Abstract
Disease-cell models that recapitulate specific molecular phenotypes are essential for the investigation of molecular pathogenesis of neurodegenerative diseases including lysosomal storage diseases (LSDs) with predominant neurological manifestations. Herein we report the development and characterization of a cell model for a rapid neurodegenerative LSDs, globoid-cell leukodystrophy (GLD), mostly known as Krabbe disease. GLD is caused by the deficiency of β-galactocerebrosidase (GALC), a lysosomal enzyme that hydrolysis two glycosphingolipids, psychosine and galactosylceramide. Unfortunately, the available culture fibroblasts from GLD patients consist in a limited research tool as these cells fail to accumulate psychosine, the central pathogenic glycosphingolipid in this LSD that results in severe demyelination. Firstly, we obtained brain samples from the Twitcher (Twi) mice (GALCtwi/twi), the natural mouse model with GALC deficiency. We immortalized the primary neuroglial cultured cells with SV40 large T antigen, generating the 145M-Twi and the 145C-Wt cell lines from the Twi and control mice, respectively. Both cell lines expressed specific oligodendrocyte markers including A2B5 and GalC. The 145M-Twi cells showed biochemical and cellular disturbances related to GLD neuropathogenesis including remarkable caspase-3 activation, release of cytochrome C into the cytosol and expansion of the lysosomal compartment. Under treatment with glycosphingolipids, 145M-Twi cells showed increased LC3B levels, a marker of autophagy. Using LC-MS/MS method we developed, the 145M-Twi cells showed significantly higher levels of psychosine. The 145M-Twi and 145C-Wt lines allowed the development of a robust throughput LC-MS/MS assay to measure cellular psychosine levels. In this throughput assay, L-cycloserine showed to significantly reduce the 145M-Twi cellular levels of psychosine. The established 145M-Twi cells is a powerful research tool to investigate neurologically relevant pathogenic pathways as well as to develop primary screening assays for the identification of therapeutic agents for GLD and potentially other glycosphingolipid disorders.
Keywords: β-galactocerebrosidase, Krabbe Disease, globoid-cell leukodystrophy, psychosine, LC-MS/MS
1. Introduction
Glycosphingolipids are complex lipids with broad structural variety and cell-dependent expression, assuming major roles in physiological functions related to membrane structure and intracellular signaling [1]. These lipids may participate in the various pathogenic disease processes [2–5]. Given that glycosphingolipids are highly expressed in the central nervous system [1], disturbances in their degradation/biosynthetic pathways are associated with numerous neurodegenerative disorders [6]. Krabbe disease (MIM#245200), originally described as globoid-cell leukodystrophy (GLD), is a classical example in which a glycosphingolipid (psychosine) is an important pathogenic molecule in the neurodegenerative process [7]. The exact mechanism of psychosine cytotoxicity is still unclear. Recent studies demonstrated that psychosine and its enantiomer have equal or greater cytotoxicity by localizing to lipid rafts, disturbing plasma membrane integrity and inhibiting protein kinase C translocation to the plasma membrane [8]. GLD is an autosomal recessive disease caused by the deficiency of β-galatocerebrosidase (GALC; EC 3.2.1.46), a lysosomal hydrolase that has galactosylceramide and psychosine as natural substrates [9]. The most common clinical form of GLD is characterized by progressive demyelination resulting in hyperirritability, hypotonia, intractable seizures and ultimately death within the first 1–2 years of age [10].
During myelination, uridine diphosphate-galactose:ceramide galactosyltransferase catalyzes the galactosylation of sphingosine and ceramide producing psychosine (galactosylsphingosine) and galactosylceramide, respectively [11]. Psychosine is promptly metabolized by GALC into sphingosine, maintaining low physiological levels of psychosine [7, 11]. In brain specimens from GLD patients however, apart from the infiltration of multinucleated PAS-positive macrophages, the “globoid-cells” [7], substantial neural cell loss is observed [12, 13]. These neuropathological findings are explained by the rapid effects of psychosine which, at elevated concentrations, becomes highly cytotoxic [14], in particular to oligodendrocytes, resulting in progressive demyelination [7]. This rapid neurodegenerative process results in, less evident, and even reduced, cellular storage, a hallmark cellular finding classically seen in several other lysosomal disorders [7].
Unfortunately, the sphingolipid and glycosphingolipid metabolism is poorly expressed in most cells other than those of neural origin. However, due to the recent advances on the technology of mass spectrometry (MS), measurement of certain glycosphingolipids is feasible in easily available specimens [15]. The non-availability of established neural cells has limited the understanding of neuropathogenic mechanisms in GLD as well as other lysosomal storage diseases (LSDs). Based on the major role of elevated psychosine levels in the neuropathogenesis of GLD, it is conceivable that the development of a neural disease-cell model will allow the identification of neurologically relevant pathogenic cascades, which can be used as targets in the discovery of therapies for this rapidly neurodegenerative disease.
Here we describe the development, characterization and potential application of a neurologically relevant cell line from the Twitcher (Twi) mouse, the classical GLD model with a naturally-occurring mutation in GALC gene [16]. Using a newly developed liquid chromatography MS (LC-MS/MS) assay, the 145M-Twi cells showed increased psychosine levels. In the 145M-Twi cells, we report novel molecular observations, which have direct implications in the pathogenesis of GLD. In addition, we demonstrate the potential of these cells to be utilized as a research tool for developing screening assays to allow the identification of therapeutic agents for this disorder.
2. Material and Methods
Cell Culture and Transformation
Primary cortical cells were obtained from dissected brain cortices from 5 pups at first day of life from Twi C57BL/6J mouse (GALCwt/wt) and 5 pups from a CB57BL/6J control mouse (GALCwt/wt). Primary cortical neuroglial cells were cultured using DMEM/F-12 medium with 1% penicillin/streptomycin and 10% dialyzed fetal bovine serum (Benchmark FBS). Cells were cultured using traditional incubators with 5% CO2 at 37°C. Cultured primary neuroglial cells were transformed using pSV3-neo plasmid containing the simian virus (SV) 40 large T cell antigen as previously described [17]. Two immortalized cell lines were established: 145M-Twi, transformed cells from the Twi mice; 145C-Wt, transformed cells from the control mice. Further information is detailed in Supplementary data.
GALC and caspase-3 enzymatic assays
The 145M-Twi and 145C-Wt cells were cultured in 75 cm2 flasks for obtaining lysates for GALC assay. On average 10 μg of protein lysate was used per assay. The GALC assays were performed with specific synthetic fluorescent 6-hexadecanoylamino-4-methylumbelliferyl-β-D-galactoside (HMUβGal) following procedures previously described [18]. After stopping assay, 260 μL aliquots from the total solution were transferred to 96-well plates for reading in a fluorescence plate reader at wavelengths of HMU (λexcitation = 404 nm; λemission = 406 nm). Assays were run in quadruplicate samples for Twitcher and control mice cell pellets. Caspase-3 assays were performed with 20 μM concentrations of N-acetyl-DEVD 7-maino-4-methylcoumarin (Ac-DEDV-AMC) synthetic fluorescent substrate for one hour as previously described [19]. 145M-Twi and 145C-Wt from Twitcher and control mice, respectively, were cultured in 6-well plates with wells treated with 15 and 30 μM of psychosine (purity >98%; Matreya LLC) and glucosyl-sphingosine (purity>98%; Matreya LLC). After 24hs, these cells were washed with PBS and harvested with 0.05% trypsin solution. After centrifugation and aspiration of PBS, cell lysate preparation and assay procedures were performed as per manufacturing protocol and as previously described [19].
Immunocytochemistry assays
First, cells were grown to 70–80% confluence on round coverslips, washed with PBS and fixed with 4% paraformoaldehyde for 15 min, following by blocking with PBS 10% goat serum for 30 min. After washing twice with PBS, cells were incubated in the presence of anti-A2B5 (1:400 in PBS 1% goat serum), anti-GalC (1:200 in PBS 1% goat serum), GFAP (1:200 in PBS 1% goat serum) and MAP2 (1:1,000 in PBS 1% goat serum), or antibodies at room temperature for 2 h. The cells were then immunostained with suitable secondary antibodies (1:200; Molecular Probes – Invitrogen Inc.) at room temperature for 1 h. Using confocal laser scanning microscopy on the Zeiss LSM 510, images were taken with 100 × 1.4 numerical apertures (NA) and 63 × 1.4 NA Apochromat objective (Zeiss). For staining for cytochrome C and LysoTracker, monoclonal antibody was purchased from BD Pharmigen™ (clone 6H2.B4) and LysoTracker Red (DND-99) probe was purchased from Molecular Probes – Invitrogen Inc.. Cells were cultured in 70–80% confluence over coverlips and exposed to 0.5 μM of LysoTracker Red for 30 min before fixation step with formoaldehyde 2% in PBS also for 30 min. For these immunofluorescence assays, the GalC and A2B5, monoclonal antibodies were purchased from Millipore Corp. (Billerica, MA). For LC3-B confocal immunofluorescence assays, LC3B (D11) XP Rabbit monoclonal antibody was purchased from Cell Signaling™ and used at 1:200 dilution. Secondary antibodies Alexa Fluror® F′(ab)2 against mouse and were purchased Invitrogen™ (Life Tech, Grand Island, NY). The blocking procedure (PBS in 10% goat serum), primary and secondary antibody staining were as described above.
