Abstract
Cells are capable of metabolizing a variety of carbon substrates, including glucose, fatty acids, ketone bodies, and amino acids. Cellular fuel choice not only fulfills specific biosynthetic needs, but also enables programmatic adaptations to stress conditions beyond compensating for changes in nutrient availability. Emerging evidence indicates that specific switches from utilization of one substrate to another can have protective or permissive roles in disease pathogenesis. Understanding the molecular determinants of cellular fuel preference may provide insights into the homeostatic control of stress responses, and unveil therapeutic targets. Here, we highlight overarching themes encompassing cellular fuel choice, its link to cell fate and function, its advantages in stress protection, and its contribution to metabolic dependencies and maladaptations in pathologic conditions.
Keywords: metabolism, nutrients, mitochondria, stress responses, biosynthesis, substrate supply
Carbon substrate choice in the programming of cell fate and function
Different cell states have specific anabolic and catabolic needs that can be met by processing distinct fuel substrates such as glucose, glutamine, amino acids, ketone bodies and fatty acids. What instructs the cells to preferentially utilize a particular carbon substrate and how does this fuel choice affect cellular fitness to respond to developmental cues, as well as to physiological and pathophysiological stress? Recent advances in metabolomics and metabolic flux analysis combined with the capacity to integrate high throughput data at the genome and proteome levels have begun to provide some answers to these questions. In the following sections, we integrate the information that has emerged from these multidisciplinary efforts in a variety of cellular systems to showcase both the complexity and common themes in the cross talk between cellular fuel choice and cell fate. Specifically, we focus on the metabolic requirements of transition in and out of quiescence, pluripotency, and proliferative state, and discuss the contribution of altered fuel choice in cellular responses to stress. The chosen examples of particular metabolic pathways or cellular systems are collectively meant to highlight the contribution of changes in cells’ fuel choice to regulation of cell fate and function in both normal and disease states.
Metabolic control of quiescent and proliferative states
Transition into and out of quiescence requires coordinate regulation of the cell cycle machinery, cellular energy status and biosynthetic needs in response to mitogens and nutrient cues. These transitions may not only be developmentally programmed but also influence the cell’s adaptive responses to stress and tissue damage. For example, entry into quiescence can protect cells from a myriad of stress stimuli, while regulated exit from quiescence may enable tissue repair and regeneration. Findings in a variety of cellular systems, including stem cells, T-lymphocytes, islet β-cells, and cancer cells have revealed that fulfillment of the anabolic and catabolic demands of these proliferative states as well as differentiation and lineage specification involve changes in carbon substrate preference.
Metabolic features of pluripotency
The metabolic profile of stem cells is shaped by their specific niche, their turnover rate, and the frequency at which they are mobilized. Hematopoietic stem cells (HSCs), neural stem cells (NSCs), and cardiac progenitors reside in hypoxic niches [1]. This is associated with increased glucose utilization, low contribution of mitochondrial oxidative phosphorylation (OXPHOS) to ATP generation, and overall reactive oxygen species (ROS) levels that are compatible with maintaining quiescence. The integrative function of several oxygen and energy sensing pathways, including HIF1α, AMPK, mTOR, and FOXO influence the maintenance of this metabolic profile, and their genetic or pharmacologic alterations can lead to premature differentiation or proliferation that ultimately exhausts the stem cell pool [2, 3]. The shift in energy metabolism away from OXPHOS also underlies the de-differentiation or re-programming of somatic cells to induced pluripotent stem cells (iPSCs) and modulation of this shift can increase or decrease the efficiency of transition to the pluripotent state [4].
The predominance of glucose utilization over OXPHOS in pluripotent cells is controlled at multiple levels, including transcriptional enrichment of glycolytic genes, downregulation of genes encoding mitochondrial respiratory chain components, inactivation of pyruvate dehydrogenase (PDH) by phosphorylation and the attendant decrease in pyruvate utilization in the tricarboxylic acid (TCA) cycle (Figure 1). These metabolic properties appear to be associated with differences in mitochondrial architecture and spatial organization within the cell marked by perinuclear localization of mitochondria and reduced cristae density [4–6]. On the other hand, morphologic remodeling of mitochondria to a tubular network with increased cristae density may facilitate stem cell differentiation, and is consistent with increased OXPHOS and reduced glycolysis [5, 7]. Whether changes in mitochondrial morphology are mainly a byproduct of the pluripotency vs. lineage specification or they directly regulate stem cell fate awaits further studies.
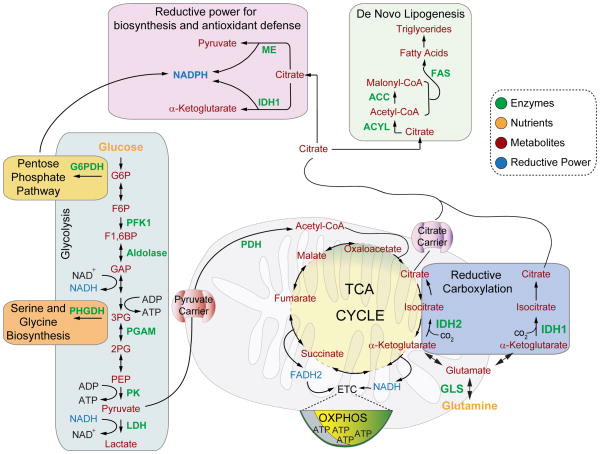
Figure 1. Schematics of select pathways in charge of glucose and glutamine metabolism, as well as metabolic outputs of citrate that relate to biosynthesis and antioxidant capacity.
Glucose-derived metabolites can be used to generate pyruvate or to supply precursors for the pentose phosphate pathway and de novo serine synthesis. Glucose-derived pyruvate can be metabolized to lactate in the cytosol or transported into mitochondria via the pyruvate carrier. Mitochondrial pyruvate can then be used for the synthesis of acetyl-CoA and citrate. Under certain circumstances, citrate can also be generated by reductive carboxylation of glutamine. Biosynthetic reactions also require reducing equivalents in the form of NADPH, which can be supplied by cytosolic citrate, via isocitrate dehydrogenase (IDH) and the malic enzyme (ME), or by the pentose phosphate pathway.
Abbreviations: FAS – Fatty acid synthase, ACC – Acetyl-CoA carboxylase, ACYL – ATP Citrate lyase, ME – Malic enzyme, IDH – Isocitrate dehydrogenase, GLS – Glutaminase, G6PDH – Glucose-6-phosphate dehydrogenase, PFK1 – Phosphofructokinase 1, PHGDH – Phosphoglycerate dehydrogenase, PGAM – Phosphoglycerate mutase, PK – Pyruvate kinase, LDH – Lactate dehydrogenase, PDH – Pyruvate dehydrogenase, ETC – Electron transport chain, OXPHOS –Oxidative phosphorylation, TCA – Tricarboxylic acid cycle.
