Abstract
Mesenchymal stromal cells (MSCs) are known to have regenerative, anti-inflammatory, and immunodulatory effects. There are extensive indications that pig MSCs function satisfactorily across species barriers. Pig MSCs might have considerable therapeutic potential, particularly in xenotransplantation, where they have several potential advantages. (i) pMSCs can be obtained from the specific organ- or cell-source donor pig or from an identical (cloned) pig. (ii) They are easy to obtain in large numbers, negating the need for prolonged ex vivo expansion. (iii) They can be obtained from genetically-engineered pigs, and the genetic modification can be related to the therapeutic goal of the MSCs. We have reviewed our own studies on MSCs from genetically-engineered pigs, and summarize them here.
We have successfully harvested and cultured MSCs from wild-type and genetically-engineered pig bone marrow and adipose tissue. We have identified several pig (p)MSC surface markers (positive for CD29, CD44, CD73, CD105, CD166, and negative for CD31, CD45), have demonstrated their proliferation and differentiation (into adipocytes, osteoblasts, and chondroblasts), and evaluated their antigenicity and immune suppressive effects on human peripheral blood mononuclear cells and CD4+T cells. They have identical or very similar characteristics to MSCs from other mammals.
Genetically-modified pMSCs are significantly less immunogenic than wild-type pMSCs, and downregulate the human T cell response to pig antigens as efficiently as do human MSCs. We hypothesized that pMSCs can immunomodulate human T cells through induction of apoptosis or anergy, or cause T cell phenotype switching with induction of regulatory T cells, but we could find no evidence for these mechanisms. However, pMSCs upregulated the expression of CD69 on human CD4+ and CD8+ T cells, the relevance of which is currently under investigation.
We conclude that MSCs from genetically-engineered pigs should continue to be investigated for their immunomodulatory (and regenerative and anti-inflammatory) effects in pig-to-nonhuman primate organ and cell transplantation models.
Keywords: Mesenchymal stromal cells, Pig, Xenotransplantation
Introduction
Mesenchymal stromal cells (MSCs) were discovered in 1968 by Friedenstein [1] as an adherent fibroblast-like population in the bone marrow capable of differentiating into bone. MSCs can be isolated from various tissues of mammals; the blood, bone marrow, and adipose tissue have to date formed the major sources. The number of cells that can be isolated from any tissue is small. For example, MSCs represent only 0.001% to 0.01% of the total nucleated cells in the bone marrow. However, they grow readily in vitro and can be expanded to significant numbers in culture. After in vivo administration, they have the ability to migrate to sites of inflammation and also to sites of allograft rejection [2].
For better characterization of MSCs, in 2006 the International Society of Cellular Therapy defined human MSCs by the following three criteria:- (i) MSCs must be adherent to plastic under standard culture conditions; (ii) MSCs must express certain cell surface markers, such as CD73, CD90, and CD105, and lack expression of other markers, including CD45, CD34, CD14, CD11b, CD79α, CD19, and HLA-DR surface molecules; and (iii) MSCs must have the capacity to differentiate into osteoblasts, adipocytes, and chondroblasts under in vitro conditions [3].
MSCs are known to have regenerative, anti-inflammatory, and immunodulatory effects. They have garnered particular attention for their potential use as regenerative therapeutic agents in a range of acute and chronic diseases. To date, the beneficial effects of MSC therapy have been more frequently linked to their potent anti-inflammatory and immune-modulating properties, rather than their ability to differentiate. Of particular interest to transplantation, it has been well-documented that MSCs possess immunomodulatory properties. They can target several subsets of lymphocytes, including CD4+ and CD8+T lymphocytes, B lymphocytes, natural killer cells, and regulatory T lymphocytes. Their effects may be mediated by several soluble factors secreted by MSCs. Furthermore, infused MSCs can induce T cell apoptosis through Fas/FasL-mediated multiple paracrine interactions and cell-cell contact, as well as promoting the generation of T regulatory cells, which may ultimately lead to immune tolerance [4].
Interest in MSCs grew rapidly and, by the beginning of 2012, the public clinical trial database showed 206 clinical trials using these cells for a wide range of therapeutic applications [5]. The successful treatment of patients with severe acute graft-vs-host disease by the administration of third-party haploid-identical human MSCs in 2004 created a surge of interest in the potential therapeutic effects of these cells [6, 7]. Their therapeutic potential is being investigated in sub-clinical rejection [8], chronic allograft nephropathy [9], and the induction of tolerance to renal allografts [10].