Western Blott
Immunoblot analysis for cleaved caspase-3 was performed using standard procedures [20]. 145M-Twi and 145C-Wt from Twitcher and control mice, respectively, were cultured to confluence and treated with different concentrations of psychosine 7.5–30 μM and glucosylsphingosine for 24hs and harvested and lysed in standard lysis buffer containing a protease inhibitor cocktail (Thermo-Fisher Inc.). Anti-α-tubulin mouse (DM1A) antibody (1:3,000) was used as a loading control and purchased by EMD Milipore™. Secondary HRP-antibody used was rabbit anti-mouse IgG at 1:10,000. Blots were developed using chemiluminescent substrate using cooled CCD camera coupled with software from Alpha Innotech Corp. (Amersham Biosciences).
Liquid chromatography mass spectrometry (LC-MS/MS)
Samples were analyzed by LC-MS/MS on the Applied Biosystems (SCIEX) API4000 (Applied Biosystems, Foster City, CA) coupled with Agilent 1100 autosampler. The LC-MSMS injections were 8μL from cellular lipid extracts. The LC column used was C8 X-Terra MS, 125 Å, 3.5μ, 1×50mm. The total run time was 2 minutes. Given that the both retention time for the psychosine and 1-acetyl-psychosine were < 1 min, the run time was further reduced to 1 min. Table S1 (Supplementary data) describes details of the psychosine LC-MS/MS conditions as well as the mobile phase A (MPA) and mobile phase B (MPB). The MRM transitions monitored were m/z 462 to 264 for psychosine and m/z 504 to 264 for 1-acetyl-psychosine, the ISD (Fig. 2a). For measurements of psychosine levels, areas under the peaks in the chromatograms were measured for both psychosine and 1-acetyl-psychosine (ISD). The pmoles of psychosine were calculated based on the ISD present in each sample 19.8 pmoles/100 μL. The initial determination of RTs of psychosine and 1-cetyl-psychosine (Fig. 2a), methanol solutions with different concentrations of these standards of psychosine and 1-acetyl-psychosine (both with purity >98%; Matreya LLC) were injected at 8 μL volume into the API4000 LC-MS/MS system. In terms of the LC-MS/MS validation assay, BEH column was used with a running time of 30 min. Two ACQUITY UPLC BEH Glycan (Waters Inc.) columns, as described above (1.7μ, 2.1×100mm and 1.7μ, 2.1×150mm), were coupled, being first 2.1×150mm and the second 2.1×100mm. Details on the conditions were detailed in Table S2 (Supplementary data).
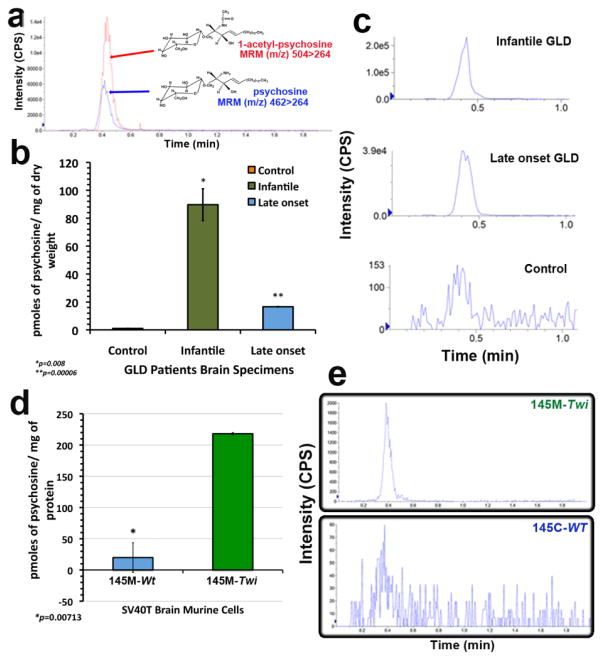
Figure 2. Development of a LC-MS/MS method to measure psychosine.
(a) Using the reverse C8 MS column in LC-MS/MS and standards for psychosine and 1-acetyl-psychosine, LC-MS/MS conditions to measure psychosine were established and respective chromatograms are shown. (b) Brain samples from infantile and late onset GLD patient were analyzed. The psychosine levels are significantly elevated in one infantile (n=3; p<0.01) and one late onset GLD brain specimens (n=3; p<0.0001), when compared to controls. (c) Chromatograms from LC-MS/MS psychosine analysis in each brain sample. (d) Lipid extracts from cell pellets were obtained from 145M-Twi and 145C-Wt (n=3). Psychosine levels were measured in these samples by the LC-MS/MS method here developed. The cellular psychosine levels were significantly elevated in Twi brain cells. (e) The LC-MS/MS chromatograms with psychosine peaks (MRM 462/264) were shown from the cell line lipid extracts. CPS, counts per second.
Brain specimens for psychosine LC-MS/MS analysis
Brain samples from one patient manifesting the infantile and another with the late onset GLD were obtained from NICHD Brain and Tissue Bank for Developmental Disorders, University of Maryland, Baltimore, MD. The brain specimens from GLD patients comprise of frozen samples from each brain region were weighted to determine the dry weight using high-precision scales to standardize the measurements of glycosphingolipids levels in further assays. The lipid extraction was performed using chloroform:methanol (2:1, v/v) as previously described [21].
Lipid extractions
For the lipid extraction from cells, lysates from 145M-Twi and 145C-Wt, derived from single clones of SV40-transformed primary neuroglial cells, received a volume of 150 μL of methanol (polar solvent) with ISD, 1-acetyl-psychosine (198.5 nM) was used. After shaking tubes for 60 min (orbital shaker), and spinning tubes (13,000 rpm) for 2–3 minutes, lipid extract solutions were passed through individual centrifuge 2 mL-tube filter (0.22 μ polypropylene. A volume of 8 μL of the filtered solution was directly injected into the API 4000. For cell cultured in 96-well plates, the 145M-Twi and 145C-Wt were cultured and then washed with PBS, polar solvent methanol with 0.5% acetic acid was dispensed directly into cells. Lipid extractions are amenable to be performed in 96-well plates [22]. A volume of 80 μL of the methanol with 0.5% acetic acid solvent containing 1-acetyl-psychosine (198.5 nM), as internal standard, was efficient for psychosine extraction in the multi-well plate. After placing the solvent, plates were sealed with 96-well polypropylene piercible round cap mat and agitated in a horizontal orbital shaker for 60 minutes. The extraction solution was then transferred to the filter plate AcroPrep™ Advance 96 (0.2 μ supor membrane) assembled to 96-well sample collection plate (1mL/well). The 96-well filter/sample collection assembly was then centrifuged at 1,400 rpm for 3 min. After the centrifugation step, the filter plate was discarded and the 96-well sample collection plate was sealed with a new 96-well polypropylene piercible round cap mat. Plates were kept in −20°C before injections into the API 4000.
3. Results
Establishment and characterization of cultured 145M-Twi cell lines from GLD mouse model
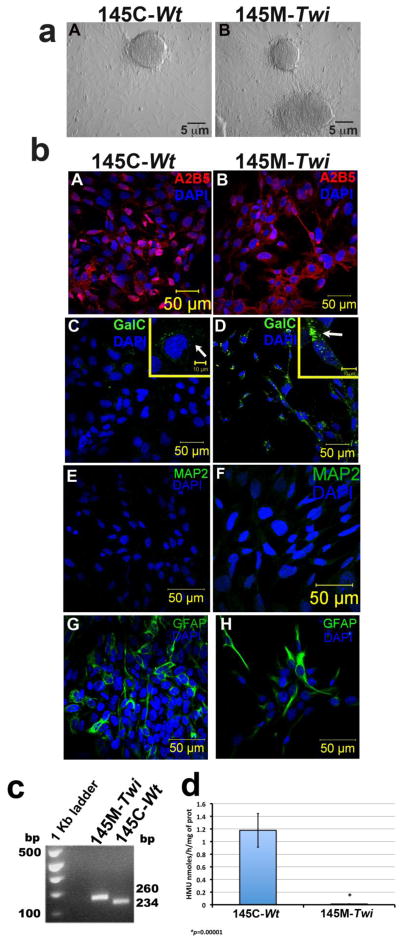
To develop an optimal disease cell-line that expresses glycosphingolipids and is representative of neurological pathogenic processes in GLD, we firstly established primary cultured neuroglial cells derived from cortices from Twitcher (Twi) GALCtwi/twi) mice at day 1 of life [16, 23] (Material and Methods). The C57BL/6J Twi carries a homozygous naturally-occurring nonsense mutation (c.1017G>A/W339X) resulting in a truncated and non-functional GALC with a clinical and neuropathological phenotype that recapitulates GLD [24]. We then successfully transformed the cortical primary neuroglial cells by transfecting the pSV3-neo plasmid containing the simian virus (SV) 40 large T-cell antigen. Subsequently, six clones from the SV-40 transformed (SV40T) cells were isolated and cultured separately (Fig. 1a). Primary neuroglial cells from the control mice, also C57BL/6J but with GALCwt/wt, were also transformed following the same procedure. Two SV40T transformed cells were generated: the 145M-Twi cell line derived from Twi (GALCtwi/twi), and the 145C-Wt derived from Wt (GALCwt/wt). In confocal cellular immunostaining assays, the transformed cells consistently expressed galactosylceramide (GalC) and A2B5, two classical oligodendrocyte markers (Fig. 1b). Interestingly, increased Galc signal was noted in the 145M-Twi when compared to the 145C-Wt controls (Fig. 1b, panels D and E). The neuronal specific marker MAP2 was absent in both 145M-Twi and 145C-Wt cell lines (Fig. 1b). These transformed cells expressing oligodendrocyte markers were named 145M-Twi those from Twi mice, and 145C-Wt those from the control mice. Few GFAP-positive cells, an astrocyte-specific marker, were noted amongst the 145M-Twi and 145C-Wt cell lines (Fig. 1b). We confirmed the presence of the homozygous nonsense mutation in the GALC gene in the 145M-Twi cells (Fig. 1c). Using the synthetic fluorescent substrate, HMUβGal, the 145M-Twi (GALCtwi/twi) showed significantly reduced GALC enzymatic activity in comparison to 145C-Wt controls (GALCwt/wt) (Fig. 1d). None of the clones showed differences in terms of the biochemical, morphological and cell growth aspects assessed in the assays above.
