Abstract
The DNA damage response factor 53BP1 functions at the intersection of two major double strand break (DSB) repair pathways – promoting non-homologous end-joining (NHEJ) and inhibiting homology-directed repair (HDR) – and integrates cellular inputs to ensure their timely execution in the proper cellular contexts. Recent work has revealed that 53BP1 controls 5′ end resection at DNA ends, mediates synapsis of DNA ends, promotes the mobility of damaged chromatin, improves DSB repair in heterochromatic regions, and contributes to lethal mis-repair of DSBs in BRCA1-deficient cells. Here we review these aspects of 53BP1 and discuss new data revealing how 53BP1 is loaded onto chromatin and uses its interacting factors Rif1 and PTIP to promote NHEJ and inhibit HDR.
Keywords: 53BP1, Rif1, PTIP, NHEJ, HDR, PARPi, telomere, CSR, V(D)J, BRCA1, resection
The choices in DSB repair
Double strand break (DSB) repair can take place through two main pathways: homology-directed repair (HDR) and classical (Ku70/80 and DNA ligase IV dependent) non-homologous end joining (c-NHEJ). The choice between HDR and c-NHEJ is regulated such that DSBs formed in S phase are preferentially repaired by HDR, whereas in G1, DSBs, including those formed in immunoglobulin loci are repaired by c-NHEJ. When this regulation fails, translocations and other genome rearrangements can result, diminishing cell viability and increasing the chance of tumorigenic changes. How the DNA damage response factor 53BP1 promotes c-NHEJ and affects the choice between HDR and c-NHEJ is the subject of this review.
53BP1 engages two modified histones at sites of DNA damage
53BP1 (TP53BP1, tumor suppressor p53 binding protein 1; see Text Box 1 and Fig. 1 for the domain structure of 53BP1) is a key regulator of DSB repair [1,2]. 53BP1 rapidly forms large foci near DNA lesions where ATM- or ATR-mediated DNA damage signaling is induced [3–5]. Similarly, 53BP1 accumulates at telomeres that have been rendered dysfunctional through removal of various components of the protective shelterin complex and have activated either the ATM or ATR kinase (or both) [6–9], forming what are referred to as Telomere dysfunction Induced Foci (TIFs). 53BP1 is also found in large entities called nuclear bodies or OPT domains, observed in G1 cells that experienced replication stress in the prior S phase [10,11].
Text Box 1. Functional domains of 53BP1.
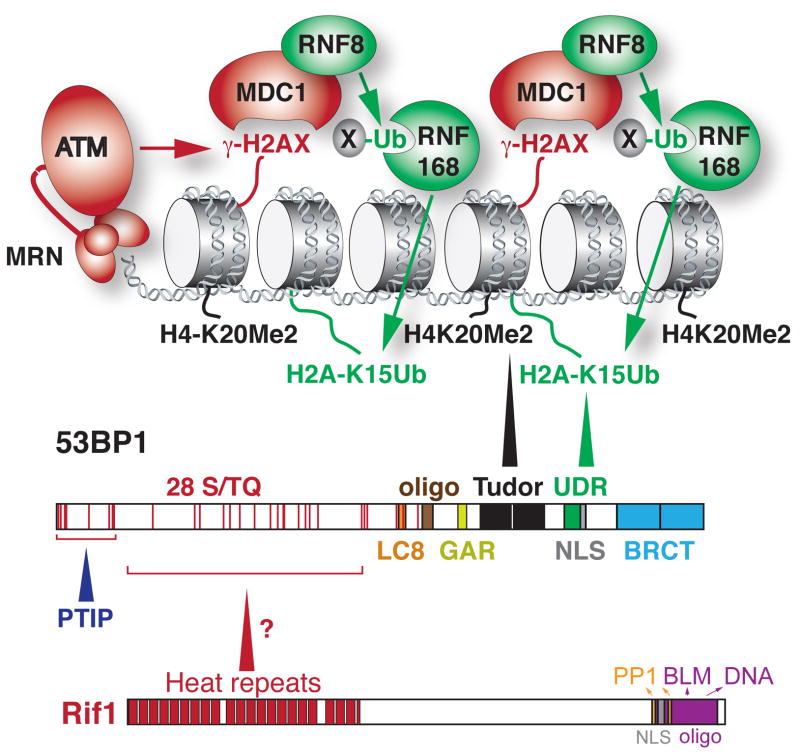
The nearly 2000 aminoacids and over 200 kDa of mammalian 53BP1 are arranged into a complex multi-domain structure [2] (Fig. 1). A large N-terminal region spanning more than a half of the 53BP1 sequence contains 28 S/TQ sites, which are phosphorylated by ATM and/or ATR upon induction of DNA damage [4,5,29,77,92,99–101]. These phosphorylation sites are not required for 53BP1 recruitment to DNA damage foci, but are necessary for DNA repair, because they bind interacting factors such as Rif1 and PTIP [29,75,77,84–87,91,99,100] (see main text). If the glutamine residues in these sites are mutated to alanines, 53BP1 fails to both block resection and increase chromatin mobility and can thus no longer mediate CSR, c-NHEJ of deprotected telomeres, or the toxic PARPi-induced mis-rejoining events in BRCA1-deficient cells [29,77,100]. The c-NHEJ defect at dysfunctional telomeres caused by the 53BP1 phosphorylation mutant is, however, milder than the one of a null allele, suggesting that other domains of 53BP1 contribute [29].
The central part of 53BP1 enables binding to damaged chromatin, because it comprises a nuclear localization signal (NLS), the tandem Tudor domains that bind to H4K20Me2 and a ubiquitin binding UDR motif that recognizes H2A(X)K15Ub [12,28]. The inactivating D1521R mutation in the Tudor domain largely disrupts binding of 53BP1 to DNA damage sites and therefore impairs its ability to facilitate c-NHEJ [29,34,77,100]. Of note, the D1521R mutant retains residual accumulation at dysfunctional telomeres [most likely due to binding to ubiquitylated H2A(X)] and is thus able to partially promote telomere fusions albeit at very low levels [29].
N-terminal of the Tudor domains 53BP1 contains an oligomerization region, which contributes to chromatin binding and is required for CSR and c-NHEJ in PARPi-treated BRCA1−/− cells [77]. Interestingly, a 53BP1 mutant lacking the oligomerization domain is fully able to block resection at dysfunctional telomeres and is only slightly impaired in promoting telomere mobility. It still, however, causes a minor telomere fusion defect [29]. The precise role of 53BP1 oligomerization remains to be determined, but it might be involved in synapsis [47].
The PRMT1-methylated, glycine-arginine rich (GAR [102]) domain and the dynein light chain 8 binding (LC8 [103]) motif that surround the oligomerization domain are not required for c-NHEJ and their functional significance is unclear. Finally, 53BP1 contains a C-terminal pair of BRCT domains, which are important for DNA repair in heterochromatin [65].
In summary, 53BP1 needs to be chromatin-bound, oligomerized and phosphorylated in order to promote c-NHEJ. Distinct protein domains enable these processes and cooperate to maintain genome integrity.
Figure 1.
The domain structures of 53BP1 and Rif1 and the mechanism by which 53BP1 is recruited to DSBs. The binding of 53BP1 requires two histone modifications. The constitutive H4K20Me2 mark (black) is bound by the Tudor domain and the DDR-induced H2A(X)K15Ub marks is bound by the UDR domain. The binding of 53BP1 to damaged chromatin is also promoted by its oligomerization domain. The N-terminal ST/Q phosphorylation sites are important for the interaction of 53BP1 with PTIP and Rif1. See text for details on the mechanism by which the 53BP1 binds to damaged chromatin and Text Box 1 for details on the domain structure of 53BP1. See Text Box 2 for how the spreading of 53BP1 from sites of DNA damage is limited.
The molecular details of how 53BP1 specifically recognizes altered chromatin near a DNA lesion have only been revealed recently (Fig. 1). A longstanding question regarding the mechanism of 53BP1 focus formation originates from the observation that 53BP1 uses its tandem Tudor domains to bind histone H4 when it is dimethylated on lysine 20 (H4K20Me2, [12]) (Fig. 1). However, the recognition of H4K20Me2, which is present throughout the genome, does not explain the ATM/ATR-dependent accumulation of 53BP1 near sites of damage. It was proposed that the methyltransferase MMSET/WHSC1, a resident of DNA damage foci, can locally increase H4K20 dimethylation and therefore 53BP1 binding [13,14], but other studies argued against this model [15,16].
