Abstract
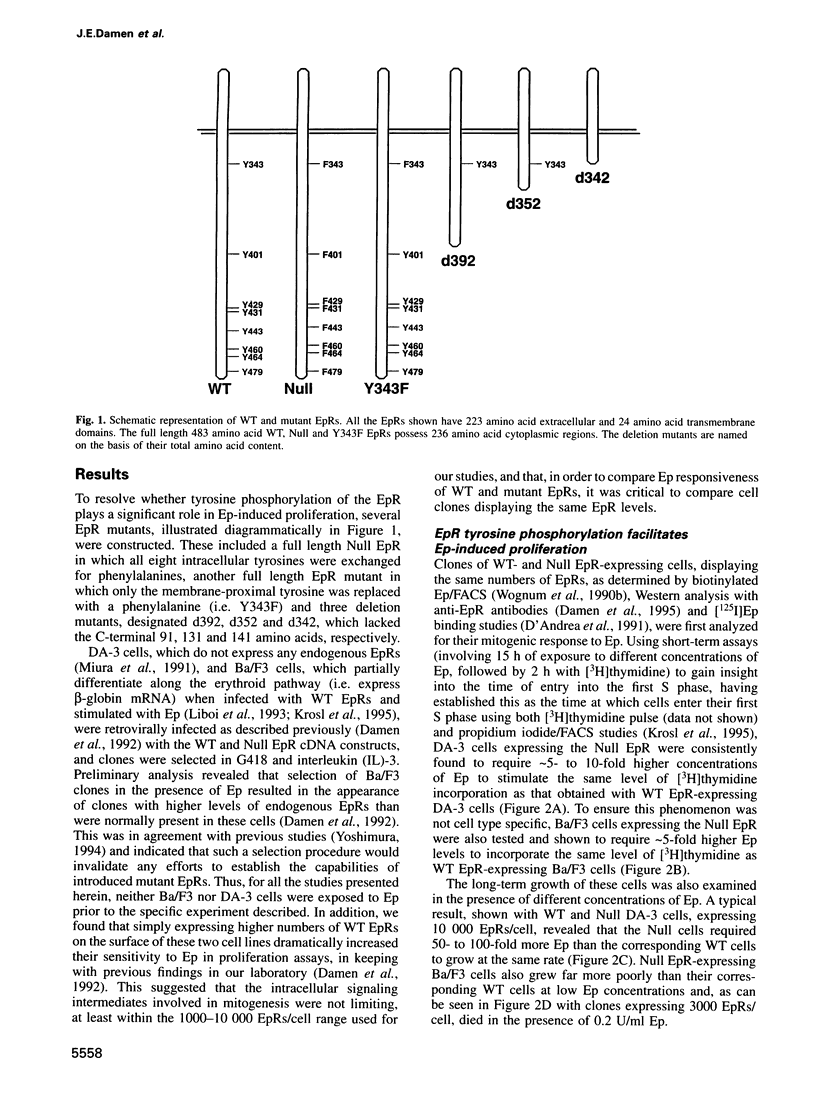
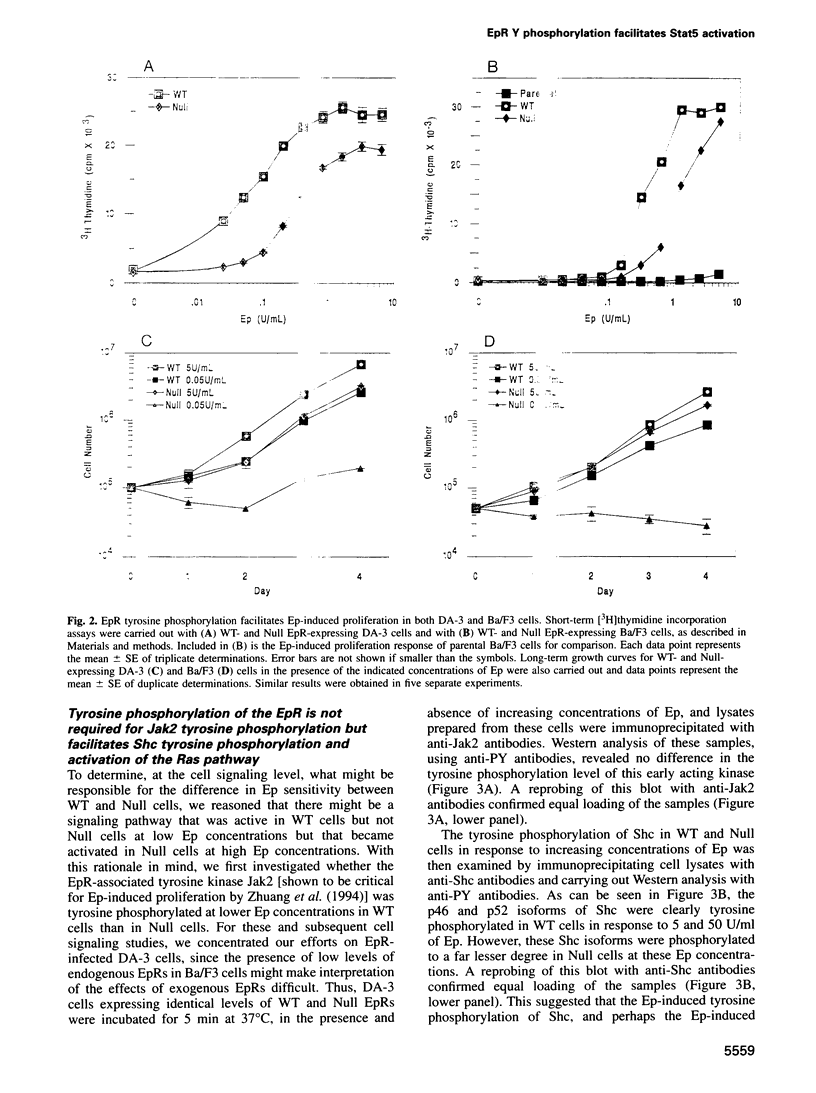
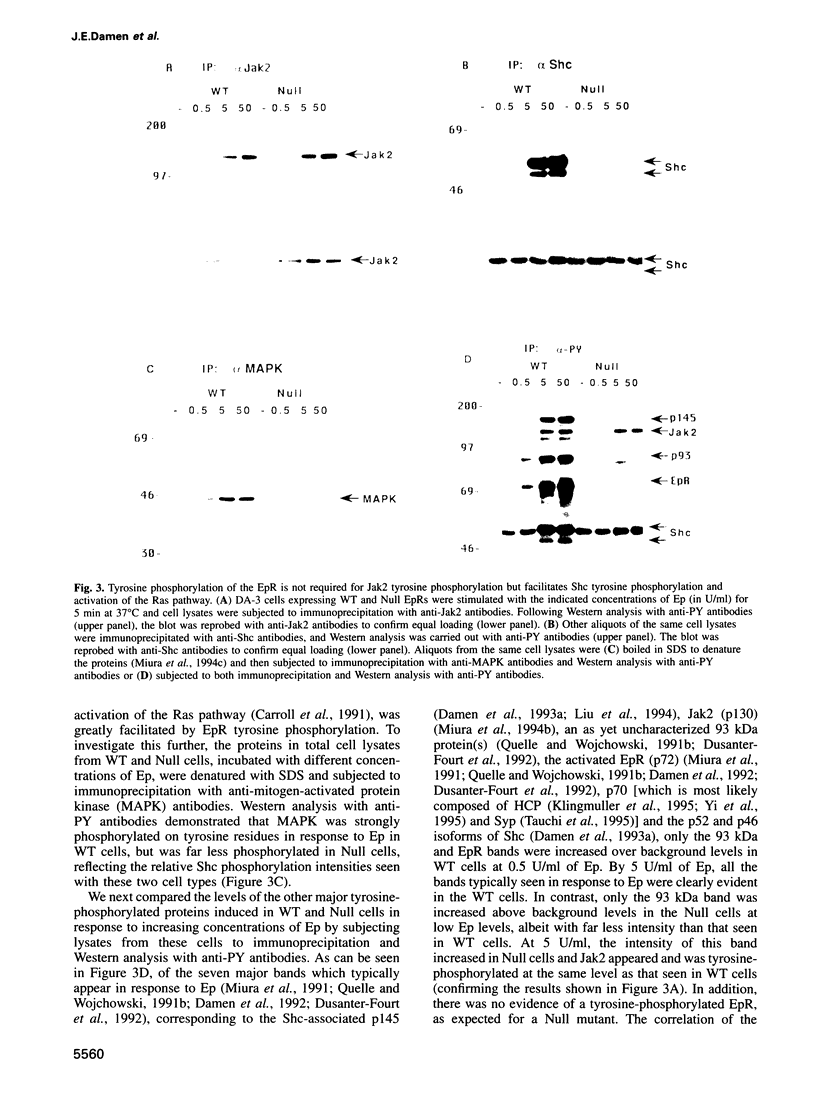
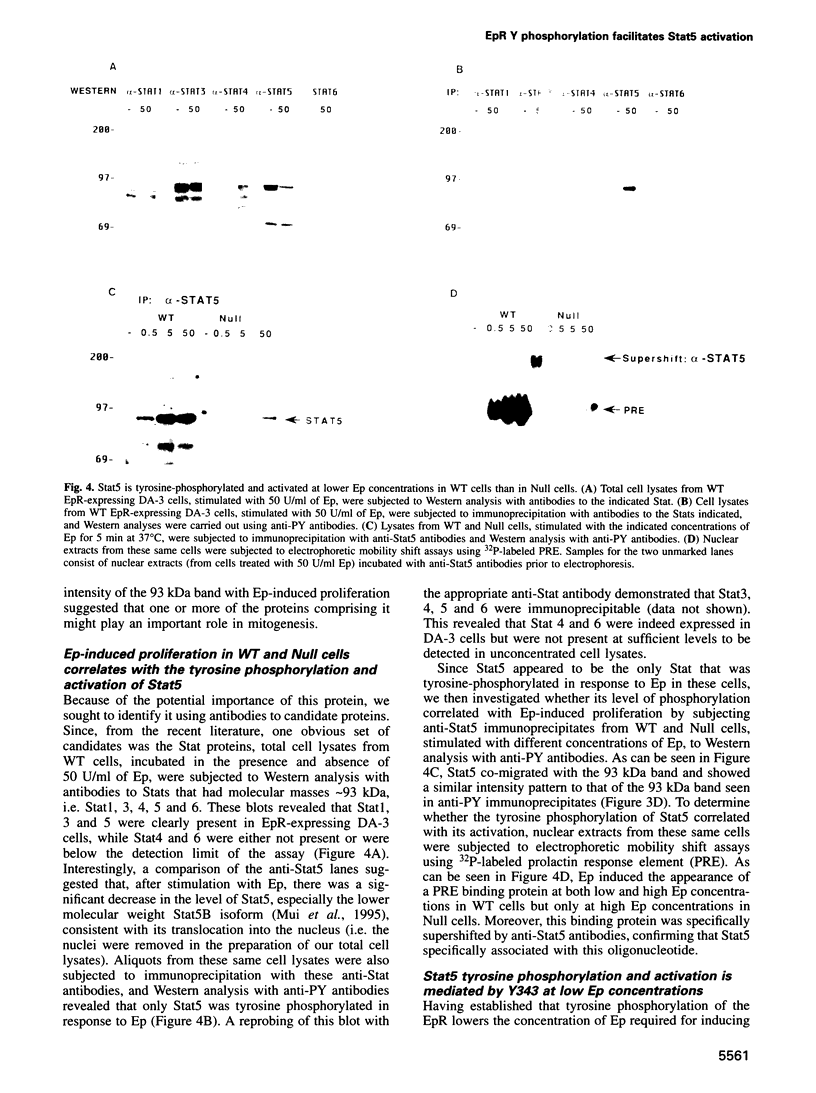
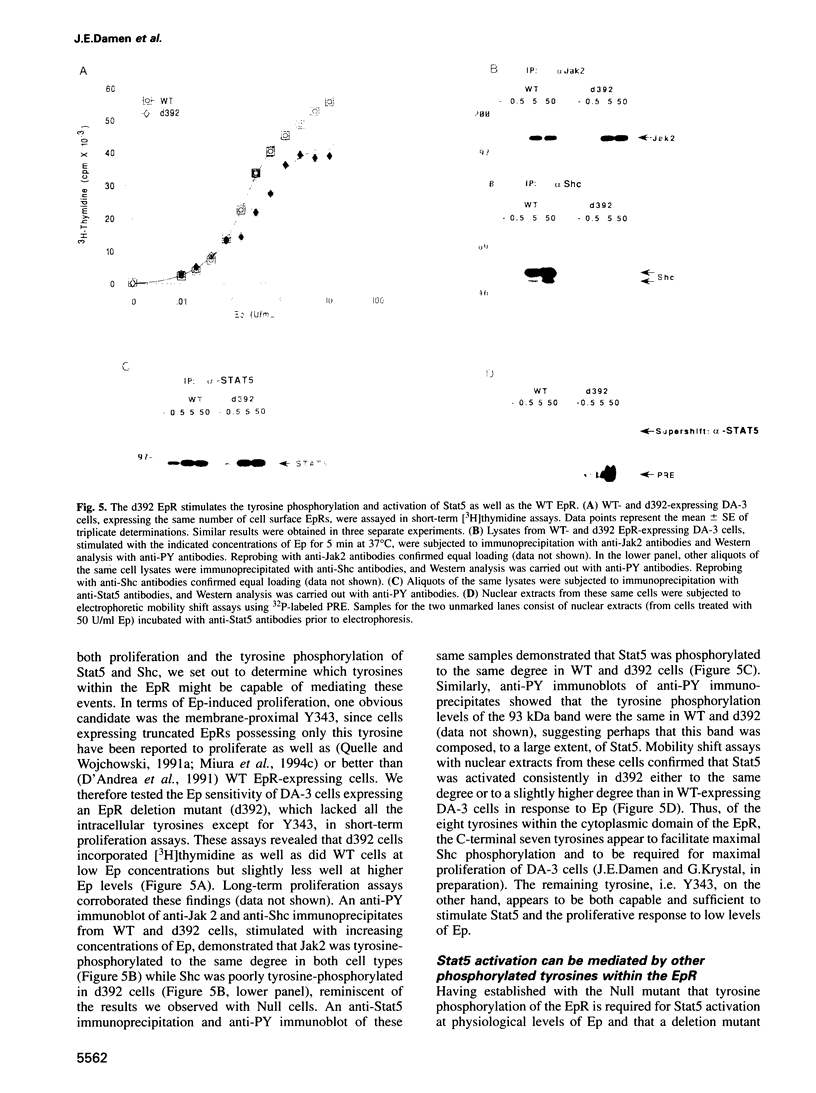
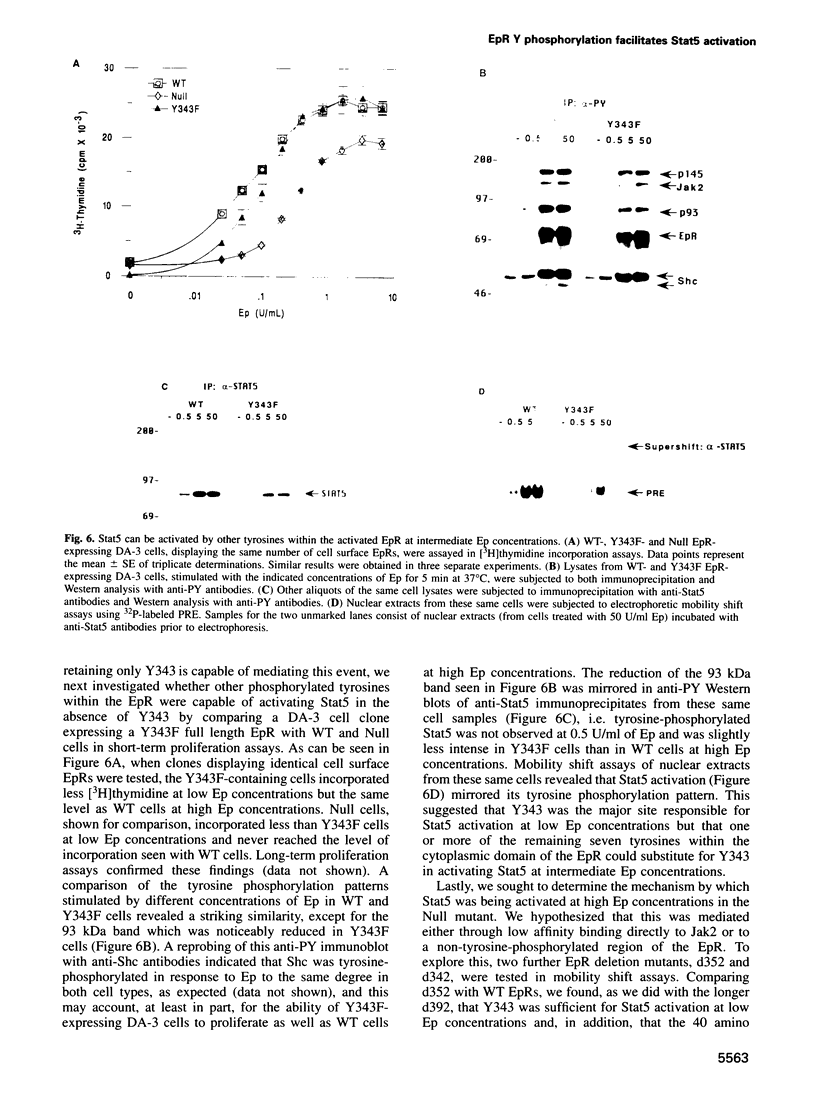
While previous studies with truncated erythropoietin receptors (EpRs) have suggested that the tyrosine phosphorylation of the EpR does not play a role in Ep-induced proliferation, we have found, using a more subtle, full length EpR mutant, designated Null, in which all eight of the intracellular tyrosines have been substituted with phenylalanine residues, that Null cells require substantially more Ep than wild-type cells in order to proliferate as efficiently. A comparison of Ep-induced proliferation with Ep-induced tyrosine phosphorylation patterns, using wild-type and Null EpR-expressing cells, revealed that Stat5 tyrosine phosphorylation and activation correlated directly with proliferation. Moreover, studies with a Y343F EpR point mutant and various EpR deletion mutants revealed that both Ep-induced proliferation and Stat5 activation were mediated primarily through Y343, but that other tyrosines within the EpR could activate Stat5 in its absence.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batzer A. G., Rotin D., Ureña J. M., Skolnik E. Y., Schlessinger J. Hierarchy of binding sites for Grb2 and Shc on the epidermal growth factor receptor. Mol Cell Biol. 1994 Aug;14(8):5192–5201. doi: 10.1128/mcb.14.8.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan J. F. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A. M., Tang T. L., Sugimoto S., Walsh C. T., Neel B. G. Protein-tyrosine-phosphatase SHPTP2 couples platelet-derived growth factor receptor beta to Ras. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7335–7339. doi: 10.1073/pnas.91.15.7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J., Guschin D., Müller M. Signal transduction. Just another signalling pathway. Curr Biol. 1994 Nov 1;4(11):1033–1035. doi: 10.1016/s0960-9822(00)00236-0. [DOI] [PubMed] [Google Scholar]
- Carroll M. P., Spivak J. L., McMahon M., Weich N., Rapp U. R., May W. S. Erythropoietin induces Raf-1 activation and Raf-1 is required for erythropoietin-mediated proliferation. J Biol Chem. 1991 Aug 15;266(23):14964–14969. [PubMed] [Google Scholar]
- Cheatham B., Vlahos C. J., Cheatham L., Wang L., Blenis J., Kahn C. R. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol Cell Biol. 1994 Jul;14(7):4902–4911. doi: 10.1128/mcb.14.7.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea A. D., Lodish H. F., Wong G. G. Expression cloning of the murine erythropoietin receptor. Cell. 1989 Apr 21;57(2):277–285. doi: 10.1016/0092-8674(89)90965-3. [DOI] [PubMed] [Google Scholar]
- D'Andrea A. D., Yoshimura A., Youssoufian H., Zon L. I., Koo J. W., Lodish H. F. The cytoplasmic region of the erythropoietin receptor contains nonoverlapping positive and negative growth-regulatory domains. Mol Cell Biol. 1991 Apr;11(4):1980–1987. doi: 10.1128/mcb.11.4.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damen J. E., Liu L., Cutler R. L., Krystal G. Erythropoietin stimulates the tyrosine phosphorylation of Shc and its association with Grb2 and a 145-Kd tyrosine phosphorylated protein. Blood. 1993 Oct 15;82(8):2296–2303. [PubMed] [Google Scholar]
- Damen J. E., Mui A. L., Puil L., Pawson T., Krystal G. Phosphatidylinositol 3-kinase associates, via its Src homology 2 domains, with the activated erythropoietin receptor. Blood. 1993 Jun 15;81(12):3204–3210. [PubMed] [Google Scholar]