The role of mitochondrial energy metabolism and substrate utilization in stem cells is, however, highly complex. For example, mitochondrial fatty acid oxidation (FAO) is required for the maintenance of HSCs [8]. The FAO program in these cells is regulated by the peroxisome proliferator-activated receptor (PPAR) δ that is in turn activated downstream of the promyelocytic leukemia (PML) protein. The exhaustion of HSC pool in the absence of PML or upon FAO inhibition appears to be associated with disruption of asymmetric cell divisions that normally produce a daughter HSC and a progenitor [8]. The exact metabolic consequences of FAO or fatty acid-derived metabolites that may control asymmetric cell divisions are not known. In addition, how FAO is maintained in HSCs, which are believed to reside in hypoxic niches [9], remains to be determined. These observations also suggest that a glycolytic phenotype in a hypoxic environment may not be the universal metabolic feature of all stem cells. Consistent with this idea, recent studies demonstrated clear metabolic distinctions between epiblast stem cells from the post-implantation epiblasts that are predominantly glycolytic, and embryonic stem cells derived from the pre-implantation inner cell mass that have active OXPHOS [10].
Mitochondria also contribute to stem cell growth by regulating threonine metabolism. Threonine dehydrogenase (TDH), a mitochondrial enzyme that catalyzes the conversion of threonine to glycine and acetyl-CoA (Figure 2), is significantly up-regulated in mouse ES cells compared with their differentiated progeny [11]. Glycine produced by the TDH reaction supports methyl-tetrahydrofolate (mTHF) and purine synthesis (Figure 2), which is consistent with the observed requirement of TDH activity for growth of these cells [11, 12]. Moreover, threonine catabolism contributes significantly to production of S-adenosyl methionine (SAM) (Figure 2). SAM is an important methyl donor for DNA and protein methylation. Threonine starvation of mouse ES cells leads to diminished trimethylation of histone H3 lysine 4 (H3K4me3), which appears to be associated with reduced pluripotency and increased differentiation propensity [13].
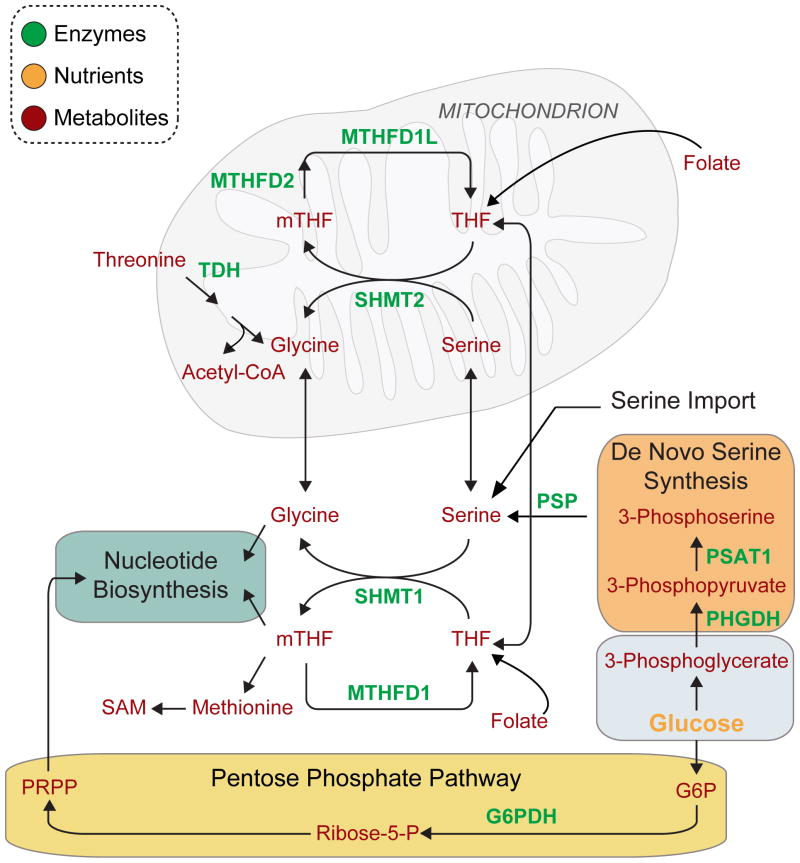
Figure 2. Nucleotide biosynthesis and the contribution of serine and glycine to one-carbon metabolism.
Nucleotide biosynthesis requires input of carbons from tetrahydrofolate (THF), glycine, and phosphoribosyl pyrophosphate (PRPP). PRPP is generated from glucose-derived carbons in the pentose phosphate pathway. Folate-derived THF can be metabolized in the cytosol or in mitochondria by the indicated enzymes. THF is converted to mTHF (methylene-tetrahydrofolate) in a cycle that is coupled to the conversion of serine to glycine. Serine, a major one-carbon donor for the synthesis of mTHF, can be imported from the extracellular milieu, synthesized from glycine, or from glucose-derived carbons. Glycine can be generated from serine or from threonine. In addition to nucleotide biosynthesis, one-carbon metabolism involving mTHF supports methionine and SAM production.
Abbreviations: SHMT1 – Serine hydroxymethyltransferase 1, SHMT2 – Serine hydroxymethyltransferase 2, MTHFDL1 – 10-formyl-tetrahydrofolate synthetase, MTHFD1 –Methylenetetrahydrofolate dehydrogenase 1, MTHFD2 – Methylenetetrahydrofolate dehydrogenase 2, PSP – Phosphoserine phosphatase, TDH – Threonine dehydrogenase, PSAT1 –Phosphoserine aminotransferase, PHGDH – 3-Phosphoglycerate dehydrogenase, mTHF –Methylene-tetrahydrofolate, THF – Tetrahydrofolate, SAM – S-Adenosyl-methionine, G6PDH –Glucose-6-phosphate dehydrogenase, PRPP – Phosphoribosyl pyrophosphate.
The transition to differentiated cell fates also involves highly regulated alterations in fuel utilization that are required for the survival and functional specialization of cell lineages. A well characterized example is the metabolic properties of T-lymphocyte subsets that parallel their specialized function in the immune system [14]. Mitochondria are the predominant source of energy in resting T cells while a switch to glycolytic metabolism marks the metabolic profile of activated T cells that undergo proliferation upon encountering their cognate antigens. Activated T cells subsequently differentiate to carry out specialized effector functions. Specifically, CD4+ T cells give rise to distinct T cell subsets with metabolic profiles that are influenced by the reciprocal activities of mTOR and AMPK [15, 16]. Among these, helper T cells subsets are involved in cell mediated and humoral immunity, and display a preferential increase in glycolytic flux even in the presence of sufficient oxygen (aerobic glycolysis) [15, 16]. Regulatory T cells (Tregs), another differentiated CD4+ T cell subset, maintain immune tolerance by suppressing other effector T cells. Tregs show higher AMPK activity and increased FAO [15]. AMPK-driven lipid oxidation is also required for the generation and survival of CD8+ memory T cells [17].
Metabolic requirements of the proliferative state
The specific needs of the proliferative state for lipids, amino acids, and nucleotides necessitate reprogramming of carbon substrate utilization and production of metabolic intermediates that are used as precursors in biosynthetic reactions [18]. Moreover, given the requisite for reducing potential to generate biomass, metabolic adaptations in the proliferative state include increased NADPH production [19]. Here, we predominantly focus on metabolic shifts that ensure provision of citrate and replenishment of the one-carbon pool for the synthesis of membrane lipids and nucleotides, respectively.
