Abstract
Although epidemiological studies from the past two decades show a link between atherosclerotic vascular disease and bone loss, that is independent of age, the mechanism is still unclear. This review focuses on evidence that suggests a role for atherogenic lipids and lipoproteins in the pathogenesis of bone loss, including direct effects of these bioactive lipids/lipoproteins on bone cells, inhibiting osteoblastic differentiation and promoting osteoclastic differentiation. It also addresses recent evidence that suggests that bioactive lipids blunt the effects of bone anabolic agents, such as teriparatide and bone morphogenetic proteins. The role of systemic and intracellular oxidant stress and inflammation in mediating the effects of bioactive lipids/lipoproteins are implicated.
Biological lipids and lipoproteins
Most biological lipids are in the forms of fatty acids, triglycerides, cholesterol, steroid hormones and phospholipids. Due to their hydrophobic nature, lipids are carried in the circulation to the peripheral tissues via lipoproteins. Dietary triglycerides are absorbed from the intestine into the circulation via chylomicrons, whereas triglycerides synthesized from the liver are carried by very low-density lipoprotein (VLDL), both of which contain apolipoprotein E (apoE). Both dietary and liver-synthesized cholesterol are carried on low-density lipoprotein (LDL) particles, which contain a single large protein, apoB 100 that is recognized by the LDL receptor. The return of fat-soluble wastes from interstitial spaces of peripheral tissues to the liver for excretion, a process known as “reverse cholesterol transport,” is carried out by another lipoprotein, high-density lipoprotein (HDL).1 Lipid levels in the circulation depend on a combination of dietary intake, cellular biosynthesis, and rate of elimination as bile acids.1 Consequently, deficiency of apoE or the LDL receptor, which reduces uptake by hepatic and peripheral cells, leads to increased levels of circulating lipoproteins, a known risk factor of atherosclerosis.
Epidemiological studies have shown that serum levels of HDL are inversely associated with risk of atherosclerotic disease.2, 3 Recent studies indicate that, in addition to facilitating reverse cholesterol transport, HDL cholesterol protects against cardiovascular disease by reducing intestinal oxidized lipid metabolism.4, 5 Among the proteins that transiently associate with HDL are paraoxonase (PON) and apolipoprotein A–I, which have antioxidant properties and are believed to confer the protective effects of HDL.6 In humans, low serum levels of paraoxonase 1 (PON1) are associated with cardiac events,7 and in animals, PON1 deficiency causes increased aortic superoxide production and leukocyte adhesion due to increased production of adhesion molecules.8 HDL-mediated protection against oxidation of LDL has been shown to correlate with levels of paraoxonase production.9 Transgenic mice expressing paraoxonase 3, another member of the PON family, also show resistance to atherosclerotic lesion formation and adiposity in response to an atherogenic diet.10 A recent study attributed the atheroprotective properties of apoA-I to conformational adaptability during reverse cholesterol transport. Transfer of apoA-I from HDL during this process has been impaired in animals fed a high fat, high cholesterol diet as well as human subjects, who carry at lest one risk factor for cardiovascular disease.11 Domain swap experiments in genetically hyperlipidemic mice demonstrated that destabilizing the amino-terminal helix bundle domain or increasing hydrophobicity of the carboxy-terminal domain of human apoA-I enhanced reverse cholesterol transport function.12
Lipids and bone
Lipids play an important role in skeletal metabolism and bone health. They restrict radial permeability of cortical bone, likely affecting the metabolic functions of bone cells.13 A relationship between lipids and biomineralization was proposed as early as 1963.14 Matrix vesicles involved in chondrocyte mineralization are enriched in cholesterol. Using solid-state NMR technique, Reid and colleagues recently identified fatty acyl lipids in calcified bone.15 The relative abundance of methyl branched fatty acids in this lipid pool suggest that they are remnants of lipoprotein particles and not of phospholipid membrane.15 In addition, lipoproteins are vehicles for carrying fat-soluble vitamins in their lipid core, such as vitamins D and K, which have key roles in bone metabolism. Vitamin D regulates serum calcium and phosphorus concentrations, while vitamin K is required for activation by gamma-carboxylation of osteocalcin (bone GLA protein) and matrix GLA protein, known regulators of mineral maturation, delimiting or slowing the mineral maturation process as a terminal step of bone tissue formation. Interestingly, in a meta-analysis of clinical studies, vitamin K supplementation does not show a significant effect on bone mineral density (BMD) in the lumbar spine or femoral neck16. Yet the relatively high bone density in mice deficient in apolipoprotein E, which is involved in LDL interaction with peripheral cells, has been attributed to inadequate delivery of vitamin K to osteoblasts.17
Hyperlipidemia, cardiovascular disease and osteoporosis
It is estimated that hyperlipidemia is present in over 30 million adults in the US (Centers for Disease Control, 2011) and over 160 million adults in China.18 Current guidelines of the American Heart Association define hyperlipidemia based on levels of total cholesterol (>240 mg/dl), LDL-cholesterol (> 160 mg/dl), or triglycerides (>200 mg/dl). Since low levels of HDL (< 40 mg/dl in men and < 50 mg/dl in women) are also considered atherogenic, the terms “dyslipidemia” and “atherogenic lipid profile” are used as well.
A correlation of osteoporosis with atherosclerosis, a pathological consequence of hyperlipidemia, has been reported.19, 20 In addition, aortic calcification, an established marker for atherosclerotic burden, correlates with osteoporosis and fracture risk.21, 22 The significance of the relationship is underscored by proposals that low BMD be used as a clinical marker for coronary artery disease.23, 24 One possible explanation for the correlation, supported by the relationship between the presence of peripheral arterial disease and osteoporosis,25 may be an indirect effect of lipids on bone – occlusive atherosclerosis in arteries supplying bone tissue may produce bone ischemia with resultant loss of bone formation and density. Another possible explanation for this correlation is a direct effect of hyperlipidemia on bone, which is supported by the evidence described in this review.