Figure 1. Establishment and characterization of 145M-Twi and 145C-Wt cells.
Primary cortical brain cells from the Twitcher (Twi, GALCtwi/twi) and control (GALCwt/wt) mice were obtained from pups (day1 of life) and transformed using the SV40 large T antigen (SV40T). (a) After SV40 transformation, clones were selected from the Twi (145M-Twi) and control (145C-Wt). (b) The 145M-Twi cells stained for two oligodendrocyte markers: A2B5 (A and B) and galactosylceramide (GalC; D and E). Increased Galc signal were noted in 145M-Twi cells as shown in a higher magnification image in panels E (145M-Twi) and D (145C-Wt). These cells did not show MAP2 marker (panels G and H) and few cells showed astrocyte GFAP marker (J and K). (c) Restriction enzyme digestion with EcoRV digestion of PCR amplified fragments confirmed the homozygous mutant murine GALC from 145M-Twi cells (n=5), which also demonstrated reduced GALC enzymatic activity (d) in comparison to 145M-Wt controls cells (n=5).
Development of a LC-MS/MS assay for measuring cellular psychosine
To determine the cellular levels of psychosine on the 145-Twi and 145C-Wt, a specific LC-MS/MS method was developed. The LC-MS/MS is a powerful method for detection and quantification of a specific glycosphingolipid through Multiple Reaction Monitoring (MRM), where a metabolite is repetitively scanned for selected precursor–product ion pairs to enhance the sensitivity and specificity of the analysis [25]. We used a C8 reserve MS X-Terra column (125 Å, 3.5 μm, 1×50 mm), whose composition of rugged particles confers injection resistance to dimethyl sulfoxide (DMSO), a common utilized drug-library solvent. Additionally, the high-mass capacity and wide pH range of this column provide the required flexibility for the development of a throughput assay. LC-MS/MS assays were done in the AB SCIEX API 4000 (Table S1). Using psychosine standards (461.64 g/mol), we were able to determine a sensitive and consistent MRM for psychosine 462/264 (Fig. 2a). The psychosine analog, 1-acetyl-psychosine (503.68 g/mol), absent in biological specimens, proved to be a reliable internal standard (ISD) for this assay with MRM 504/264 and similar extraction recovery and retention time (RT) of 0.42 min (Fig. 2a). The carry-over of psychosine and 1-acetyl-psychosine varies from 0.1 to 1.6% and from 0.1 to 0.43%, respectively. Using this methodology, both psychosine and 1-acetyl-psychosine showed RTs < 1 min, which is encouraging for throughput assay development.
Initially, we examined psychosine levels in brain samples from patients affected with GLD, which were previously shown to have significant higher levels of psychosine [26] (Fig. 2b and 2c). Brain samples from one male patient manifesting the infantile GLD (early onset of symptoms and rapidly progressive disease who died at 5 months of age) and from another adult male patient with the late onset GLD (protracted disease course who died at 39 years of age) were used in this assay. The brain samples were obtained from the Brain and Tissue Bank for Developmental Disorders at the University of Maryland, Baltimore, MD. The lipid extraction was performed in dried samples from the white matter of regions from each patient and controls as previously described [27]. The measurements of psychosine in GLD patient brains were significantly higher than controls (Fig. 2b and 2c). The highest levels of psychosine were present in the brain from the infantile GLD patient. The psychosine levels present in lipid extracts from the brain of the late onset GLD patient were significantly elevated but lower than those detected in the patient with the infantile and more severe form of GLD (Fig. 2b). Given the sensitivity of LC-MS/MS method, a small level of psychosine was detectable in brain samples from age-matched controls (Fig. 2b and 2c).
Cultured 145M-Twi and 145C-Wt cells were harvested (~3.2×106 cells) and underwent lipid extraction with methanol, a polar lipid solvent. 1-acetyl-psychosine (198.5 nM) was used as an ISD (Material and Methods). Envision throughput applications, nitrogen evaporation and the use of solid-phase extraction (SPE) columns were avoided. Lipid extraction solutions were directly filtered in individuals 0.22 μ–tube-filters. The 145M-Twi showed significantly elevated levels of psychosine when compared to the 145C-Wt control cells (Fig. 2d).
The 145M-Twi cells showed increased caspase-3 activation and altered cytochrome C distribution
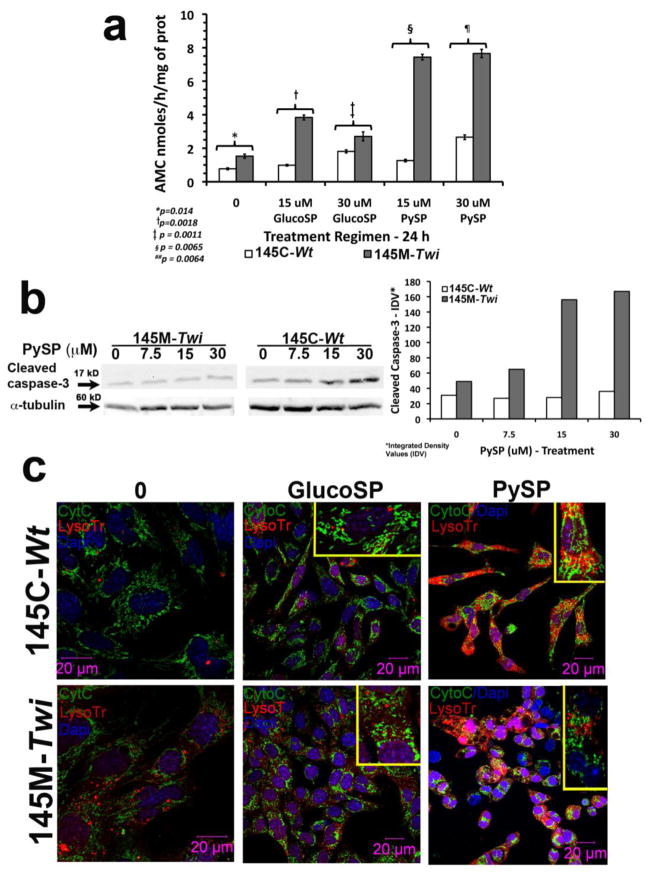
To assess susceptibility to apoptosis, we measured the caspase-3 activity in both 145M-Twi and 145C-Wt cells before and after treatment with different concentrations of psychosine in culture media, and its isomer glucosylsphingosine, which also showed a degree of cytotoxicity in previous studies [28] (Fig. 3a). Using the Ac-DEVD-AMC, specific fluorescent substrate for caspase-3, the 145M-Twi showed increased caspase-3 activation, which was significantly enhanced under 24h-treatment with different concentrations of psychosine and glucosylsphingosine (Fig. 3). Psychosine produced a more robust activation of caspase-3 when compared to the glucosylphingosine (Fig. 3a). The 24h-treatment with glucosylsphingosine (15 and 30 μM) produced 1.8±0.6 and 2.1±0.4 fold increases in the caspase-3 activity in 145M-Twi and 145C-Wt cells, respectively (Fig. 3a). Whereas, after the 24h-treatment with psychosine at the same concentrations, the activation of caspase-3 was 1.9±1.2 fold in 145C-Wt cells and, 3.9±1.6 fold in 145M-Twi cells (p=0.04). Even in the absence of exogenous complex lipids, 145M-Twi cells showed increased caspase-3 activation (p=0.014; Fig. 3a). Confirming the caspase-3 activation measured by specific enzymatic assays, the immunoblots showed increased levels of cleaved and activated caspase-3 in 145M-Twi cells in a dose-response manner (Fig. 3b). In addition, the 145M-Twi cells showed a larger lysosomal compartment, which was not evident in the 145C-Wt control cells (Fig. 3c). Under treatment with psychosine, expansion of lysosomal compartment was more pronounced in 145M-Twi cells (Fig. 3c). Interestingly, in the control 145C-Wt cells, the lysosomal compartment was not evident until cells were treated with psychosine (Fig. 3c). Another important observation is the pattern of cytochrome c (Cyto C) staining in 145M-Twi cells. In 145M-Twi cells treated with glucosylsphingosine, and especially psychosine, the long and string Cyto C pattern became punctuated and more disorganized, indicating fragmented mitochondria and release of Cyto C into the cytosol was observed. In control 145C-Wt cells also treated with the two glycosphingolipids, the Cyto C staining showed consistently the long and string Cyto C pattern, indicating normal and intact mitochondria (Fig. 3c). It is important to emphasize that both 145M-Twi and 145C-Wt have normal lysosomal glucocerebrosidase activity. In addition, the levels of both complex lipids used in the culture medium were higher than cellular endogenous levels.
Figure 3. Caspase-3 activation, lysosomal compartment expansion and cytochrome C expression pattern in 145M-Twi from Twitcher.
