Abstract
Objective
To understand the role of genetic variation in the catecholamine biosynthetic pathway for control of human heart rate (HR).
Background
Human HR is an integrated cardiovascular trait predictive of morbidity and survival. Since the autonomic pathway exerts rapid control over the heart, we probed the role of heredity in control of HR, focusing on a component of the autonomic sympathetic pathway already predictive of outflow responses: Cytochrome b561 (CYB561), the electron shuttle in catecholamine vesicle membranes for transmitter biosynthesis.
Methods
We studied hereditary control of HR with the twin pair design, at rest and during environmental (cold) stress. SNP disruption of a micro-RNA recognition motif in the human CYB561 3’-UTR was identified computationally, and its differential effect on gene expression was demonstrated in a transfected luciferase reporter / 3’-UTR variant. We exposed of stem-cell-derived human embryoid bodies to the micro-RNA mimic or antagomir oligonucleotides, and observed effects on contraction rate in proto-hearts.
Results
Substantial heritability (h2) was demonstrated, by twin pair variance components, for both basal/resting HR (h2=50.9±6.4% of trait variation, p=2.47E-10) and stress-augmented HR (h2=55.1±5.9%, p=8.79E-13), and the two HR traits shared genetic determination (genetic covariance ρG=0.747±0.058, p=2.85E-09). CYB561 displayed one common genetic variant in the transcript region: A+1485G (rs3087776), in the 3’-UTR, 1485 bp downstream of the termination codon, in a conserved region, with the A-allele ancestral in primates. In a twin/sibling sample (n=576), A+1485G influenced HR, both at rest (p=0.010) and after environmental stress (p=0.002), with the minor (A) allele displaying a recessive effect with lower HR. The effect of A+1485G on HR was extended by meta-analysis into two additional population samples (total n=2579), and the influence remained directionally consistent and significant (p=0.007). A+1485G disrupted a micro-RNA (hsa-miR-1294) recognition motif in the 3’-UTR (with AG) was demonstrated in a transfected luciferase reporter / human 3’-UTR variant system in two different neuronal/neuroendocrine cell types, and the micro-RNA effect was further documented by co-transfection of a hsa-miR-1294 mimic, yielding an exaggerated decline in expression of the A-allele (better match) reporter (p=4.3E-05). Similar findings of differential 3’-UTR allelic susceptibility to miR-1294 were noted during expression of the full-length human CYB561 mRNA with its cognate 3’-UTR. Finally, exposure of stem-cell-derived human embryoid bodies to miR-1294 mimic or antagomir oligonucleotides yielded directionally opposite effects on contraction rate in proto-hearts.
Conclusions
HR is a substantially heritable trait, with genetic influence by variation in the adrenergic pathway, here shown for mRNA translational control at the CYB561 step of transmitter formation. The results have implications for potentially modifiable autonomic pathways that influence this risk trait in the population.
Keywords: Cytochrome b561, catecholamine, chromaffin, micro-RNA, heart rate
INTRODUCTION
Human heart rate (HR) is an integrated cardiovascular trait that has assumed epidemiogical importance, since HR may be predictive of premature mortality (both cardiovascular and non-cardiovascular) and consequently lifespan (1,2). The HR trait is complex, reflecting not only cardiovascular but also metabolic adjustments (2). Rapid control of HR is achieved by the autonomic nervous system, in both its sympathetic (catecholaminergic, stimulatory) (3) and parasympathetic (vagal, inhibitory) branches. The role of heredity in control of HR has not been exhaustively examined, but genetic variation in the sympathetic pathway, including “tagging” (intronic) variation at the cytochrome b561 (CYB561) locus reportedly influences cardiovascular responses to sympathetic activation (4). Indeed, naturally occurring genetic variation at every other point in the adrenergic pathway – GCH1 (5), TH (6), DBH (7), PNMT (8) , and ADRB1 (9) – has already been associated with altered HR control, yet the interaction of CYB561 and HR is still unexplored.
Cytochrome b561 (CYB561) is an electron transfer protein unique to catecholamine and neuropeptide secretory vesicles of the adrenal medulla, pituitary gland and other neuroendocrine tissues (10,11). The 30-kDa protein may comprise as much as ~15% of the hormone storage vesicle membrane protein (12) and its role is to supply reducing equivalents to two monooxygenases, dopamine beta-hydroxylase in chromaffin granules and peptidylglycine alpha-amidating monooxygenase in neurosecretory vesicles (13). The cytochrome fulfills this role by catalyzing the transfer of electrons from a cytoplasmic donor, ascorbate, across a phospholipid bilayer to the luminal acceptor, semidehydroascorbate, in the interior of the vesicles. The continuously regenerated ascorbate within these vesicles is the immediate donor for the monooxygenases within the neuroendocrine secretory vesicles. Thus, cytochrome b561 is a transmembrane electron channel.
Since the sympathetic system exerts substantial control over HR, we first used the twin pair approach to ask whether HR responses were heritable, since twin studies have successfully revealed hereditary contributions to cardiovascular stress traits, including HR (14). We then probed the role of genetic variation at the CYB561 locus in such responses. Later, we used a transfected reporter system to examine the role of common variation in the 3’-UTR of the gene, which disrupted a micro-RNA recognition motif, a potential control point for mRNA translation. Finally, we explored whether the micro-RNA influenced contraction rate in cultured human embryoid bodies.
Micro-RNAs are emerging as a widespread endogenous mechanism for control of gene expression at the post-translational level (15), wherein the ~22-nucleotide mature/processed micro-RNAs bind to particular motifs in the 3’-UTRs of target mRNAs, inhibiting translation by catalyzing transcript scission/degradation, or by steric hindrance. Indeed, the human genome harbors over 1000 micro-RNA-encoding loci <http://www.microrna.org>.
METHODS
Subjects and characterization
Subjects were volunteers drawn largely from southern California, and each subject gave informed, written consent; the protocol was approved by the institutional review board. Recruitment procedures, definitions and confirmation of subject diagnoses are according to previous reports from our group.
Initial: Twins and siblings
From 235 nuclear families, 576 individuals from twin and sibling pairs were recruited to conduct the following study. Zygosity was confirmed by extensive microsatellite and SNP genotyping, as described (16). Twins ranged in age from 15-84 years. Individuals of Caucasian (European-American, 87%) or Hispanic (Mexican-American, 13%) biogeographic ancestry/ethnicity (by self-identification) were included here.
Physiological phenotyping in vivo (twins and siblings): Environmental (cold) stress
To probe the functional significance of common variation at CYB561, we examined the potential influence of 1 common CYB561 polymorphisms on the HR before and during environmental (cold) stress testing (17) on 576 twin (MZ or DZ) and sibling individuals. Resting heart was recorded continuously for ~5 min prior to cold stress. During the stressor, the subject immersed the non-dominant hand into ice (zero °C) water for one minute, with averaged measurements of HR stable over at least 3 beats prior to and then again towards the end of the 60-sec procedure.
Extension: Primary care population samples
From a database of over 53,000 people in a primary care (Kaiser Permanente) database in southern California, we ascertained two samples (Kaiser-1, Kaiser-2) of European-ancestry individuals, of both sexes (18). Evaluation included physical examination (with vital signs), blood chemistries, hemogram, and medical history questionnaire. HR was measured by manual palpation of a radial artery for 30 seconds (then multiplying by two, to obtain beats/min) in seated, resting subjects, just prior to the BP measurement.