- Damen J., Mui A. L., Hughes P., Humphries K., Krystal G. Erythropoietin-induced tyrosine phosphorylations in a high erythropoietin receptor-expressing lymphoid cell line. Blood. 1992 Oct 15;80(8):1923–1932. [PubMed] [Google Scholar]
- Darnell J. E., Jr, Kerr I. M., Stark G. R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994 Jun 3;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Dusanter-Fourt I., Casadevall N., Lacombe C., Muller O., Billat C., Fischer S., Mayeux P. Erythropoietin induces the tyrosine phosphorylation of its own receptor in human erythropoietin-responsive cells. J Biol Chem. 1992 May 25;267(15):10670–10675. [PubMed] [Google Scholar]
- Gouilleux F., Pallard C., Dusanter-Fourt I., Wakao H., Haldosen L. A., Norstedt G., Levy D., Groner B. Prolactin, growth hormone, erythropoietin and granulocyte-macrophage colony stimulating factor induce MGF-Stat5 DNA binding activity. EMBO J. 1995 May 1;14(9):2005–2013. doi: 10.1002/j.1460-2075.1995.tb07192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouilleux F., Wakao H., Mundt M., Groner B. Prolactin induces phosphorylation of Tyr694 of Stat5 (MGF), a prerequisite for DNA binding and induction of transcription. EMBO J. 1994 Sep 15;13(18):4361–4369. doi: 10.1002/j.1460-2075.1994.tb06756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpur A. G., Andres A. C., Ziemiecki A., Aston R. R., Wilks A. F. JAK2, a third member of the JAK family of protein tyrosine kinases. Oncogene. 1992 Jul;7(7):1347–1353. [PubMed] [Google Scholar]
- He T. C., Jiang N., Zhuang H., Quelle D. E., Wojchowski D. M. The extended box 2 subdomain of erythropoietin receptor is nonessential for Jak2 activation yet critical for efficient mitogenesis in FDC-ER cells. J Biol Chem. 1994 Jul 15;269(28):18291–18294. [PubMed] [Google Scholar]