Studies also found direct associations between osteoporosis and hyperlipidemia. Orozco and colleagues found that postmenopausal women with atherogenic lipid profiles (fasting state) have lower BMD at the lumbar, total hip and femoral neck bones than those with normal lipid profile.26, 27 In another study, lumbar and whole body BMD correlated inversely with lipid levels (above the atherogenic threshold) in postmenopausal women, but not in premenopausal women or postmenopausal women on hormone replacement therapy (HRT),28 suggesting that HRT use should be taken into consideration due to the known anti-inflammatory effects of estrogen. When the subjects were not fasted prior to blood collection or when the lipid levels were below the atherogenic threshold, a weak or even a positive association between serum lipid levels and BMD was found.29, 30 Variability in the outcome among the studies may be attributed to the study design parameters, including bone site (lumbar, femoral neck, etc.), lipid level thresholds (atherogenic vs. normal profile), exclusion criteria (hormone replacement therapy, hyperparathyroidism), and/or technical aspects (fasting or non-fasting blood samples for lipid measurements). It has been proposed that the true determinant of the impact of hyperlipidemia may be -- not current lipid levels --but total or long-term, duration of exposure to abnormal lipid levels, which has been measured in cholesterol-years.31 Furthermore, subendothelial accumulation of lipids, which depends not only on hyperlipidemia, but also on the rate of clearance through the lymphatic flow,32 may be the culprit in the inflammatory reaction. It should be noted that a relationship between lipids and bone density may be missed in any cohorts in which subjects with cardiovascular disease are excluded. If there is genetic susceptibility to general tissue damage from hyperlipidemia, then the subjects whose bone is vulnerable to hyperlipidemic damage may be the same ones who develop cardiovascular disease from hyperlipidemia. Thus, excluding subjects with hyperlipidemic heart disease may also exclude subjects with hyperlipidemic bone disease. Lastly, the measurements of bone density by x-ray densitometric scanning, particularly for the lumbar and femoral areas, may be affected to some degree by the overlap with aortic, iliac and femoral artery calcification, which are also positively associated with lipid levels.33
Animal models of hyperlipidemia
Animal models of hyperlipidemia are based on genetic and/or dietary interventions. Genetic models of hyperlipidemia involve disruption of normal lipoprotein regulation and metabolism. Among mouse strains, C57BL/6 mice are the most susceptible, whereas C3H/HeJ mice are the most resistant to atherosclerosis.34 C57BL/6 mice that are on an atherogenic, high-fat diet or deficient in receptor (LDL receptor) or ligand (apoE) develop hyperlipidemia and atherosclerosis due to reduced clearance of non-HDL lipoproteins. Two high-fat diets are widely used to induce atherosclerosis: the atherogenic “Paigen” diet, which contains 1% cholesterol, 15% fat, and, importantly, 0.5% cholic acid; and the Western diet, which contains 21% fat and 0.15% cholesterol, but no cholic acid. Results differ with the two diets, and the differences are often attributed to liver inflammation related to cholate.35
In rodents, significant relationships have been reported between BMD and LDL- cholesterol levels. Diet-induced hyperlipidemia has been shown to adversely affect bone growth and BMD in C57BL6 background mice.36–39 In mice with normal lipid levels prior to the diet, the bone loss occurs after 7 months. In contrast, in genetically modified mice with hyperlipidemia prior to the diet, the bone loss is accelerated (approximately 3 months) by the same diet,38, 39 suggesting a pathogenic role of lipids on bone loss. It also reduces mechanical strength and measures of structural integrity in both cortical and trabecular bone.39–41 In addition, bone regeneration, in a cranial-defect model, is also blunted in mice fed an atherogenic diet.39
In C3H/HeJ mice, which are genetically resistant to tissue disorders (such as atherosclerosis) caused by abnormal lipid levels,42, 43 are also resistant to hyperlipidemic bone loss.38 This resistance has been attributed to reduced lipid retention in the subendothelial space of the artery wall as well as reduced expression of inflammatory and oxidant stress-response genes in endothelial cells in response to oxidized lipids in these two strains.42, 43 In an interesting twist, the genetically hyperlipidemic apoE-deficient mouse has unexpectedly greater BMD than wild type mice.44 This finding raises questions about the effect of hyperlipidemia on bone, but it has been attributed to the function of apoE in delivering fat-soluble vitamins, such as vitamins D and K to osteoblasts.44 This is based on the reasoning that vitamin K is required for gamma-carboxylation of osteocalcin, an inhibitory regulator of mineralization, and its deficiency would release opposition to bone formation, leading to higher BMD in apoE-deficient mice. The strength of this effect appears to outweigh any effect of hyperlipidemia on bone.
Inflammatory lipids/lipoproteins
The primary mechanism of hyperlipidemia’s role in atherogenesis is the formation of oxidatively modified forms of LDL and phospholipids. In the context of an atherogenic lipid profile and/or reduced lymphatic flow (e.g., sedentary lifestyle), LDL particles tend to accumulate in the subendothelial matrices of arteries, and possibly other tissues. There, in the presence of metabolically active cells, which produce oxygen radicals, they undergo varying degrees of non-enzymatic, free radical-induced oxidation of arachidonate-containing phospholipids resulting in modified forms of LDL, such as minimally modified LDL (partially oxidized; MM-LDL) and highly oxidized LDL (ox-LDL).45 These modified LDL particles induce potent inflammatory responses that are not associated with native LDL, such as recruitment of monocytes to the arterial wall, induction of chemotactic factors in endothelial cells, and adhesion of monocytes to endothelial cells, thereby initiating pathogenic events of atherosclerotic lesion formation.46 Importantly, since arachidonates are precursors of prostaglandins47 that mediate inflammatory responses,48, 49 arachidonate-containing bioactive lipids may further amplify inflammation. A significant advance has been the identification of oxidized forms of 1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphocholine (ox-PAPC), the biologically active phospholipid components of MM-LDL, and demonstration of their atherogenic potential in vitro and in vivo. Ox-PAPC induces inflammatory gene expression in cultured bone marrow-derived macrophages, and intraperitoneal injection of ox-PAPC causes inflammation and oxidative tissue damage in livers.50 These modified biologically active lipoproteins (MM-LDL, ox-LDL) and phospholipids (ox-PAPC) are also known as “bioactive inflammatory lipids.” Emerging evidence suggests that these pro-inflammatory molecules are also deleterious to bone health, as described below.