Do mesenchymal stem cells function across species barriers?
For the purposes of xenotransplantation, it is important to know whether MSCs function across species barriers. An extensive survey of the literature indicated that, by the end of 2011, there had been 94 reports of in vivo cross-species administration of MSCs [11]. In 88 studies (93.6%) there was evidence that the MSCs engrafted and functioned across the species barrier, and in only 6 cases (6.4%) was there evidence of failure to function. Human-derived MSCs were demonstrated to function in no fewer than seven different recipient species, including mouse, rat, sheep, hamster, dog, rabbit, and pig. However, to date there are no in vivo data from a pig-to-primate model.
A large range of animal disease models (n=90) were chosen for testing MSC function across species. In nine studies, MSCs co-transplanted with hematopoietic stem cells, umbilical cord blood, skin or liver, influenced graft survival. Five different routes of administration, including intravenous, intraperitoneal, subcutaneous, and intratrachael administration, or local injection, have been proved successful.
There are, therefore, extensive indications that pig MSCs would function satisfactorily in a different species, for example, humans. In our own laboratory we have had the opportunity of studying MSCs from various genetically-engineered pigs, and we here review our initial experience.
Pig mesenchymal stromal cells (pMSCs)
At our own center, we have successfully harvested and cultured MSCs from pig bone marrow and adipose tissue [12–15]. We identified a fibroblast-like morphology of genetically-engineered porcine adipose-derived MSCs (Figure 1). We have also demonstrated differentiation of these pig (p)MSCs into adipocytes, osteoblasts, and chondroblasts (Figure 1). We have identified several pMSC surface markers, have demonstrated differentiation and proliferation of the MSCs, and evaluated their antigenicity and immune suppressive effects on human peripheral blood mononuclear cells (PBMC) and CD4+ T cells. We have successfully isolated MSCs from three different genetically-engineered pigs (of which there are now more than 20 different genetic variations and which are reviewed in 16, 17) and have confirmed their phenotype and differentiation capacity. They have identical or very similar characteristics to MSCs from other mammals.
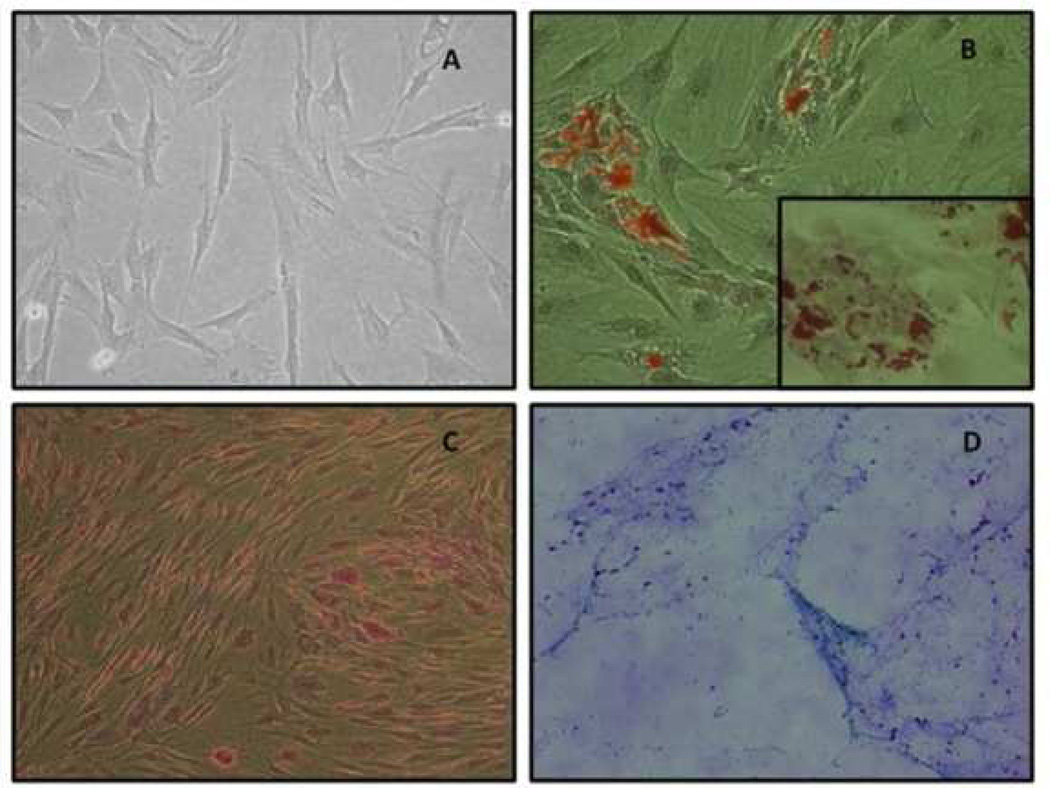
Figure 1. Differentiation of pMSCs.