(a) Culture 145M-Twi and 145C-Wt showed baseline caspase-3 activity, key component orchestrating apoptosis. Under 24h treatment with 15 and 30 μM of glucosylsphingosine (GlucoSP) and psychosine (PySP), the caspase-3 activation was significantly higher in the 145M-Twi cells. Triplicates were analyzed on each concentration tested. (b) Cleaved caspase-3 immunoblots (17 kD) from 145M-Twi and 145C-Wt cells treated at increasing concentrations of PySP are shown. α-tubulin (60 kD) was used as a loading control. The histogram shows the integrated density values (IDV) from the immunoblot for cleaved caspase-3. (c) Confocal immunocellfluorescence shows 145M-Twi and 145C-Wt stained with cytochrome C (Cyto C - green) and lysosomes labeled with lysosomal marker LysoTracker (LysoTr - red). Cell nuclei were stained with DAPI. The 145M-Twi and 145C-Wt were treated for 24h-period with 30 μM of glucosylsphingosine and psychosine. Increased lysosomal compartment (LysoTr –red) was noted in 145M-Twi cells. Under treatment with both glycosphingolipids, both 145M-Twi and 145C-Wt cells showed enhancement of lysosomal compartment, which was more evident in the 145M-Twi cells. Additionally, the 145M-Twi cells showed alterations in the Cyto C staining under glucosylphingosine and psychosine treatment. The long string pattern of Cyto C, representing intact mitochondria, became punctuated and dispersed, indicating the Cyto C translocation from the inner mitochondrial space into the cytosol. Higher magnification showing the Cyto C pattern is shown on the upper right corner of each panel of treated murine 145M-Twi and 145C-Wt cells.
Increased LC3B, an autophagy marker in 145M-Twi cells
Along with apoptosis, autophagy is another vacuolar stress-mechanism responsible for the removal of long-lived proteins and sequestration of cytoplasmic damaged organelles into double-membrane bound organelles known as autophagosomes, and their subsequent delivery to lysosomes for degradation and recycling. Additionally, autophagy also function as an “adaptive” cell survival mechanism to maintain the levels of nutrients and energy levels during periods of stress [29]. Autophagy has been shown to be involved in the pathogenesis of some LSDs [30]. To investigate whether the 145M-Twi cells demonstrate any alteration in autophagy, using confocal immunofluorescence, we evaluated the levels of cleaved LC3B, a phospatidylethanolamine-protein which is the key marker for autophagy induction [31]. Autophagy was assessed in the presence of multiple concentrations of exogenous psychosine and glucosyl-sphingosine during 24h period. The 145M-Twi cells showed increased cleaved LC3B levels under treatment with psychosine, which was elevated in comparison to 145C-Wt controls (Fig. 4). In 145M-Twi cells, the cleaved LC3B autophagy protein is localized in lysosomes (Fig. 4). The cleaved LC3B levels were mildly increased under treatment with glucosylsphingosine at 7.5 and 15 μM, but remarkably increased in 145M-Twi cells treated with correspondent concentrations of psychosine (Fig. 4).
Figure 4. Autophagy induction marker in 145M-Twi cells.
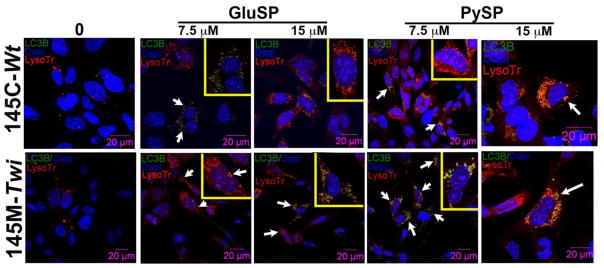
The 145M-Twi and 145C-Wt cells were treated for 24h-period with two different concentrations of glucosyl-sphingosine (GluSP) and psychosine (PySP). These two sphingolipids caused expansion of lysosome number and size labeled with LysoTracker Red (Lyso Tr - red). The elevated levels of cleaved LC3B (green), the autophagy inducer protein, were mostly noticeable in the 145M-Twi cells (arrows). The cleaved LC3B (green) showed to co-localize with the lysosomes (red) in both Twi and control cells (yellow signal - merge). 145M-Twi cells however showed the highest levels of cleaved LC-3B in both concentrations of psychosine (yellow). Higher magnification of one cell is shown on the right-hand corner of the each panel to highlight the labeled lysosomes and LC3B.
Distinguishing of psychosine from glucosylsphingosine in 145M-Twi cells
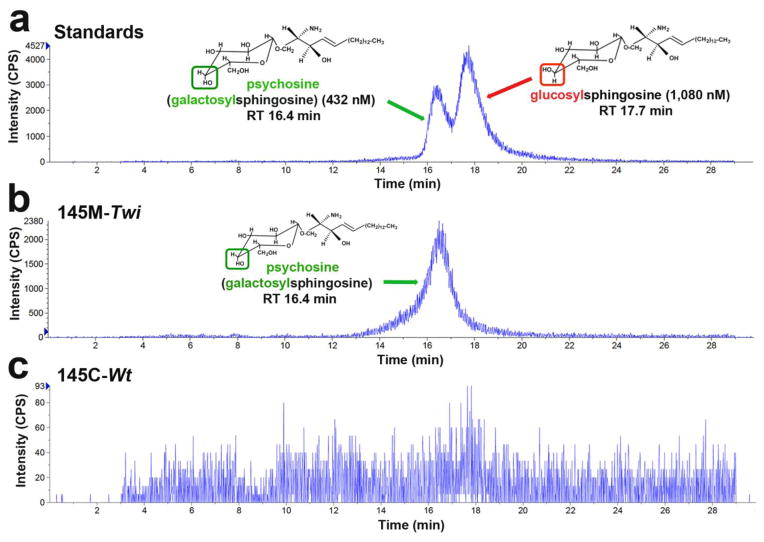
Glucosylsphingosine is an isomer of psychosine (galactosylsphingosine) with identical molecular weight (461.64 g/mol), but with a different orientation of hydroxyl residue bound to C4 carbon of the hexagonal carbohydrate ring (Fig. 2a). To confirm that the LC-MS/MS short-run (<1 min) using C8 reverse MS column specifically detects and quantifies psychosine from the cell-lipid extracts, we measured psychosine from the same lipid extracts using the two coupled Bridged Ethyl Hybrid (BEH) Glycan columns (2.1×150mm, 1.7μ and 2.1×100 mm, 1.7 μ - Waters Inc. - Table S2). With the BEH columns, which are commonly applied to identify complex N- and 0-linked structures composed of frequently similar and repeating sugar moieties [32], we showed a distinct separation of psychosine from glucosylsphingosine (Fig. 5a–c). In fact, lipid extracts from 145M-Twi cells showed consistently an evident peak corresponding to psychosine, which was not present in the lipid extracts from 145C-Wt control cells (Fig. 5b and 5c). Similar results were obtained when analyzing extracted lipids from brain samples from GLD patients and controls (Fig. S1).
Figure 5. Specificity of LC-MS/MS assay to measure psychosine.
(a) A mixture solution of psychosine (432 nM) and glucosyl-psychosine (1,080 nM) standards was prepared in methanol with 0.5% acetic acid and injected to the LC-MS/MS. The BEH Glycan column generated a doublet peak. The early peak corresponds to psychosine and the late peak to glucosylsphingosine. Psychosine and glucosyl-sphingosine are molecular isomers. (b) Lipid extracts from 145M-Twi cells showed a single peak corresponding to psychosine retention time (RT). (c) Lipid extract from the 145C-Wt showed no peak matching either psychosine or glucosyl-sphingosine.
Development of throughput LC-MS/MS assay to measure cellular psychosine in 145M-Twi
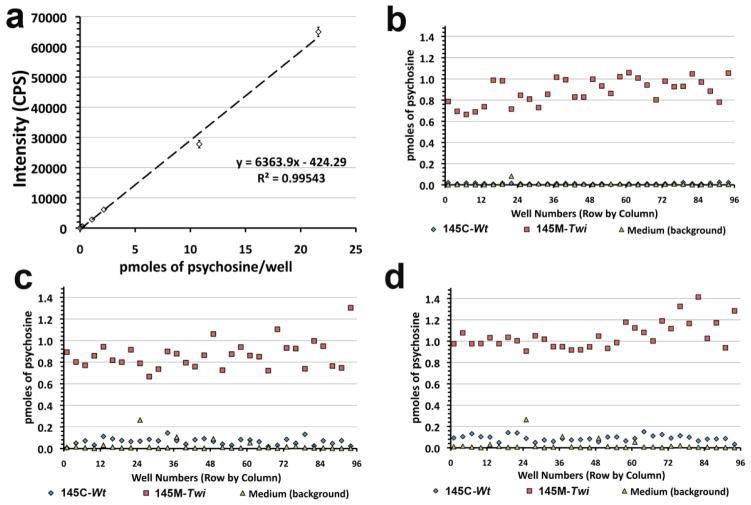
Based on the increased levels of psychosine in the 145M-Twi cells, we evaluated the potential of these cells to be used in a throughput assay, which can be ultimately used in drug screen assays. The 145M-Twi and 145C-Wt were cultured in 96-well plates (32–34 × 103 cells/well) followed by lipid extractions. In order to increase feasibility for a throughput assay, methanol (99.5%) was used as the sole solvent adding a small percentage of acetic acid (0.5%) to maximize the extraction of polar sphingolipids as psychosine. This solvent allowed the lipid extraction to be performed directly in the 96-well polystyrene tissue culture plates. Using psychosine standards, the sensitivity analysis of the LC-MS/MS assay for psychosine was determined. The standard psychosine was diluted in the lipid extraction solvent in a broad range of concentrations with a final volume of 150 μL dispensed in each well of a 96-well collection plate. The LC-MS/MS method showed high sensitivity and remarkable linearity in both lower and higher psychosine concentrations (Fig. 6a). A volume of 80 μL per well of the methanol:acetic acid (99.5:0.5) solvent containing 1-acetyl-psychosine, as ISD (198.5 nM), resulted in efficient cellular psychosine extraction (Fig. 6b and Table S2).