Lipid accumulation in bone
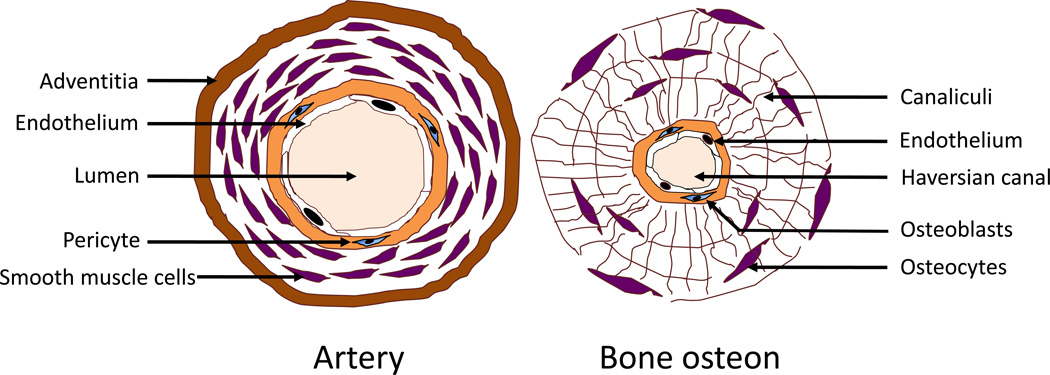
During bone formation, vascularization plays an important role, supplying nutrients, scaffolds, and osteogenic precursor cells, which remain in close association with the vessels. Cortical bone osteons are each centered on a vessel, which is lined by a subendothelial matrix, and trabecular bone is adjacent to the subendothelial matrix of marrow sinusoidal vessels. Since modified lipoproteins and bioactive lipids are also systemically delivered to bone tissue compartments, lipoproteins and lipids may accumulate in the subendothelial matrix of human bone vessels51–53 as they do in the vascular endothelium. Since osteoblast progenitor cells are primarily adjacent to the subendothelial spaces, they may be exposed to inflammatory lipoproteins and lipids (Figure 1).
Figure 1. Schematic comparing the anatomic structure of an artery and bone osteon.
Both the artery and osteon are centered on a blood lumen, which is surrounded by a single layer of endothelium. This, in turn, is surrounded by a basement membrane housing immature mesenchymal cells. In arteries, the immature cells are pericytes and/or smooth muscle cells, whereas, in bone, they are pericytes and/or preosteoblasts in the bone. (Modified from Parhami et al., Arteriosclerosis, Thrombosis, and Vascular Biology, 1997; 17:680–687).
In osteoporotic human bone, interstitial lipid accumulation has been shown by histochemical staining in the subendothelial spaces of Haversian canal vessels.53 Interestingly, lipid accumulation in osteocytes, and osteocyte apoptosis have also been associated with alcohol-induced bone loss.54 The lipid accumulation in tissues may result in part from dysfunction of lymphatic vessels, causing reduced clearance of lipids and lipoproteins.32 Their prolonged accumulation in interstitial spaces exposes them to non-enzymatic modifications, such as oxidation and glycation, which make them biologically active in inducing inflammatory responses as occurs in atherosclerotic lesions.55
Notably, osteoblasts produce and secrete various forms of lipids including triglycerides, cholesterol and phospholipids.56, 57 It has been shown that osteoblasts, like vascular cells, are participants in the pathological cascade as they also cause nonenzymatic oxidation of lipoproteins58 -- presumably through release of metabolic factors. Thus, bone can produce its own oxidized lipids, which would lead to an inflammatory microenvironment, if those lipids accumulate for a prolonged period in the extracellular milieu. The combination of these locally produced oxidized lipids together with the systemic insult of lipids from the circulation may exacerbate the progression of pathology. Of note, this lipid accumulation in the subendothelial space is extracellular, i.e. in the interstitial spaces; hence, it is a distinct process from lipid storage in adipose tissue, which consists of intracellular neutral lipid synthesized by the adipocytes.
Oxidant stress
Reactive oxygen species (ROS), including superoxide anion, hydroxyl radical, thiol, and nitric oxide generated as by-products of cellular metabolism, most likely contribute to the production of lipid peroxidation products.45 Ordinarily, ROS probably dissipate or are scavenged by radical scavenger enzymes. However, by oxidatively modifying adjacent lipids, the biological activity of ROS may be, in effect, prolonged. Lymphatic stagnation of the tissue increases exposure time to reactive species, and hyperlipidemia increases the numbers of lipid molecules exposed. Lipid oxidation products in the interstitium, in turn, promote intracellular oxidant stress in osteoblasts,59–61 further exacerbating and amplifying an oxidative stress response in bone. An atherogenic, high-fat diet has been shown to increase ROS formation by inducing liver expression of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complexes, an ROS-producing enzyme.62 In mice, this diet results in accumulation of lipid oxidation products in serum, liver, and bone marrow.39, 53, 62
Lipid oxidation products and bone cells
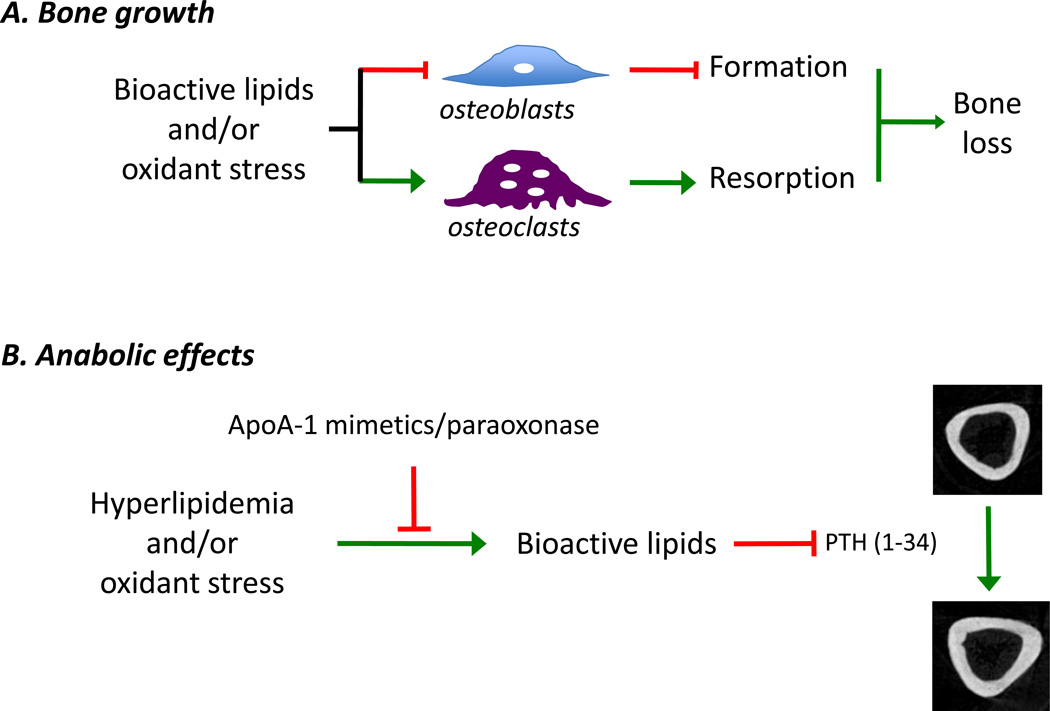
Evidence suggests that inflammatory bioactive lipids induce bone loss, by inhibiting differentiation of bone forming osteoblasts, and promoting differentiation of bone resorbing osteoclasts (Figure 2A). In vitro studies show that modified lipids and lipoproteins directly inhibit expression of osteoblastic differentiation markers, including expression and activity of alkaline phosphatase, processing of type I collagen and matrix calcium deposition,63 and that their effects are mediated by intracellular ROS.59, 64 Bioactive lipids, however, promote adipogenic differentiation of bone marrow stromal cells, in part through the activation of peroxisome proliferator-activated receptor-alpha63 and Wnt signaling,59 suggesting that lipid oxidation products promote bone loss by directing progenitor stem cells in the bone marrow along the adipogenic lineage rather than the osteoblastic lineage. Of note, hyperglycemia also promotes adipogenic induction of bone marrow stromal cells via the pathway involving ERK/PI3K/Akt signaling.65 Given the prevalence of diabetes in hyperlipidemic subjects, one may postulate that individuals with metabolic syndrome have increased risk for bone loss and fracture. However, due to the general positive association between obesity/body weight and bone mineral density, epidemiological studies do not show a clear pattern of association between metabolic syndrome and fractures or bone mineral density.66
Figure 2. Schematic of putative actions of bioactive lipids.