(A)Fibroblast-like morphology of GTKO/hCD46 pAdMSC (light microscopy 10×) (B) Adipogenic differentiation of GTKO/hCD46 pAdMSC – Oil red stain (20×). (Insert –fat droplets stained with oil red-40×). (C) Osteogenic differentiation of GTKO/hCD46 pAdMSC – Von Kossa stain (20×), showing morphological changes in pAdMSC and linear calcium deposition (black). (D) Chondrogenic differentiation of GTKO/hCD46 pAdMSC – Alcian blue stain (10×), showing strongly acidic mucosubstance (blue) and goblet cells (red nuclei). (Reproduced from Kumar G, et al, Cytotherapy 2012; 14:494–504 [14], with permission).
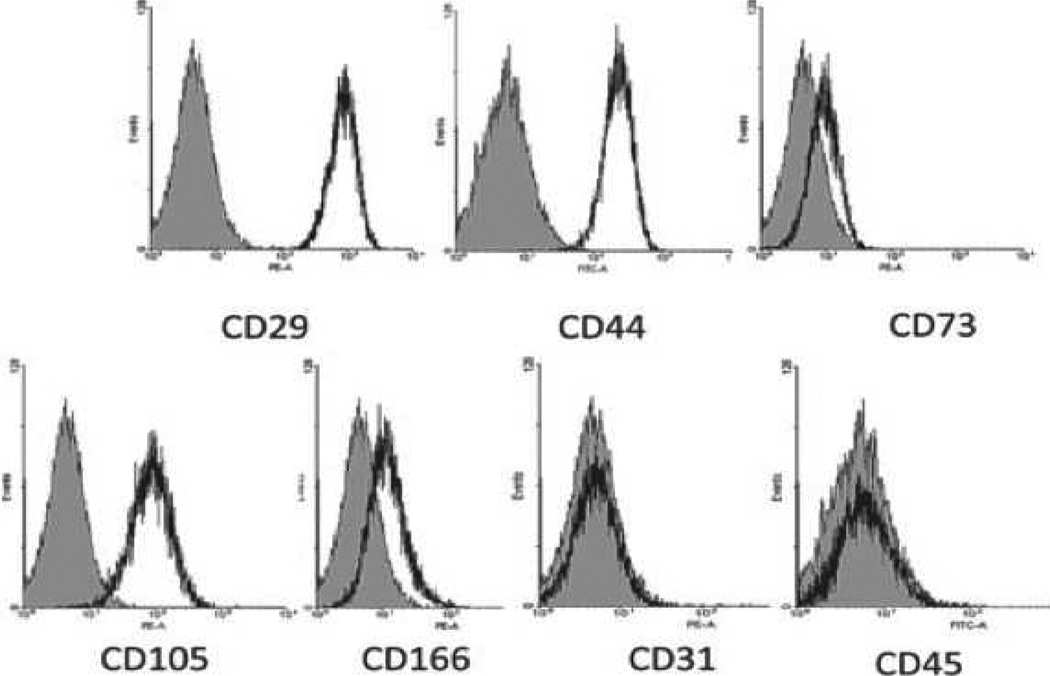
By flow cytometry, wild-type pMSCs were demonstrated to be positive for expression of the important galactose-α1,3-galactose (Gal) antigen (against which humans have natural antibodies) and MSCs from α1,3-galactosyltransferase gene-knockout (GTKO) pigs (in which this antigen has been deleted) were negative [18, 19]. GTKO pMSCs were positive for CD29, CD44, CD73, CD105, and CD166, and negative for CD31 and CD45 (Figure 2).
Figure 2. Pig MSC surface markers.
By flow cytometry, GTKO pMSC were positive for CD29, CD44, CD73, CD105, and CD166, and negative for CD31 and CD45 (black); isotype control (gray). (Reproduced from Ezzelarab M, et al. Xenotransplantation 2011; 18:183–195 [13] with permission).