An important clue came from the finding that efficient retention of 53BP1 at DNA lesions is dependent on the E3 ubiquitin ligase RNF168 [17,18]. The recruitment of RNF168 itself to sites of DNA damage relies on MDC1, which binds to the histone variant H2AX in chromatin when phosphorylated by ATM or ATR (Fig. 1). MDC1 first recruits the ubiquitin E3 ligase complex RNF8/HERC2/UBC13 and the ubiquitin-activating enzyme UBA1, which then allow RNF168 to bind to K63-linked ubiquitin chains on an unknown target protein [19–23]. Despite the clear involvement of RNF168, the mechanism by which chromatin ubiquitylation could help 53BP1 recognize damaged chromatin remained elusive. One proposal was that histone ubiquitylation might modify (“open”) the local chromatin structure, increasing the accessibility of H4K20Me2 [12,24]. Another mechanism may be that histone ubiquitylation releases the polycomb group proteins L3MBTL1 and JMJD2A/JMJD2B from H4K20Me2, thus allowing 53BP1 to bind [25–27].
This riddle of 53BP1 localization to sites of DNA damage was recently solved by the demonstration that 53BP1 not only binds to H4K20Me2 but also engages a ubiquitylated form of H2A, H2AK15Ub [28]. The ubiquitylation of H2A (or H2AX) on lysine 15 is a specific function of RNF168 and therefore occurs only in chromatin near DNA lesions where ATM or ATR signaling is activated [23]. 53BP1 recognizes H2AK15Ub using a conserved Ubiquitin-Dependent Recruitment (UDR) motif located close to the tandem Tudor domain (Fig. 1). Thus, the stable accumulation of 53BP1 at sites of DNA damage requires recognition of two histone modifications, one that is constitutive and one induced by DNA damage signaling. Consistent with this finding, a 53BP1 mutant with an inactive Tudor domain, while strongly diminished in its ability to localize to sites of DNA damage, can still bind near deprotected telomeres [29].
Finally, the accumulation of 53BP1 at sites of DNA damage is in part dependent on its oligomerization domain [30,31] (Fig. 1). Although an oligomerization deficient mutant of 53BP1 can accumulate fairly efficiently at dysfunctional telomeres, oligomerization does help in maximizing 53BP1 binding at DSBs [29,30]. The primary function of the oligomerization domain in chromatin binding may be to form either dimers or tetramers, since the domain can be replaced with ectopic dimerization and tetramerization motifs without compromising 53BP1 localization [28,31]. Moreover, a recombinant 53BP1 fragment spanning the oligomerization and Tudor domains eluted from a size-exclusion column with an apparent molecular weight suggestive of tetramers [31].
However, the studies discussed above do not take into account the observation that 53BP1 can form transient foci in cells lacking H2AX [32]. Furthermore, the c-NHEJ deficiencies in absence of 53BP1 (discussed below) are more pronounced than those found in the absence of H2AX, which is inconsistent with 53BP1 binding requiring H2AX phosphorylation [33–35]. Therefore it is likely that there is an H2AX-independent mechanism by which 53BP1 can accumulate at sites of DNA damage. Perhaps the Mre11-Rad50-Nbs1 (MRN) complex can provide the alternative means of 53BP1 recruitment, because the formation of 53BP1 foci in H2AX−/− cells is impaired when the MRN subunits (but not MDC1 or RNF8) are knocked down [36]. Indeed, 53BP1 has been shown to associate with Nbs1 [37]. The existence of H2AX-independent 53BP1 recruitment is further supported by the finding that H2AX is dispensable for formation of the 53BP1 containing OPT domains in G1 cells [38].
Interestingly, 53BP1 cannot form DNA damage foci in mitosis, despite normal accumulation of γH2AX, MDC1, and MRN [39,40]. This mitosis-specific exclusion of 53BP1 is likely caused by cell cycle-mediated changes in histone ubiquitylation. H2A is deubiquitylated upon mitotic entry [41] and DNA-damage dependent histone ubiquitylation in mitosis is not possible, because the recruitment of RNF8 and RNF168 is hindered [39]. The exact mechanism that blocks RNF8 binding to DNA damage sites in mitosis is unknown, but might involve specific posttranslational modifications [39]. In addition to the exclusion of 53BP1 from mitotic chromatin, 53BP1 is also negatively regulated in terms of how far it can spread from the site of DNA damage (see Text Box 2).
Text Box 2. Negative regulation of 53BP1 spreading.
There are several mechanisms that limit accumulation of 53BP1 and other DNA repair factors at sites of DNA damage, presumably preventing self-perpetuating activation of DNA damage checkpoints and excessive spreading of DNA repair factors to undamaged chromatin. Several deubiquitylating enzymes (DUBs) have been found to counteract DNA damage-dependent histone ubiquitylation and the assembly of 53BP1 as well as other repair factors, including BRCA1 at sites of DNA damage. These include BRCC36, USP3, POH1 and USP44 [104–107]. Another DUB, called OTUB1, restricts histone ubiquitylation and 53BP1 loading, but in a non-catalytic manner that involves binding to UBC13 and hindering the ubiquitin ligase activity of RNF8 and RNF168 [108,109]. USP16 and USP28 have been also shown to modulate the DNA damage response and repair pathways. USP16 mediates histone deubiquitylation-dependent transcription silencing at DSB sites [110], whereas USP28 possibly regulates 53BP1 stability [111]. Another mechanism that prevents excessive spreading of the DNA damage-induced ubiquitylation to loci distant from the DSB is catalyzed by the TRIP12 and UBR5 E3 ubiquitin ligases. These enzymes target RNF168 for degradation, effectively dampening the DNA damage response [112].
In addition, histone acetylation is thought to play a role in specifically inhibiting 53BP1 chromatin binding. The acetyltransferase Tip60/Kat5 can acetylate histone H4 on Lys 16, which interferes with the binding of 53BP1 to the nearby H4K20Me2 [16,113]. Importantly, H4K16 acetylation concomitantly increases BRCA1 recruitment to DNA damage foci and knockdown of Tip60/Kat5 or chemical inhibition of histone deacetylases (HDACs) can rescue the HR defect of BRCA1-deficient cells, similarly to the absence of 53BP1 [53,54,113]. The H4K16Ac histone mark might therefore be one of the determinants of the DSB repair pathway choice.
53BP1 stimulates c-NHEJ in specific contexts
Although 53BP1 was initially implicated in DNA damage signaling [42–44], this function is minor compared to its role in DSB repair, specifically in promoting Ku70/80- and DNA ligase IV-dependent c-NHEJ. The ability of 53BP1 to promote c-NHEJ is only apparent in certain settings including class switch recombination (CSR), V(D)J recombination, telomere dysfunction, BRCA1-deficient cells, and centromeric heterochromatin (Fig. 2).
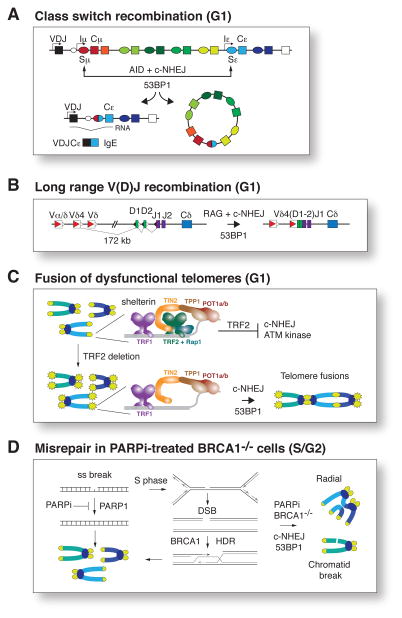
Figure 2.