(A) The putative actions of bioactive lipids on bone growth, as shown. These include generation of oxidant stress and inhibition of osteoblastic differentiation that leads to decreased bone formation, and promotion of osteoclastic differentiation which in turn leads to increased bone resorption, ultimately resulting in bone loss. (B) Bioactive lipids may also affect the anabolic effects of intermittent parathyroid hormone treatment [PTH(1–34), teriparatide], including generation of bioactive lipids, which may be blocked by apolipoprotein peptide mimetics and/ or antioxidant enzymes.
With respect to bone resorption, bioactive lipids promote differentiation of osteoclastic cells by inducing enzyme activity of tartrate-resistant acid phosphatase, promoting multinucleation, and resorption of bone matrix.67 The increased resorption is likely due to stimulatory effects of bioactive lipids on production of interleukin-668 as well as receptor activator of NF-kB ligand (RANKL) by bone marrow stromal/osteoblast cells67 and by T lymphocytes.69 In mice, an atherogenic, high-fat diet causes high serum levels of RANKL36 and increased differentiation potential of preosteoclasts in the bone marrow.53
Osteocytes have emerged as an additional source of in vivo RANKL production. They serve as biomechanically sensitive sentinels and are the most abundant cells in bone constituting over 90–95% of cells.70 They regulate bone formation and resorption by inhibiting or promoting osteoclastic activity according to need.71 The role of osteocytes in the control of bone resorption is evidenced by the high levels of both of the osteoclastogenic cytokines, macrophage colony-stimulating factor (M-CSF) and RANKL in the osteocytic cell line MLO-Y4.70 This is further supported by in vivo work of Xiong and colleagues, who generated mice with osteocyte-specific deletion of RANKL and found that, although the bones developed normally by 5 weeks of age, the adult mice were resistant to unloading-induced bone loss.72
In skeletally mature adults, bone mass homeostasis is maintained by the tightly coupled activities of bone forming osteoblasts and bone resorbing osteoclasts.73 Therefore, even though bioactive lipids have inhibitory effects on both osteoclastic and osteoblastic function, it is possible that one of the two effects is direct and primary whereas the other is indirect and secondary, due to the effects of coupling.73
Effects on bone anabolic agents
Adverse effects of inflammatory lipoproteins and lipids extend to the efficacy of bone anabolic agents. The first and only anabolic agent used to treat osteoporosis is parathyroid hormone (PTH), which is administered by intermittent injection. This 84-amino acid peptide critically maintains calcium homeostasis by stimulating either bone formation or resorption, necessary to keep serum calcium levels within the physiological range. Sustained serum PTH elevation stimulates bone resorption, whereas a transient rise in serum PTH, as achieved by intermittent injections of recombinant human PTH (1–34; teriparatide), stimulates bone formation.74 In both young and old, and in mice and humans, intermittent PTH(1–34) treatment markedly enhances mineral volume, microarchitecture and structure.75 Bone anabolic actions of PTH(1–34) have been attributed to driving progenitor cells toward osteogenic fate and decreased apoptosis of osteoprogenitor cells.,76 Effects of PTH(1–34) on the osteogenic commitment appear to be in part through stimulation of insulin-like growth factor (IGF)-dependent mechanisms.77
Bioactive lipids also have adverse effects on bone anabolic agents. In vitro cell culture studies show that bioactive phospholipids inhibit PTH(1–34) induction of immediate early genes and bone morphogenetic protein 2 (BMP-2)–induced osteoblastic differentiation.78 In vivo, hyperlipidemic Ldlr−/− mice have a blunted bone anabolic response to teriparatide (PTH 1–34).79 The inhibitory mechanism appears to be through the protein kinase A (PKA) pathway,78 as well as Wnt and/or IGF-I-dependent mechanisms.39 Reducing oxidant stress by oral administration of an apoA-I mimetic peptide (D-4F) or overexpression of the antioxidant enzyme, paraoxonase 1, restores bone anabolic effects of PTH(1–34) in hyperlipidemic mice by affecting both bone formation and resorption (Figure 2B.80, 81 A recent study in transgenic mice show that intermittent PTH(1–34)-induced promoter activity driving green fluorescent protein expression in preosteoblasts, but not osteocytes, is blunted by hyperlipidemia, suggesting that hyperlipidemia-induced PTH(1–34) resistance depends on osteoblast developmental stage.61 In support of the animal studies, a recent retrospective, longitudinal study demonstrated that lumbar bone mineral density correlates negatively with levels of total cholesterol and positively with levels of HDL cholesterol in patients, who received teriparatide.82
Concluding remarks and future perspectives
Epidemiological studies suggest a link between atherosclerosis and bone loss; and growing evidence from both tissue culture and animal studies now show that the same bioactive lipids, lipoproteins, and phospholipids that promote atherosclerosis also adversely affect bone. Importantly, their effects depend of the type of bone cells: they inhibit differentiation of bone forming cells while promoting differentiation of bone resorbing cells and upregulating expression of osteoclastogenic cytokines, such as IL-6 and RANKL, by osteoblasts and T lymphocytes. Recent studies in mice now demonstrate that the bone anabolic actions of teriparatide are also blunted by the same bioactive lipids, effects of which are, in turn, attenuated by antioxidants, suggesting that blocking formation of lipid oxidation products may be necessary for teriparatide anabolism.