- He T. C., Zhuang H., Jiang N., Waterfield M. D., Wojchowski D. M. Association of the p85 regulatory subunit of phosphatidylinositol 3-kinase with an essential erythropoietin receptor subdomain. Blood. 1993 Dec 15;82(12):3530–3538. [PubMed] [Google Scholar]
- Heim M. H., Kerr I. M., Stark G. R., Darnell J. E., Jr Contribution of STAT SH2 groups to specific interferon signaling by the Jak-STAT pathway. Science. 1995 Mar 3;267(5202):1347–1349. doi: 10.1126/science.7871432. [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Yokota T., Arai K., Miyajima A. Suppression of apoptotic death in hematopoietic cells by signalling through the IL-3/GM-CSF receptors. EMBO J. 1995 Jan 16;14(2):266–275. doi: 10.1002/j.1460-2075.1995.tb07000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingmüller U., Lorenz U., Cantley L. C., Neel B. G., Lodish H. F. Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell. 1995 Mar 10;80(5):729–738. doi: 10.1016/0092-8674(95)90351-8. [DOI] [PubMed] [Google Scholar]
- Komatsu N., Adamson J. W., Yamamoto K., Altschuler D., Torti M., Marzocchini R., Lapetina E. G. Erythropoietin rapidly induces tyrosine phosphorylation in the human erythropoietin-dependent cell line, UT-7. Blood. 1992 Jul 1;80(1):53–59. [PubMed] [Google Scholar]
- Krantz S. B. Erythropoietin. Blood. 1991 Feb 1;77(3):419–434. [PubMed] [Google Scholar]
- Krosl J., Damen J. E., Krystal G., Humphries R. K. Erythropoietin and interleukin-3 induce distinct events in erythropoietin receptor-expressing BA/F3 cells. Blood. 1995 Jan 1;85(1):50–56. [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Nishimura R., Kashishian A., Batzer A. G., Kim W. J., Cooper J. A., Schlessinger J. A new function for a phosphotyrosine phosphatase: linking GRB2-Sos to a receptor tyrosine kinase. Mol Cell Biol. 1994 Jan;14(1):509–517. doi: 10.1128/mcb.14.1.