53BP1 contributes to c-NHEJ in four contexts. 53BP1 is involved in the c-NHEJ in G1 in three specialized contexts: (A) CSR, (B) long range V(D)J recombination, and (C) the fusion of dysfunctional telomeres deprived of TRF2 protection. In (A) the Switch regions where AID induces DSBs are shown by ovals and their associated Constant regions by rectangles with the same color. Joining Sμ to Sε results in productive CSR, yielding IgE and a deleted segment (circle). Iμ and Iε are transcription start sites that promote switching. In (B), and example of long-range V(D)J recombination is given where RAG-dependent recombination joins a Variable (V) region to a D/J region 172 kb away (adapted from [47]). In (C), the shelterin complex at telomeres is shown before and after the deletion of TRF2, which results in ATM signaling and c-NHEJ of the dysfunctional telomeres in G1. These fusions are visualized as the chromosome-type fusions (drawn) in the following metaphase. In (D), the role of 53BP1 in BRCA1-deficient cells is shown. In S/G2, 53BP1 promotes the formation of mis-rejoined chromosome aberrations and inhibits HDR, leading to radial chromosomes and chromatid breaks as shown. This role of 53BP1 is only observed in BRCA1-deficient cells and is enhanced by treatment with PARP1 inhibitors, which lead to DSB formation in S/G2 (as depicted). Not shown is the fifth setting, DSB repair in heterochromatin, where 53BP1 acts through an unknown mechanism that involves its BRCT domains, which are not required for the processes shown in A–D.
The contribution of 53BP1 to c-NHEJ is apparent during CSR, a rearrangement involving c-NHEJ of AID-induced DSBs in B-cells, which is reduced approximately 5–20 fold when 53BP1 is absent [33,45] (Fig. 2A). Furthermore, in absence of 53BP1, the AID-induced breaks in the immunoglobulin switch regions often generate translocations rather than being joined correctly [46], indicating that 53BP1 affects the proper joining event rather than DSB formation. 53BP1 can also promote V(D)J recombination but this effect is only observed when the joining events involve RAG cleavage sites that are at a considerable distance [47,48] (Fig. 2B). These findings are consistent with an earlier report showing that 53BP1−/− chicken DT40 cells have a deficiency in c-NHEJ [49] and with the observation that a dominant negative fragment of 53BP1 can inhibit NHEJ and stimulate HDR as measured using I-SceI reporter constructs [50]. However, a second report on DT40 KO cells placed 53BP1 outside the Ku70/80-dependent DSB repair [51]. Thus, the role of 53BP1 in c-NHEJ can be detected in the context of DSB formation occurring in lymphocyte development and, to a lesser extent, during repair of exogenous DSBs.
In the context of telomere dysfunction, c-NHEJ appears almost completely dependent on 53BP1 (Fig. 2C). The system in which this critical contribution of 53BP1 was revealed involves deletion of the shelterin protein TRF2 from mouse cells. The resulting deprotected telomeres activate the ATM kinase signaling cascade, become coated with 53BP1, and are processed by c-NHEJ [52]. Because the telomere fusions primarily occur in G1 and are duplicated in the following S phase, the resulting metaphase spreads show chromosomes that are fused at both chromatids (Fig. 2C). When 53BP1 is absent, the rate of these telomere fusions is reduced by approximately 100-fold [34].
Furthermore, 53BP1 is responsible for the formation of toxic chromosome aberrations when BRCA1-deficient cells experience DSBs in S phase [53–55] (Fig. 2D). Absence of the hereditary breast and ovarian cancer predisposition gene BRCA1 (Breast cancer 1, early onset) results in 5–6 fold reduction in HDR and a deficiency in Rad51 focus formation [56–60]. Their HDR defect sensitizes BRCA1-deficient cells to inhibitors of the poly(ADP-ribose) polymerase PARP1. PARP1 inhibitors (PARPi) are therefore thought to be of potential use for the treatment of BRCA1 (or BRCA2) negative breast and ovarian cancers ([61,62] reviewed in [63]). The basis for the effect of PARPi is that upon inhibition of PARP1, unrepaired single-stranded nicks are converted into DSBs in S phase and then require BRCA1-dependent HDR for repair (Fig. 2). In absence of BRCA1, DSB repair is inappropriately channeled towards NHEJ, forming radial chromosomes and other lethal chromosome aberrations. Absence of 53BP1 rescues embryonic lethality, DNA damage sensitivity, PARPi sensitivity, and formation of PARPi-induced lethal chromosomal aberrations associated with BRCA1 deficiency [53–55]. In PARPi-treated BRCA1-deficient cells, 53BP1 appears to promote c-NHEJ-dependent (mis−) rejoining of the unrepaired DSBs. Consistent with this, loss of 53BP1 causes resistance to PARPi treatment in BRCA1-deficient mouse mammary tumors [64] and 53BP1 deficiency is positively correlated with triple negative status and poor survival in breast cancer patients [54].
53BP1 also plays a role in the repair of DSB breaks occurring in centromeric heterochromatin. These sites of DNA damage are repaired more slowly than euchromatic DSBs, resulting in the delayed disappearance of heterochromatic γ-H2AX foci (referred to as the ‘late phase’ of repair) [1,65]. In the absence of 53BP1, this type of repair is severely impaired. Unlike its effect in the other c-NHEJ processes, 53BP1 requires its BRCT domains (see Fig. 1) to enhance heterochromatic DSB repair [65,66]. The current model of 53BP1 action in heterochromatin involves its ability to promote the phosphorylation of KAP-1 by the ATM kinase, which is needed for the repair of heterochromatic DSBs [67]. In addition, the role of 53BP1 at heterochromatic DSBs may involve the chromatin decondensation activity of EXPAND1/MUM1, which is recruited to DSBs by the BRCT domain of 53BP1 [37]. In conclusion, the c-NHEJ events catalyzed by 53BP1 are diverse and involve physiological processes such as productive CSR or heterochromatic DSB repair on one hand, as well as pathological end-joining of deprotected telomeres and DSBs in BRCA1-deficient cells on the other.
53BP1 promotes c-NHEJ by blocking 5′ resection
The ability of 53BP1 to promote c-NHEJ is in part explained by its ability to block the 5′ end resection at DSBs (Fig. 3). Whereas DSB resection is required for the initiation of HDR, it is not needed for c-NHEJ and will most likely impede the engagement of Ku70/80. Exposed DNA ends can undergo 5′ end resection by the Exo1 exonuclease and the combined action of the BLM RecQ helicase and the DNA2 endonuclease (reviewed in [68]). This process requires an initial cleavage step of poorly defined nature that involves CtIP and the MRN complex [69].
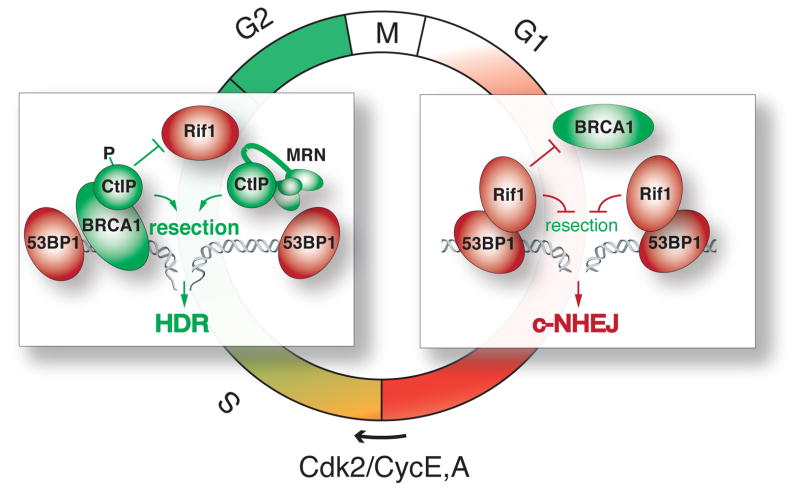
Figure 3.
Cell cycle-dependent mutual exclusion of BRCA1 and Rif1 from DSBs. In G1, Rif1 prevents the accumulation of BRCA1 at DSBs and acts downstream of 53BP1 to block resection, thereby promoting c-NHEJ. In S/G2, phosphorylated CtIP blocks Rif1 from binding to 53BP1, thereby allowing resection and promoting HDR. CtIP also plays a role in promoting resection but this attribute appears to be independent of its binding to BRCA1.