Potential advantages of pig MSCs over human MSCs
Pig MSCs might have considerable therapeutic potential, particularly in xenotransplantation [12–15], but also possibly in allotransplantation and even autoimmune disease. In regard to xenotransplantation there are several potential advantages of pMSCs:-
In the case of organ or cell transplantation, pMSCs can be obtained from the specific organ- or cell-source donor pig or from an identical (cloned) pig. This would enable the MSCs to be genetically identical to the organ or cells to be transplanted. There would be no need for third-party donors, as is the case with most human MSCs. For example, in pig islet transplantation into primates, the pMSCs could be obtained from the same islet “donor”, i.e., the same islet-source pig, and could possibly provide a preferable microenvironment for the islets, resulting in more rapid stimulation of local growth factors and revascularization, and even regeneration of the islets [20, 21].
pMSCs are easy to obtain in large numbers from either adipose tissue or bone marrow. There is, therefore, much less need for prolonged ex vivo expansion of pMSCs with its disadvantages, which include cell senescence and the risk of malignant change [22, 23].
pMSCs can be obtained from genetically-engineered pigs, which is clearly not possible for hMSCs, and the genetic modification can be related to the therapeutic goal of the MSCs. In particular, even though the immunogenicity of MSCs from wild-type pigs is already weak compared with other pigs cells, e.g., pig aortic endothelial cells (pAECs), genetic modifications reduce the immunogenicity further. For example, as we have shown for other pig cells (e.g., pAECs), the absence of expression of Gal on GTKO pMSCs reduces both human antibody binding and the proliferative cellular response of human immune cells (Figure 3) [13]. The data suggest that in vitro observations on the efficacy of pMSCs in down-regulating the strength of the human T cell response to pig antigens would likely be reproduced in vivo in pre-clinical large animal models and in clinical trials.
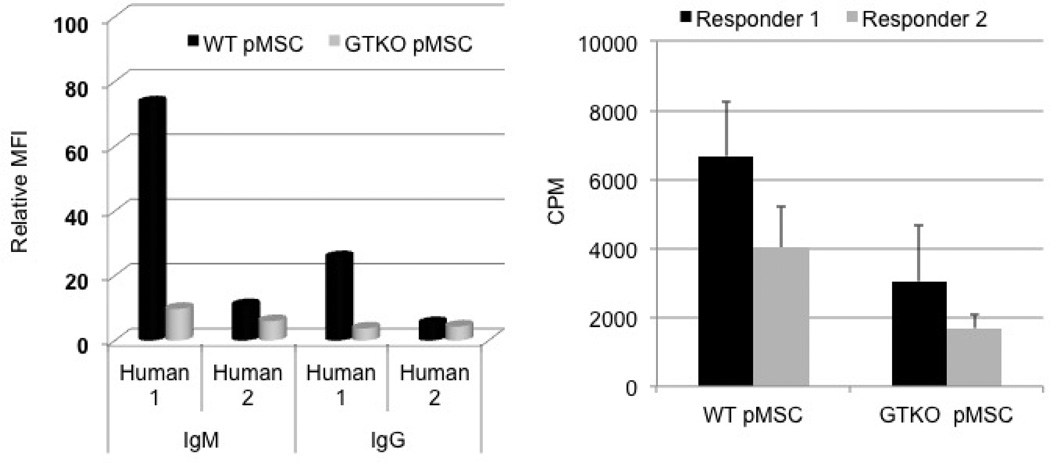
Figure 3. Immunogenicity of pMSCs.
(Left) Using serum from two healthy human volunteers (one with high [Human 1] and one with low [Human 2] levels of anti-pig antibodies), IgM/IgG antibody binding to WT and GTKO pMSC was measured by flow cytometry. IgM/IgG binding to GTKO pMSC was less than that to WT pMSC. Relative mean immunoflorescence intensity (MFI) was calculated by dividing the MFI of the tested serum sample by the MFI of the background (secondary antibody binding to target cells). Data are representative of three different experiments.
(Right) Proliferative responses of PBMC from two different healthy humans to WT and GTKO pMSC in mixed lymphocyte reaction (responder:stimulator ratio 1:10). In both cases, the response to GTKO pMSC was weaker than that to WT pMSC (p<0.01 vs. WT pMSC).
(Reproduced from Ezzelarab M, et al. Xenotransplantation 2011; 18:183–195 [13] with permission).