De novo fatty acid synthesis is dependent on the coordinate function of acetyl-coA carboxylase (ACC) and fatty acid synthase (FAS). ACC catalyzes the carboxylation of acetyl-CoA to malonyl-CoA and FAS combines malonyl-CoA and acetyl-CoA for fatty acid synthesis (Figure 1). Acute regulation of malonyl-CoA levels and FAS expression were recently implicated in the proliferation of neural stem and progenitor cells (NSPCs) in the adult brain. Compared with low proliferating NSPCs that have low ACC activity and FAS expression, NSPCs in neurogenic areas of the brain switch to higher FAS expression and activate ACC by down-regulating Spot14, an ACC inhibitor that keeps the enzyme inactive in the low proliferating NSPCs [20]. Differentiation of NSPCs in vitro in turn involves decreased lipogenesis. Notably, the switch to increased malonyl-CoA levels and FAS activity does not affect the proliferation of non-myelinating Schwann cells, indicating cell-type specific mechanisms that fulfill the anabolic needs of the proliferative state [20].
Cytosolic acetyl-CoA used for fatty acid synthesis is produced from citrate through the reaction catalyzed by ATP citrate lyase (ACLY) (Figure 1). Citrate is initially generated in the mitochondrion from oxaloacetate and acetyl-CoA, and subsequently transported to the cytosol by the mitochondrial citrate carrier [21]. The availability of acetyl-CoA for citrate synthesis is dependent on regulated transport of pyruvate into mitochondria and the activity of PDH (Figure 1 and Box 1). In certain cancers, elevated PDH activity is a metabolic adaptation that can support citrate production [22, 23]. Under certain conditions such as glucose deprivation or hypoxia, where normal substrate oxidation is diminished or PDH activity is low, citrate is synthesized via reductive carboxylation, which involves conversion of glutamine-derived α-ketoglutarate to isocitrate by the reverse reactions of the NADPH dependent IDH isoforms (IDH1 & IDH2) [24, 25] (Figure 1). Reductive carboxylation of glutamine is also relevant for proliferation of cells that harbor mutations in fumarate hydratase (FH), succinate dehydrogenase (SDH), or components of the mitochondrial electron transport chain [26] (Figure 1).
Box 1. Mitochondrial compartmentalization of pyruvate.
As a metabolite central to glucose, amino acids, and lipid metabolism, subcellular localization of pyruvate is an important determinant of its metabolic fate. It is therefore not surprising that multiple regulatory mechanisms control pyruvate fate, including post-translational and allosteric regulation of PDH. Characterization of small molecule inhibitors of the mitochondrial pyruvate carrier (MPC) [81] as well as the recent discovery of its evolutionarily conserved protein components, MPC1 and MPC2 [82, 83], indicate additional mechanisms that influence pyruvate compartmentalization. MPC does not share the characteristics of the mitochondrial carrier family proteins [84], and is a large oligomeric complex composed of MCP 1 and 2 in the inner mitochondrial membrane [82, 83], the stoichiometry and structural details of which await future studies. Thiazolidinediones, a class of insulin sensitizers used to slow the progression of type 2 diabetes, were recently shown to specifically inhibit MPC [85], indicating a role for mitochondrial pyruvate transport in the regulation of glucose homeostasis. Understanding MPC regulation will provide important insights into metabolic adaptation to nutrient status with relevance to cancer, diabetes and seizure disorders.
Nucleotide synthesis is another anabolic requirement for transition out of quiescence as well as rapid cell proliferation. Purine synthesis requires input of carbons from 5-phosphoribosyl-α-pyrophosphate (PRPP), glycine, and N10-formyl-tetrahydrofolate (N10-formyl-THF). PRPP is the activated form of ribose-5-phosphate derived from the pentose phosphate pathway (Figure 2). N10-formyl-THF is a one-carbon donor that can be synthesized from glycine and serine. The mitochondrial glycine biosynthetic pathway and glycine cleavage enzymes provide a significant portion of the one-carbon pool required for de novo purine biosynthesis [27] (Figure 2). Global metabolite profiling in the NCI-60 cancer cell lines indicated that glycine uptake and the mitochondrial glycine biosynthetic pathway contribute significantly to cancer cell proliferation through de novo purine biosynthesis [28].
Serine can also contribute to purine biosynthesis by influencing glycine and the one-carbon pool (Figure 2), and serine deprivation has been shown to induce a p53-dependent cell cycle arrest associated with diminished purine biosynthesis [29]. De novo serine synthesis via glucose contributes to tumor cell proliferation in breast cancer and melanoma [30, 31]. This pathway is modulated by multiple enzymes, including phosphoglycerate dehydrogenase (PHGDH), phosphoserine aminotransferase (PSAT) 1, phosphoglycerate mutase (PGAM) 1, and the M2 isoform of pyruvate kinase (PKM2) [30–33] (Figures 1 and 2). A preferential switch to the less active PKM2 isoform over the constitutively active PKM1 enzyme in rapidly proliferating cells under nutrient rich conditions allows glycolytic intermediates to be used for biosynthetic reactions such as serine production, rather than pyruvate synthesis.
Metabolic features of the quiescent state
Unlike proliferating cells, the metabolism of quiescent cells is not massively directed towards biosynthesis of lipids, nucleotides and proteins. However, the precise metabolic characteristics of quiescent cells appear to be cell type specific. Studies in many experimental systems, including B and T lymphocytes demonstrated reduced glycolytic rate and metabolic activity during quiescence [14]. However, recent work in primary human fibroblast unexpectedly revealed high metabolic activity, including marked increase in the pentose phosphate pathway (PPP) flux and NADPH-generating reactions in quiescent fibroblasts despite reduced rates of nucleotide synthesis and comparable rates of glucose consumption in quiescent and proliferating fibroblasts [34]. Remarkably, inhibition of PPP was shown to be highly toxic to quiescent fibroblasts [34], indicating survival benefits are derived from this pathway, perhaps through increased ROS detoxification capacity via NADPH produced by PPP and/or increased fatty acid synthesis, which consumes NADPH.
The cross talk between cell cycle machinery and metabolism
Transition in and out of quiescence requires coordinate fine-tuning of nutrient availability/metabolism and the cell cycle machinery. In certain cell types such as insulin producing β-cells, increased flux through glycolysis can trigger exit out of quiescence by regulating the core components of the cell cycle machinery. This is mediated, at least in part, by glucose-dependent elevation in Ca2+ flux and the attendant up-regulation of cyclin D2 expression [35]. Replicated β-cells normally enter a short period of quiescence prior to cell cycle re-entry. Glucose can shorten this period by expediting the build up of cyclin D2. Transcriptional regulation of cyclin D2 and other components of the cell cycle machinery is further controlled by the carbohydrate response element binding protein (ChREBP) that is regulated by several glucose-derived metabolites, including glucose-6-phosphate (G6P), xylulose-5-phosphate (X5P), and Fructose 2,6 bisphosphate (F2,6BP) [36–38] (Figure 1). Additional gluco-regulatory mechanisms that converge on ChREBP for the control of cell cycle transition in β-cells include post-translational modifications via O-GlcNAcylation and acetylation that modulate ChREBP protein stability and DNA binding capacity [39]. The mitogenic effect of glucose on β-cell replication has major implications for both physiologic expansion of β-cell mass in face of increased demand for insulin secretion, as well as β-cell mass regeneration upon injury [40]. In addition to receiving input from the metabolic status of the cell, the cell cycle machinery is also capable of in turn regulating glucose and glutamine metabolism [41].