Resection at DSBs is promoted by the ATM kinase and likely involves BRCA1, which forms a complex with CtIP and MRN in S phase (the BRCA1 C complex) and ubiquitylates CtIP [70,71]. Indeed, in the absence of BRCA1, resection at DSBs is impaired and the Rad51 recombinase fails to form foci [57,72,73], the latter explaining the HDR defect in BRCA1-deficient cells [56,60]. However, these phenotypes are not due to the absence of the BRCA1 C complex alone since CtIP mutant mouse cells, in which the interaction with BRCA1 is disrupted, show normal resection at DSBs and do not recapitulate the BRCA1 HDR defect [74]. A more likely explanation was recently deduced from the observation (discussed below) that BRCA1 promotes resection by inhibiting the accumulation of the 53BP1 partner Rif1 at sites of DNA damage in S/G2.
Recent experiments revealed that 53BP1 can block the CtIP-dependent step in DSB resection. When 53BP1 is removed from BRCA1-deficient cells, their ability to execute 5′ end resection is restored, as determined from accumulation of the trimeric single-stranded DNA binding protein RPA (Replication Protein A) at sites of DNA damage and the phosphorylation of the RPA32 subunit [53]. Furthermore, Rad51 foci form and HDR, measured using a I-SceI-GFP reporter, improves in the 53BP1/BRCA1 double knockout cells, while the formation of toxic mis-rejoined chromosomes is reduced [53]. Deletion of DNA ligase IV does not rescue the defect in IR-induced RPA32 phosphorylation in BRCA1-deficient cells, suggesting that the lack of c-NHEJ per se is not responsible for the restoration of resection in the absence of 53BP1. 53BP1 was also shown to block resection in two other specialized NHEJ events: Long range V(D)J recombination and in an engineered CSR system employing two I-SceI induced DSBs in the murine IgH locus [47,48,53]. Also in these settings, absence of 53BP1 leads to increased ATM- and CtIP-dependent nucleolytic processing of the DNA ends before they are joined.
Dysfunctional telomeres have provided the opportunity to directly observe 5′ resection, which results in an extended 3′ overhang at the telomere terminus [29,75,76]. When TRF2 is removed from cells that cannot execute c-NHEJ, the terminal structure of the dysfunctional telomeres remains largely unaltered. In contrast, removal of TRF2 from 53BP1 null cells renders telomeres sensitive to 5′ end resection and the amount of ssDNA at the telomere end is doubled [29,75]. This resection is abrogated by inhibition of ATM signaling or depletion of CtIP [29]. When the whole shelterin complex is removed from 53BP1 null cells, the telomere terminus undergoes more extensive resection, leading to a 5–15 fold increase of the ssDNA [76]. As in the case of the TRF2 deletion, resection at the shelterin-free telomeres is mediated by CtIP and in addition was shown to involve BLM and Exo1. It is therefore now clear that the 53BP1-dependent resection block occurs in each context where 53BP1 promotes c-NHEJ.
53BP1 recruits Rif1 to block 5′ end resection
In each setting – telomere fusion, CSR, and events in BRCA1-deficient cells – 53BP1 can only repress 5′ end resection when it retains its N-terminal S/TQ sites, which are targets for the ATM and ATR kinases [29,77] (see Fig. 1). The 53BP1 ST/Q sites are not required for localization of 53BP1 to sites of DNA damage but mediate interactions with critical effectors of 53BP1 function.
Rif1, the mammalian ortholog of the yeast Rap1-interacting factor 1, is the first factor identified to block resection downstream of 53BP1 (Fig. 1). Rif1 plays diverse roles in DNA metabolism in yeast and mammals (reviewed in [78,79]). Although it is a diverged ortholog of a yeast telomere binding protein, mammalian Rif1 is not part of the telomeric complex [80–82]. Instead, it is recruited to DNA damage foci induced by various insults, including irradiation, replication stress, and telomere deprotection [81–83]. This recruitment is strictly dependent on ATM (or ATR) signaling and on phosphorylation of a subset of 53BP1’s ST/Q sites(Fig. 1) [75,82,84–87]. The accumulation of Rif1 at sites of DNA damage is more strongly influenced by ATM signaling levels than that of 53BP1 [82,84,86], consistent with Rif1 requiring ATM-mediated phosphorylation of 53BP1 for its localization. Rif1 does not contain known phosphopeptide-binding motifs, so it is possible that an unknown factor mediates its interaction with 53BP1.
In CSR, a process that requires a block to resection in G1, Rif1 deficiency results in the same phenotypes as absence of 53BP1 – increased resection and diminished productive end joining [84,86,87]. In the context of genome wide DSBs, absence of Rif1 results in diminished cell survival [82,85,86], impaired DSB repair as gauged based on the disappearance of γ-H2AX foci, a failure in c-NHEJ [84,86], and increased accumulation of RPA [75,84–86]. Comparison of the effects of Rif1−/−, 53BP1−/−, and Rif1−/−53BP1−/− MEFs indicates that Rif1 and 53BP1 are epistatic with regard to the repression of resection at DSBs and suggest that Rif1 is the main effector of this 53BP1 function [75,86]. Similarly, in the context of telomere dysfunction induced by deletion of TRF2, Rif1 appears to be the only component downstream of 53BP1 needed to block resection since the increase in telomeric ssDNA is the same in Rif1−/− and 53BP1−/− cells [75]. However, the rate at which the dysfunctional telomeres join is higher in Rif1−/− cells compared to 53BP1−/− cells [75] suggesting that another factor acts downstream of 53BP1 to stimulate c-NHEJ of dysfunctional telomeres. Additionally, loss of Rif1 diminishes the formation of mis-rejoined chromosomes after PARP1 inhibition in BRCA1-deficient cells [75,86]. The effect of Rif1 deficiency in this context is less than in 53BP1 null cells, suggesting again, that another downstream factor acts with 53BP1 [75]. In contrast Rif1 appears to be the only partner of 53BP1 required for CSR [86, 87].
How Rif1 acts is currently unknown. It can interact with the BLM helicase [88], harbors a protein-phosphatase 1 binding motif [89] and binds to ssDNA in vitro [88] (see Fig. 1), but the relevance of these attributes to Rif1’s ability to block resection is unclear. Rif1 has a large N-terminal domain, composed of HEAT/Armadillo-like repeats [88]. This domain is required for localization of Rif1 to DNA damage foci [84], but could also potentially allow Rif1 to recruit a critical factor needed to control resection.
Cell cycle regulated mutual exclusion of BRCA1 and Rif1
The ability of 53BP1/Rif1 to inhibit DSB resection in BRCA1-deficient cells is counterintuitive since it takes place in S/G2, when resection-dependent HDR is the predominant DSB repair pathway [68]. However, the ability of Rif1 to block end-resection in G1, at dysfunctional telomeres and in CSR, is appropriate since G1 DSBs are preferentially repaired by c-NHEJ. Thus, in wild type cells, Rif1 must be regulated to block resection in G1 but not in S/G2 and this regulation is likely altered in BRCA1-deficient cells.
How the inhibition of Rif1 in S/G2 is achieved and why it is lost in BRCA1-deficient cells is clear from the observation that BRCA1 and CtIP regulate the accumulation of Rif1 at DSBs (Fig. 3). In S/G2, but not in G1, BRCA1 strongly diminishes the presence of Rif1 at DSBs [84–86]. In contrast, BRCA1 only has a mild effect on 53BP1 focus formation [90]. The effect of BRCA1 on Rif1 is dependent on CtIP, whose depletion also results in greater accumulation of Rif1 at DSBs in S/G2 [84]. The CDK (cyclin dependent kinase)-dependent phosphorylation of T847 on CtIP is required for its ability to affect Rif1, explaining the S/G2 specific repression of Rif1 focus formation [84]. Thus, the repression of end-resection in BRCA1-deficient cells is likely due to inappropriate accumulation of Rif1 at DSBs in S/G2 phase.
Conversely, Rif1 blocks BRCA1 from accumulating at sites of DNA damage in G1 [75,84,85] (Fig. 3). Although BRCA1 was originally thought to be absent from G1 cells, it is actually present but kept from accumulating at sites of DNA damage. When either 53BP1 or Rif1 is deleted, BRCA1 is able to form foci at DSBs in G1. This mutual exclusion between BRCA1 and 53BP1/Rif1 and the CDK regulated switch from dominant 53BP1/Rif1 to BRCA1/CtIP ensures that DSBs are shuttled towards c-NHEJ in G1 and preferentially repaired by HDR in S/G2 (Fig. 3).