The data available in the literature from other animal models suggest that pMSCs might also have a regenerative effect if administered to humans [24–26].
In vitro studies using pMSCs
We initially investigated bone marrow-derived pMSCs from both wild-type and GTKO pigs [12,13]. Before activation with porcine interferon-gamma (pIFN-γ), <% of bone marrow-derived GTKO pMSCs expressed swine leukocyte antigen (SLA) class II, compared to 2.5% of GTKO pAECs. After activation, only 49% of GTKO pMSCs upregulated SLA class II expression, compared to 99% of GTKO pAECs. Without activation, only 3% of GTKO pMSCs expressed CD80 compared to 80% of GTKO pAECs. After activation, GTKO pAECs upregulated the CD86 mRNA level much more strongly than GTKO pMSCs. The human CD4+ T cell response to GTKO pMSCs was significantly weaker than to GTKO pAECs, even after activation.
More than 99% of MSCs from GTKO pigs transgenic for the human complement-regulatory protein CD46 expressed hCD46. Human PBMC and CD4+ T cell responses to GTKO and GTKO/CD46 pMSCs were comparable to those to hMSCs, and all were significantly lower than to GTKO pAECs. GTKO/CD46 pMSCs downregulated human T cell proliferation as efficiently as hMSCs. Reduced levels of proinflammatory cytokines, IL-2, IFN-γ, TNF-α, and soluble CD154 (CD40L) correlated with the downregulation of human T cell proliferation by co-culture with pMSCs.
We also investigated adipose-derived pMSCs (AdMSCs) from GTKO/hCD46 pigs [14]. These pMSCs (i) did not express Gal, but did express hCD46, (ii) differentiated into chondroblasts, osteocytes, and adipocytes, (iii) expressed stem cell markers, (iv) expressed lower levels of SLA class I, SLA class IIDR, and CD80 than pAECs before and after pIFN-γ stimulation. The proliferative responses of human (h)PBMCs to GTKO/hCD46 pAdMSC and hAdMSC stimulators were similar, and both were significantly lower than to GTKO pAECs. The proliferation of hPBMCs to GTKO pAECs was equally suppressed by GTKO/hCD46 pAdMSCs and hAdMSCs. The supernatant from GTKO/hCD46 pAdMSCs did not suppress the human xenoresponse to GTKO pAECs which must therefore be dependent on cell-cell contact.
From these and other data, we concluded that genetically-modified pMSCs are significantly less immunogenic than wild-type pMSCs. Furthermore, GTKO/CD46 pMSCs downregulate the human T cell response to pig antigens as efficiently as do hMSCs, which will be advantageous in therapeutic pig cell xenotransplantation.
We further investigated the immunosuppressive effect of pMSCs on the human cellular immune response to pig antigens [13,14]. When a mixed lymphocyte reaction (MLR) was set up using hPBMCs as responder cells, and GTKO pAECs or pMSCs as stimulators, hPBMC responses were significantly weaker to pMSCs than to pAECs (Figure 4). When GTKO pAECs were mixed with pMSCs, the pMSCs significantly reduced the hPBMC proliferative response to GTKO pAECs (Figure 4). The response of hPBMCs to GTKO pMSCs was not significantly different from that to hMSCs, both of which were significantly lower than to allogeneic PBMCs.
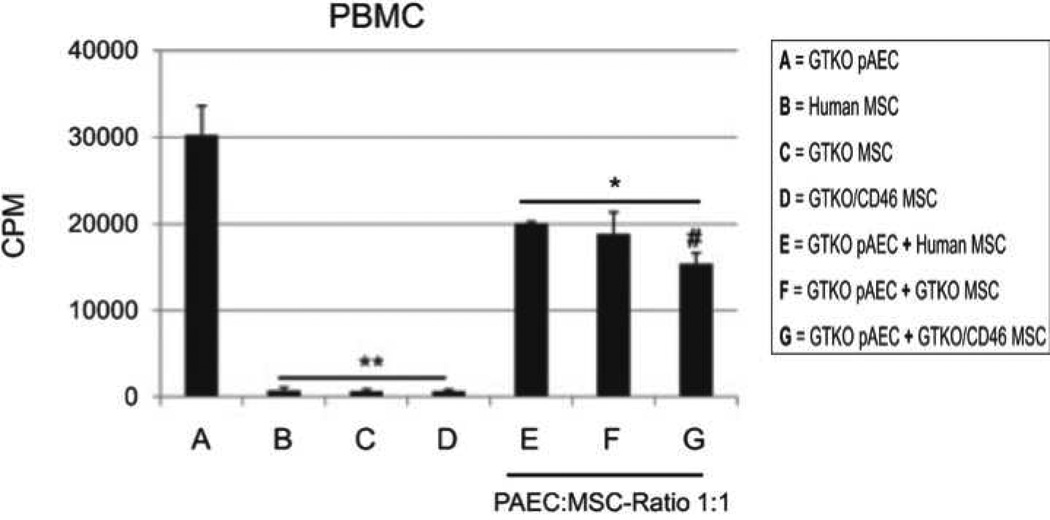
Figure 4. Downregulation of human PBMC responses to GTKO pig (p)AEC by GTKO pMSC, GTKO/CD46 pMSC, and human (h)MSC.