Genotyping CYB561 variants
Bioinformatics of polymorphism
The extent of polymorphism at the CYB561 locus was visualized at NCBI (locus ID 1534), though GeneView at dbSNP <http://www.ncbi.nlm.nih.gov/SNP/>. The 3003-bp CYB561 mRNA (RefSeq clone NM_001915.3), which encodes a 251 amino acid protein product (Uniprot P49447), exhibits only one common (MAF>5%) variant: rs3087776 A/G, located in the 3’-UTR, 1485 bp downstream from the stop codon (A+1485G), at MAF=49.9%, heterozygosity = 0.499 (Figure 1A). Sequence diversity results emerged from inspection of: 1000-Genomes project <http://www.1000genomes.org/> (chromosomes represented: 120 CEU [at 5.1x sequence coverage], 118 YRI, 120 CHB+JPT); and the HapMap <http://hapmap.ncbi.nlm.nih.gov/> (chromosomes represented: 226 CEU, 226 YRI, 86 CHB, 172 JPT). No common non-synonymous (amino acid replacement at >1% frequency) variation has been observed at CYB561.
Figure 1. Human CYB561 genomics, common 3’-UTR variant A+1485G (rs3087776), and micro-RNA hsa-miR-1294.
1A. Location on chromosome 17q23, with exon/intron structure and position of 3’-UTR variant.
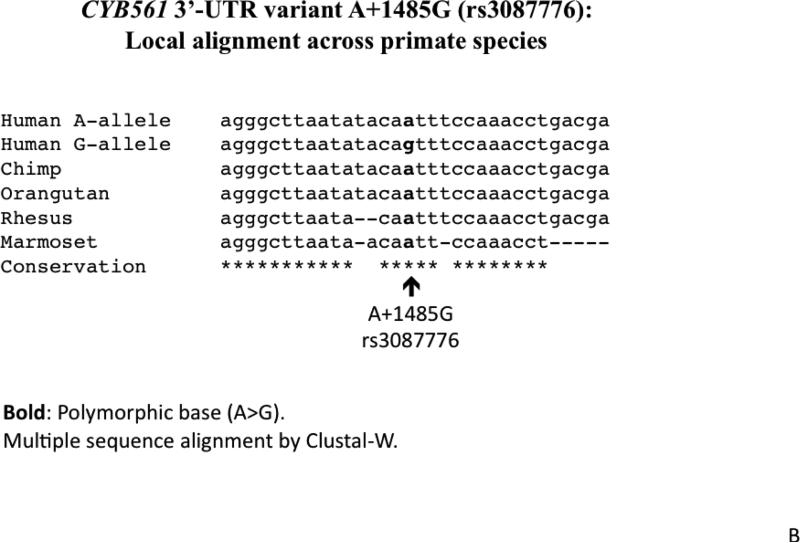
1B. Local alignment across primate species. The local sequence is evolutionally conserved across most primates, while the ancestral allele (based upon chimpanzee and other primate sequences) seems to be the A-allele.
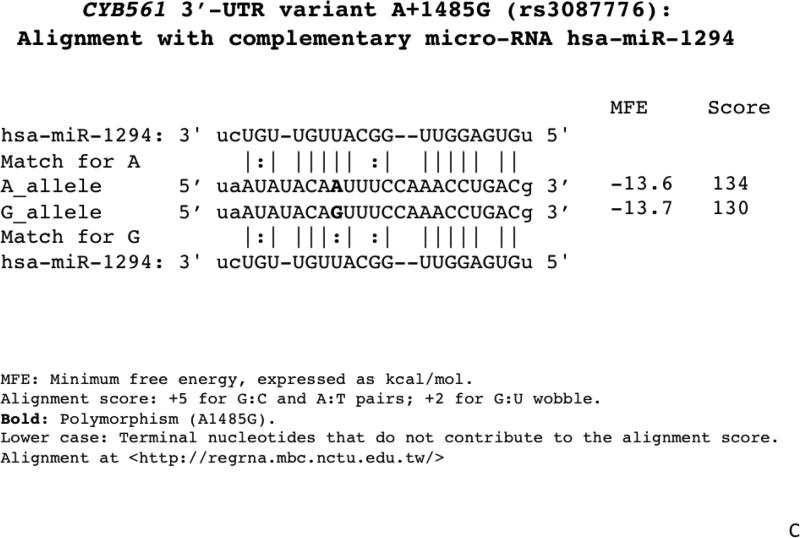
1C. Alignment of the A+1485G region with the micro-RNA hsa-miR-1294 motif. The A-allele provides a superior match.
Experimental
Genomic DNA of each individual was prepared from leukocytes in EDTA-anticoagulated blood, using PureGene extraction columns (Gentra Biosystems, Minnesota). The 3’-UTR polymorphism rs3087776 A+1485G diploid genotypes was scored by TaqMan technology (Applied Biosystems; Foster City, CA) with a 5 μl reaction using 384 well plates: DNA, 5 ng; probe (40x), 0.0625 μl; and master mix (2x), 2.5 μl. The PCR profile was: 95 °C (strand separation) for 10 minutes, followed by 40 cycles of 92 °C for 15 seconds and 60 °C for 1 minute. Twins and siblings were genotyped with the Illumina-610-Quad array, encompassing ~590K SNP genotypes.
Computation and statistics
Heritability of phenotype expression in vivo
In twin pairs, heritability (or the fraction of trait variance accounted for by genetic variance, h2=VG/VP), as well as the genetic covariance (shared environmental determination or pleiotropy; parameter Rho_G or rG) and environmental covariance (shared environmental determination; Rho_E or rE), were estimated by variance components in SOLAR (Sequential Oligogenic Linkage Routines)(19), available at <http://www.txbiomed.org/departments/genetics>.
Marker-on-trait association
To test SNP on phenotype effects with explicit accounting for family structure, MERLIN v1.1.2 (http://www.sph.umich.edu/csg/abecasis/merlin/) was used. As an additional QC step, unlikely genotypes based on expected inheritance patterns were removed using MERLIN's Pedwipe procedure. During the SNP association, subjects were categorized according to diploid genotype at the bi-allelic SNP locus. To control for additional genetic background heterogeneity in this predominantly Caucasian cohort we performed a multidimensional scaling analysis (MDS) using PLINK (20) including all autosomal SNPs. We then included the first MDS dimension as covariate in the association analysis, which corresponded to the Native American admixture of Hispanic subjects (21,22).
Descriptive and inferential statistics
For twin/sibling analyses, descriptive (genotype-specific mean ± SEM) and inferential (chi-square, p value) statistics were computed across all of the twins, using generalized estimating equations (GEE) in SPSS-17 (Chicago, IL), to account for correlated trait values within each sibship, using an exchangeable correlation matrix (23). After inspection of clustering of the descriptive statistics (mean±SEM), heterozygotes and minor/major allele homozygotes could be grouped together, to test dominant/recessive models. Estimates are stated as mean value ± one standard error. For unrelated individuals, two-way ANOVA or multivariable general linear modeling, as well as post-hoc corrections, were performed in SPSS-17 to evaluate the significance of a single variant.
Meta-analyses
The coefficient of genotype effect (Beta), its SE and p-values were obtained by regression analysis in SPSS-17. Meta-analyses were carried out with the command META, testing fixed effect (i.e., genotype) models in STATA-12 (College Station, TX), after individual study regression analysis in SPSS, incorporating individual study data to derive significance as well as pooled genotype effect size (beta, or slope per allele) and its SE. Analyses in twins were adjusted for age and sex (as well as ancestry), while results in the Kaiser subjects were adjusted for age and sex.