53PB1-mediated chromatin movement and synapsis
Compared to Rif1, 53BP1 has a much greater effect on DSB repair, fusion of dysfunctional telomeres, and formation of radial chromosomes in PARPi treated BRCA1−/− cells [75,85,87]. One possibility is that there is a second factor that helps repress resection, such as Pax transactivation domain-interacting protein (PTIP) (see below). However, at least in the context of telomeres, this explanation does not hold since the block to resection by 53BP1 can be fully explained based on the action of Rif1 [75].
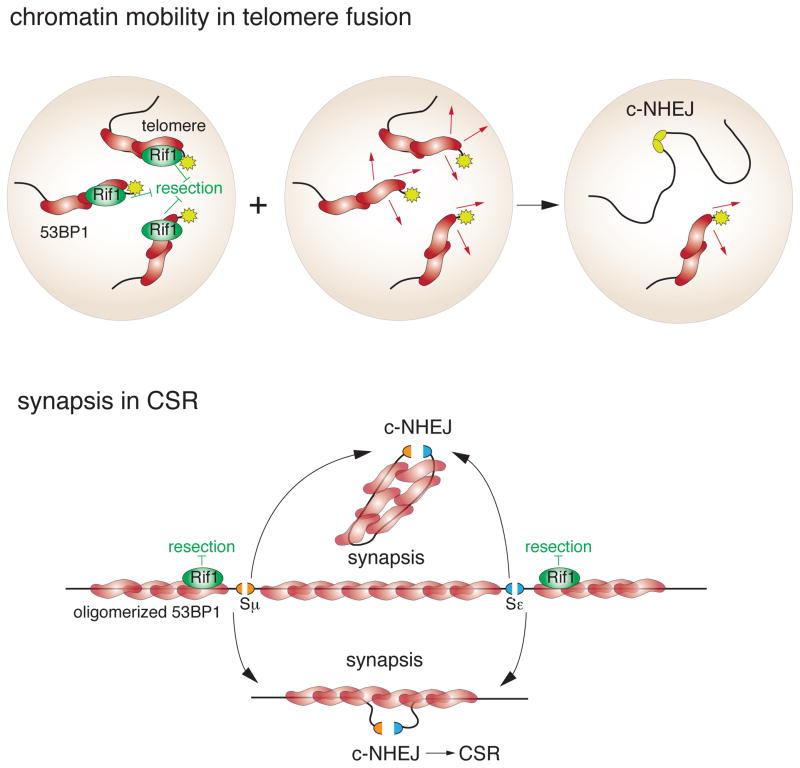
A more likely explanation is found in the effect of 53BP1 on telomere mobility (Fig. 4). After telomeres become deprotected through deletion of TRF2, the mobility of the dysfunctional telomeres increases and the telomeres sample greater territories in the nucleus [29,34]. However, when 53BP1 is absent the dysfunctional telomeres are much less mobile. In contrast, Rif1 has no effect on this process [75]. The increased mobility of the damaged telomeres can explain why 53BP1 stimulates c-NHEJ because the fusion of telomeres takes place in G1 when the telomeres are not in close proximity and require movement to find a fusion partner. How 53BP1 promotes telomere mobility is of obvious interest. Domain analysis has shown that the BRCT, GAR, and the dynein LC8 light chain interface are dispensable for this process and the requirement for oligomerization is minimal [29]. However, the N-terminal ST/Q sites are strictly required, suggesting that an interacting factor (other than Rif1) is at work [29].
Figure 4. 53BP1 stimulates chromatin mobility and mediates synapsis.
Top: 53BP1 is required for the increased mobility of dysfunctional telomeres. Rif1 inhibits resection at the dysfunctional telomeres while a Rif1-independent 53BP1 function promotes the mobility of the damaged telomeric chromatin. Both processes are thought to enhance the c-NHEJ of dysfunctional telomeres in G1. Bottom: 53BP1 is proposed to mediate synapsis of distal DNA ends during CSR (shown) and long-range V(D)J (not shown). Oligomerization of 53BP1 is required for this function. 53BP1 may act by synapsis of the intervening DNA fragment thereby removing the competing DNA ends and promoting productive CSR. Alternatively, 53BP1 may be acting by bringing the DNA ends together that will lead to productive CSR.
In the context of CSR and long range V(D)J, it was proposed that 53BP1 mediates the synapsis of the distal DNA ends involved in the recombination [47] (Fig. 4). How synapsis works is not clear but one possibility proffered here (Fig. 4) is that the 53BP1 covered domain in between the two AID-induced breaks is altered in such a way that its DNA ends no longer compete for joining with the other two ends. Consistent with the synapsis proposal, CSR is severely impaired if 53BP1 lacks its ability to oligomerize [77]. Given that the oligomerization domain has a minimal effect on the ability of 53BP1 to alter telomere movements, the simplest explanation is that synapsis and the mobility of dysfunctional telomeres differ mechanistically [29].
In summary, 53BP1 can aid c-NHEJ in CSR, long range V(D)J, and at dysfunctional telomeres by regulating long-range chromatin alterations. However, it seems unlikely that 53BP1 has evolved its ability to promote synapsis of distal DNA ends for the purpose of CSR and similarly, the 53BP1-mediated chromatin mobility is unlikely to be selected for to allow dysfunctional telomeres to fuse. It will therefore be important to understand how these long-range effects of 53BP1 contribute to ‘normal’ DSB repair and how the induction of chromatin mobility and DNA end synapsis impact the formation of radial chromosomes in PARPi-treated BRCA1-deficient cells.
Potential roles for the 53BP1 binding partner PTIP
Recently, a second 53BP1 interacting protein, PTIP, has been invoked in the control of c-NHEJ. PTIP is involved in the regulation of transcription and DNA repair ([91–95]; reviewed in [96]). Like Rif1, PTIP relies on the phosphorylation of the N-terminal ST/Q sites to bind to 53BP1 [91–93,97], but its binding interface is distinct from that of Rif1 [91] (Fig. 1). Mutation of the PTIP binding sites of 53BP1 does not affect CSR, but abrogates illicit NHEJ in BRCA1-deficient cells treated with PARPi [91]. Deletion of PTIP recapitulates these phenotypes and results in elevated Rad51 and RPA foci formation, suggesting increased 5′ end resection [91]. Although the lack of an effect of PTIP on CSR might suggest that PTIP, unlike Rif1, can only act in S/G2, another G1 c-NHEJ event, the fusion of dysfunctional telomeres, is also diminished in PTIP−/− cells, which is not consistent with an S/G2 specific role of PTIP.
A challenge in evaluating the role of PTIP is that the interplay between 53BP1 and PTIP may be complex. Although a recent study showed that the accumulation of PTIP at DNA damage foci is severely diminished when 53BP1 is absent [91], earlier work indicated the independent localization of 53BP1 and PTIP to damaged chromatin [92–94] and in one study, the conditional knockout of PTIP completely abolished 53BP1 focus formation [98].
Another point of interest is why deletion of PTIP completely abolishes radial formation in PARPi-treated BRCA1−/− cells since this would argue that PTIP is the only factor downstream of 53BP1 in this pathway [91]. Yet deletion of Rif1, which presumably acts independently of PTIP, also diminishes radial formation in this setting [75,86]. It will also be important to determine why the resection of DSBs in G1, unleashed in the absence of PTIP, does not affect CSR. A detailed epistasis analysis of the relationships between 53BP1, Rif1, and PTIP will be useful to assess the individual contributions of these factors to NHEJ in different contexts.
Concluding remarks
53BP1 has received much attention because of its role in the choice between c-NHEJ and HDR and its relevance to the treatment of BRCA1 negative breast cancer. Important questions remain, however. We do not understand how 53BP1 binds Rif1 and how this affects resection. We also do not know how and where 53BP1 mediates synapsis, through what mechanism 53BP1 induces chromatin mobility, and what the role is of these phenomena in general DSB repair. In addition, the interplay between 53BP1, Rif1, and PTIP requires further clarification. Finally, much needs to be learned about the mechanism by which BRCA1 and Rif1 exclude each other from sites of DNA damage. Clearly, work on 53BP1 and its binding partners will continue to be a fertile ground for new discoveries.