Although some progress has been made, much remains to be learned. The intracellular molecular mediators of the bioactive lipids on bone cells are not known. Although the cellular targets, such as osteoprogenitors, osteoblasts, and osteoclasts have been investigated in vitro, their effects in vivo and effects on other bone cells, such as osteocytes, which also regulate bone functions by producing RANKL, prostaglandins, and sclerostin are not known. Elucidation of the cellular targets and the molecular mediators of these bioactive lipids may allow for preventative as well as new therapeutic options. Additional important translational questions arise from the current data, in that osteoporotic patients, who have hyperlipidemia, may not be receiving the full benefits of anabolic therapy. Such clinical studies may be warranted to investigate the efficacy of PTH therapy in patients with disorders of lipid metabolism.
Box 1: Emerging Questions and Trends.
Questions
How do bioactive lipids act on bone cells?
What is the receptor(s) responsible for mediating effects of bioactive lipids on bone cells?.
Do osteocytes play a role in hyperlipidemia-induced bone resorption? Do bioactive inflammatory lipids have direct effects on osteocytic functions that control bone formation and resorption?
Trends
The effects of highly oxidized LDL are mediated by lectin-like oxLDL receptor (LOX-1) in vascular endothelial cells,83 and T lymphocytes,69 and by CD36 in macrophages.84 Effects of ox-PAPC are mediated by toll-like receptor 2 (TLR2) in macrophages and in liver.50 Although expression of LOX-1 and TLR2 is found in bone marrow derived mesenchymal cells85 and osteoblasts,86 the receptor mediating actions of bioactive lipids in bone cells remains to be investigated.
Growing evidence indicates that osteocytes regulate bone resorption during bone remodeling in skeletally mature adults.72 Osteocytic cells secrete RANKL in response to inflammatory cytokines87 and osteocyte-induced bone resorption may occur in hyperlipidemic conditions. Lipid accumulation is found juxtaposed to osteons.51–53 Serum bone resorption markers are induced in mice fed an atherogenic diet.39 Pretreatment of hyperlipidemic mice with apoA-I mimetic peptide, D-4F, prior to intermittent PTH(1–34) treatment, attenuates serum bone resorption markers.88 Mice overexpressing paraoxonase 1 had decreased numbers of osteoclasts in their endocortical envelopes.80
HIGHLIGHTS.
A link between atherosclerotic vascular disease and bone loss is discussed
Lipids/lipoproteins have direct effects on the differentiation of osteoblasts and osteoclasts
Lipids/lipoproteins blunt the bone anabolic effects of PTH (1–34)
Oxidant stress plays a role in hyperlipidemic bone disease
Acknowledgements
This research was supported by funding from National Institute of Health (DK081346, HL 109628, and HL114709).
GLOSSARY
- ApoA-1 mimetic peptide
a short peptide whose sequence does not contain the exact amino acid sequence, but is largely based on the amphipathic helical structure of apoA-l. D-4F is one such peptide.
- Apoptosis
a type of cell death that is programmed and occurs during embryogenesis and cellular injury.
- Bioactive inflammatory lipids
are lipids that have biological activity such as inducing genes that are involved in inflammation.
- Bone mineral density (BMD)
it refers to the amount of mineral matter per square centimeter of bones and is used in clinical practice as an indirect indicator of osteoporosis and fracture risk.
- Cholesterol-years
is a unit of measure describing duration of exposure to abnormal lipid levels.
- Dyslipidemia
A condition in which blood levels of total cholesterol, LDL cholesterol, and triglycerides are elevated and/or HDL cholesterol is reduced.
- Hyperlipidemia
A condition in which blood levels of total cholesterol, LDL cholesterol and triglycerides are elevated.
- Matrix GLA protein
is a small, secreted protein that inhibits BMP-2 and/or mineralization. Post-translational carboxylation of gluatmate residues is required for its optimal function, and this process requires a cofactor, vitamin K.
- Matrix vesicles
are cell-derived vesicles located within the extracellular matrix. They initiate mineralization of the matrix in a variety of tissues, including bone, cartilage, and dentin.
- Osteoblasts
are cells that form bone (mineralized matrix) by producing a matrix called osteoid that is permissive for mineralization.
- Osteocalcin
is a vitamin K-dependent protein secreted by cells during their differentiation. Its function is to limit mineral maturation.
- Osteoclasts
are cells that resorb mineralized matrix. Osteoclastic differentiation requires macrophage colony stimulating factor (M-CSF) and RANKL.
- Osteocytes
are former osteoblasts that become buried in mineralized matrix. Although osteocytes stop proliferating, by mechanosensation, they are actively involved in maintenance of bone by regulating bone formation and resorption.
- Osteon
also known as Haversian system is a roughly cylindrical, fundamental, functional unit of cortical bone tissue. It consists of a Haversian canal surrounded by osteocytes that connect to each other via a network of small transverse canals called canaliculi.
- Ox-PAPC
is the biologically active phospholipid component of MM-LDL and can be synthesized.
- Paraoxonase
is an enzyme with arylesterase activity and is synthesized in liver. It associates with HDL and confers anti-oxidant properties of HDL
- Receptor activator of nuclear factor kappa-B ligand (RANKL)
a member of the tumor necrosis factor (TNF) cytokine family. It is produced by osteoblasts, osteocytes, hypertrophic chondrocytes and T lymphocytes. It activates osteoclastic cell differentiation and dendritic cell maturation.
- Reactive oxygen species (ROS)
are oxygen-containing, short-lived molecules that are chemically highly reactive. Although ROS have important roles in signaling and homeostasis, excess production causes oxidative stress to nearby molecules such as proteins and DNA.
- Reverse cholesterol transport
is a process in which cholesterol is removed from peripheral tissues and returned to the liver.