CYB561 cis-eQTL
We queried human mRNA expression data from the GTEx (Genotype Tissue Expression) program at its eQTL browser <http://www.ncbi.nlm.nih.gov/gtex/GTEX2/gtex.cgi>, focusing on the effects of CYB561 genetic variation on CYB561 mRNA expression in neuronal tissues, originally available from (24). CYB561 3’-UTR variant A+1485G (rs3087776; chr-17, position 61,510,277) was tested by linear regression (assoc function within PLINK, with an additive genotype model) as a determinant of CYB561 mRNA abundance in a series of n=143 human brain (frontal cortex) samples from neurologically normal Caucasian subjects, with available transcriptome (Illumina Human Ref-8 Expression bead-chips with probes for 22,184 mRNA transcripts; CYB561 probe ILMN_1771179, chr-17 position 60,214,268) and GWAS data (Illumina Infinium HumanHap 550 bead-chips; 561,466 SNPs). Before regression, raw intensity values for mRNA expression were transformed using the rank invariant normalization method.
CYB561 3’-UTR/luciferase reporter, and activity assays
The CYP561 3’-UTR (2079 bp) was PCR-amplified from genomic DNA of known homozygotes, and ligated into the unique XbaI site just downstream (3’) of the firefly luciferase reporter in plasmid pGL3-Promoter (Promega, Madison, WI), in which eukaryotic transcription is driven by the SV40 early promoter (Supplementary Figure 1). The variant 3’-UTR mutant G/A was created by site-directed mutagenesis (QuikChange, Stratagene), and verified by dideoxy sequencing. Cells were transfected (at ~50-60% confluence in 15.6 mm polystyrene dishes) with 500 ng of supercoiled plasmid by the liposome method (Transfectin; Bio-Rad, Hercules, CA). After transfection and cell growth over a 12-48 hour time course, cells were treated with passive lysis buffer (Cat#: E194A, Promega) for sequential measurement of luciferase enzymatic activity and protein concentration, and the results were expressed as the ratio of firefly luciferase/protein, as described previously (25,26). Each cellular experiment was repeated a minimum of four times. Results were expressed as mean ± SEM. Statistical significance (p<0.05) was calculated by ANOVA.
Cell and molecular biology
Neuronal or neuroendocrine cells, including rat pheochromocytoma cells (PC12), human HEK-293T cells, or human neuroblastoma cells (SH-SY5Y), were grown at ~50-60% confluence in 15.6 mm polystyrene dishes, prior to transfection. HEK-293T cells were chosen because of their prominent neuronal gene expression phenotypes (27). mRNA (e.g., CYB561) abundance was quantified by qRT-PCR, and normalized to expression of endogenous “housekeeping” RNAs (GAPDH, beta-actin, or 18S rRNA) in the same sample, by the ΔΔCt method (28). Endogenous micro-RNA (i.e., hsa-miR-1294) abundance was also quantified by qRT-PCR, but normalized to an endogenous small RNA (SNORD61). The full-length human CYB561 cDNA (NM_001915; including the 753 bp [251 amino acid] ORF with 2079 bp 3’-UTR) was obtained from Open Biosystems (Huntsville, AL) in the eukaryotic (pCMV) expression plasmid pSPORT6; site-directed mutagenesis (QuikChange, Stratagene) created the 3’-UTR G/A variant, verified by dideoxy sequencing. These plasmids were then transfected into PC12 or HEK-293T cells, with or without co-transfected hsa-miR-1294 oligonucleotides (double-stranded mimic or control), to test whether expression of the human CYB561 mRNA was influenced by the A+1485G variant in its cognate 3’-UTR.
Evaluation of 3’-UTR micro-RNA (hsa-miR-1294) motif and function
Computational
Inter-species sequence alignments were done by Clustal-W. 3’-UTR micro-RNA (miR) motifs were predicted at RegRNA (29) (http://regrna.mbc.nctu.edu.tw/). The effects of the variant on RNA hybrid structures and predicted minimum folding energies were analyzed using BiBiServ (30) (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/submission.html).
Experimental
An initial strategy of miR over-expression was adopted to explore miRNA function in cells. A Dharmacon (Lafayette, CO) miRIDIAN 22-mer (UGUGAGGUUGGCAUUGUUGUCU) double-stranded RNA mimic for human micro-RNA-1294 (hsa-miR-1294, Cat#: C-301348-00) was co-transfected (Transfectin; Bio-Rad, Hercules, CA) to increase that miRNA abundance, while a pre-designed negative control (No. 1; Cat#: CN-001000-01-05) was co-transfected to control for off-target effects. Later, we proceeded to hsa-miR-1294 miR antagonist (antagomir, antisense version) studies, with a single-stranded oligonucleotide (Dharmacon IH-301348-01-0005). The identities of synthetic oligonucleotides were verified by MALDI-TOF mass spectrometry, with confirmation of duplex integrity by PAGE. Synthetic oligonucleotides were applied to a final concentration of 15 nM, and co-transfected into PC12 or HEK-293T cells, along with wild-type or variant CYB561 3’-UTR/luciferase reporters, by the liposome method described above. For studies in human embryoid bodies, we also transfected (Transfectin; Bio-Rad, Hercules, CA) a synthetic single-stranded hsa-miR-1294 antagomir (antisense version, or inhibitor; Dharmacon IH-301348-01-0005).
Human embryoid body cultures (for contraction rate effects of hsa-miR-1294 in vivo)
Human embryonic stem cells (ESC; line H9) were grown on MEFs in WiCell hESC medium (with 12 ng/mL bFGF). ESCs were differentiated to cardiomyocytes in embryoid bodies (EB) as described (31). After 6 weeks, cells were transfected for 24 hours with either 20 nM hsa-miR-1294 double-stranded mimic, single-stranded antagomir (inhibitor), or control oligonucleotide (8 replicate wells per condition), using the liposome method (Transfectin; Bio-Rad, Hercules, CA). The contraction rate for each embryoid body was counted immediately prior to transfection and again 24 hours post transfection. Due to the usual heterogeneity in contraction rate among EBs in all conditions, contraction rate was compared for each EB pre- and post-transfection. Since contraction rate values were not normally distributed, results were evaluated by the non-parametric Mann-Whitney test.
RESULTS
CYB561 genomics
Human cytochrome b-561 (CYB561) gene
Located on chromosome 17q23, the CYB561 locus spans ~14 kbp, with only one common (MAF >5%) variant in the entire encoded transcript: 3’-UTR polymorphism A+1485G (rs3087776), with a MAF ~49% (Figure 1A). Linkage disequilibrium (LD) across the locus, reflecting substantial marker-on-marker correlations, indicates that the entire gene is contained within a single block (Supplementary Figure 1).
CYB561 3’-UTR variant A+1485G (rs3087776) conserved across primate species
The A+1485G variant is located in a relatively conserved 3’-UTR region across primate species (human, chimpanzee, orangutan, rhesus and marmoset; Figure 1B). The minor (less frequent) A-allele seems to be ancestral, since it is present in other primates, including chimpanzee. The A-allele displays a better match for the hsa-miR-1294 motif than does the G-allele (Figure 1C).