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liboi E., Carroll M., D'Andrea A. D., Mathey-Prevot B. Erythropoietin receptor signals both proliferation and erythroid-specific differentiation. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11351–11355. doi: 10.1073/pnas.90.23.11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Damen J. E., Cutler R. L., Krystal G. Multiple cytokines stimulate the binding of a common 145-kilodalton protein to Shc at the Grb2 recognition site of Shc. Mol Cell Biol. 1994 Oct;14(10):6926–6935. doi: 10.1128/mcb.14.10.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura O., D'Andrea A., Kabat D., Ihle J. N. Induction of tyrosine phosphorylation by the erythropoietin receptor correlates with mitogenesis. Mol Cell Biol. 1991 Oct;11(10):4895–4902. doi: 10.1128/mcb.11.10.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura O., Nakamura N., Ihle J. N., Aoki N. Erythropoietin-dependent association of phosphatidylinositol 3-kinase with tyrosine-phosphorylated erythropoietin receptor. J Biol Chem. 1994 Jan 7;269(1):614–620. [PubMed] [Google Scholar]
- Miura O., Nakamura N., Quelle F. W., Witthuhn B. A., Ihle J. N., Aoki N. Erythropoietin induces association of the JAK2 protein tyrosine kinase with the erythropoietin receptor in vivo. Blood. 1994 Sep 1;84(5):1501–1507. [PubMed] [Google Scholar]
- Miura Y., Miura O., Ihle J. N., Aoki N. Activation of the mitogen-activated protein kinase pathway by the erythropoietin receptor. J Biol Chem. 1994 Nov 25;269(47):29962–29969. [PubMed] [Google Scholar]