Highlights.
53BP1 regulates the choice between two major double strand break repair pathways
53BP1 binds damaged chromatin through multiple histone modifications
53BP1 promotes classical non-homologous end joining in various specialized contexts
53BP1 blocks 5′ end resection and facilitates mobility and synapsis of chromatin
53BP1 recruits interacting partners Rif1 and PTIP to block resection
Acknowledgments
We thank Simon Boulton for discussion and remarkable collegiality during the course of our work on Rif1. We thank the de Lange lab for comments on this review. Our work on telomeres is supported by grants from the NIH (5R37GM49046 and 5RO1AG16642). The work on Rif1 was made possible by generous support from the Breast Cancer Research Foundation. TdL is an American Cancer Society Research Professor. MZ is supported by a Brno PhD talent fellowship and by a grant from the Czech Science Foundation to Dr. Ctirad Hofr (P205/12/0550).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Noon AT, Goodarzi AA. 53BP1-mediated DNA double strand break repair: insert bad pun here. DNA Repair (Amst) 2011;10:1071–1076. doi: 10.1016/j.dnarep.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Adams MM, Carpenter PB. Tying the loose ends together in DNA double strand break repair with 53BP1. Cell Div. 2006;1:19. doi: 10.1186/1747-1028-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultz LB, et al. p53 binding protein 1 (53BP1) is an early participant in the cellular response to DNA double-strand breaks. J Cell Biol. 2000;151:1381–1390. doi: 10.1083/jcb.151.7.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rappold I, et al. Tumor suppressor p53 binding protein 1 (53BP1) is involved in DNA damage-signaling pathways. J Cell Biol. 2001;153:613–620. doi: 10.1083/jcb.153.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson L, Henderson C, Adachi Y. Phosphorylation and rapid relocalization of 53BP1 to nuclear foci upon DNA damage. Mol Cell Biol. 2001;21:1719–1729. doi: 10.1128/MCB.21.5.1719-1729.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Curr Biol. 2003;13:1549–1556. doi: 10.1016/s0960-9822(03)00542-6. [DOI] [PubMed] [Google Scholar]
- 7.Denchi EL, de Lange T. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. 2007;448:1068–1071. doi: 10.1038/nature06065. [DOI] [PubMed] [Google Scholar]
- 8.Celli GB, de Lange T. DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion. Nat Cell Biol. 2005;7:712–718. doi: 10.1038/ncb1275. [DOI] [PubMed] [Google Scholar]
- 9.Takai KK, et al. Telomere protection by TPP1/POT1 requires tethering to TIN2. Mol Cell. 2011;44:647–659. doi: 10.1016/j.molcel.2011.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrigan JA, et al. Replication stress induces 53BP1-containing OPT domains in G1 cells. J Cell Biol. 2011;193:97–108. doi: 10.1083/jcb.201011083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lukas C, et al. 53BP1 nuclear bodies form around DNA lesions generated by mitotic transmission of chromosomes under replication stress. Nat Cell Biol. 2011;13:243–253. doi: 10.1038/ncb2201. [DOI] [PubMed] [Google Scholar]
- 12.Botuyan MV, et al. Structural basis for the methylation state-specific recognition of histone H4-K20 by 53BP1 and Crb2 in DNA repair. 2006;127:1361–1373. doi: 10.1016/j.cell.2006.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pei H, et al. MMSET regulates histone H4K20 methylation and 53BP1 accumulation at DNA damage sites. 2011;470:124–128. doi: 10.1038/nature09658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hajdu I, et al. Wolf-Hirschhorn syndrome candidate 1 is involved in the cellular response to DNA damage. Proc Natl Acad Sci U S A. 2011;108:13130–13134. doi: 10.1073/pnas.1110081108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartlerode AJ, et al. Impact of histone H4 lysine 20 methylation on 53BP1 responses to chromosomal double strand breaks. 2012;7:e49211. doi: 10.1371/journal.pone.0049211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsiao KY, Mizzen CA. Histone H4 deacetylation facilitates 53BP1 DNA damage signaling and double-strand break repair. J Mol Cell Biol. 2013;5:157–165. doi: 10.1093/jmcb/mjs066. [DOI] [PubMed] [Google Scholar]
- 17.Doil C, et al. RNF168 binds and amplifies ubiquitin conjugates on damaged chromosomes to allow accumulation of repair proteins. 2009;136:435–446. doi: 10.1016/j.cell.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 18.Stewart GS, et al. The RIDDLE syndrome protein mediates a ubiquitin-dependent signaling cascade at sites of DNA damage. 2009;136:420–434. doi: 10.1016/j.cell.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 19.Mailand N, et al. RNF8 ubiquitylates histones at DNA double-strand breaks and promotes assembly of repair proteins. 2007;131:887–900. doi: 10.1016/j.cell.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 20.Moudry P, et al. Ubiquitin-activating enzyme UBA1 is required for cellular response to DNA damage. Cell Cycle. 2012;11:1573–1582. doi: 10.4161/cc.19978. [DOI] [PubMed] [Google Scholar]
- 21.Huen MS, et al. RNF8 transduces the DNA-damage signal via histone ubiquitylation and checkpoint protein assembly. 2007;131:901–914. doi: 10.1016/j.cell.2007.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolas NK, et al. Orchestration of the DNA-damage response by the RNF8 ubiquitin ligase. 2007;318:1637–1640. doi: 10.1126/science.1150034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattiroli F, et al. RNF168 ubiquitinates K13–15 on H2A/H2AX to drive DNA damage signaling. 2012;150:1182–1195. doi: 10.1016/j.cell.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Sanders SL, et al. Methylation of histone H4 lysine 20 controls recruitment of Crb2 to sites of DNA damage. 2004;119:603–614. doi: 10.1016/j.cell.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Mallette FA, et al. RNF8- and RNF168-dependent degradation of KDM4A/JMJD2A triggers 53BP1 recruitment to DNA damage sites. EMBO J. 2012;31:1865–1878. doi: 10.1038/emboj.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meerang M, et al. The ubiquitin-selective segregase VCP/p97 orchestrates the response to DNA double-strand breaks. Nat Cell Biol. 2011;13:1376–1382. doi: 10.1038/ncb2367. [DOI] [PubMed] [Google Scholar]
- 27.Acs K, et al. The AAA-ATPase VCP/p97 promotes 53BP1 recruitment by removing L3MBTL1 from DNA double-strand breaks. Nat Struct Mol Biol. 2011;18:1345–1350. doi: 10.1038/nsmb.2188. [DOI] [PubMed] [Google Scholar]
- 28.Fradet-Turcotte A, et al. 53BP1 is a reader of the DNA-damage-induced H2A Lys 15 ubiquitin mark. 2013;499:50–54. doi: 10.1038/nature12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lottersberger F, et al. Role of 53BP1 oligomerization in regulating double-strand break repair. Proc Natl Acad Sci U S A. 2013;110:2146–2151. doi: 10.1073/pnas.1222617110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward I, et al. The tandem BRCT domain of 53BP1 is not required for its repair function. J Biol Chem. 2006;281:38472–38477. doi: 10.1074/jbc.M607577200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zgheib O, et al. An oligomerized 53BP1 tudor domain suffices for recognition of DNA double-strand breaks. Mol Cell Biol. 2009;29:1050–1058. doi: 10.1128/MCB.01011-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Celeste A, et al. Histone H2AX phosphorylation is dispensable for the initial recognition of DNA breaks. Nat Cell Biol. 2003;5:675–679. doi: 10.1038/ncb1004. [DOI] [PubMed] [Google Scholar]