Human HR: Heritability, stress responses, and influence of CYB561 3’-UTR variant
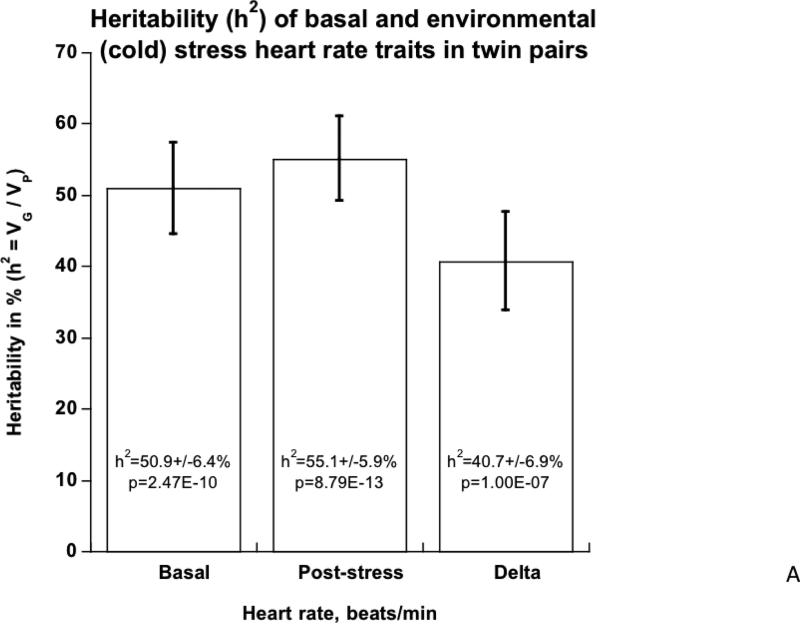
HR trait heritability (h2) in twins (Table 1; Figure 2A)
Table 1. CYB561 3'-UTR variant A+1485G (rs3087776): Effect on heritable stress heart rate traits in twins and sibs.
Heritability (h2) and genetic covariance were estimated from variance components in twin pairs (MZ versus DZ), with SOLAR. Significance was estimated by MERLIN in twins and sibs, while descriptive statistics were computed by GEE in SAS.
| 1a. Change in heart rate (HR, beats/min) during cold stress (n=576). Bold: p<0.05. | ||
|---|---|---|
| Trait | Value (beats/min) | Change from basal (p-value). |
| Basal/resting HR | 72.0±0.50 | - |
| Post-stress HR | 75.4±0.53 | - |
| Delta (pre→post) HR | 3.2±0.39 | p=1.92E-13 |
| 1b. Heart rate (HR) traits in twin pairs: Heritability and shared genetic determination (pleiotropy). | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Heart rate (HR) trait (beats/min) | Trait heritability (h^2) in twins (n=384) | Correlation with basal HR | Shared genetic determination (pleiotropy) with basal HR | Shared environmental determination with basal HR | |||||||
| Spearman | |||||||||||
| h^2 | SEM | P-value | ρ | P-value | Rho_G | SEM | P-value | Rho_E | SEM | P-value | |
| Basal/resting HR | 0.509 | 0.064 | 2.47E-10 | - | - | - | - | - | - | - | - |
| During-stress HR | 0.551 | 0.059 | 8.79E-13 | 0.682 | 2.73E-75 | 0.747 | 0.058 | 2.85E-09 | 0.603 | 0.055 | 8.61E-16 |
| Delta (pre→during) HR | 0.407 | 0.069 | 1.00E-07 | −0.299 | 1.94E-13 | −0.265 | 0.122 | 0.056 | −0.443 | 0.068 | 2.46E-08 |
| 1c. Effects of CYB561 3'-UTR variant on HR traits. | ||||
|---|---|---|---|---|
| Category/Trait | 3’-UTR A+1485G diploid genotype (n=576) | P-value | ||
| A/A(122) | A/G (289) | G/G (165) | ||
| Heart rate, beats/min | ||||
| Before environmental (cold) stress | 70.0±1.2 | 72.6±0.8 | 73.1±1.1 | 0.010 |
| During environmental (cold) stress | 72.0±1.3 | 76.4±0.8 | 75.7±1.2 | 0.002 |
Bold: p<0.05. “()”: n.
Heritability (h^2), Rho_G, and Rho_E were estimated from twin pair (MZ versus DZ) variance components in SOLAR. Bold: p<0.05.
Model: A_allele recessive; covariates: age, sex, ancestry. Bold: p<0.05.
Figure 2. CYB561 3’-UTR variant A+1485G and human physiology.
Analyses in twin and sibling pairs were adjusted for age and sex.
2A. Heritability of basal and environmental (cold) stress HR traits in twin pairs.
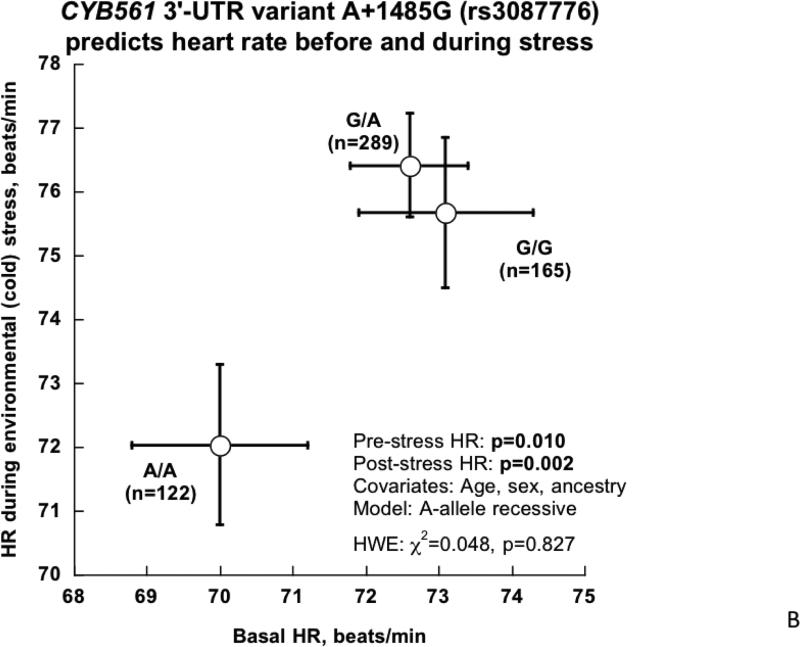
2B. CYB561 3’-UTR variant A+1485G and HR before and during environmental (cold) stress in twins and siblings.
In the overall group, HR rose by an average of 3.2±0.39 beats/min (p=1.92E-13) (Table 1a). Variance component analyses (Table 1b) indicated that basal (resting) HR was under substantial genetic control, with h2 =50.9±6.4% of trait variance (p=2.47E-10), as was HR post-stress, at h2=55.1±5.9% (p=8.71E-13), while the change during stress was somewhat less heritable, though still highly significant (h2 =40.7±6.9%, p=1.00E-07). While basal and post-stress HR were directly correlated (Spearman r=0.682, p=2.73E-75) and shared substantial genetic co-determination (or pleiotropy; rG=0.747±0.058, p=2.85E-09). By contrast, change in HR was inversely correlated with basal HR (Spearman r=−0.299, p=1.94E-13), and the two shared principally environmental co-determination (rE=−0.443±0.068, p=2.46E-08).
CYB561 3’-UTR polymorphism A+1485G (rs3087776): Effects on heritable HR traits (Table 1, Figure 2B)
In the twin/sibling sample, the 3’-UTR A+1485G polymorphism predicted both basal (p=0.010) and post-stress (p=0.002) HR (Table 1c, Figure 2B). Minor allele (A/A) homozygotes displayed clearly lower values for both basal and stressed HR (Figure 2B), suggesting a recessive mode of inheritance of the A-allele on HR traits. Change in HR (delta, pre→post stress) was not influenced by A+1485G (p=0.752).