The choice of carbon substrates and cellular responses to stress
The ability of cells to process alternate carbon substrates such as carbohydrates, amino acids, fatty acids, and ketone bodies can influence their capacity to sense and respond to stress stimuli. Below, we highlight examples demonstrating how fuel preference can alter the capacity of cells to meet their bioenergetic and anabolic demands under metabolic stress. Within this context, changing “cells’ appetite” for different nutrients may not only be a component of homeostatic responses to stress, but may produce targetable metabolic alterations in disease contexts.
Fatty acid oxidation in cell survival and adaptation to metabolic stress
Emerging evidence in several cancer models underscores the relevance of FAO in tumor cell survival [22, 42–48]. In ovarian cancers that metastasize to the omental fat pad of the abdomen, increased FAO is supported by lipids supplied by neighboring adipocytes. Elevated FAO in these cells is dependent on AMPK-mediated inhibition of ACC and the attendant increase in fatty acid import into the mitochondria via carnitine palmitoyl transferase 1 (CPT1) [45] (Figure 3). The survival-promoting effect of FAO has also been reported in breast cancer and acute myeloid leukemia (AML) [42, 44]. Furthermore, FAO is required for the survival of a subset of diffuse large B-cell lymphoma (DLBCL) that harbors the gene signature of mitochondrial oxidative phosphorylation (OxPhos DLBCL) and shows a preference for fatty acids as fuel substrates [22]. OxPhos DLBCLs are distinct from B-cell receptor (BCR) dependent DLBCLs that are primarily glycolytic (“Warburg-type”) [22]. Remarkably, inhibition of BCR signaling in Warburg-type DLBCLs shifts their fuel preference to FAO, indicating that fuel preference among DLBCL subtypes can be regulated through a switch-like mechanism in a BCR-dependent manner [22]. Elevated FAO in OxPhos DLBCLs as well as breast cancer cells with increased expression of PML is, in part, dependent on the PPAR signaling pathway [22, 42]. Thus, targeted inhibition of FAO and/or the PPAR signaling pathway may be an attractive therapeutic strategy in these tumors.
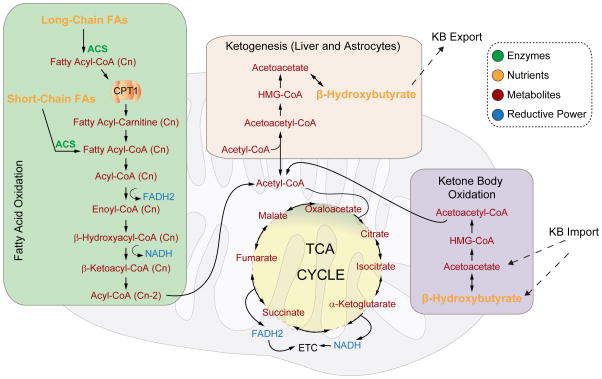
Figure 3. Fatty acid oxidation and ketone body metabolism.
Long chain fatty acids (FAs) are imported into the mitochondria in a CPT-1 dependent manner after activation to their fatty acyl-CoA derivatives, while short chain FAs diffuse across the mitochondrial membranes. They are then oxidized to acetyl-CoA units so that the FA chain length is reduced by two carbons in every cycle of β-oxidation. Fatty acid-derived acetyl-CoA units can then enter the TCA cycle and be completely oxidized to generate ATP through OXPHOS. In the liver and astrocytes, excess acetyl-CoA can be diverted for the synthesis of ketone bodies (KB), such as β-hydroxybutyrate and acetoacetate. Liver-produced ketone bodies are exported for use by other tissues. Ketone bodies are also locally produced in the brain by astrocytes and can serve as fuel for neurons. Ketone bodies are oxidized to acetyl-CoA in the mitochondria.
Abbreviations: ACS – Acyl-CoA synthetase, CPT1 – Carnitine palmitoyl transferase 1, ETC – Electron transport chain. Cn denotes fatty acid chain length, which is reduced by 2 carbons with every cycle of acetyl-CoA production.
Metabolic determinants of cellular responses to oxidative and genotoxic stress
Adaptive responses to oxidative stress and DNA damage involve production of NADPH and nucleotides downstream of a preferential increase in the PPP flux. As such, redox-sensing mechanisms can influence PPP directly. For example, direct oxidation of Cys358 in PKM2 inhibits its activity so that glycolytic intermediates are diverted to PPP [49]. Given its importance in the supply of NADPH and nucleotide precursors, the PPP is regulated by key guardians of the genome; p53 [50] and ATM [51]. In response to DNA damage, ATM phosphorylates the heat shock protein p27, which in turn binds and activates glucose-6-phosphate dehydrogenase (G6PDH), the rate-limiting enzyme of the PPP [51]. In contrast, the binding of p53 to G6PDH inhibits its activity and thereby blunts utilization of glucose-derived metabolites in the PPP [50]. Recent studies also showed that concomitant with increased PPP intermediates, inhibition of mitochondrial glutamine metabolism is required for a proper response to DNA damage [52].
Cells can also generate NADPH from non-glucose substrates. For example, under glucose-deprived conditions, AMPK-mediated increase in FAO leads to NADPH production via malate and citrate, while AMPK-mediated inhibition of ACC prevents consumption of NADPH by fatty acid synthesis [48]. Citrate produced via FAO can be exported to the cytosol and serve as a substrate for NADPH-producing reactions catalyzed by the malic enzyme (ME) and isocitrate dehydrogenase (IDH) (Figure 1). As noted in the previous section, FAO may be an inherent metabolic feature of certain cancers. In addition, FAO is associated with resistance to rapamycin [43], as well as chemotherapy- and radiation-induced toxicity [53]. The protective effect of FAO under conditions of metabolic stress relates, in part, to its contribution to NADPH synthesis and antioxidant capacity [46–48]. The metabolic control of the cellular antioxidant response can also be mediated by distinct metabolites that regulate gene expression and signaling pathways. For example, the TCA cycle intermediate, fumarate, can trigger an antioxidant gene expression program through activation of the Nrf2 transcription factor [54]. This is likely mediated by fumarate-dependent succination and inhibition of Keap1, a negative regulator that normally binds and inhibits Nrf2 [55].
Glucose-to-ketone body fuel switch in regulation of neuronal excitation
In response to metabolic challenges such as extreme fasting and hypoxia/ischemia, ketone bodies such as β-hydroxybutyrate and acetoacetate can serve as an alternative energy source in the brain. However, the metabolic benefits of ketone bodies extend beyond energy production. For example, substrate switching from glucose to ketone bodies substantially alters neuronal excitability. Ketogenic diets (KD), which reduce glucose metabolism and promote the breakdown of fatty acids to generate ketone bodies (Figure 3), have anticonvulsant effects in pharmacoresistant epilepsy [56]. While the exact mechanism underlying the effect of a glucose-to-ketone-body fuel switch is not fully understood, changes in ATP, amino acids, ROS, and glutathione have been implicated [57]. Increased ketone body consumption in the brain can further modulate gene expression programs. In particular, β-hydroxybutyrate can inhibit class I HDAC activity and selectively induce the expression of genes involved in the antioxidant response [58]. This may underlie, at least in part, the protective role of β-hydroxybutyrate against oxidative stress in neurons [59]. The full spectrum of changes in gene expression triggered by a glucose-to-ketone body fuel switch is likely broader and may include induction of mitochondrial biogenesis genes [60] and suppression of brain-derived neurotrophic factor [61].