- Mui A. L., Wakao H., O'Farrell A. M., Harada N., Miyajima A. Interleukin-3, granulocyte-macrophage colony stimulating factor and interleukin-5 transduce signals through two STAT5 homologs. EMBO J. 1995 Mar 15;14(6):1166–1175. doi: 10.1002/j.1460-2075.1995.tb07100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi T., Masuda M., Ruscetti S. K. Induction of sequence-specific DNA-binding factors by erythropoietin and the spleen focus-forming virus. Blood. 1995 Mar 15;85(6):1454–1462. [PubMed] [Google Scholar]
- Quelle D. E., Wojchowski D. M. Localized cytosolic domains of the erythropoietin receptor regulate growth signaling and down-modulate responsiveness to granulocyte-macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4801–4805. doi: 10.1073/pnas.88.11.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quelle F. W., Wojchowski D. M. Proliferative action of erythropoietin is associated with rapid protein tyrosine phosphorylation in responsive B6SUt.EP cells. J Biol Chem. 1991 Jan 5;266(1):609–614. [PubMed] [Google Scholar]
- Sakamaki K., Yonehara S. Serum alleviates the requirement of the granulocyte-macrophage colony-stimulating factor (GM-CSF)-induced Ras activation for proliferation of BaF3 cells. FEBS Lett. 1994 Oct 17;353(2):133–137. doi: 10.1016/0014-5793(94)01024-2. [DOI] [PubMed] [Google Scholar]
- Sasaoka T., Rose D. W., Jhun B. H., Saltiel A. R., Draznin B., Olefsky J. M. Evidence for a functional role of Shc proteins in mitogenic signaling induced by insulin, insulin-like growth factor-1, and epidermal growth factor. J Biol Chem. 1994 May 6;269(18):13689–13694. [PubMed] [Google Scholar]
- Sawada K., Krantz S. B., Dai C. H., Koury S. T., Horn S. T., Glick A. D., Civin C. I. Purification of human blood burst-forming units-erythroid and demonstration of the evolution of erythropoietin receptors. J Cell Physiol. 1990 Feb;142(2):219–230. doi: 10.1002/jcp.1041420202. [DOI] [PubMed] [Google Scholar]