We previously reported (4) that a “tagging” genetic variant at CYB561 (rs2058203, intron-1) predicted the human vasoconstrictive response to endogenous in vivo catecholamine release. This CYB561 tagging variant (rs2058203, intron-1) also predicted HR in the current study, with identical p-value to rs3087776 (3’-UTR), perhaps not surprising in that the two SNPs are separated by only 12,726 bp, and are in perfect (100%, R^2=1.0) linkage disequilibrium in the current subjects.
Extension studies of CYB561 rs3087776 on basal HR in additional population samples
Meta-analysis combining the twin/sibling pairs and two additional independent population samples (Kaiser-1 and Kaiser-2) indicated allelic effects consistent in direction (- sign on slope) across groups; the overall slope of the meta-analysis regression (beta) = −0.899, with SE (of beta) = 0.327, achieved significance at p=0.007 (Table 2). Congruent with the consistent negative slope in each group, there was no demonstrable heterogeneity across the groups (Q=2.212, df=2 [i.e., n-1], p=0.331); nonetheless, inspection of individual study p-values (Table 2) suggests that the twin/sibling study is the most important contributor to the overall meta-analysis results.
Table 2. Effects of CYB561 3'-UTR variant G+1485A on basal/resting heart rate in multiple independent groups: Meta-analysis.
Effect sizes (beta, SE) were determined by regression (SPSS) in each of the three contributory groups, with meta-analysis in STATA.
| Group | Alleles, Minor/Major | Minor allele frequency | N | Trait | Model | Beta (slope per allele) | SE (of beta) | P-value |
|---|---|---|---|---|---|---|---|---|
| Twins/sibs | A/G | 0.475 | 576 | Heart rate | Additive | −1.636 | 0.627 | 0.009265 |
| Kaiser-1 | A/G | 0.454 | 926 | Heart rate | Additive | −0.412 | 0.544 | 0.449 |
| Kaiser-2 | A/G | 0.486 | 1077 | Heart rate | Additive | −1.30 | 0.863 | 0.136 |
| Meta-result | A/G | - | 2579 | Heart rate | Additive | −0.899 | 0.327 | 0.007 |
Groups: Kaiser-1, Kaiser-2: Independent samples (−1,−2) from a large white primary care
Bold: meta-result. Analyses in twins were adjusted for age and sex (as well as ancestry), while results in the Kaiser subjects were adjusted for age and sex.
Functional studies of the trait-associated variant: Human CYB561 3’-UTR variant and expression in transfected cells
CYB561 3’-UTR reporter activity assays
To probe the functional significance of common variant rs3087776, we inserted each of the two versions (G versus A alleles) into the luciferase reporter plasmid pGL3-Promoter, just downstream (3’) from the luciferase open reading frame (Supplementary Figure 2). After transfection into rat chromaffin (PC12) cells, the two alleles yielded substantially different luciferase reporter activities (A<G) at each time point (Figure 3A).
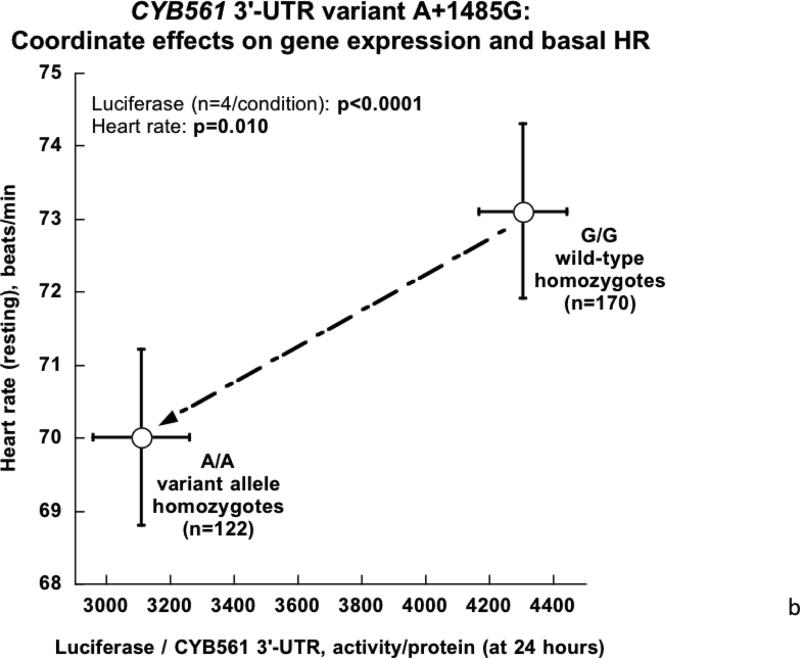
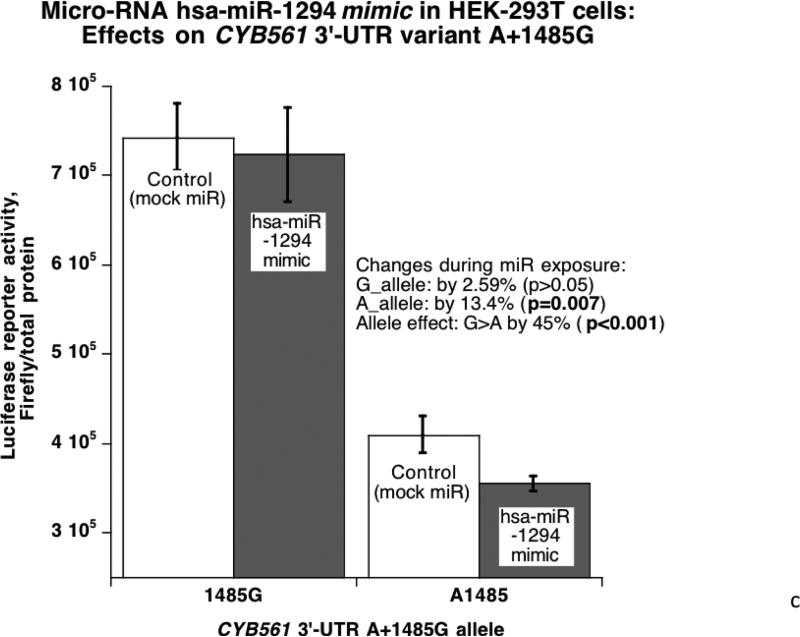
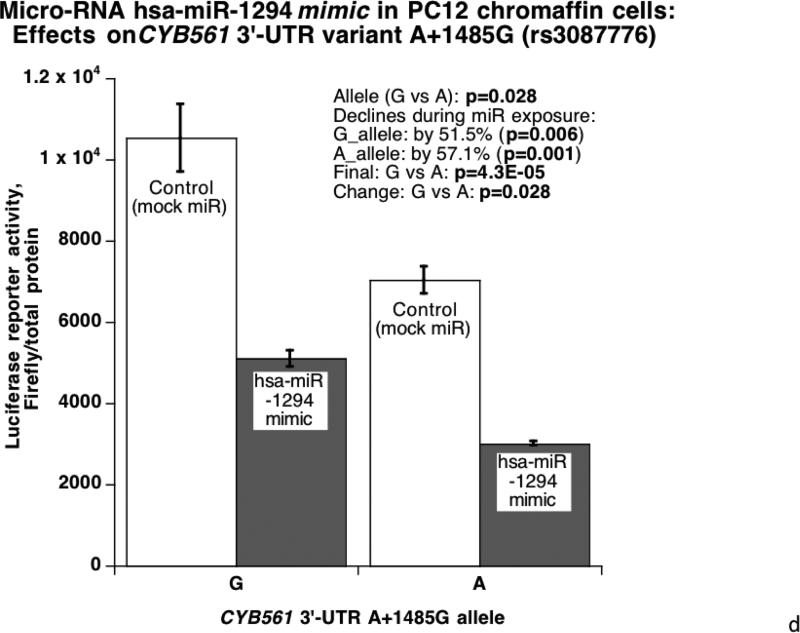
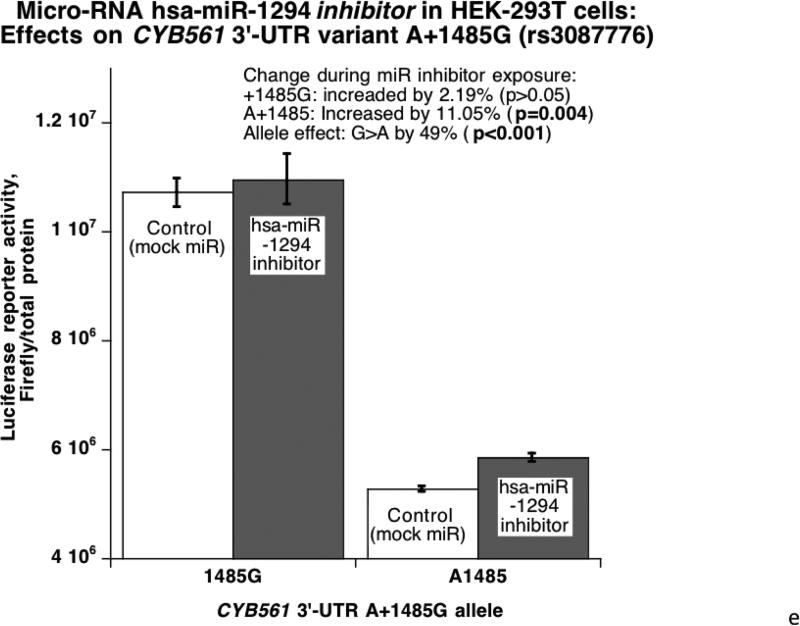
Figure 3. Human CYB561 3’-UTR variant A+1485G: Functional studies in cells transfected with a chimeric luciferase / human CYB561 3’-UTR reporter.