In mixed lymphocyte reaction, human PBMC proliferative responses to GTKO pAEC alone (A), hMSC alone (B), GTKO pMSC alone (C), and GTKO/CD46 pMSC alone (D) were measured. The human PBMC response was significantly weaker to all three types of MSC (B, C, and D) than to GTKO pAEC (A). When GTKO pAEC were mixed with hMSC (E), GTKO pMSC (F), or GTKO/CD46 pMSC (G) at a 1:10 responder:stimulator ratio, each type of MSC significantly reduced the human PBMC proliferative response, with GTKO/CD46 pMSC reducing the response more significantly than hMSC. Data representative of three different experiments (*p<0.01 and **p<0.001 vs. GTKO pAEC [A]; #p<0.05 vs. GTKO pAEC + hMSC [E]). (Reproduced from Ezzelarab M, et al. Xenotransplantation 2011;18:183–195 [13] with permission).
We proceeded to (i) further evaluate the immunomodulatory effect of pMSCs on human T cells, especially CD8+ T cells (which we had not investigated previously); (ii) explore the mechanism of how pMSCs affect human T cells in vitro; and (iii) focus on the direct interplay of pMSCs and human CD4+ and human CD8+ T cells.
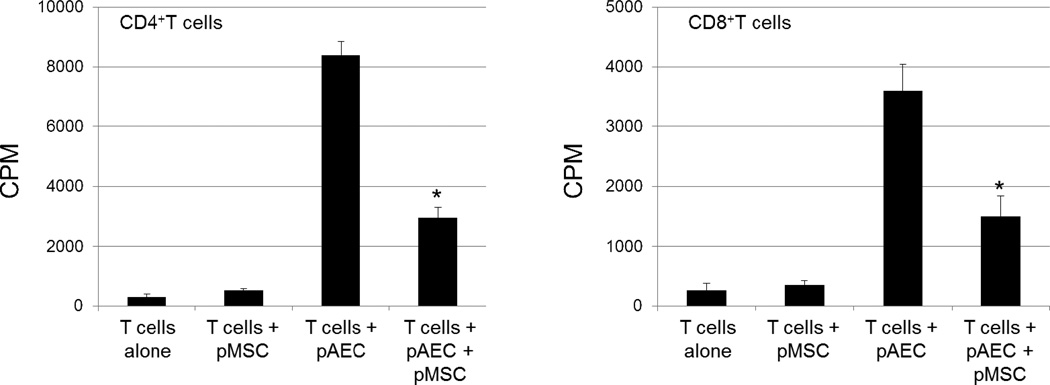
We hypothesized that pMSCs can immunomodulate human T cells through induction of apoptosis or anergy, or possibly cause T cell phenotype switching with induction of regulatory T cells. To test this hypothesis, adipose tissue was harvested from GTKO/hCD46 pigs, and pAECs were harvested from GTKO pigs. MLRs demonstrated that pAdMSCs clearly suppress the hCD4+T cell response to pAECs (Figure 5). GTKO/CD46 pAdMSCs had low immunogenicity, and significantly decreased the hCD4+ T cell proliferative response to GTKO pAECs in a dose-dependent manner. Similarly, although hCD8+T cells displayed a lower proliferative response to GTKO/CD46 pAdMSCs, the pAdMSCs displayed the same immunosuppressive effect on hCD8+T cells as they did on hCD4+T cells, again in a dose-dependent manner (Figure 5). Furthermore, pAdMSCs downregulated granzyme B expression on human CD4+ and CD8+T cells. They did not induce the development of T regulatory cells, and did not induce human T cell apoptosis.