- Schmitt-Ney M., Doppler W., Ball R. K., Groner B. Beta-casein gene promoter activity is regulated by the hormone-mediated relief of transcriptional repression and a mammary-gland-specific nuclear factor. Mol Cell Biol. 1991 Jul;11(7):3745–3755. doi: 10.1128/mcb.11.7.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl N., Farruggella T. J., Boulton T. G., Zhong Z., Darnell J. E., Jr, Yancopoulos G. D. Choice of STATs and other substrates specified by modular tyrosine-based motifs in cytokine receptors. Science. 1995 Mar 3;267(5202):1349–1353. doi: 10.1126/science.7871433. [DOI] [PubMed] [Google Scholar]
- Taniguchi T. Cytokine signaling through nonreceptor protein tyrosine kinases. Science. 1995 Apr 14;268(5208):251–255. doi: 10.1126/science.7716517. [DOI] [PubMed] [Google Scholar]
- Tauchi T., Feng G. S., Shen R., Hoatlin M., Bagby G. C., Jr, Kabat D., Lu L., Broxmeyer H. E. Involvement of SH2-containing phosphotyrosine phosphatase Syp in erythropoietin receptor signal transduction pathways. J Biol Chem. 1995 Mar 10;270(10):5631–5635. doi: 10.1074/jbc.270.10.5631. [DOI] [PubMed] [Google Scholar]
- Wakao H., Gouilleux F., Groner B. Mammary gland factor (MGF) is a novel member of the cytokine regulated transcription factor gene family and confers the prolactin response. EMBO J. 1994 May 1;13(9):2182–2191. doi: 10.1002/j.1460-2075.1994.tb06495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakao H., Harada N., Kitamura T., Mui A. L., Miyajima A. Interleukin 2 and erythropoietin activate STAT5/MGF via distinct pathways. EMBO J. 1995 Jun 1;14(11):2527–2535. doi: 10.1002/j.1460-2075.1995.tb07250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. D., Wong K., Wood W. I. Intracellular tyrosine residues of the human growth hormone receptor are not required for the signaling of proliferation or Jak-STAT activation. J Biol Chem. 1995 Mar 31;270(13):7021–7024. doi: 10.1074/jbc.270.13.7021. [DOI] [PubMed] [Google Scholar]