3A. Effect of 3’-UTR allele on gene expression in PC12 chromaffin cells. The A-allele displays lower gene expression at each time point (p<0.001).
3B. CYB561 3’-UTR variant A+1485G: Coordinate effects on gene expression in cells as well as HR in humans in vivo. Cellular data are from transfection (Figure 3A), while in vivo data are from human HR in twin pairs (Figure 2). Results are plotted for an A-allele recessive model.
3C. Micro-RNA hsa-miR-1294 mimic and CYB561: Effects on 3’-UTR variant A+1485G in HEK-293T cells. The A-allele, with a better match for the hsa-miR-1294 motif, experiences a more substantial decline of gene expression when co-transfected with the hsa-miR-1294 mimic.
3D. Micro-RNA hsa-miR-1294 mimic and CYB561: Effects on 3’-UTR variant A+1485G in PC12 chromaffin cells. The experimental protocol proceeds as in Figure 3C.
3E. Micro-RNA hsa-miR-1294 inhibition and CYB561: Effects on 3’-UTR variant A+1485G in HEK-293T cells. The A-allele, with a better match for the hsa-miR-1294 motif, experienced a more substantial increase of reporter activity when co-transfected with the hsa-miR-1294 inhibitor.
CYB5613’-UTR variant A+1485G: Coordinate directional functions in cells and in vivo (twins/sibling pairs)
Taking advantage of in vivo (Figure 2B) and cellular (Figure 3A) data, we observe that the A/A genotype, which predicts lower HR in the twins/sibling pairs, also displays lower gene expression (Figure 3B).
Micro-RNA effects: hsa-miR-1294 mimicry and inhibition on luciferase reporter/human CYB561 3’-UTR transfections
Since A+1485G disrupted a hsa-miR-1294 recognition motif with a superior match for the A-allele (Figure 1C), we probed the functional significance of the match with a specific miR mimic and inhibitor (versus control oligonucleotide) cotransfected with the luciferase/human 3’-UTR allele reporter plasmids into HEK-293T or PC12 cells. In HEK-293T cells (Figure 3C), the alleles were expressed differentially (G>A, p<0.001), and exogenous/cotransfected hsa-miR-1294 mimic decreased reporter expression more effectively on the A-allele (p=0.007). Likewise PC12 chromaffin cells (Figure 3D) displayed an expression difference between alleles (G>A, p=0.028), and the difference was amplified on the A-allele (57.1% decline [p=0.001] versus 51.5% for the G-allele [p=0.006]). The substantial (indeed magnified) difference in expression after exposure of each allele to the micro-RNA (p=4.3E-05) further documents the role of hsa-miR-1294. Upon exposure of transfected HEK-293T cells to a miR-1294 inhibitor (antagomir) (Figure 3E), reporter expression was increased more effectively on the A-allele (by 11%, p=0.004).
Micro-RNA effects: hsa-miR-1294 mimicry on human CYB561 mRNA expression with its cognate 3’-UTR
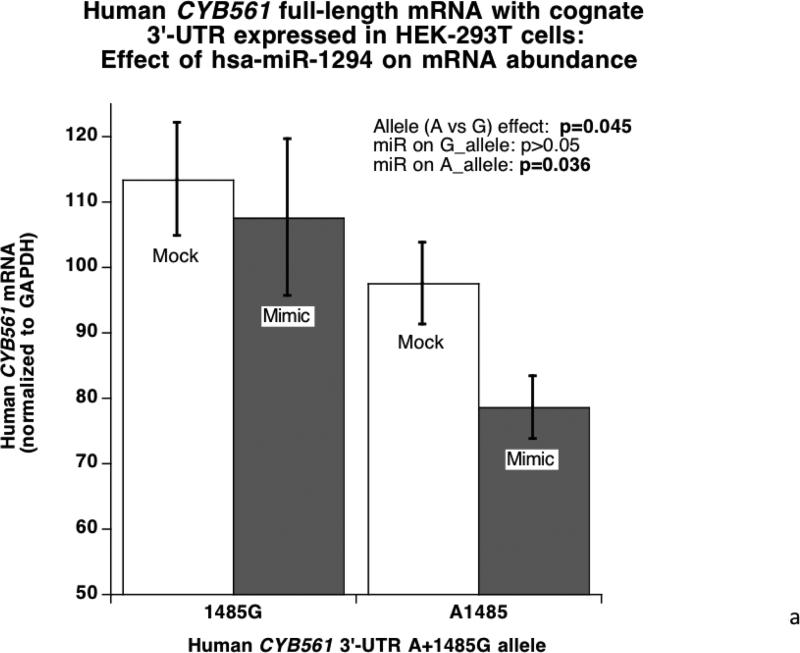
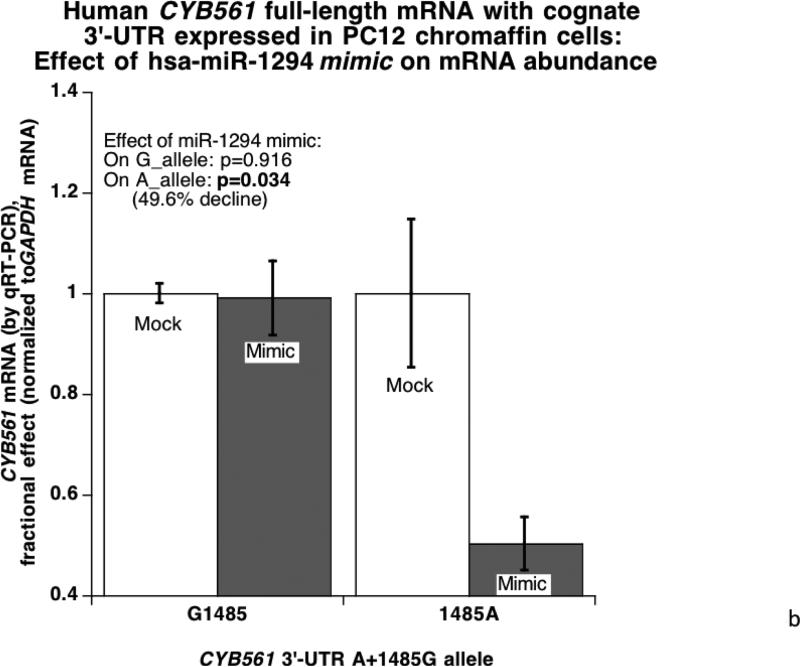
When the full-length human CYB561 with its cognate 3’-UTR was expressed in HEK-293T cells (Figure 4A), the 3’-UTR alleles were expressed differentially (G>A, p=0.045), and the mir-1294 mimic preferentially inhibited the A-allele (p=0.036). Likewise, upon expression in PC12 chromaffin cells (Figure 4B) the miR-1294 mimic preferentially inhibited the 3’-UTR A-allele (p=0.034), though basal expression of the two alleles was similar.