- 33.Ward IM, et al. 53BP1 is required for class switch recombination. J Cell Biol. 2004;165:459–464. doi: 10.1083/jcb.200403021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dimitrova N, et al. 53BP1 promotes non-homologous end joining of telomeres by increasing chromatin mobility. 2008;456:524–528. doi: 10.1038/nature07433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dimitrova N, de Lange T. Cell cycle dependent role of MRN at dysfunctional telomeres: ATM signaling-dependent induction of NHEJ in G1 and resection-mediated inhibition of NHEJ in G2. Mol Cell Biol. 2009;29:5552–5563. doi: 10.1128/MCB.00476-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan J, Chen J. MRE11-RAD50-NBS1 complex dictates DNA repair independent of H2AX. J Biol Chem. 2010;285:1097–1104. doi: 10.1074/jbc.M109.078436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huen MS, et al. Regulation of chromatin architecture by the PWWP domain-containing DNA damage-responsive factor EXPAND1/MUM1. Mol Cell. 2010;37:854–864. doi: 10.1016/j.molcel.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen WT, et al. Systematic identification of functional residues in mammalian histone H2AX. Mol Cell Biol. 2013;33:111–126. doi: 10.1128/MCB.01024-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giunta S, Belotserkovskaya R, Jackson SP. DNA damage signaling in response to double-strand breaks during mitosis. J Cell Biol. 2010;190:197–207. doi: 10.1083/jcb.200911156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nelson G, Buhmann M, von Zglinicki T. DNA damage foci in mitosis are devoid of 53BP1. Cell Cycle. 2009;8:3379–3383. doi: 10.4161/cc.8.20.9857. [DOI] [PubMed] [Google Scholar]
- 41.Joo HY, et al. Regulation of cell cycle progression and gene expression by H2A deubiquitination. 2007;449:1068–1072. doi: 10.1038/nature06256. [DOI] [PubMed] [Google Scholar]
- 42.DiTullio RA, Jr, et al. 53BP1 functions in an ATM-dependent checkpoint pathway that is constitutively activated in human cancer. Nat Cell Biol. 2002;4:998–1002. doi: 10.1038/ncb892. [DOI] [PubMed] [Google Scholar]
- 43.Wang B, et al. 53BP1, a mediator of the DNA damage checkpoint. 2002;298:1435–1438. doi: 10.1126/science.1076182. [DOI] [PubMed] [Google Scholar]
- 44.Fernandez-Capetillo O, et al. DNA damage-induced G2-M checkpoint activation by histone H2AX and 53BP1. Nat Cell Biol. 2002;4:993–997. doi: 10.1038/ncb884. [DOI] [PubMed] [Google Scholar]
- 45.Manis JP, et al. 53BP1 links DNA damage-response pathways to immunoglobulin heavy chain class-switch recombination. Nat Immunol. 2004;5:481–487. doi: 10.1038/ni1067. [DOI] [PubMed] [Google Scholar]
- 46.Franco S, et al. H2AX prevents DNA breaks from progressing to chromosome breaks and translocations. Mol Cell. 2006;21:201–214. doi: 10.1016/j.molcel.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 47.Difilippantonio S, et al. 53BP1 facilitates long-range DNA end-joining during V(D)J recombination. 2008;456:529–533. doi: 10.1038/nature07476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bothmer A, et al. 53BP1 regulates DNA resection and the choice between classical and alternative end joining during class switch recombination. J Exp Med. 2010;207:855–865. doi: 10.1084/jem.20100244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakamura K, et al. Genetic dissection of vertebrate 53BP1: a major role in non-homologous end joining of DNA double strand breaks. DNA Repair (Amst) 2006;5:741–749. doi: 10.1016/j.dnarep.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 50.Xie A, et al. Distinct roles of chromatin-associated proteins MDC1 and 53BP1 in mammalian double-strand break repair. Mol Cell. 2007;28:1045–1057. doi: 10.1016/j.molcel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iwabuchi K, et al. 53BP1 contributes to survival of cells irradiated with X-ray during G1 without Ku70 or Artemis. Genes Cells. 2006;11:935–948. doi: 10.1111/j.1365-2443.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 52.Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 53.Bunting SF, et al. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. 2010;141:243–254. doi: 10.1016/j.cell.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bouwman P, et al. 53BP1 loss rescues BRCA1 deficiency and is associated with triple-negative and BRCA-mutated breast cancers. Nat Struct Mol Biol. 2010;17:688–695. doi: 10.1038/nsmb.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cao L, et al. A selective requirement for 53BP1 in the biological response to genomic instability induced by Brca1 deficiency. Mol Cell. 2009;35:534–541. doi: 10.1016/j.molcel.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moynahan ME, et al. Brca1 controls homology-directed DNA repair. Mol Cell. 1999;4:511–518. doi: 10.1016/s1097-2765(00)80202-6. [DOI] [PubMed] [Google Scholar]
- 57.Bhattacharyya A, et al. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin. J Biol Chem. 2000;275:23899–23903. doi: 10.1074/jbc.C000276200. [DOI] [PubMed] [Google Scholar]
- 58.Scully R, et al. Dynamic changes of BRCA1 subnuclear location and phosphorylation state are initiated by DNA damage. 1997;90:425–435. doi: 10.1016/s0092-8674(00)80503-6. [DOI] [PubMed] [Google Scholar]
- 59.Chen JJ, et al. BRCA1, BRCA2, and Rad51 operate in a common DNA damage response pathway. Cancer Res. 1999;59:1752s–1756s. [PubMed] [Google Scholar]
- 60.Snouwaert JN, et al. BRCA1 deficient embryonic stem cells display a decreased homologous recombination frequency and an increased frequency of non-homologous recombination that is corrected by expression of a brca1 transgene. 1999;18:7900–7907. doi: 10.1038/sj.onc.1203334. [DOI] [PubMed] [Google Scholar]
- 61.Farmer H, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 62.Bryant HE, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 63.Helleday T. DNA repair as treatment target. Eur J Cancer. 2011;47(Suppl 3):S333–S335. doi: 10.1016/S0959-8049(11)70192-7. [DOI] [PubMed] [Google Scholar]
- 64.Jaspers JE, et al. Loss of 53BP1 causes PARP inhibitor resistance in Brca1-mutated mouse mammary tumors. Cancer Discov. 2013;3:68–81. doi: 10.1158/2159-8290.CD-12-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Noon AT, et al. 53BP1-dependent robust localized KAP-1 phosphorylation is essential for heterochromatic DNA double-strand break repair. Nat Cell Biol. 2010;12:177–184. doi: 10.1038/ncb2017. [DOI] [PubMed] [Google Scholar]
- 66.Lee JH, et al. 53BP1 promotes ATM activity through direct interactions with the MRN complex. EMBO J. 2010;29:574–585. doi: 10.1038/emboj.2009.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goodarzi AA, et al. ATM signaling facilitates repair of DNA double-strand breaks associated with heterochromatin. Mol Cell. 2008;31:167–177. doi: 10.1016/j.molcel.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 68.Symington LS, Gautier J. Double-strand break end resection and repair pathway choice. Annu Rev Genet. 2011;45:247–271. doi: 10.1146/annurev-genet-110410-132435. [DOI] [PubMed] [Google Scholar]
- 69.Sartori AA, et al. Human CtIP promotes DNA end resection. 2007;450:509–514. doi: 10.1038/nature06337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu X, et al. BRCA1 ubiquitinates its phosphorylation-dependent binding partner CtIP. Genes Dev. 2006;20:1721–1726. doi: 10.1101/gad.1431006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen L, et al. Cell cycle-dependent complex formation of BRCA1.CtIP.MRN is important for DNA double-strand break repair. J Biol Chem. 2008;283:7713–7720. doi: 10.1074/jbc.M710245200. [DOI] [PubMed] [Google Scholar]
- 72.Huber LJ, et al. Impaired DNA damage response in cells expressing an exon 11-deleted murine Brca1 variant that localizes to nuclear foci. Mol Cell Biol. 2001;21:4005–4015. doi: 10.1128/MCB.21.12.4005-4015.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schlegel BP, Jodelka FM, Nunez R. BRCA1 promotes induction of ssDNA by ionizing radiation. Cancer Res. 2006;66:5181–5189. doi: 10.1158/0008-5472.CAN-05-3209. [DOI] [PubMed] [Google Scholar]