- Teriparatide
a recombinant form of parathyroid hormone and the only FDA-approved drug for osteoporosis. Intermittent treatment with teriparatide increases bone mineral density and reduces fractures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Annema W, Tietge UJ. Regulation of reverse cholesterol transport - a comprehensive appraisal of available animal studies. Nutr Metab (Lond) 2012;9:25. doi: 10.1186/1743-7075-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Angelantonio E, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewington S, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. lancet. 2007;370:1829–1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 4.Navab M, et al. D-4F-mediated reduction in metabolites of arachidonic and linoleic acids in the small intestine is associated with decreased inflammation in low-density lipoprotein receptor-null mice. J Lipid Res. 2012;53:437–445. doi: 10.1194/jlr.M023523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navab M, et al. High-density lipoprotein and 4F peptide reduce systemic inflammation by modulating intestinal oxidized lipid metabolism: novel hypotheses and review of literature. Arterioscler Thromb Vase Biol. 2012;32:2553–2560. doi: 10.1161/ATVBAHA.112.300282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Y, et al. Myeloperoxidase, paraoxonase-1, and HDL form a functional ternary complex. J Clin invest. 2013;123:3815–3828. doi: 10.1172/JCI67478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhattacharyya T, et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA. 2008;299:1265–1276. doi: 10.1001/jama.299.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng DS, et al. Paraoxonase-1 deficiency in mice predisposes to vascular inflammation, oxidative stress, and thrombogenicity in the absence of hyperlipidemia. Cardiovasc Pathol. 2008;17:226–232. doi: 10.1016/j.carpath.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Gong M, et al. Quercetin up-regulates paraoxonase 1 gene expression with concomitant protection against LDL oxidation. Biochem Biophys Res Commun. 2009;379:1001–1004. doi: 10.1016/j.bbrc.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Shih DM, et al. Decreased obesity and atherosclerosis in human paraoxonase 3 transgenic mice. Circ Res. 2007;100:1200–1207. doi: 10.1161/01.RES.0000264499.48737.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borja MS, et al. HDL-apoA-l Exchange: Rapid Detection and Association with Atherosclerosis. PLoS One. 2013;8:e71541. doi: 10.1371/journal.pone.0071541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander ET, et al. Influence of apolipoprotein A–I domain structure on macrophage reverse cholesterol transport in mice. Arterioscler Thromb Vase Biol. 2011;31:320–327. doi: 10.1161/ATVBAHA.110.216226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen D, et al. Lipids and collagen matrix restrict the hydraulic permeability within the porous compartment of adult cortical bone. Ann Biomed Eng. 2010;38:558–569. doi: 10.1007/s10439-009-9858-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irving JT. Calcification of the Organic Matrix of Enamel. Arch Oral Biol. 1963;8:773–774. doi: 10.1016/0003-9969(63)90010-4. [DOI] [PubMed] [Google Scholar]
- 15.Reid DG, et al. Lipids in biocalcification: contrasts and similarities between intimal and medial vascular calcification and bone by NMR. J Lipid Res. 2012;53:1569–1575. doi: 10.1194/jlr.M026088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang Y, et al. Effect of vitamin K on bone mineral density: a meta-analysis of randomized controlled trials. J Bone Miner Metab. 2012;30:60–68. doi: 10.1007/s00774-011-0287-3. [DOI] [PubMed] [Google Scholar]
- 17.Niemeier A, et al. The role of apolipoprotein E in bone metabolism. Bone. 2012;50:518–524. doi: 10.1016/j.bone.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Jiang G, et al. Coronary heart disease mortality in China: age, gender, and urban-rural gaps during epidemiological transition. Rev Panam Salud Publico. 2012;31:317–324. doi: 10.1590/s1020-49892012000400008. [DOI] [PubMed] [Google Scholar]
- 19.Tamaki J, et al. Low bone mass is associated with carotid atherosclerosis in postmenopausal women: the Japanese Population-based Osteoporosis (JPOS) Cohort Study. Osteoporos int. 2009;20:53–60. doi: 10.1007/s00198-008-0633-z. [DOI] [PubMed] [Google Scholar]
- 20.Hmamouchi I, et al. Low bone mineral density is related to atherosclerosis in postmenopausal Moroccan women. BMCPublic Health. 2009;9:388. doi: 10.1186/1471-2458-9-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naves M, et al. Progression of vascular calcifications is associated with greater bone loss and increased bone fractures. Osteoporos Int. 2008;19:1161–1166. doi: 10.1007/s00198-007-0539-1. [DOI] [PubMed] [Google Scholar]
- 22.Szulc P, et al. Calcifications in the abdominal aorta predict fractures in men: MINOS study. J Bone Miner Res. 2008;23:95–102. doi: 10.1359/jbmr.070903. [DOI] [PubMed] [Google Scholar]
- 23.Tanko LB, et al. Low bone mineral density in the hip as a marker of advanced atherosclerosis in elderly women. Calcif Tissue Int. 2003;73:15–20. doi: 10.1007/s00223-002-2070-x. [DOI] [PubMed] [Google Scholar]
- 24.Barengolts EI, et al. Osteoporosis and coronary atherosclerosis in asymptomatic postmenopausal women. Calcif Tissue Int. 1998;62:209–213. doi: 10.1007/s002239900419. [DOI] [PubMed] [Google Scholar]
- 25.Collins TC, et al. Peripheral arterial disease is associated with higher rates of hip bone loss and increased fracture risk in older men. Circulation. 2009;119:2305–2312. doi: 10.1161/CIRCULATIONAHA.108.820993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim YH, et al. Low bone mineral density is associated with dyslipidemia in South Korean men: The 2008–2010 Korean National Health and Nutrition Examination Survey. Endocr J. 2013 doi: 10.1507/endocrj.ej13-0224. [DOI] [PubMed] [Google Scholar]
- 27.Saghafi H, Hossein-nezhad A, Rahmani M, Larijani B. Relationship between Lipid Profile and Bone Turnover in Pre and Postmenopausal Women. Iranian J Publ Health, A supplementary issue on Osteoporosis and Bone Turnover. 2008;1:23–29. [Google Scholar]
- 28.Makovey J, et al. Association between serum cholesterol and bone mineral density. Bone. 2009;44:208–213. doi: 10.1016/j.bone.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 29.Brownbill RA, Ilich JZ. Lipid profile and bone paradox: higher serum lipids are associated with higher bone mineral density in postmenopausal women. J Womens Health (Larchmt) 2006;15:261–270. doi: 10.1089/jwh.2006.15.261. [DOI] [PubMed] [Google Scholar]