Figure 4. CYB561 mRNA: Effects of miR-1294.
4A. Micro-RNA effects in HEK-293T cells: hsa-miR-1294 mimicry on human CYB561 mRNA expression with its cognate 3’-UTR. The full-length human CYB561 mRNA, with its cognate 3’-UTR, was expressed under control of a eukaryotic pCMV promoter in HEK-293T cells, with or without co-transfection of a miR-1294 mimic. CYB561 mRNA abundance was quantified by qRT-PCR, and normalized to GAPDH mRNA.
4B. Micro-RNA effects in PC12 chromaffin cells: hsa-miR-1294 mimicry on human CYB561 mRNA expression with its cognate 3’-UTR. The experimental protocol proceeds as in Figure 4A.
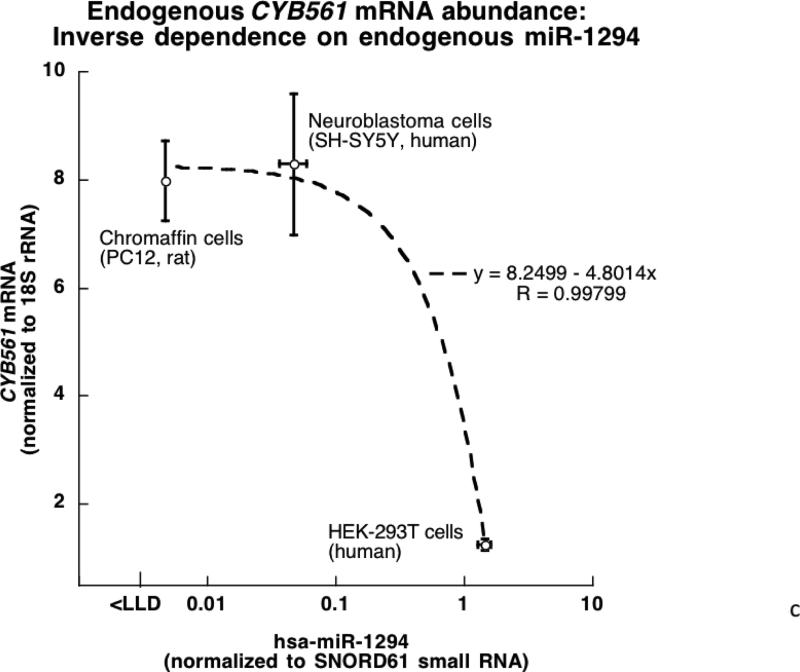
4C. Endogenous RNAs in model neuronal/neuroendocrine cells: CYB561 mRNA and miR-1294 small RNA. RNAs were quantified by qRT-PCR, normalizing mRNA results to 18S rRNA and miR results to small RNA SNORD61. The lower limit of detection (LLD) is ~10 molecules per qRT-PCR reaction.
Endogenous RNAs in model neuronal/neuroendocrine cells: CYB561 mRNA and miR-1294 small RNA
We quantified endogenous abundance of the pertinent RNAs by qRT-PCR in untransfected neural/neuroendocrine cells (PC12, SH-SY5Y, and HEK-293T), normalizing mRNA results to 18S rRNA and miR results to small RNA SNORD61 (Figure 4C). In general there was an inverse proportionality for the mRNA and miR (R=0.998, p=0.0001), with the mRNA reaching a plateau at low miR abundance, suggesting the expected dependence of target mRNA expression on the specific miR.
CYB561 cis-eQTL
3’-UTR variant A+1485G (rs3087776) predicted CYB561 mRNA mRNA expression in human brain frontal cortex, at p=3.14×10−08, accounting for ~20% of the variance of mRNA abundance in the 143 samples (R2=0.1958). No trans-QTLs were detected for CYB561, even down to a permissive p<0.01 threshold.
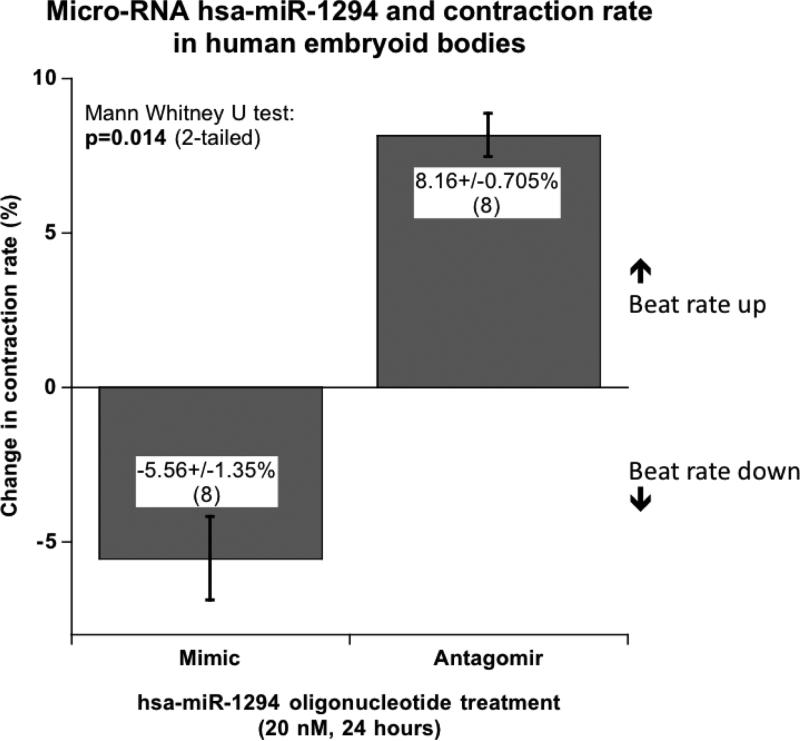
Contraction rate effects of hsa-miR-1294 in vivo: Human embryoid body cultures
When exposed to 20 nM miR-1294 mimic oligonucleotide for 24 hours, the proto-hearts decreased contraction rate by 5.6±1.4%, while the miR-1294 antagomir (inhibitor, again at 20 nM) elevated contraction rate by 8.2±0.7%, resulting in a significant difference between treatments (p=0.014, Figure 5).
Figure 5. Contraction rate effects of hsa-miR-1294 in vivo: Human embryoid body cultures.