- 74.Reczek CR, et al. The interaction between CtIP and BRCA1 is not essential for resection-mediated DNA repair or tumor suppression. J Cell Biol. 2013;201:693–707. doi: 10.1083/jcb.201302145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zimmermann M, et al. 53BP1 regulates DSB repair using Rif1 to control 5′ end resection. 2013;339:700–704. doi: 10.1126/science.1231573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sfeir A, de Lange T. Removal of shelterin reveals the telomere end-protection problem. 2012;336:593–597. doi: 10.1126/science.1218498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bothmer A, et al. Regulation of DNA end joining, resection, and immunoglobulin class switch recombination by 53BP1. Mol Cell. 2011;42:319–329. doi: 10.1016/j.molcel.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Buonomo SB. Heterochromatin DNA replication and Rif1. Exp Cell Res. 2010;316:1907–1913. doi: 10.1016/j.yexcr.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 79.Yamazaki S, Hayano M, Masai H. Replication timing regulation of eukaryotic replicons: Rif1 as a global regulator of replication timing. Trends Genet. 2013 doi: 10.1016/j.tig.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 80.Hardy CF, Sussel L, Shore D. A RAP1-interacting protein involved in transcriptional silencing and telomere length regulation. Genes Dev. 1992;6:801–814. doi: 10.1101/gad.6.5.801. [DOI] [PubMed] [Google Scholar]
- 81.Xu L, Blackburn EH. Human Rif1 protein binds aberrant telomeres and aligns along anaphase midzone microtubules. J Cell Biol. 2004;167:819–830. doi: 10.1083/jcb.200408181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Silverman J, et al. Human Rif1, ortholog of a yeast telomeric protein, is regulated by ATM and 53BP1 and functions in the S-phase checkpoint. Genes Dev. 2004;18:2108–2119. doi: 10.1101/gad.1216004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Buonomo S, et al. Mammalian Rif1 contributes to replication stress survival and homology-directed repair. J Cell Biol. 2009;187:385–398. doi: 10.1083/jcb.200902039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Escribano-Diaz C, et al. A cell cycle-dependent regulatory circuit composed of 53BP1-RIF1 and BRCA1-CtIP controls DNA repair pathway choice. Mol Cell. 2013;49:872–883. doi: 10.1016/j.molcel.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 85.Feng L, et al. RIF1 Counteracts BRCA1-mediated End Resection during DNA Repair. J Biol Chem. 2013;288:11135–11143. doi: 10.1074/jbc.M113.457440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chapman JR, et al. RIF1 is essential for 53BP1-dependent nonhomologous end joining and suppression of DNA double-strand break resection. Mol Cell. 2013;49:858–871. doi: 10.1016/j.molcel.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Di Virgilio M, et al. Rif1 prevents resection of DNA breaks and promotes immunoglobulin class switching. 2013;339:711–715. doi: 10.1126/science.1230624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu D, et al. Rif1 provides a new DNA-binding interface for the Bloom syndrome complex to maintain normal replication. EMBO J. 2010;29:3140–3155. doi: 10.1038/emboj.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sreesankar E, et al. Functional diversification of yeast telomere associated protein, Rif1, in higher eukaryotes. BMC Genomics. 2012;13:255. doi: 10.1186/1471-2164-13-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chapman JR, et al. BRCA1-associated exclusion of 53BP1 from DNA damage sites underlies temporal control of DNA repair. J Cell Sci. 2012;125:3529–3534. doi: 10.1242/jcs.105353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Callen E, et al. 53BP1 Mediates Productive and Mutagenic DNA Repair through Distinct Phosphoprotein Interactions. 2013;153:1266–1280. doi: 10.1016/j.cell.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jowsey PA, Doherty AJ, Rouse J. Human PTIP facilitates ATM-mediated activation of p53 and promotes cellular resistance to ionizing radiation. J Biol Chem. 2004;279:55562–55569. doi: 10.1074/jbc.M411021200. [DOI] [PubMed] [Google Scholar]
- 93.Munoz IM, et al. Phospho-epitope binding by the BRCT domains of hPTIP controls multiple aspects of the cellular response to DNA damage. Nucleic Acids Res. 2007;35:5312–5322. doi: 10.1093/nar/gkm493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gong Z, et al. Accumulation of Pax2 transactivation domain interaction protein (PTIP) at sites of DNA breaks via RNF8-dependent pathway is required for cell survival after DNA damage. J Biol Chem. 2009;284:7284–7293. doi: 10.1074/jbc.M809158200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang X, Takenaka K, Takeda S. PTIP promotes DNA double-strand break repair through homologous recombination. Genes Cells. 2010;15:243–254. doi: 10.1111/j.1365-2443.2009.01379.x. [DOI] [PubMed] [Google Scholar]
- 96.Munoz IM, Rouse J. Control of histone methylation and genome stability by PTIP. EMBO Rep. 2009;10:239–245. doi: 10.1038/embor.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Manke IA, et al. BRCT repeats as phosphopeptide-binding modules involved in protein targeting. 2003;302:636–639. doi: 10.1126/science.1088877. [DOI] [PubMed] [Google Scholar]
- 98.Wu J, et al. PTIP regulates 53BP1 and SMC1 at the DNA damage sites. J Biol Chem. 2009;284:18078–18084. doi: 10.1074/jbc.M109.002527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Harding SM, Bristow RG. Discordance between phosphorylation and recruitment of 53BP1 in response to DNA double-strand breaks. Cell Cycle. 2012;11:1432–1444. doi: 10.4161/cc.19824. [DOI] [PubMed] [Google Scholar]
- 100.Rai R, et al. The function of classical and alternative non-homologous end-joining pathways in the fusion of dysfunctional telomeres. EMBO J. 2010;29:2598–2610. doi: 10.1038/emboj.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xia Z, et al. Negative cell cycle regulation and DNA damage-inducible phosphorylation of the BRCT protein 53BP1. J Biol Chem. 2001;276:2708–2718. doi: 10.1074/jbc.M007665200. [DOI] [PubMed] [Google Scholar]
- 102.Adams MM, et al. 53BP1 oligomerization is independent of its methylation by PRMT1. Cell Cycle. 2005;4:1854–1861. doi: 10.4161/cc.4.12.2282. [DOI] [PubMed] [Google Scholar]
- 103.Lo KW, et al. The 8-kDa dynein light chain binds to p53-binding protein 1 and mediates DNA damage-induced p53 nuclear accumulation. J Biol Chem. 2005;280:8172–8179. doi: 10.1074/jbc.M411408200. [DOI] [PubMed] [Google Scholar]
- 104.Butler LR, et al. The proteasomal de-ubiquitinating enzyme POH1 promotes the double-strand DNA break response. EMBO J. 2012;31:3918–3934. doi: 10.1038/emboj.2012.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mosbech A, et al. The Deubiquitylating Enzyme USP44 Counteracts the DNA Double-strand Break Response Mediated by the RNF8 and RNF168 Ubiquitin Ligases. J Biol Chem. 2013;288:16579–16587. doi: 10.1074/jbc.M113.459917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nicassio F, et al. Human USP3 is a chromatin modifier required for S phase progression and genome stability. Curr Biol. 2007;17:1972–1977. doi: 10.1016/j.cub.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 107.Shao G, et al. The Rap80-BRCC36 de-ubiquitinating enzyme complex antagonizes RNF8-Ubc13-dependent ubiquitination events at DNA double strand breaks. Proc Natl Acad Sci U S A. 2009;106:3166–3171. doi: 10.1073/pnas.0807485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nakada S, et al. Non-canonical inhibition of DNA damage-dependent ubiquitination by OTUB1. 2010;466:941–946. doi: 10.1038/nature09297. [DOI] [PubMed] [Google Scholar]
- 109.Sato Y, et al. Molecular basis of Lys-63-linked polyubiquitination inhibition by the interaction between human deubiquitinating enzyme OTUB1 and ubiquitin-conjugating enzyme UBC13. J Biol Chem. 2012;287:25860–25868. doi: 10.1074/jbc.M112.364752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shanbhag NM, et al. ATM-dependent chromatin changes silence transcription in cis to DNA double-strand breaks. 2010;141:970–981. doi: 10.1016/j.cell.2010.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang D, et al. A role for the deubiquitinating enzyme USP28 in control of the DNA-damage response. 2006;126:529–542. doi: 10.1016/j.cell.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 112.Gudjonsson T, et al. TRIP12 and UBR5 suppress spreading of chromatin ubiquitylation at damaged chromosomes. 2012;150:697–709. doi: 10.1016/j.cell.2012.06.039. [DOI] [PubMed] [Google Scholar]
- 113.Tang J, et al. Acetylation limits 53BP1 association with damaged chromatin to promote homologous recombination. Nat Struct Mol Biol. 2013;20:317–325. doi: 10.1038/nsmb.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]