- 30.Jeong IK, et al. Lipid profiles and bone mineral density in pre- and postmenopausal women in Korea. Calcif Tissue Int. 2010;87:507–512. doi: 10.1007/s00223-010-9427-3. [DOI] [PubMed] [Google Scholar]
- 31.Nozue T, et al. Cholesterol-years score is associated with development of senile degenerative aortic stenosis in heterozygous familial hypercholesterolemia. J Atheroscler Thromb. 2006;13:323–328. doi: 10.5551/jat.13.323. [DOI] [PubMed] [Google Scholar]
- 32.Lim HY, et al. Hypercholesterolemic mice exhibit lymphatic vessel dysfunction and degeneration. Am J Pathol. 2009;175:1328–1337. doi: 10.2353/ajpath.2009.080963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Awan Z, et al. Vascular calcifications in homozygote familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2008;28:777–785. doi: 10.1161/ATVBAHA.107.160408. [DOI] [PubMed] [Google Scholar]
- 34.Rowlan JS, et al. Atherosclerosis susceptibility Loci identified in an extremely atherosclerosis-resistant mouse strain. J Am Heart Assoc. 2013;2:e000260. doi: 10.1161/JAHA.113.000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desai MS, et al. Atherogenic diet-induced hepatitis is partially dependent on murine TLR4. J Leukoc Biol. 2008;83:1336–1344. doi: 10.1189/jlb.0607390. [DOI] [PubMed] [Google Scholar]
- 36.Graham LS, et al. Bone density and hyperlipidemia: the T-lymphocyte connection. J Bone Miner Res. 2010;25:2460–2469. doi: 10.1002/jbmr.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirasawa H, et al. ApoE gene deficiency enhances the reduction of bone formation induced by a high-fat diet through the stimulation of p53-mediated apoptosis in osteoblastic cells. J Bone Miner Res. 2007;22:1020–1030. doi: 10.1359/jbmr.070330. [DOI] [PubMed] [Google Scholar]
- 38.Parhami F, et al. Atherogenic high-fat diet reduces bone mineralization in mice. J Bone Miner Res. 2001;16:182–188. doi: 10.1359/jbmr.2001.16.1.182. [DOI] [PubMed] [Google Scholar]
- 39.Pirih F, et al. Adverse effects of hyperlipidemia on bone regeneration and strength. J Bone Miner Res. 2012;27(2):309–318. doi: 10.1002/jbmr.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ionova-Martin SS, et al. Reduced size-independent mechanical properties of cortical bone in high-fat diet-induced obesity. Bone. 2010;46:217–225. doi: 10.1016/j.bone.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soares EA, et al. Effect of hyperlipidemia on femoral biomechanics and morphology in low-density lipoprotein receptor gene knockout mice. J Bone Miner Metab. 2012;30:419–425. doi: 10.1007/s00774-011-0345-x. [DOI] [PubMed] [Google Scholar]
- 42.Brown MD, et al. Lipid retention in the arterial wall of two mouse strains with different atherosclerosis susceptibility. J Lipid Res. 2004;45:1155–1161. doi: 10.1194/jlr.M400092-JLR200. [DOI] [PubMed] [Google Scholar]
- 43.Shi W, et al. Endothelial responses to oxidized lipoproteins determine genetic susceptibility to atherosclerosis in mice. Circulation. 2000;102:75–81. doi: 10.1161/01.cir.102.1.75. [DOI] [PubMed] [Google Scholar]
- 44.Schilling AF, et al. Increased bone formation in mice lacking apolipoprotein E. J Bone Miner Res. 2005;20:274–282. doi: 10.1359/JBMR.041101. [DOI] [PubMed] [Google Scholar]
- 45.Smith WL, Murphy RC. Oxidized lipids formed non-enzymatically by reactive oxygen species. J Biol Chem. 2008;283:15513–15514. doi: 10.1074/jbc.R800006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gleissner CA, et al. Effects of native and modified low-density lipoproteins on monocyte recruitment in atherosclerosis. Hypertension. 2007;50:276–283. doi: 10.1161/HYPERTENSIONAHA.107.089854. [DOI] [PubMed] [Google Scholar]
- 47.Gutierrez LL, et al. Atherosclerosis: a redox-sensitive lipid imbalance suppressible by cyclopentenone prostaglandins. Biochem Pharmacol. 2008;75:2245–2262. doi: 10.1016/j.bcp.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 48.Spanbroek R, et al. Expanding expression of the 5-lipoxygenase pathway within the arterial wall during human atherogenesis. Proc Natl Acad Sci U S A. 2003;100:1238–1243. doi: 10.1073/pnas.242716099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aoki T, Narumiya S. Prostaglandins and chronic inflammation. Trends Pharmacol Sci. 2012;33:304–311. doi: 10.1016/j.tips.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 50.Kadl A, et al. Oxidized phospholipid-induced inflammation is mediated by Tolllike receptor 2. Free Radic Biol Med. 2011;51:1903–1909. doi: 10.1016/j.freeradbiomed.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kawai K, et al. Fat necrosis of osteocytes as a causative factor in idiopathic osteonecrosis in heritable hyperlipemic rabbits. Clin Orthop Relat Res. 1980:273–282. [PubMed] [Google Scholar]
- 52.Watanabe Y, et al. Histopathology of femoral head osteonecrosis in rheumatoid arthritis: the relationship between steroid therapy and lipid degeneration in the osteocyte. Rheumatol Int. 1989;9:25–31. doi: 10.1007/BF00270286. [DOI] [PubMed] [Google Scholar]
- 53.Tintut Y, et al. Hyperlipidemia promotes osteoclastic potential of bone marrow cells ex vivo. Arterioscler Thromb Vasc Biol. 2004;24:e6–e10. doi: 10.1161/01.ATV.0000112023.62695.7f. [DOI] [PubMed] [Google Scholar]
- 54.Maurel DB, et al. Osteocyte apoptosis and lipid infiltration as mechanisms of alcohol-induced bone loss. Alcohol Alcohol. 2012;47:413–422. doi: 10.1093/alcalc/ags057. [DOI] [PubMed] [Google Scholar]
- 55.Gordon S. Macrophage heterogeneity and tissue lipids. J Clin Invest. 2007;117:89–93. doi: 10.1172/JCI30992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wuthier RE. Lipids of matrix vesicles. Fed Proc. 1976;35:117–121. [PubMed] [Google Scholar]
- 57.Dirksen TR, Marinetti GV. Lipids of bovine enamel and dentin and human bone. Calcif Tissue Res. 1970;6:1–10. doi: 10.1007/BF02196179. [DOI] [PubMed] [Google Scholar]
