–310. doi: 10.1016/0006-8993(74)90870-1. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol. 1983;45:229–242. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Mierzwiak DS, Wildenthal K, Willis WD, Jr, Smith AM. Effect on left ventricular performance of stimulation of an afferent nerve from muscle. Circ Res. 1968;22:507–516. doi: 10.1161/01.res.22.4.507. [DOI] [PubMed] [Google Scholar]
- Mo J, Maizels M, Ding M, Ahn AH. Does throbbing pain have a brain signature? Pain. 2013;154:1150–1155. doi: 10.1016/j.pain.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr M, Krustrup P, Nielsen JJ, Nybo L, Rasmussen MK, Juel C, Bangsbo J. Effect of two different intense training regimens on skeletal muscle ion transport proteins and fatigue development. Am J Physiol Regul integr Comp Physiol. 2007;292:R1594–1602. doi: 10.1152/ajpregu.00251.2006. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Immke DC, Fierro L, Pare M, Rice FL, McCleskey EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol pain. 2005;1:35. doi: 10.1186/1744-8069-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naves lA, McCleskey EW. An acid-sensing ion channel that detects ischemic pain. Braz J Med Biol Res. 2005;38:1561–1569. doi: 10.1590/s0100-879x2005001100001. [DOI] [PubMed] [Google Scholar]
- Reeh PW, Kress M. Molecular physiology of proton transduction in nociceptors. Curr Opin Pharmacol. 2001;1:45–51. doi: 10.1016/s1471-4892(01)00014-5. [DOI] [PubMed] [Google Scholar]
- Reinohl J, Hoheisel U, Unger T, Mense S. Adenosine triphosphate as a stimulant for nociceptive and non-nociceptive muscle group IV receptors in the rat. Neurosci Lett. 2003;338:25–28. doi: 10.1016/s0304-3940(02)01360-5. [DOI] [PubMed] [Google Scholar]
- Rotto DM, Kaufman MP. Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol. 1988;64:2306–2313. doi: 10.1152/jappl.1988.64.6.2306. [DOI] [PubMed] [Google Scholar]
- Rybicki KJ, Waldrop TG, Kaufman MP. Increasing gracilis muscle interstitial potassium concentrations stimulate group III and IV afferents. J Appl Physiol. 1985;58:936–941. doi: 10.1152/jappl.1985.58.3.936. [DOI] [PubMed] [Google Scholar]
- Secher NH, Amann M. Human investigations into the exercise pressor reflex. Exp Physiol. 2012;97:59–69. doi: 10.1113/expphysiol.2011.057679. [DOI] [PubMed] [Google Scholar]
- Semmler JG, Tucker KJ, Allen TJ, Proske U. Eccentric exercise increases EMG amplitude and force fluctuations during submaximal contractions of elbow flexor muscles. J Appl Physiol. 2007;103:979–989. doi: 10.1152/japplphysiol.01310.2006. [DOI] [PubMed] [Google Scholar]
- Sinoway LI, Hill JM, Pickar JG, Kaufman MP. Effects of contraction and lactic acid on the discharge of group III muscle afferents in cats. J Neurophysiol. 1993;69:1053–1059. doi: 10.1152/jn.1993.69.4.1053. [DOI] [PubMed] [Google Scholar]
- Smith SA, Williams MA, Mitchell JH, Mammen PP, Garry MG. The capsaicin-sensitive afferent neuron in skeletal muscle is abnormal in heart failure. Circulation. 2005;111:2056–2065. doi: 10.1161/01.CIR.0000162473.10951.0A. [DOI] [PubMed] [Google Scholar]
- Steinhagen C, Hirche HJ, Nestle HW, Bovenkamp U, Hosselmann I. The interstitial pH of the working gastrocnemius muscle of the dog. Pflugers Arch. 1976;367:151–156. doi: 10.1007/BF00585151. [DOI] [PubMed] [Google Scholar]
- Sutherland SP, Benson CJ, Adelman JP, McCleskey EW. Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc Natl Acad Sci USA. 2001;98:711–716. doi: 10.1073/pnas.011404498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimm F, Baum K. Response of chemosensitive nerve fibers of group III and IV to metabolic changes in rat muscle. Pflugers Arch. 1987;410:143–152. doi: 10.1007/BF00581907. [DOI] [PubMed] [Google Scholar]
- Yagi J, Wenk HN, Naves LA, McCleskey EW. Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ Res. 2006;99:501–509. doi: 10.1161/01.RES.0000238388.79295.4c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.