Figure 5. Suppression of human CD4+ and CD8+ T cell proliferation by genetically-modified GTKO/CD46 pMSC.
In MLR, human CD4+ (left) or CD8+ (right) T cell proliferation was measured after coculture with GTKO/CD46 pMSC, or with GTKO pAEC (at a responder:stimulator ratio of 1:10). Also, human T cells were cocultured with GTKO/CD46 pMSC mixed with GTKO pAEC at a 1:1 ratio. GTKO/CD46 pMSC significantly downregulated the responses of CD4+ or CD8+ T cells in response to GTKO pAEC. The proliferation was measured after 5 days of culture, and 16h after addition of 3H-thymidine (1µCi/well) using a beta-scintillation counter (PerkinElmer, Waltham, MA). The results are represented as counts per minute (CPM). Data represent 2 different experiments, and are presented as mean ± SD of triplicates. * p<0.01
One interesting finding that does not appear to have been reported previously is that pAdMSCs upregulated the expression of CD69 on hCD4+ and hCD8+ T cells. The relevance of this upregulation of CD69 is currently under investigation.
Conclusions
From the studies summarized in this brief review, and from other studies [12–15], we have concluded that:- (i) isolation of MSCs from genetically-modified pigs is efficient; (ii) expression of surface MSC markers and poor expression of SLA and costimulatory molecules can be demonstrated; (iii) tri-lineage differentiation can be achieved; (iv) pMSCs have low xenoantigenicity and also an inhibitory effect on human T cell xenoresponses; (v) GTKO/CD46 pMSCs downregulate human T cell proliferation as efficiently as hMSCs; and (vi) cell-cell contact appears to be required for the MSCs to have their immunomodulatory effects.
In summary, therefore, we believe that genetically-modified pMSCs may have considerable potential in xenotransplantation. Not only are they available in large numbers, which reduces the necessity for prolonged ex vivo expansion, but they can be obtained from a herd of identical pigs, thus significantly reducing the variation seen when they are obtained from pooled human sources. This may be a significant advantage over hMSCs. Although not yet tested, their anti-inflammatory and regenerative capacities in humans are likely to be no different from allogeneic MSCs.
ACKNOWLEDGEMENTS
Jiang Li is funded by the China Scholarship Council. Mohamed Ezzelarab is supported in part by the Joseph A. Patrick Research Fellowship in Transplantation of the Thomas E. Starzl Transplantation Institute of the University of Pittsburgh. Studies on xenotransplantation at the Thomas E. Starzl Transplantation Institute are supported in part by CMRF (competitive medical research funding) from the University of Pittsburgh Medical Center, NIH grants #1U19AI090959-01 and #1PO1 HL107152, and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Inc., Blacksburg, VA.
ABBREVIATIONS
- AdMSC
adipose-derived mesenchymal stromal cells
- AECs
aortic endothelial cells
- Gal
galactose-α1,3-galactose
- GTKO
α1,3-galactosyltransferase gene-knockout
- MLR
mixed lymphocyte reaction
- MSC
mesenchymal stromal cells
- PBMC
peripheral blood mononuclear cells
- SLA
swine leukocyte antigen
Footnotes
CONFLICT OF INTEREST
DA is an employee of Revivicor Inc., Blacksburg, VA. The other authors report no conflicts of interest.