- 58.Brodeur MR, et al. Influence of oxidized low-density lipoproteins (LDL) on the viability of osteoblastic cells. Free Radic Biol Med. 2008;44:506–517. doi: 10.1016/j.freeradbiomed.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 59.Almeida M, et al. Increased lipid oxidation causes oxidative stress, increased peroxisome proliferator-activated receptor-gamma expression, and diminished pro-osteogenic Wnt signaling in the skeleton. J Biol Chem. 2009;284:27438–27448. doi: 10.1074/jbc.M109.023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maziere C, et al. Oxidized low density lipoprotein inhibits phosphate signaling and phosphate-induced mineralization in osteoblasts. Involvement of oxidative stress. Biochim Biophys Acta. 2010;1802:1013–1019. doi: 10.1016/j.bbadis.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 61.Li X, et al. Mechanism of hyperlipidemia-induced parathyroid hormone (PTH) resistance: A role of PTH receptor and reactive oxygen species in preosteoblasts. J Cell Biochem. 2013 doi: 10.1002/jcb.24648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsuzawa N, et al. Lipid-induced oxidative stress causes steatohepatitis in mice fed an atherogenic diet. Hepatology. 2007;46:1392–1403. doi: 10.1002/hep.21874. [DOI] [PubMed] [Google Scholar]
- 63.Parhami F, et al. Atherogenic diet and minimally oxidized low density lipoprotein inhibit osteogenic and promote adipogenic differentiation of marrow stromal cells. J Bone Miner Res. 1999;14:2067–2078. doi: 10.1359/jbmr.1999.14.12.2067. [DOI] [PubMed] [Google Scholar]
- 64.Arai M, et al. Effects of reactive oxygen species (ROS) on antioxidant system and osteoblastic differentiation in MC3T3-E1 cells. IUBMB Life. 2007;59:27–33. doi: 10.1080/15216540601156188. [DOI] [PubMed] [Google Scholar]
- 65.Chuang CC, et al. Hyperglycemia enhances adipogenic induction of lipid accumulation: involvement of extracellular signal-regulated protein kinase 1/2, phosphoinositide 3-kinase/Akt, and peroxisome proliferator-activated receptor gamma signaling. Endocrinology. 2007;148:4267–4275. doi: 10.1210/en.2007-0179. [DOI] [PubMed] [Google Scholar]
- 66.Hernandez JL, et al. Metabolic syndrome, fractures and gender. Maturitas. 2011;68:217–223. doi: 10.1016/j.maturitas.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 67.Tintut Y, et al. 8-Isoprostaglandin E2 enhances receptor-activated NFkappa B ligand (RANKL)-dependent osteoclastic potential of marrow hematopoietic precursors via the cAMP pathway. J Biol Chem. 2002;277:14221–14226. doi: 10.1074/jbc.M111551200. [DOI] [PubMed] [Google Scholar]
- 68.Tseng W, et al. Regulation of interleukin-6 expression in osteoblasts by oxidized phospholipids. J Lipid Res. 2010;51:1010–1016. doi: 10.1194/jlr.M001099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Graham LS, et al. Oxidized lipids enhance RANKL production by T lymphocytes: implications for lipid-induced bone loss. Clin Immunol. 2009;133:265–275. doi: 10.1016/j.clim.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26:229–238. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tatsumi S, et al. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5:464–475. doi: 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 72.Xiong J, et al. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235–1241. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003;423:349–355. doi: 10.1038/nature01660. [DOI] [PubMed] [Google Scholar]
- 74.Cipriani C, et al. Safety of osteoanabolic therapy: a decade of experience. J Bone Miner Res. 2012;27:2419–2428. doi: 10.1002/jbmr.1800. [DOI] [PubMed] [Google Scholar]
- 75.Chen P, et al. Increases in BMD correlate with improvements in bone microarchitecture with teriparatide treatment in postmenopausal women with osteoporosis. J Bone Miner Res. 2007;22:1173–1180. doi: 10.1359/jbmr.070413. [DOI] [PubMed] [Google Scholar]
- 76.Wang YH, et al. Effects of transient PTH on early proliferation, apoptosis, and subsequent differentiation of osteoblast in primary osteoblast cultures. Am J Physiol Endocrinol Metab. 2007;292:E594–E603. doi: 10.1152/ajpendo.00216.2006. [DOI] [PubMed] [Google Scholar]
- 77.Wang Y, et al. IGF-I receptor is required for the anabolic actions of parathyroid hormone on bone. J Bone Miner Res. 2007;22:1329–1337. doi: 10.1359/jbmr.070517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang MS, et al. Atherogenic phospholipids attenuate osteogenic signaling by BMP-2 and parathyroid hormone in osteoblasts. J Biol Chem. 2007;282:21237–21243. doi: 10.1074/jbc.M701341200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang MS, et al. Hyperlipidemia impairs osteoanabolic effects of PTH. J Bone Miner Res. 2008;23:1672–1679. doi: 10.1359/JBMR.080513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu J, et al. Role of paraoxonase-1 in bone anabolic effects of parathyroid hormone in hyperlipidemic mice. Biochem Biophys Res Commun. 2013 doi: 10.1016/j.bbrc.2012.12.114. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sage AP, et al. Hyperlipidemia induces resistance to PTH bone anabolism in mice via oxidized lipids. J Bone Miner Res. 2011;26:1197–1206. doi: 10.1002/jbmr.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jeon YK, et al. The Anabolic Effect of Teriparatide is Undermined by Low Levels of High-Density Lipoprotein Cholesterol. Calcif Tissue Int. 2013 doi: 10.1007/s00223-013-9772-0. [DOI] [PubMed] [Google Scholar]
- 83.Chu CS, et al. Electronegative Low-Density Lipoprotein Increases C-Reactive Protein Expression in Vascular Endothelial Cells through the LOX-1 Receptor. PLoS One. 2013;8:e70533. doi: 10.1371/journal.pone.0070533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Collot-Teixeira S, et al. CD36 and macrophages in atherosclerosis. Cardiovasc Res. 2007;75:468–477. doi: 10.1016/j.cardiores.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 85.Zhang F, et al. Lectin-like oxidized LDL receptor-1 expresses in mouse bone marrow-derived mesenchymal stem cells and stimulates their proliferation. Exp Cell Res. 2013;319:1054–1059. doi: 10.1016/j.yexcr.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 86.Matsumoto C, et al. Toll-like receptor 2 heterodimers, TLR2/6 and TLR2/1 induce prostaglandin E production by osteoblasts, osteoclast formation and inflammatory periodontitis. Biochem Biophys Res Commun. 2012;428:110–115. doi: 10.1016/j.bbrc.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 87.Kulkarni RN, et al. Mechanical loading prevents the stimulating effect of IL-1beta on osteocyte-modulated osteoclastogenesis. Biochem Biophys Res Commun. 2012;420:11–16. doi: 10.1016/j.bbrc.2012.02.099. [DOI] [PubMed] [Google Scholar]
- 88.Sage AP, et al. Hyperphosphatemia-induced nanocrystals upregulate the expression of bone morphogenetic protein-2 and osteopontin genes in mouse smooth muscle cells in vitro. Kidney Int. 2010 doi: 10.1038/ki.2010.390. [DOI] [PMC free article] [PubMed] [Google Scholar]