In addition to dietary manipulations, cell-intrinsic molecular modulators that can simultaneously attenuate glucose metabolism and enhance ketone body consumption also exist. The BCL-2 family protein BAD was recently shown to impart reciprocal effects on mitochondrial handling of glucose vs. ketone bodies in neural cells independent of dietary treatment. Modifications in BAD that induce a glucose-to-ketone body fuel switch, produce resistance to behavioural and electrographic seizures that are mediated by the KATP channel [62]. The existence of cell intrinsic modulators of a switch to ketone body metabolism also implies mechanisms that supply neurons with ketone bodies in the absence of any dietary manipulations. These include systemic supply via liver, which is the major site of ketone body production, as well as local ketogenesis by astrocytes [63] (Figure 3).
Regulation of carbon substrate utilization at the level of substrate supply and substrate access
In addition to transcriptional and post-transcriptional mechanisms that control metabolic flux, metabolite transport and spatio-temporal organization of metabolic pathways can influence the choice of fuel substrates. Below, we highlight recent findings that point to the contribution of mitochondrial architecture, spatial assembly and organization of metabolic enzymes, and regulated metabolite transport to fuel utilization.
Mitochondrial architecture in the control of fuel access
The functional quality of mitochondria and the efficiency of fuel utilization are intimately linked to the mitochondrial architecture, which is dynamically shaped by fusion and fission events. The mitochondrial fusion and fission machinery is orchestrated by GTPases at the outer and inner mitochondrial membranes [64]. While early investigations implicated a role for the fragmented vs. connected state of the mitochondrial network in regulation of cellular life/death balance, recent findings increasingly point to a broader role for mitochondrial dynamics in homeostatic control of nutrient utilization and energy expenditure [65]. For example, fission ensures segregation and removal of damaged mitochondria by autophagy [66], and links the mitochondrial energetic capacity, mitochondrial architecture, and adaptive responses to changes in nutrient availability [67–69] (Box 2).
Box 2. Mitochondrial quality control through mitophagy.
Mitophagy, the selective targeting of mitochondria to autophagosomes, is an important homeostatic adaptation that ensures the turnover of damaged mitochondria. As such, mitophagy is an important quality control process that can influence the organelle’s fitness for fuel oxidation. Briefly, mitophagy is mediated by the general autophagic machinery and specific sequestration of damaged mitochondria in autophagosomes that are subsequently fused with lysosomes and degraded [86, 87]. While the process by which damaged mitochondria are selectively recognized or tagged for recruitment into autophagosomes is an evolving area of investigation, genetic and biochemical studies have revealed several molecular players that act in tissue- and context-specific manner. These include the Parkinson’s disease associated gene products, the PINK1 kinase and the E3 ubiquitin protein ligase Parkin, which work in succession to phosphorylate and ubiquitinate mitochondrial outer-membrane (MOM) proteins such as mitofusin (MFN) 2 in a manner dependent upon mitochondrial energetic status [86, 88, 89]. Ubiquitination of MOM proteins is believed to mark mitochondria for recruitment to the autophagosome. In conjunction with Parkin, the E2 ubiquitin conjugating enzyme RAD6A was recently shown to be required for ubiquitination of MOM proteins [90]. Notably, RAD6A deficiency is associated with mitochondrial defects and synaptic dysfunction in patients with X-linked intellectual dysfunction. Other regulators of mitochondrial recruitment to autophagosomes include the mitochondrial resident proteins NIX, BNIP3 and FUNDC1, as well as the NIX-interacting GTPase Rheb [91–93].
In addition to Parkinson’s disease, defects in mitophagy can manifest in multiple other pathologies [88, 90, 93]. For example, in the liver, where metabolic flexibility in fuel choice plays a prominent role in adaptive responses to fed and fasted nutritional states, defects in mitophagy result in hepatic accumulation of mitochondria with low membrane potential and respiratory capacity. This is in turn associated with reduced fatty acid oxidation and an attendant diminution of hepatic glucose production during fasting [93]. These and other examples of tissue-specific contributions of mitochondrial turnover to fuel utilization also suggest that the molecular modulators of mitophagy must be poised to receive input from a diverse array of signals, including nutrient and energy sensing as well as stress response pathways. Consistent with this idea, recent reports indicate that changes in FUNDC1 and MFN2 phosphorylation can modulate the turnover of damaged mitochondria [88, 92]. It is likely that additional mechanisms for integrating mitophagy with cellular energy and nutrient status exist.
It is likely that modulation of nutrient utilization capacity by mitochondrial architecture occurs through multiple control points, including the organization and assembly of electron transport chain components, the cristae structure, as well as mitochondrial nutrient access and import. The precise regulation of these processes by the mitochondrial network is not fully understood. In HeLa cells, fuel switching from glucose to glutamine is associated with increased number of cristae and an interconnected mitochondrial network reminiscent of increased fusion [70]. Whether these morphologic changes are permissive for glutamine utilization or a byproduct of glucose-to-glutamine fuel switch remains to be determined. Cristae density is similarly increased upon general nutrient deprivation [67], indicating an intimate and dynamic link between mitochondrial architecture and nutrient availability.
Spatial organization of mitochondria may also affect substrate access. This level of regulation may be especially relevant when fatty acids (FAs) are the preferred substrates. Once imported into the cell, FAs can be esterified and become incorporated into lipid droplets as triglycerides or be activated to their fatty acyl-CoA derivatives and oxidized in the mitochondria (Figure 3). The FA utilization capacity is regulated by the net balance of lipid storage in lipid droplets and lipolysis. The metabolic dialogue between mitochondria and lipid droplets is facilitated by their physical association. In oxidative cells and tissues such as cardiomyocytes, the lipid scaffolding protein perilipin 5 (Plin5, LSDP5) was shown to recruit mitochondria to the vicinity of lipid droplets [71]. Under low cellular energy demand, Plin5 inhibits lipolysis in favor of FA storage. In the presence of cellular signals that augment cAMP levels and trigger PKA activation, Plin5 favors lipolysis and the supply of FA to mitochondria [71]. In addition to physical proximity of mitochondria to FAs, increased mitochondrial fatty acid oxidation (FAO) entails several regulatory mechanisms that ensure increased FA activation and enhanced FA import into mitochondria (Box 3).
Box 3. Mitochondrial uptake of fatty acids.
Activation of fatty acids (FAs) to their acyl-CoA derivatives is differentially regulated depending on FA chain length. Long-chain (FAs) are first activated to fatty acyl-CoAs by long-chain acyl-CoA synthetase (ACSL) and converted to fatty acyl carnitine by CPT1 prior to entry into the mitochondrion via the carnitine shuttle (Figure 3). In addition to AMPK signaling, which regulates CPT1 activity, recent studies indicate additional control mechanisms that converge on CPT1 through a hetero-oligomeric complex containing the mitochondrial voltage-dependent anion channel (VDAC), CPT1, ACSL in the outer mitochondrial membrane [94, 95]. This might allow juxtaposition of FA activation by ACSL with VDAC-mediated import of fatty acyl-CoAs into mitochondria and generation of acyl-carnitines by CPT1. Mitochondrial architecture may influence this hetero-oligomeric complex by supporting VDAC-mediated import of fatty acyl-CoAs or the efficiency of CPT1-catalyzed reaction. The connection between mitochondrial architecture and FAO is also underscored by recent genetic evidence that shifting the balance of mitochondrial fusion and fission towards fusion decreases the efficiency of brown adipose tissues to oxidize FAs [96].