References
- 1.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–247. [PubMed] [Google Scholar]
- 2.Sordi V. Mesenchymal stem cell homing capacity. Transplantation. 2009;87:S42–S45. doi: 10.1097/TP.0b013e3181a28533. [DOI] [PubMed] [Google Scholar]
- 3.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 4.Wang L, Zhao Y, Shi S. Interplay between mesenchymal stem cells and lymphocytes: implications for immunotherapy and tissue regeneration. Journal of Dental Research. 2012;91:1003–1010. doi: 10.1177/0022034512460404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang S, Qu X, Zhao RC. Clinical applications of mesenchymal stem cells. Journal of Hematology and Oncology. 2012;5:19. doi: 10.1186/1756-8722-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 7.Le Blanc K, Rasmusson I, Gotherstrom C, Seidel C, Sundberg B, Sundin M, et al. Mesenchymal stem cells inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scandinavian Journal of Immunology. 2004;60:307–315. doi: 10.1111/j.0300-9475.2004.01483.x. [DOI] [PubMed] [Google Scholar]
- 8.Reinders ME, Fijter JW, Roelofs H, Bajema IM, Vries DK, Schaapherder AF, et al. Autologous bone marrow-derived mesenchymal stromal cells for the treatment of allograft rejection after renal transplantation: results of a phase I study. Stem Cells Translational Medicine. 2013;2:107–111. doi: 10.5966/sctm.2012-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mesenchymal stem cell transplantation in the treatment of chronic allograft nephropathy. (Under investigation, ClinicalTrials.gov Identifier: NCT00659620)
- 10.Perico N, Casiraghi F, Introna M, Gotti E, Todeschini M, Cavinato RA, et al. Autologous mesenchymal stromal cells and kidney transplantation: a pilot study of safety and clinical feasibility. Clinical Journal of the American Society of Nephrology. 2011;6:412–422. doi: 10.2215/CJN.04950610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Ezzelarab MB, Cooper DK. Do mesenchymal stem cells function across species barriers? Relevance for xenotransplantation. Xenotransplantation. 2012;19:273–285. doi: 10.1111/xen.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ezzelarab M, Ayares D, Cooper DK. The potential of genetically-modified pig mesenchymal stromal cells in xenotransplantation. Xenotransplantation. 2010;17:3–5. doi: 10.1111/j.1399-3089.2009.00567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ezzelarab M, Ezzelarab C, Wilhite T, Kumar G, Hara H, Ayares D, et al. Genetically-modified pig mesenchymal stromal cells: xenoantigenicity and effect on human T-cell xenoresponses. Xenotransplantation. 2011;18:183–195. doi: 10.1111/j.1399-3089.2011.00635.x. [DOI] [PubMed] [Google Scholar]
- 14.Kumar G, Hara H, Long C, Shaikh H, Ayares D, Cooper DK, et al. Adipose-derived mesenchymal stromal cells from genetically modified pigs: immunogenicity and immune modulatory properties. Cytotherapy. 2012;14:494–504. doi: 10.3109/14653249.2011.651529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Andreyeva O, Chen M, Marcoa M, Iwasea H, Long C, et al. Human T cells upregulate CD69 after coculture with xenogeneic genetically-modified pig mesenchymal stromal cells. Cellular Immunology. 2013 doi: 10.1016/j.cellimm.2013.08.004. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper DK, Ekser B, Burlak C, Ezzelarab M, Hara H, Paris L, et al. Clinical lung xenotransplantation--what donor genetic modifications may be necessary? Xenotransplantation. 2012;19:144–158. doi: 10.1111/j.1399-3089.2012.00708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ekser B, Ezzelarab M, Hara H, van der Windt DJ, Wijkstrom M, Bottino R, et al. Clinical xenotransplantation: the next medical revolution? Lancet. 2012;379:672–683. doi: 10.1016/S0140-6736(11)61091-X. [DOI] [PubMed] [Google Scholar]
- 18.Hara H, Long C, Lin YJ, Tai HC, Ezzelarab M, Ayares D, et al. In vitro investigation of pig cells for resistance to human antibody-mediated rejection. Transplant International. 2008;21:1163–1174. doi: 10.1111/j.1432-2277.2008.00736.x. [DOI] [PubMed] [Google Scholar]
- 19.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brusko TM. Mesenchymal stem cells: a potential border patrol for transplanted islets? Diabetes. 2009;58:1728–1729. doi: 10.2337/db09-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solari MG, Srinivasan S, Boumaza I, Unadkat J, Harb G, Garcia-Ocana A, et al. Marginal mass islet transplantation with autologous mesenchymal stem cells promotes long-term islet allograft survival and sustained normoglycemia. Journal of Autoimmunity. 2009;32:116–124. doi: 10.1016/j.jaut.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Rosland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H, et al. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Research. 2009;69:5331–5339. doi: 10.1158/0008-5472.CAN-08-4630. [DOI] [PubMed] [Google Scholar]
- 23.Vacanti V, Kong E, Suzuki G, Sato K, Canty JM, Lee T. Phenotypic changes of adult porcine mesenchymal stem cells induced by prolonged passaging in culture. Journal of Cellular Physiology. 2005;205:194–201. doi: 10.1002/jcp.20376. [DOI] [PubMed] [Google Scholar]
- 24.Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. The International Journal of Biochemistry & Cell Biology. 2004;36:568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nature Reviews Immunology. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 26.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. The Journal of Clinical Investigation. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]