Figure 6. Psychosine standard curves and plate assessment assays in cells cultured in 96-well plates.
(a) Standard psychosine were diluted in the solvent solution (methanol:acetic acid; 99.5:0.5) at concentrations ranging from 0.02 to 21 pmoles/well. Means of measured pmoles of psychosine from 4 wells were plotted in graphs according the intensity Signals (CPS). (b–d) Plate uniformity assessment was performed using 145M-Twi and 145C-Wt cells and control (CTRL) mice cultured in three 96-well plates. 145M-Twi and 145C-Wt cells were displayed in interleaved-signal plate format including background (medium), i.e. alternating column-positions as three independent experiments, each in a different 96-well plate.
Importantly, no nitrogen evaporation or SEP column steps were needed for sample preparation pre-injection. In order to assure the samples were clean prior to LC-MS/MS injection, a filter plate AcroPrep™ Advance 96 (0.2 μ) assembled to 96-well sample collection plate was used following “on-plate” cellular lipid extraction. Using the Agilent 1100 autosampler (Waters Inc.), samples from the 96-well collection plate were automatically injected at 8 μL/well (80 μL sample volume/well). To test the robustness of the assay in 96-well plates, an interleaved uniformity plate-assessment assay was performed (Fig. 6b). The 145M-Twi, 145C-Wt and wells containing no cells, with only medium (background) were allocated in alternating column positions as previously described [33]. The assessment demonstrated feasibility and robustness of the throughput LC-MS/MS cellular psychosine assay (Fig. 6b–c). The assay-window ratio of psychosine levels in 145M-Twi cells to controls are of ~ 9–16 with a Z’ score factor of 0.40–0.57 (Table 1).
Table 1.
Plate assessment for throughput LC-MS/MS cellular psychosine assay.
| Plate Statistical Parameters
|
Mean (pmoles of psychosine/well) | SD | CV* (%) | Z’ factor | Signal window |
|---|---|---|---|---|---|
| SV40-transformed cells | |||||
|
|
|||||
| Plate 1
|
|||||
| 145M-Twi | 886 × 10−3 | 120 × 10−3 | 1.38 | 0.57 | 16.03 |
| 145C-Wt | 12 × 10−3 | 5 × 10−3 | 4.49 | - | - |
| Medium (background) | 7 × 10−3 | 14 × 10−3 | 19.63 | - | - |
| Plate 2
|
|||||
| 145M-Twi | 865 × 10−3 | 129 × 10−3 | 1.52 | - | - |
| 145C-Wt | 65 × 10−3 | 31 × 10−3 | 4.98 | 0.40 | 2.43 |
| Medium (background) | 44 × 10−3 | 46 × 10−3 | 10.82 | - | - |
| Plate 3
|
|||||
| 145M-Twi | 1,053 × 10−3 | 125 × 10−3 | 1.21 | 0.52 | 3.32 |
| 145C-Wt | 91 × 10−3 | 28 × 10−3 | 3.16 | - | - |
| Medium (background) | 54 × 10−3 | 40 × 10−3 | 7.67 | - | - |
CV (coefficient of variation), standard deviation (SD).
Using 145M-Twi cells for a throughput LC-MS/MS cellular psychosine assay
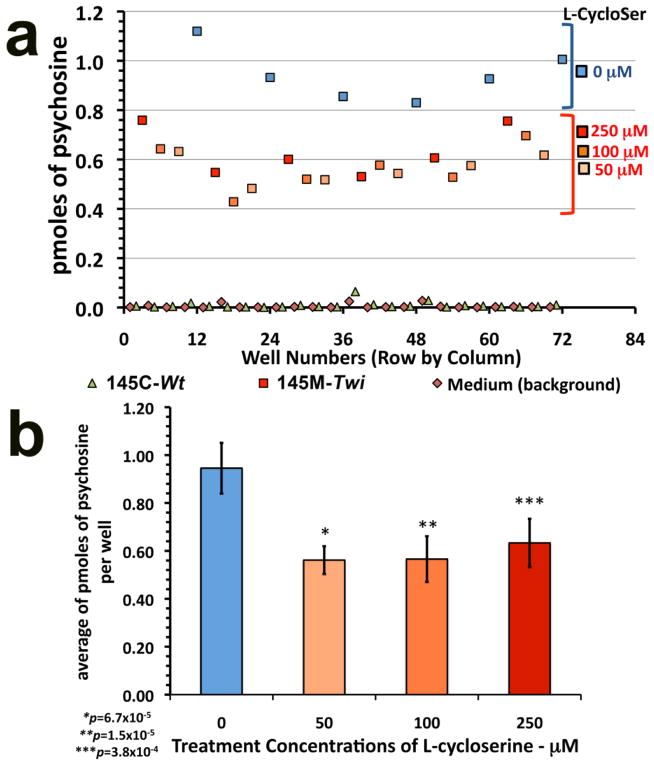
To confirm the potential of the developed cell-based throughput LC-MS/MS assay to detect small molecules that reduce cellular psychosine, 145M-Twi cells cultured in 96-well plates were treated with L-cycloserine, a small molecule previously shown to reduce psychosine levels in vivo [34]. At culture medium concentrations ranging from 50–250 μM over 9-day period, L-cycloserine significantly reduced psychosine levels in 145M-Twi cells (Fig. 7). On the other hand, no alterations of the already small levels of psychosine in the 145C-Wt control cells were observed (Fig. 7).
Figure 7. Testing throughput LC-MS/MS assay against a known psychosine-reducing agent.
(a) In 96-well plates, the 145M-Twi and 145C-Wt derived from the Twi and respective control mice were cultured and treated for 9 days with L-cycloserine at 50 μM, 100 μM and 250 μM before measuring cellular psychosine levels by LC-MS/MS. (b) Significant reductions of psychosine were observed in 145M-Twi cells with L-cycloserine in cultured medium at various concentrations (n=6). No effect of L-cycloserine was observed in the 145C-Wt (CTRL).
4. Discussion
Globoid-cell leukodystrophy (GLD), mostly known as Krabbe disease, is typical representative of neurological LSDs. GLD is caused by the deficiency of GALC, which is a soluble lysosomal hydrolase that catalyzes the removal of galactose-containing glycosphingolipids including galactosylceramide and psychosine [35]. During myelination, galactosylation of sphingosine produces psychosine, a reaction catalyzed by UDP-galactose:ceramide galactosyltransferase – known as the Cleland and Kennedy pathway [11]. Under physiological conditions, the produced psychosine is rapidly degraded by GALC, resulting in low and, almost undetectable, levels of this sphingolipid in brain of healthy individuals [7]. In the setting of GALC deficiency, however, psychosine levels are elevated in the brain of patients with GLD [26]. At high levels, psychosine becomes cytotoxic, particularly to oligodendrocytes, the myelin-forming cells, resulting in apoptosis and extensive demyelination classically observed in brain imaging studies of GLD patients. The degree of demyelination correlates with disease progression [7].
Most current therapies for LSDs have been limited to treatment of non-neurological symptoms. Few enzyme replacement treatment (ERT) agents are FDA-approved and used to successfully treat and control symptoms of some LSDs [36]. Unfortunately, being large molecular-weight molecules, ERT agents are unable to cross the blood-brain barrier (BBB). By inhibiting serine palmitoyltransferase, a key step in the biosynthesis sphingolipids, L-cycloserine showed to delay weight loss and prolong survival of the Twi mouse, the murine model of GLD [34, 37]. However, because of its detrimental effects secondary to the generalized sphingolipid deprivation, L-cycloserine has failed to reach clinical use. Therefore, a specific inhibitor of UDP-galactosylceramide transferase would be desirable. Hematopoietic stem cell transplantation (HSCT) is the treatment offered for the most severe and early-onset clinical form of GLD [38].
Although HSCT prevents the typical fulminant course of the infantile form of GLD, presymptomatic patients who receive HSCT eventually present with debilitating neurological symptoms, indicating disease progression [39]. The advent of newborn screening programs for GLD [40] and other LSDs urge the development of novel types of therapies that can tackle the neuronal pathogenesis and alter the natural disease course. Small molecules, which are more likely to cross the BBB and reach the affected neural cells, have become a more attractive approach.