Spatial organization of metabolic enzymes
Organization of metabolic enzymes in distinct protein complexes has been proposed as a mechanism for metabolic compartmentalization. For example, PFK and aldolase, which catalyze two sequential steps in glycolysis (Figure 1), form a heterotetrameric complex that enables increased activity of both enzymes compared with the homodimer of each enzyme [72]. Spatial organization of metabolic enzymes may also provide functionally separate pools of metabolites within the cells. For example, the glycolytic enzyme glucokinase (GK, hexokinase IV) exists in several cellular locations, including the cytosol, the nucleus, and the mitochondrial surface, and is regulated by distinct binding interactions (reviewed in [73]). This may indicate potentially separate pools of glucose-6-phosphate and/or regulatory mechanisms that converge on GK activity. For example, the nuclear pool of GK in liver, which is normally sequestered and inhibited by the GK regulatory protein (GKRP), serves as a stable reserve that can be released in response to elevated glucose levels and plays a dominant role in glycogen synthesis [74]. The freely diffusible cytosolic pool of GK is retained, stabilized and activated by the dual enzyme 6-phosphofructo-2 kinase/fructose 2,6-bisphosphatase (PFK-2/FBPAse-2) [73]. The mitochondrial pool of GK can be modulated by the BCL-2 family protein BAD [75, 76] and GKRP [38]. While the exact metabolic contributions of the mitochondria-tethered GK are not fully understood, at this location, GK may serve to couple glycolysis and mitochondrial metabolism or determine the fate of pyruvate within cells.
Metabolic compartmentalization may have important signaling functions. In astrocytes, compartmentalization of the glutamate transporter (GLT1), the Na+/K+ ATPase, glycolytic enzymes, and mitochondria is proposed to increase the efficiency of glutamate transport and/or clearance, which is an energy demanding process [77]. This predicts localization of mitochondria and the glycolytic machinery in the vicinity of the plasma membrane where GLT1 and the Na+/K+ ATPase are located. Mitochondria have been implicated as the main source of ATP for sustaining information processing in neurons in the form of pre-synaptic potentials, neurotransmitter release, post-synaptic currents and post-synaptic potentials [78]. The preferential use of ATP generated from glycolysis vs. mitochondrial oxidative phosphorylation in neurons and other cell types appears to be related to the particular function for which ATP is required, indicating a functional coupling of ATP production to its use in a particular cellular compartment. For example, fast axonal transport (FAT) of vesicles in neurons is dependent on glycolytically-derived ATP [79]. The glycolytic enzyme GAPDH co-localizes with motile vesicles and its molecular depletion leads to a decrease in FAT. On the other hand, mitochondria transport in axons, which is dependent on mitochondrially produced ATP, is unaffected by GAPDH depletion [79]. In addition to mechanisms that juxtapose ATP-generating reactions with ATP-consuming processes within cells, regulated ATP storage and release through the creatine kinase system is an important means of spatio-temporal modulation of phospho-transfer reactions [80].
Concluding remarks
Recent resurgence of research interest in the intersection of metabolism and stress responses has uncovered important roles for cellular fuel choice. Cells can fulfill specific energy and biosynthetic needs by preferentially metabolizing a given carbon substrate over another. It is therefore not surprising that the cells’ fuel choice can alter their fate and function by influencing transitions between quiescence and proliferation, producing resistance or sensitivity to oxidative stress, facilitating DNA and tissue repair, and allowing metabolic adaptations to changes in nutrient availability. Furthermore, metabolism of particular carbon substrates can affect cell identity and behavior through programmatic changes in gene expression and epigenetic modifications. While switches in cells’ “appetite” for different carbon substrates can have homeostatic benefits by triggering appropriate stress responses, they can be disadvantageous in disease context. Understanding how these fuel switches are controlled and defining their specific metabolic outputs will provide a molecular handle on modulating cell behavior in normal physiology and in pathologic conditions (Box 4).
Box 4. Outstanding questions.
Is the reprogramming of cellular fuel choice a cause or a consequence of transitions in and out of quiescence, lineage specification, and cellular adaptation to stress?
Beyond genetically-encoded metabolic programs, how is the cell instructed to preferentially metabolize a particular carbon substrate when it has access to multiple fuels? What are the mechanisms underlying “fuel competition”?
How are signaling pathways altered by individual metabolites? Beyond serving as metabolic substrates, products or co-factors, can metabolites have roles analogous to second messengers?
How does mitochondrial architecture influence the cell’s fuel utilization capacity and fuel preference?
What is the contribution of metabolic compartmentalization or channeling to the cellular fuel choice?
What is the extent of heterogeneity in the metabolic response of individual cells within a population under stress conditions? What contributes to this metabolic heterogeneity?
Highlights.
Cells can use different metabolic fuels as an adaptive advantage to stress
Proliferation and differentiation are accompanied by metabolic rewiring
Mitochondrial architecture and metabolic compartmentalization influence fuel choice
Acknowledgments
We thank members of the Danial laboratory for helpful discussions. This work was supported by grants from the National Institutes of Health [F31 CA171400 (IAS), R56 NS072142 (N.N.D.)], the Portuguese Foundation for Science and Technology (FCT) grant SFRH/BD/51200/2010 (S.M.R.), and the Swedish Society for Medical Research (SSMF) (E.N.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eliasson P, Jönsson JI. The hematopoietic stem cell niche: Low in oxygen but a nice place to be. Journal of Cellular Physiology. 2010;222:17–22. doi: 10.1002/jcp.21908. [DOI] [PubMed] [Google Scholar]
- 2.Rafalski VA, Brunet A. Energy metabolism in adult neural stem cell fate. Progress in Neurobiology. 2011;93:182–203. doi: 10.1016/j.pneurobio.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Folmes, Clifford DL, et al. Metabolic Plasticity in Stem Cell Homeostasis and Differentiation. Cell Stem Cell. 2012;11:596–606. doi: 10.1016/j.stem.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Folmes, Clifford DL, et al. Somatic Oxidative Bioenergetics Transitions into Pluripotency-Dependent Glycolysis to Facilitate Nuclear Reprogramming. Cell Metabolism. 2011;14:264–271. doi: 10.1016/j.cmet.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lonergan T, et al. Differentiation-related changes in mitochondrial properties as indicators of stem cell competence. Journal of Cellular Physiology. 2006;208:149–153. doi: 10.1002/jcp.20641. [DOI] [PubMed] [Google Scholar]
- 6.Varum S, et al. Energy Metabolism in Human Pluripotent Stem Cells and Their Differentiated Counterparts. PLoS ONE. 2011;6:e20914. doi: 10.1371/journal.pone.0020914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CT, et al. Coordinated Changes of Mitochondrial Biogenesis and Antioxidant Enzymes During Osteogenic Differentiation of Human Mesenchymal Stem Cells. STEM CELLS. 2008;26:960–968. doi: 10.1634/stemcells.2007-0509. [DOI] [PubMed] [Google Scholar]