- Witthuhn B. A., Quelle F. W., Silvennoinen O., Yi T., Tang B., Miura O., Ihle J. N. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993 Jul 30;74(2):227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- Wognum A. W., Krystal G., Eaves C. J., Eaves A. C., Lansdorp P. M. Increased erythropoietin-receptor expression on CD34-positive bone marrow cells from patients with chronic myeloid leukemia. Blood. 1992 Feb 1;79(3):642–649. [PubMed] [Google Scholar]
- Wognum A. W., Lam V., Goudsmit R., Krystal G. A specific in vitro bioassay for measuring erythropoietin levels in human serum and plasma. Blood. 1990 Oct 1;76(7):1323–1329. [PubMed] [Google Scholar]
- Wognum A. W., Lansdorp P. M., Humphries R. K., Krystal G. Detection and isolation of the erythropoietin receptor using biotinylated erythropoietin. Blood. 1990 Aug 15;76(4):697–705. [PubMed] [Google Scholar]
- Yi T., Zhang J., Miura O., Ihle J. N. Hematopoietic cell phosphatase associates with erythropoietin (Epo) receptor after Epo-induced receptor tyrosine phosphorylation: identification of potential binding sites. Blood. 1995 Jan 1;85(1):87–95. [PubMed] [Google Scholar]
- Yoshimura A. Second subunit of Epo receptor? Nature. 1994 Nov 10;372(6502):137–138. doi: 10.1038/372137b0. [DOI] [PubMed] [Google Scholar]
- Zhuang H., Patel S. V., He T. C., Sonsteby S. K., Niu Z., Wojchowski D. M. Inhibition of erythropoietin-induced mitogenesis by a kinase-deficient form of Jak2. J Biol Chem. 1994 Aug 26;269(34):21411–21414. [PubMed] [Google Scholar]