The investigation of affected tissue and, especially with live cells obtained from in vivo models, has allowed the understanding of the pathogenic mechanisms of the disease. In the study of neurological diseases, the establishment of brain cell lines is important to understanding of mechanisms causing and contributing to the neurological processes. Because glycosphingolipids, as psychosine and several other sphingolipids, are poorly expressed in cells and tissues other than those originated from the nervous system, the 145M-Twi (GALCtwi/twi) several characteristics of an optimal cell-line for the study of different pathways related to the lysosomal dysfunction with implications in the neuropathogenesis of GLD [16]. To have sufficient cells for the experiments designed, we successfully transformed these cells using SV 40 large T-cell antigen. Post-transformation, six clones were selected from the 145M-Twi and 145C-Wt, the background control (Fig. 1a). Subsequently, these cells behaved as stable immortalized cells. Amongst the different isolated clones from each cell line, 145M-Twi and 145C-Wt, no differences were noted regarding the psychosine levels, morphology and rate of cell growth. Confocal cellular immunostaining showed that both 145M-Twi and 145C-Wt consistently express galactosylceramide (GalC) and A2B5, two classical oligodendrocyte markers (Fig. 1b). The 145M-Twi cell line showed increased accumulation of galactosylceramide as expected (Fig. 1b). No MAP2-positive cells were observed and a few GFAP-positive cells were noted (Fig. 1b). Several human and murine oligodendrocyte cell lines have been generated using SV40 large T antigen [41–45]. The morphology of the both 145M-Twi and 145C-Wt were similar to the immortalized oligodendrocytes 158N and 158JP, generated from the normal (wild type) and jimpy mice, which is an X-linked dysmyelination model carrying a A>G mutation at the splice site acceptor site of exon 5 of the PLP/DM20 gene [45, 46]. The cellular phenotypic characteristics, in addition to the elevated levels of psychosine detected in the 145M-Twi cells, suggest that both immortalized 145M-Twi and 145C-Wt have oligodendrocyte origin. In terms of cell maintenance, no extra growth factors were required to maintain these cells, which were cultured in regular DMEM/F-12 medium with fetal bovine serum (10%). A previous study reported the generation of spontaneously immortalized Schwann cells from Twi mice [47]. These previously described cells were distinct from the ones here described. Firstly, they were originated from peripheral nervous system, i.e. Schwann cells (TwS1) which were present in specimens collected from the dorsal root ganglia and peripheral nerves of 3-week-old Twi mice [47]. Secondly, these TwS1 cells require more laborious tissue culture procedures [48]. For these reasons, the immortalized 145M-Twi cells reported bring advantages from a standpoint of tissue culture procedures and being obtained directly from CNS.
The 145M-Twi cells showed several characteristics to be a disease-cell model for GLD. This cell line showed reduced GALC enzymatic activity and confirmed to carry the nonsense mutation in GALC gene (Fig. 1c and 1d). These cells also showed a remarkable susceptibility to apoptosis. When treating the 145M-Twi cells with exogenous psychosine for a relative short period (24h), dramatic activation of caspase-3 was observed (Fig. 3a and 3b). These findings are consistent with increased cytotoxicity of psychosine, which is significantly elevated in 145M-Twi cells in comparison to controls as demonstrated by the cellular LC-MS/MS assay (Fig. 2d). Similar observations were reported in earlier studies [49, 50]. Under treatment with glucosylsphingosine and psychosine, expansion of the lysosomal compartment was noted, mostly in the 145M-Twi cells (Fig. 3c). Given its localization in the inner mitochondrial space, cyto C staining shows a long and string pattern, reflecting intact mitochondria [51]. Under treatment with both glucosylsphingosine and psychosine, the disrupted and punctuated cyto C staining, indicating its release into the cytoplasm, was only observed in the 145M-Twi cells (Fig. 3c), which also demonstrated significantly higher caspase-3 activation (Fig. 3a and 3b). These results taken together explain the increased susceptibility of the 145M-Twi cells to apoptosis, which is in alignment with the rapid neurodegeneration observed in GLD. Since autophagy was potentially dysfunctional in LSDs [52] and also related to apoptosis, we assessed the levels of cleaved LC3B autophagy marker in comparison to controls. Under treatment with psychosine and its isomer, glucosylsphingosine, the 145M-Twi cells showed elevated levels of cleaved LC3B, which co-localizes with lysosomes (Fig. 4). The increased in LC3B levels can be present as part of the autophagy process, or a disruption of the autophagy flow caused by the impairment on the lysosomal system caused by the primary GALC deficiency [29]. The concentrations of psychosine and glucosylsphingosine used to treat these cells were much higher than their endogenous levels in normal and mutant cell lines. Some hydrolysis of the exogenous substrates is expected to occur. However, given the multiple pathogenic cascades secondary to a specific lysosomal enzyme, in this case GALC, it is conceivable that the 145M-Twi cells become more susceptible to these two cytotoxic complex lipids.
Using psychosine standards in the initial LC-MS/MS studies, we were able to determine a sensitive and consistent MRM for psychosine 462/264 (Fig. 2a). The psychosine analog, 1-acetyl-psychosine, which is absent in biological specimens, proved a reliable ISD with MRM of 504/264, and similar extraction recovery and RT (~ 0.42 min) (Fig. 2a). In MRM, the first mass analyzer is set to pass a specific precursor ion mass-charge ratio (m/z), and the second mass analyzer is set to pass a specific product ion m/z. Therefore, only ions that meet both precursor and product ion m/z conditions simultaneously will be transmitted to the detector [53]. Furthermore, because essentially all of the instrument time is spent detecting ions of interest, the detected signal intensities are greatly enhanced. The major advantage of developing a throughput assay based on LC-MS/MS is its greater sensitivity and robust throughput. The increased sensitivity of the MRM pairs for psychosine along with its short RT (Fig. 2a) allowed the development of a practical throughput LC-MS/MS assay. This throughput assay for psychosine showed remarkable linearity for a broad concentration range (0.02–21 pmoles/well) in a 96-well plate (Fig. 6a). This linearity was also reflected in the robustness and reliability of the cellular psychosine LC-MS/MS assay performed in multi-well plates, which was confirmed by the statistical parameters generated from plate assessment (Fig. 6 and Table 1). This assessment was performed in 3 independent 96-well plates containing 145M-Twi and 145C-Wt cells and also wells with only medium (background), as per guidelines from NIH-National Chemical Genomics Center (Fig. 6) [33]. Confirming the robustness of this cellular LC-MS/MS assay, we were able to identify 145M-Twi cells in wells treated with the L-cycloserine (Fig. 7), which showed reduction of psychosine levels in comparison to wells containing non-treated 145M-Twi cells. It is important to emphasize that the establishment of these immortalized neurologically relevant cell lines were crucial to allow the development and miniaturization of the cellular psychosine LC-MS/MS assay.
5. Conclusions
The 145M-Twi cells showed biochemical and molecular characteristics that correlates to the GLD pathogenesis. Relevant molecular pathogenic findings involving apoptosis and autophagy were also identified in the 145M-Twi cells. Using a newly developed LC-MS/MS, we showed that the 145M-Twi cells have increased levels of psychosine, the central neuropathogenic glycosphingolipid in GLD. Given the robustness of the 145M-Twi cells and the LC-MS/MS assay here developed, cellular psychosine levels were measurable from cells cultured in 96-well plates. The 145M-Twi cells showed to recapitulate several aspects of the molecular pathogenesis of GLD and can be used as a powerful tool for the identification of therapeutic agents for this devastating neurological disease.
Supplementary Material
Highlights.
A disease-cell model for globoid-cell leukodystrophy (GLD) is described.
From brain specimens of GLD mouse model, the 145M-Twi cell line was established.
The 145M-Twi cells revealed molecular aspects of the neuropathogenesis of GLD.
The 145M-Twi cells are potential research tools for drug discovery screens.
Acknowledgments
We thank Drs. Akira Sawa MD, PhD and Shin-ichi Kano MD, PhD for providing primary cortical neuroglial cells from the C57BL/6J (GALCwt/wt), from which the 145C-Wt was established. We thank Drs. Craig Hendrix MD, PhD and Theresa Shapiro MD, PhD for making available the equipments and infra-structure of the Mass Spectrometry Laboratory in the Div. of Clinical Pharmacology at The Johns Hopkins University School of Medicine. We are also thankful for the suggestions from Dr. Joseph Orsini PhD. Funding for the mouse resources were from grant RNS065808A from NIH (E.R.B.). Funding for LC-MS/MS instrumentation were from grant 1S10 RR16798 from NIH (W.C.H). G.H.B.M. is supported currently from NIH-NIMH 1R03MH098689, NIH-NINDS 1R01NS079655 and National MPS Society. G.H.B.M. receives also funds fro clinical and educational studies from Genzyme-Sanofi Corp., Shire HGT, Biomarin Pharm. Corp. and Protalix Biotherapeutics. Human tissue was obtained from the NICHD Brain and Tissue Bank for Developmental Disorders at the University of Maryland, Baltimore, MD. The role of the NICHD Brain and Tissue Bank is to distribute tissue, and therefore, cannot endorse the studies performed or the interpretation of results. NICHD Brain and Tissue Bank for Developmental Disorders and NICHD Contract No. N01- HD-4-3368 and NO1-HD-4-3383.