- 8.Ito K, et al. A PML-PPAR-[delta] pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nat Med. 2012;18:1350–1358. doi: 10.1038/nm.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simsek T, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7:380–390. doi: 10.1016/j.stem.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou W, et al. HIF1alpha induced switch from bivalent to exclusively glycolytic metabolism during ESC-to-EpiSC/hESC transition. EMBO J. 2012;31:2103–2116. doi: 10.1038/emboj.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, et al. Dependence of Mouse Embryonic Stem Cells on Threonine Catabolism. Science. 2009;325:435–439. doi: 10.1126/science.1173288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander PB, et al. Targeted killing of a mammalian cell based upon its specialized metabolic state. Proceedings of the National Academy of Sciences. 2011;108:15828–15833. doi: 10.1073/pnas.1111312108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shyh-Chang N, et al. Influence of Threonine Metabolism on S-Adenosylmethionine and Histone Methylation. Science. 2013;339:222–226. doi: 10.1126/science.1226603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacIver NJ, et al. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259–283. doi: 10.1146/annurev-immunol-032712-095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michalek RD, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delgoffe GM, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Windt GJ, et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 19.Locasale JW, Cantley LC. Metabolic flux and the regulation of mammalian cell growth. Cell Metab. 2011;14:443–451. doi: 10.1016/j.cmet.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knobloch M, et al. Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis. Nature. 2013;493:226–230. doi: 10.1038/nature11689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gnoni GV, et al. The mitochondrial citrate carrier: metabolic role and regulation of its activity and expression. IUBMB Life. 2009;61:987–994. doi: 10.1002/iub.249. [DOI] [PubMed] [Google Scholar]
- 22.Caro P, et al. Metabolic Signatures Uncover Distinct Targets in Molecular Subsets of Diffuse Large B Cell Lymphoma. Cancer Cell. 2012;22:547–560. doi: 10.1016/j.ccr.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marin-Valencia I, et al. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab. 2012;15:827–837. doi: 10.1016/j.cmet.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metallo CM, et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2012;481:380–384. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wise DR, et al. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci U S A. 2011;108:19611–19616. doi: 10.1073/pnas.1117773108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mullen AR, et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2012;481:385–388. doi: 10.1038/nature10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tibbetts AS, Appling DR. Compartmentalization of Mammalian folate-mediated one-carbon metabolism. Annu Rev Nutr. 2010;30:57–81. doi: 10.1146/annurev.nutr.012809.104810. [DOI] [PubMed] [Google Scholar]
- 28.Jain M, et al. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science. 2012;336:1040–1044. doi: 10.1126/science.1218595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maddocks OD, et al. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature. 2013;493:542–546. doi: 10.1038/nature11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Possemato R, et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 2011;476:346–350. doi: 10.1038/nature10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Locasale JW, et al. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat Genet. 2011;43:869–874. doi: 10.1038/ng.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaneton B, et al. Serine is a natural ligand and allosteric activator of pyruvate kinase M2. Nature. 2012;491:458–462. doi: 10.1038/nature11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye J, et al. Pyruvate kinase M2 promotes de novo serine synthesis to sustain mTORC1 activity and cell proliferation. Proc Natl Acad Sci U S A. 2012;109:6904–6909. doi: 10.1073/pnas.1204176109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemons JM, et al. Quiescent fibroblasts exhibit high metabolic activity. PLoS Biol. 2010;8:e1000514. doi: 10.1371/journal.pbio.1000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salpeter SJ, et al. Glucose regulates cyclin D2 expression in quiescent and replicating pancreatic beta-cells through glycolysis and calcium channels. Endocrinology. 2011;152:2589–2598. doi: 10.1210/en.2010-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Metukuri MR, et al. ChREBP mediates glucose-stimulated pancreatic beta-cell proliferation. Diabetes. 2012;61:2004–2015. doi: 10.2337/db11-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diaz-Moralli S, et al. Target metabolomics revealed complementary roles of hexose- and pentose-phosphates in the regulation of carbohydrate-dependent gene expression. Am J Physiol Endocrinol Metab. 2012;303:E234–242. doi: 10.1152/ajpendo.00675.2011. [DOI] [PubMed] [Google Scholar]
- 38.Arden C, et al. Fructose 2,6-bisphosphate is essential for glucose-regulated gene transcription of glucose-6-phosphatase and other ChREBP target genes in hepatocytes. Biochem J. 2012;443:111–123. doi: 10.1042/BJ20111280. [DOI] [PubMed] [Google Scholar]
- 39.Guinez C, et al. O-GlcNAcylation increases ChREBP protein content and transcriptional activity in the liver. Diabetes. 2011;60:1399–1413. doi: 10.2337/db10-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salpeter SJ, et al. Glucose and aging control the quiescence period that follows pancreatic beta cell replication. Development. 2010;137:3205–3213. doi: 10.1242/dev.054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moncada S, et al. Fulfilling the metabolic requirements for cell proliferation. Biochem J. 2012;446:1–7. doi: 10.1042/BJ20120427. [DOI] [PubMed] [Google Scholar]
- 42.Carracedo A, et al. A metabolic prosurvival role for PML in breast cancer. The Journal of Clinical Investigation. 2012;122:3088–3100. doi: 10.1172/JCI62129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zaugg K, et al. Carnitine palmitoyltransferase 1C promotes cell survival and tumor growth under conditions of metabolic stress. Genes Dev. 2011;25:1041–1051. doi: 10.1101/gad.1987211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samudio I, et al. Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. The Journal of Clinical Investigation. 2010;120:142–156. doi: 10.1172/JCI38942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nieman KM, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pike LS, et al. Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochim Biophys Acta. 2011;1807:726–734. doi: 10.1016/j.bbabio.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 47.Schafer ZT, et al. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature. 2009;461:109–113. doi: 10.1038/nature08268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeon SM, et al. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485:661–665. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anastasiou D, et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334:1278–1283. doi: 10.1126/science.1211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang P, et al. p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat Cell Biol. 2011;13:310–316. doi: 10.1038/ncb2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cosentino C, et al. ATM activates the pentose phosphate pathway promoting anti-oxidant defence and DNA repair. EMBO J. 2011;30:546–555. doi: 10.1038/emboj.2010.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeong, Seung M, et al. SIRT4 Has Tumor-Suppressive Activity and Regulates the Cellular Metabolic Response to DNA Damage by Inhibiting Mitochondrial Glutamine Metabolism. Cancer Cell. 2013;23:450–463. doi: 10.1016/j.ccr.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harper ME, et al. Characterization of a novel metabolic strategy used by drug-resistant tumor cells. FASEB J. 2002;16:1550–1557. doi: 10.1096/fj.02-0541com. [DOI] [PubMed] [Google Scholar]
- 54.Ashrafian H, et al. Fumarate is cardioprotective via activation of the Nrf2 antioxidant pathway. Cell Metab. 2012;15:361–371. doi: 10.1016/j.cmet.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adam J, et al. Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: roles for fumarate in KEAP1 succination and Nrf2 signaling. Cancer Cell. 2011;20:524–537. doi: 10.1016/j.ccr.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thiele EA. Assessing the efficacy of antiepileptic treatments: the ketogenic diet. Epilepsia. 2003;44(Suppl 7):26–29. doi: 10.1046/j.1528-1157.44.s7.4.x. [DOI] [PubMed] [Google Scholar]
- 57.Lutas A, Yellen G. The ketogenic diet: metabolic influences on brain excitability and epilepsy. Trends Neurosci. 2013;36:32–40. doi: 10.1016/j.tins.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shimazu T, et al. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339:211–214. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jarrett SG, et al. The ketogenic diet increases mitochondrial glutathione levels. J Neurochem. 2008;106:1044–1051. doi: 10.1111/j.1471-4159.2008.05460.x. [DOI] [PubMed] [Google Scholar]
- 60.Bough KJ, et al. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006;60:223–235. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- 61.Garriga-Canut M, et al. 2-Deoxy-D-glucose reduces epilepsy progression by NRSF-CtBP-dependent metabolic regulation of chromatin structure. Nat Neurosci. 2006;9:1382–1387. doi: 10.1038/nn1791. [DOI] [PubMed] [Google Scholar]
- 62.Giménez-Cassina A, et al. BAD-Dependent Regulation of Fuel Metabolism and KATP Channel Activity Confers Resistance to Epileptic Seizures. Neuron. 2012;74:719–730. doi: 10.1016/j.neuron.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blázquez C, et al. Role of Carnitine Palmitoyltransferase I in the Control of Ketogenesis in Primary Cultures of Rat Astrocytes. Journal of Neurochemistry. 1998;71:1597–1606. doi: 10.1046/j.1471-4159.1998.71041597.x. [DOI] [PubMed] [Google Scholar]
- 64.Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol. 2010;11:872–884. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- 65.Liesa M, Shirihai OS. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013;17:491–506. doi: 10.1016/j.cmet.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Twig G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gomes LC, et al. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol. 2011;13:589–598. doi: 10.1038/ncb2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Molina AJ, et al. Mitochondrial networking protects beta-cells from nutrient-induced apoptosis. Diabetes. 2009;58:2303–2315. doi: 10.2337/db07-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rambold AS, et al. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci U S A. 2011;108:10190–10195. doi: 10.1073/pnas.1107402108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rossignol R, et al. Energy substrate modulates mitochondrial structure and oxidative capacity in cancer cells. Cancer Res. 2004;64:985–993. doi: 10.1158/0008-5472.can-03-1101. [DOI] [PubMed] [Google Scholar]
- 71.Wang H, et al. Perilipin 5, a lipid droplet-associated protein, provides physical and metabolic linkage to mitochondria. J Lipid Res. 2011;52:2159–2168. doi: 10.1194/jlr.M017939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marcondes MC, et al. Muscle-type 6-phosphofructo-1-kinase and aldolase associate conferring catalytic advantages for both enzymes. IUBMB Life. 2011;63:435–445. doi: 10.1002/iub.464. [DOI] [PubMed] [Google Scholar]
- 73.Baltrusch S, Tiedge M. Glucokinase Regulatory Network in Pancreatic β-Cells and Liver. Diabetes. 2006;55:S55–S64. [Google Scholar]
- 74.Agius L. Glucokinase and molecular aspects of liver glycogen metabolism. Biochem J. 2008;414:1–18. doi: 10.1042/BJ20080595. [DOI] [PubMed] [Google Scholar]
- 75.Danial NN, et al. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature. 2003;424:952–956. doi: 10.1038/nature01825. [DOI] [PubMed] [Google Scholar]
- 76.Danial NN, et al. Dual role of proapoptotic BAD in insulin secretion and beta cell survival. Nat Med. 2008;14:144–153. doi: 10.1038/nm1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Genda EN, et al. Co-compartmentalization of the astroglial glutamate transporter, GLT-1, with glycolytic enzymes and mitochondria. J Neurosci. 2011;31:18275–18288. doi: 10.1523/JNEUROSCI.3305-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hall CN, et al. Oxidative Phosphorylation, Not Glycolysis, Powers Presynaptic and Postsynaptic Mechanisms Underlying Brain Information Processing. The Journal of Neuroscience. 2012;32:8940–8951. doi: 10.1523/JNEUROSCI.0026-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zala D, et al. Vesicular glycolysis provides on-board energy for fast axonal transport. Cell. 2013;152:479–491. doi: 10.1016/j.cell.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 80.Wallimann T, et al. The creatine kinase system and pleiotropic effects of creatine. Amino Acids. 2011;40:1271–1296. doi: 10.1007/s00726-011-0877-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thomas AP, Halestrap AP. Identification of the protein responsible for pyruvate transport into rat liver and heart mitochondria by specific labelling with [3H]N-phenylmaleimide. Biochem J. 1981;196:471–479. doi: 10.1042/bj1960471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bricker DK, et al. A Mitochondrial Pyruvate Carrier Required for Pyruvate Uptake in Yeast, Drosophila, and Humans. Science. 2012;337:96–100. doi: 10.1126/science.1218099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Herzig S, et al. Identification and Functional Expression of the Mitochondrial Pyruvate Carrier. Science. 2012;337:93–96. doi: 10.1126/science.1218530. [DOI] [PubMed] [Google Scholar]
- 84.Palmieri F, Pierri CL. Mitochondrial metabolite transport. Essays Biochem. 2010;47:37–52. doi: 10.1042/bse0470037. [DOI] [PubMed] [Google Scholar]
- 85.Divakaruni AS, et al. Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proc Natl Acad Sci U S A. 2013;110:5422–5427. doi: 10.1073/pnas.1303360110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gottlieb RA, Gustafsson AB. Mitochondrial turnover in the heart. Biochim Biophys Acta. 2011;1813:1295–1301. doi: 10.1016/j.bbamcr.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Twig G, Shirihai OS. The interplay between mitochondrial dynamics and mitophagy. Antioxid Redox Signal. 2011;14:1939–1951. doi: 10.1089/ars.2010.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen Y, Dorn GW., 2nd PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. 2013;340:471–475. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Narendra D, et al. Mitochondrial quality control mediated by PINK1 and Parkin: links to parkinsonism. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Haddad DM, et al. Mutations in the intellectual disability gene ube2a cause neuronal dysfunction and impair parkin-dependent mitophagy. Mol Cell. 2013;50:831–843. doi: 10.1016/j.molcel.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 91.Melser S, et al. Rheb regulates mitophagy induced by mitochondrial energetic status. Cell Metab. 2013;17:719–730. doi: 10.1016/j.cmet.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 92.Liu L, et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat Cell Biol. 2012;14:177–185. doi: 10.1038/ncb2422. [DOI] [PubMed] [Google Scholar]
- 93.Glick D, et al. BNip3 regulates mitochondrial function and lipid metabolism in the liver. Mol Cell Biol. 2012;32:2570–2584. doi: 10.1128/MCB.00167-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee K, et al. Mitochondrial carnitine palmitoyltransferase 1a (CPT1a) is part of an outer membrane fatty acid transfer complex. J Biol Chem. 2011;286:25655–25662. doi: 10.1074/jbc.M111.228692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hoppel C, et al. Rat liver mitochondrial contact sites and carnitine palmitoyltransferase-I. Arch Biochem Biophys. 2001;392:321–325. doi: 10.1006/abbi.2001.2463. [DOI] [PubMed] [Google Scholar]
- 96.Quiros PM, et al. Loss of mitochondrial protease OMA1 alters processing of the GTPase OPA1 and causes obesity and defective thermogenesis in mice. EMBO J. 2012;31:2117–2133. doi: 10.1038/emboj.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]