Non-standard abbreviations
- Ac-DEDV-AMC
N-acetyl-DEVD 7-amido-4-methylcoumarin
- BEH
Bridged Ethyl Hybrid
- GALC
β-galactocerebrosidase
- GalC
galactosylceramide
- GFAP
glial fibrillary acidic protein
- GLD
globoid-cell leukodystrophy
- HMUβGal
6-hexadecanoylamino-4-methylumbelliferyl-β-D-galactoside
- ISD
internal standard
- LSD
lysosomal storage disease
- MAP-2
microtubule-associated protein 2
- MPA
mobile phase A
- MPB
mobile phase B
- MS
mass spectrometry
- RT
retention time
- SPE
solid-phase extraction
- SV40T
SV40-tranformation
- Twi
Twitcher
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Futerman AH, Riezman H. The ins and outs of sphingolipid synthesis. Trends Cell Biol. 2005;15:312–318. doi: 10.1016/j.tcb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Lee JM, Lee YK, Mamrosh JL, Busby SA, Griffin PR, Pathak MC, Ortlund EA, Moore DD. A nuclear-receptor-dependent phosphatidylcholine pathway with antidiabetic effects. Nature. 2011;474:506–510. doi: 10.1038/nature10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Contreras FX, Ernst AM, Haberkant P, Bjorkholm P, Lindahl E, Gonen B, Tischer C, Elofsson A, von Heijne G, Thiele C, Pepperkok R, Wieland F, Brugger B. Molecular recognition of a single sphingolipid species by a protein’s transmembrane domain. Nature. 2012;481:525–529. doi: 10.1038/nature10742. [DOI] [PubMed] [Google Scholar]
- 4.Carette JE, Raaben M, Wong AC, Herbert AS, Obernosterer G, Mulherkar N, IKuehne A, Kranzusch PJ, Griffin AM, Ruthel G, Dal Cin P, Dye JM, Whelan SP, Chandran K, Brummelkamp TR. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature. 2011;477:340–343. doi: 10.1038/nature10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, Hubbard WC, Berdyshev EV, Tuder RM. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med. 2005;11:491–498. doi: 10.1038/nm1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fantini J, Yahi N. Molecular insights into amyloid regulation by membrane cholesterol and sphingolipids: common mechanisms in neurodegenerative diseases. Expert Rev Mol Med. 2010;12:e27. doi: 10.1017/S1462399410001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki K. Twenty five years of the “psychosine hypothesis”: a personal perspective of its history and present status. Neurochem Res. 1998;23:251–259. doi: 10.1023/a:1022436928925. [DOI] [PubMed] [Google Scholar]
- 8.Hawkins-Salsbury JA, Parameswar AR, Jiang X, Schelsinger PH, Bongarzone ER, Ory DS, Demchenko AV, Sands MS. Psychosine, the cytotoxic sphingolipid that accumulates in Globoid Cell Leukodystrophy, alters membrane architecture. J Lipid Res. 2013 doi: 10.1194/jlr.M039610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki Y, Suzuki K. Krabbe’s globoid cell leukodystrophy: deficiency of glactocerebrosidase in serum, leukocytes, and fibroblasts. Science. 1971;171:73–75. doi: 10.1126/science.171.3966.73. [DOI] [PubMed] [Google Scholar]
- 10.Wenger DA, Rafi MA, Luzi P. Molecular genetics of Krabbe disease (globoid cell leukodystrophy): diagnostic and clinical implications. Hum Mutat. 1997;10:268–279. doi: 10.1002/(SICI)1098-1004(1997)10:4<268::AID-HUMU2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 11.Cleland WW, Kennedy EP. The enzymatic synthesis of psychosine. J Biol Chem. 1960;235:45–51. [PubMed] [Google Scholar]
- 12.Miyatake T, Suzuki K. Globoid cell leukodystrophy: additional deficiency of psychosine galactosidase. Biochem Biophys Res Commun. 1972;48:539–543. doi: 10.1016/0006-291x(72)90381-6. [DOI] [PubMed] [Google Scholar]
- 13.Taketomi T, Nishimura K. Physiological Activity of Psychosine Jpn. J Exp Med. 1964;34:255–265. [PubMed] [Google Scholar]
- 14.Igisu H, Suzuki K. Progressive accumulation of toxic metabolite in a genetic leukodystrophy. Science. 1984;224:753–755. doi: 10.1126/science.6719111. [DOI] [PubMed] [Google Scholar]
- 15.Chuang WL, Pacheco J, Zhang XK, Martin MM, Biski CK, Keutzer JM, Wenger DA, Caggana M, Orsini JJ., Jr Determination of psychosine concentration in dried blood spots from newborns that were identified via newborn screening to be at risk for Krabbe disease. Clin Chim Acta. 2013;419:73–76. doi: 10.1016/j.cca.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi T, Yamanaka T, Jacobs JM, Teixeira F, Suzuki K. The Twitcher mouse: an enzymatically authentic model of human globoid cell leukodystrophy (Krabbe disease) Brain Res. 1980;202:479–483. doi: 10.1016/0006-8993(80)90159-6. [DOI] [PubMed] [Google Scholar]
- 17.Chatelut M, Harzer K, Christomanou H, Feunteun J, Pieraggi MT, Paton BC, Kishimoto Y, O’Brien JS, Basile JP, Thiers JC, Salvayre R, Levade T. Model SV40-transformed fibroblast lines for metabolic studies of human prosaposin and acid ceramidase deficiencies. Clin Chim Acta. 1997;262:61–76. doi: 10.1016/s0009-8981(97)06527-3. [DOI] [PubMed] [Google Scholar]
- 18.Wiederschain G, Raghavan S, Kolodny E. Characterization of 6-hexadecanoylamino-4-methylumbelliferyl-beta-D- galactopyranoside as fluorogenic substrate of galactocerebrosidase for the diagnosis of Krabbe disease. Clin Chim Acta. 1992;205:87–96. doi: 10.1016/s0009-8981(05)80003-8. [DOI] [PubMed] [Google Scholar]
- 19.Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 20.Nestal de Moraes G, Carvalho E, Maia RC, Sternberg C. Immunodetection of caspase-3 by Western blot using glutaraldehyde. Anal Biochem. 2011;415:203–205. doi: 10.1016/j.ab.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 21.Kaneko T, Okita H, Nakajima H, Iijima K, Ogasawara N, Miyagawa Y, Katagiri YU, Nakagawa A, Kiyokawa N, Sato T, Fujimoto J. Neuroblastoma cells can be classified according to glycosphingolipid expression profiles identified by liquid chromatography-tandem mass spectrometry. Int J Oncol. 2010;37:1279–1288. doi: 10.3892/ijo_00000779. [DOI] [PubMed] [Google Scholar]
- 22.Stahlman M, Ejsing CS, Tarasov K, Perman J, Boren J, Ekroos K. High-throughput shotgun lipidomics by quadrupole time-of-flight mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2664–2672. doi: 10.1016/j.jchromb.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 23.White AB, Galbiati F, Givogri MI, Lopez Rosas A, Qiu X, van Breemen R, Bongarzone ER. Persistence of psychosine in brain lipid rafts is a limiting factor in the therapeutic recovery of a mouse model for Krabbe disease J Neurosci Res. 2011;89:352–364. doi: 10.1002/jnr.22564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakai N, Inui K, Tatsumi N, Fukushima H, Nishigaki T, Taniike M, Nishimoto J, Tsukamoto H, Yanagihara I, Ozono K, Okada S. Molecular cloning and expression of cDNA for murine galactocerebrosidase and mutation analysis of the twitcher mouse, a model of Krabbe’s disease. J Neurochem. 1996;66:1118–1124. doi: 10.1046/j.1471-4159.1996.66031118.x. [DOI] [PubMed] [Google Scholar]
- 25.Scherer M, Leuthauser-Jaschinski K, Ecker J, Schmitz G, Liebisch G. A rapid and quantitative LC-MS/MS method to profile sphingolipids. J Lipid Res. 2010;51:2001–2011. doi: 10.1194/jlr.D005322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Svennerholm L, Vanier MT, Mansson JE. Krabbe disease: a galactosylsphingosine (psychosine) lipidosis. J Lipid Res. 1980;21:53–64. [PubMed] [Google Scholar]
- 27.Li YT, Li SC, Buck WR, Haskins ME, Wu SW, Khoo KH, Sidransky E, Bunnell BA. Selective extraction and effective separation of galactosylsphingosine (psychosine) and glucosylsphingosine from other glycosphingolipids in pathological tissue samples. Neurochem Res. 2011;36:1612–1622. doi: 10.1007/s11064-010-0348-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schueler UH, Kolter T, Kaneski CR, Blusztajn JK, Herkenham M, Sandhoff K, Brady RO. Toxicity of glucosylsphingosine (glucopsychosine) to cultured neuronal cells: a model system for assessing neuronal damage in Gaucher disease type 2 and 3. Neurobiol Dis. 2003;14:595–601. doi: 10.1016/j.nbd.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 29.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Settembre C, Fraldi A, Rubinsztein DC, Ballabio A. Lysosomal storage diseases as disorders of autophagy. Autophagy. 2008;4:113–114. doi: 10.4161/auto.5227. [DOI] [PubMed] [Google Scholar]
- 31.Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, Bamber BA, Bassham DC, Bergamini E, Bi X, Biard-Piechaczyk M, Blum JS, Bredesen DE, Brodsky JL, Brumell JH, Brunk UT, Bursch W, Camougrand N, Cebollero E, Cecconi F, Chen Y, Chin LS, Choi A, Chu CT, Chung J, Clarke PG, Clark RS, Clarke SG, Clave C, Cleveland JL, Codogno P, Colombo MI, Coto-Montes A, Cregg JM, Cuervo AM, Debnath J, Demarchi F, Dennis PB, Dennis PA, Deretic V, Devenish RJ, Di Sano F, Dice JF, Difiglia M, Dinesh-Kumar S, Distelhorst CW, Djavaheri-Mergny M, Dorsey FC, Droge W, Dron M, Dunn WA, Jr, Duszenko M, Eissa NT, Elazar Z, Esclatine A, Eskelinen EL, Fesus L, Finley KD, Fuentes JM, Fueyo J, Fujisaki K, Galliot B, Gao FB, Gewirtz DA, Gibson SB, Gohla A, Goldberg AL, Gonzalez R, Gonzalez-Estevez C, Gorski S, Gottlieb RA, Haussinger D, He YW, Heidenreich K, Hill JA, Hoyer-Hansen M, Hu X, Huang WP, Iwasaki A, Jaattela M, Jackson WT, Jiang X, Jin S, Johansen T, Jung JU, Kadowaki M, Kang C, Kelekar A, Kessel DH, Kiel JA, Kim HP, Kimchi A, Kinsella TJ, Kiselyov K, Kitamoto K, Knecht E, Komatsu M, Kominami E, Kondo S, Kovacs AL, Kroemer G, Kuan CY, Kumar R, Kundu M, Landry J, Laporte M, Le W, Lei HY, Lenardo MJ, Levine B, Lieberman A, Lim KL, Lin FC, Liou W, Liu LF, Lopez-Berestein G, Lopez-Otin C, Lu B, Macleod KF, Malorni W, Martinet W, Matsuoka K, Mautner J, Meijer AJ, Melendez A, Michels P, Miotto G, Mistiaen WP, Mizushima N, Mograbi B, Monastyrska I, Moore MN, Moreira PI, Moriyasu Y, Motyl T, Munz C, Murphy LO, Naqvi NI, Neufeld TP, Nishino I, Nixon RA, Noda T, Nurnberg B, Ogawa M, Oleinick NL, Olsen LJ, Ozpolat B, Paglin S, Palmer GE, Papassideri I, Parkes M, Perlmutter DH, Perry G, Piacentini M, Pinkas-Kramarski R, Prescott M, Proikas-Cezanne T, Raben N, Rami A, Reggiori F, Rohrer B, Rubinsztein DC, Ryan KM, Sadoshima J, Sakagami H, Sakai Y, Sandri M, Sasakawa C, Sass M, Schneider C, Seglen PO, Seleverstov O, Settleman J, Shacka JJ, Shapiro IM, Sibirny A, Silva-Zacarin EC, Simon HU, Simone C, Simonsen A, Smith MA, Spanel-Borowski K, Srinivas V, Steeves M, Stenmark H, Stromhaug PE, Subauste CS, Sugimoto S, Sulzer D, Suzuki T, Swanson MS, Tabas I, Takeshita F, Talbot NJ, Talloczy Z, Tanaka K, Tanida I, Taylor GS, Taylor JP, Terman A, Tettamanti G, Thompson CB, Thumm M, Tolkovsky AM, Tooze SA, Truant R, Tumanovska LV, Uchiyama Y, Ueno T, Uzcategui NL, van der Klei I, Vaquero EC, Vellai T, Vogel MW, Wang HG, Webster P, Wiley JW, Xi Z, Xiao G, Yahalom J, Yang JM, Yap G, Yin XM, Yoshimori T, Yu L, Yue Z, Yuzaki M, Zabirnyk O, Zheng X, Zhu X, Deter RL. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mellors JS, Jorgenson JW. Use of 1.5-microm porous ethyl-bridged hybrid particles as a stationary-phase support for reversed-phase ultrahigh-pressure liquid chromatography. Anal Chem. 2004;76:5441–5450. doi: 10.1021/ac049643d. [DOI] [PubMed] [Google Scholar]
- 33.Sittampalam G, Gal-Edd N, Weidner J, Auld D, Glicksman M, Arkin M, Napper A, Inglese J. Assay Guidance Manual. Eli Lilly & Company and the National Center for Advancing Translational Sciences; Bethesda, MD: 2004. [PubMed] [Google Scholar]
- 34.LeVine SM, Pedchenko TV, Bronshteyn IG, Pinson DM. L-cycloserine slows the clinical and pathological course in mice with globoid cell leukodystrophy (twitcher mice) J Neurosci Res. 2000;60:231–236. doi: 10.1002/(SICI)1097-4547(20000415)60:2<231::AID-JNR12>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki K. Globoid cell leukodystrophy (Krabbe’s disease): update. J Child Neurol. 2003;18:595–603. doi: 10.1177/08830738030180090201. [DOI] [PubMed] [Google Scholar]
- 36.Grabowski GA, Hopkin RJ. Enzyme therapy for lysosomal storage disease: principles, practice, and prospects. Annu Rev Genomics Hum Genet. 2003;4:403–436. doi: 10.1146/annurev.genom.4.070802.110415. [DOI] [PubMed] [Google Scholar]
- 37.Biswas S, LeVine SM. Substrate-reduction therapy enhances the benefits of bone marrow transplantation in young mice with globoid cell leukodystrophy. Pediatr Res. 2002;51:40–47. doi: 10.1203/00006450-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Escolar ML, Poe MD, Provenzale JM, Richards KC, Allison J, Wood S, Wenger DA, Pietryga D, Wall D, Champagne M, Morse R, Krivit W, Kurtzberg J. Transplantation of umbilical-cord blood in babies with infantile Krabbe’s disease. N Engl J Med. 2005;352:2069–2081. doi: 10.1056/NEJMoa042604. [DOI] [PubMed] [Google Scholar]
- 39.Duffner PK, Caviness VS, Jr, Erbe RW, Patterson MC, Schultz KR, Wenger DA, Whitley C. The long-term outcomes of presymptomatic infants transplanted for Krabbe disease: report of the workshop held on July 11 and 12, 2008, Holiday Valley, New York. Genet Med. 2009;11:450–454. doi: 10.1097/GIM.0b013e3181a16e04. [DOI] [PubMed] [Google Scholar]
- 40.Duffner PK, Caggana M, Orsini JJ, Wenger DA, Patterson MC, Crosley CJ, Kurtzberg J, Arnold GL, Escolar ML, Adams DJ, Andriola MR, Aron AM, Ciafaloni E, Djukic A, Erbe RW, Galvin-Parton P, Helton LE, Kolodny EH, Kosofsky BE, Kronn DF, Kwon JM, Levy PA, Miller-Horn J, Naidich TP, Pellegrino JE, Provenzale JM, Rothman SJ, Wasserstein MP. Newborn screening for Krabbe disease: the New York State model. Pediatr Neurol. 2009;40:245–252. doi: 10.1016/j.pediatrneurol.2008.11.010. discussion 253–245. [DOI] [PubMed] [Google Scholar]
- 41.Buntinx M, Vanderlocht J, Hellings N, Vandenabeele F, Lambrichts I, Raus J, Ameloot M, Stinissen P, Steels P. Characterization of three human oligodendroglial cell lines as a model to study oligodendrocyte injury: morphology and oligodendrocyte-specific gene expression. J Neurocytol. 2003;32:25–38. doi: 10.1023/a:1027324230923. [DOI] [PubMed] [Google Scholar]
- 42.Bongarzone ER, Foster LM, Byravan S, Schonmann V, Campagnoni AT. Temperature-dependent regulation of PLP/DM20 and CNP gene expression in two conditionally-immortalized jimpy oligodendrocyte cell lines. Neurochem Res. 1997;22:363–372. doi: 10.1023/a:1027339222720. [DOI] [PubMed] [Google Scholar]
- 43.Verity AN, Bredesen D, Vonderscher C, Handley VW, Campagnoni AT. Expression of myelin protein genes and other myelin components in an oligodendrocytic cell line conditionally immortalized with a temperature-sensitive retrovirus. J Neurochem. 1993;60:577–587. doi: 10.1111/j.1471-4159.1993.tb03188.x. [DOI] [PubMed] [Google Scholar]
- 44.Feutz AC, Bellomi I, Allinquant B, Schladenhaufen Y, Ghandour MS. Isolation and characterization of defective jimpy oligodendrocytes in culture. J Neurocytol. 1995;24:865–877. doi: 10.1007/BF01179985. [DOI] [PubMed] [Google Scholar]
- 45.Feutz AC, Pham-Dinh D, Allinquant B, Miehe M, Ghandour MS. An immortalized jimpy oligodendrocyte cell line: defects in cell cycle and cAMP pathway. Glia. 2001;34:241–252. doi: 10.1002/glia.1058. [DOI] [PubMed] [Google Scholar]
- 46.Baarine M, Ragot K, Genin EC, El Hajj H, Trompier D, Andreoletti P, Ghandour MS, Menetrier F, Cherkaoui-Malki M, Savary S, Lizard G. Peroxisomal and mitochondrial status of two murine oligodendrocytic cell lines (158N, 158JP): potential models for the study of peroxisomal disorders associated with dysmyelination processes. J Neurochem. 2009;111:119–131. doi: 10.1111/j.1471-4159.2009.06311.x. [DOI] [PubMed] [Google Scholar]
- 47.Shen JS, Watabe K, Meng XL, Ida H, Ohashi T, Eto Y. Establishment and characterization of spontaneously immortalized Schwann cells from murine model of globoid cell leukodystrophy (twitcher) J Neurosci Res. 2002;68:588–594. doi: 10.1002/jnr.10247. [DOI] [PubMed] [Google Scholar]
- 48.Yim SH, Szuchet S, Polak PE. Cultured oligodendrocytes. A role for cell-substratum interaction in phenotypic expression. J Biol Chem. 1986;261:11808–11815. [PubMed] [Google Scholar]
- 49.Zaka M, Wenger DA. Psychosine-induced apoptosis in a mouse oligodendrocyte progenitor cell line is mediated by caspase activation. Neurosci Lett. 2004;358:205–209. doi: 10.1016/j.neulet.2003.12.126. [DOI] [PubMed] [Google Scholar]
- 50.Giri S, Khan M, Rattan R, Singh I, Singh AK. Krabbe disease: psychosine-mediated activation of phospholipase A2 in oligodendrocyte cell death. J Lipid Res. 2006;47:1478–1492. doi: 10.1194/jlr.M600084-JLR200. [DOI] [PubMed] [Google Scholar]
- 51.Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22:1577–1590. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vitner EB, Platt FM, Futerman AH. Common and uncommon pathogenic cascades in lysosomal storage diseases. J Biol Chem. 2010;285:20423–20427. doi: 10.1074/jbc.R110.134452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lam MP, Scruggs SB, Kim TY, Zong C, Lau E, Wang D, Ryan CM, Faull KF, Ping P. An MRM-based workflow for quantifying cardiac mitochondrial protein phosphorylation in murine and human tissue. J Proteomics. 2012;75:4602–4609. doi: 10.1016/j.jprot.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.