Embryoid bodies (with visibly beating proto-hearts) were transfected at 20 nM for 24 hours (with 8 replicate wells per condition), with either miR-1294 double-stranded mimic, single-stranded antagomir, or control oligonucleotide. The control oligonucleotide did not alter contraction rate (−1.9±2.9% change, p not significant).
DISCUSSION
Overview
HR not only predicts survival (1,2) and reflects cardiovascular performance, but also serves as an integrated indicator of autonomic nervous (sympathetic and parasympathetic/vagal) system activity and metabolic rate (2). Genome wide association studies (GWAS) have now identified up to 21 genetic loci that contribute significantly to resting HR (32); although two of these loci are in the classical parasympathetic pathway (ACHE, CHRM2), none of the genes encode catecholamine biosynthetic enzymes or adrenergic receptors, and the identified loci so far account for only ~0.9% of trait variance. By contrast, twin studies indicate that heredity contirbutes up to ~50% of HR variability; indeed, our twin results suggest HR heritability of 50.9±64% of trait variance (p=2.47E-10, Figure 2A). The discrepancy between the two approaches is sometimes referred to as the “missing heritability problem” (33), and prompts a continuing search for responsible loci, in this case exploring the role of genetic variation in a contributory physiological pathway.
The autonomic system serves as a “master regulator” of diverse organ function, and autonomic dysregulation can manifest in a variety of ways. Since increased sympathetic tone may contribute to HR elevation (3), and “tagging” genetic variation at the CYB561 locus may predict responses to pre-synaptic stimulation in humans (4), here we examined the association of sympathetic pathway gene CYB561 with HR traits, focusing on functional (rather than simply “tagging”) genetic variation. Already, it was known that genetic variation across the adrenergic pathway – GCH1 (5), TH (6), DBH (7), PNMT (8), and ADRB1 (9) – predicted control of HR; thus, we focused here on CYB561. We found that common genetic variation in the CYB561 was confined to the 3’-UTR at A+1485G, which disrupted a micro-RNA recognition motif. In twin pairs, A+1485G predicted both basal and stress-augmented HR, two highly heritable traits (Figure 2).
CYB561 (cytochrome b561)
Cytochrome b561 (Uniprot P49447; named because of its optical absorbance at 561 nm) is a monomeric, 251-amino acid ~28 kDa protein with 6 trans-membrane-spanning domains, which functions as an electron shuttle for dopamine beta-hydroxylase (DBH) in the catecholamine vesicle (chromaffin granule) membrane, wherein it is essential to catecholamine biosynthesis and hence the effects of these amines (34). CYB561 subserves catecholamine biosynthesis by accepting electrons from semidehydroascorbate, and thus regenerating ascorbate for its role as electron acceptor (as enzyme cofactor) during oxidative conversion of dopamine to norepinephrine by dopamine beta-hydroxylase (DBH) within catecholamine storage vesicles. Since the ascorbate/dehydroascorbate shuttle also subserves the action of the endocrine secretory vesicle enzyme peptidyl-alpha-amidating monooxygenase (PAM), the synthesis of carboxy-terminal amidated peptide transmitters (such as neuropeptide Y [NPY], pancreastatin, PHI [peptide histidine-isoleucine], peptide tyrosine-tyrosine [PYY], and NPK) is also dependent upon CYB561 (35). CYB561 has also been described as an electron shuttle in macrophage lysosomal membranes (36).
The di-heme-binding CYB561 amino acid sequence is highly conserved across species (37,38) with 6 transmembrane helices of which helices 2-6 are located closely in the same regions of the 26 sequences in the alignment. Indeed, we observed that the local region of the CYB561 3’-UTR harboring the hsa-miR-1294 motif is also substantially conserved (Figure 1B).
Role of micro-RNA miR-1294 motif in the CYB561 3’-UTR
Isolation of the CYB561 3’-UTR variant onto a luciferase reporter allowed functional studies, confirming the role of A+1485G in differential responses to hsa-miR-1294 (Figure 3), thus providing a mechanistic basis for the clinical findings, and the differential allelic effects were confirmed during eukaryotic expression of full-length human CYB561 mRNA with its cognate 3’-UTR (Figure 4). The apparent recessive effect of A/A homozygosity on HR traits (Figure 2B) suggests that the A-allele may be rate-limiting in determining the formation or effect of CYB561_mRNA:miR-1294 complexes, a proposal consistent with the superior match of the A-allele motif with miR-1294.
Differential 3’-UTR allelic (A/G) effects on CYB561 mRNA abundance (Figures 4A&4B), despite the use of identical promoters (pCMV) for cDNA/mRNA expression, suggest that a decline in mRNA stability is likely for the A-allele, in the face of the enhanced miR:mRNA match for the A-allele; alterations in mRNA translation by the miR are also likely, as a result of steric hindrance of the translational machinery by the mRNA-bound miR. The RNA components of this system (CYB561 mRNA, and miR-1294 small RNA) were endogenously co-expressed in model cell lines. Finally, exposure of human embryoid bodies to miR-1294 mimic or antagomir oligonucleotides yielded directionally opposite effects on contraction rate in beating proto-hearts (Figure 5).
Advantages and limitations of this study
We took advantage of the twin pair approach to establish heritability of HR traits (Table 1, Figure 2), and then characterize the effect of sympathetic pathway variation at the CYB561 locus on such traits. We then observed directionally similar CYB561-on-trait effects in two additional population samples, with preserved significance (Table 2). Later we were able to confirm the functional effects of 3’-UTR variation upon responses to a particular micro-RNA, using a transfected chimeric 3’-UTR reporter system (Figure 3), as well as the cognate (CYB561) mRNA (Figure 4), and finally we observed reciprocal effects of miR-1294 mimic and antagomir on contraction rate in human embryoid bodies in vivo (Figure 5). Nonetheless, additional questions arise during this work. For example, CYB561 acts as an electron shuttle in service to not only DBH (dopamine beta-hydroxylase), but also PAM (peptidyl alpha-amidating monooxygenase), and hence might exert effects through peptidergic in addition to catecholaminergic pathways. In addition, we have not yet documented the effects of miR-1294 on heart rate in freely living organisms. Finally, hsa-miR-1294 likely has additional mRNA targets in the transcriptome; indeed, algorithms such as TargetScan <http://www.targetscan.org/> requiring only very short miR:mRNA matches at 7-8 bp miR “seed sequences” (39), identify many potential mRNA targets for hsa-miR-1294; the functional importance of such very short, partial matches at these loci has not been explored.
Conclusions and perspectives
In conclusion, a very common (MAF ~45-49%) genetic variant in the CYB561 3’-UTR alters responses to a specific micro-RNA (hsa-miR-1294), ultimately leading to heritable changes in both basal and stress-augmented HR. Supplementary Figure 3 presents a hypothetical schema outlining a step-by-step pathway in series that may unify our observations, as well as suggest avenues for further experimentation.
Supplementary Material
Acknowledgments
Support/funding: National Institutes of Health [HL58120; DK094894; UL1RR031980 (UCSD Clinical and Translational Research Institute); MD000220 (UCSD Comprehensive Research Center in Health Disparities, CRCHD); 5U01HL107442], Department of Veterans Affairs.
Abbreviations
- A+1485G
SNP (rs3087776) in the human CYB561 3’-UTR
- CYB561
Cytochrome b561 (electron shuttle for dopamine beta-hydroxylase)
- DZ
Dizygotic (twin)
- GWAS
Genome Wide Association Study
- hsa-miR-1294
Human micro-RNA-1294
- h2
Heritability (fraction or % of phenotypic variance accounted for by genetic variance)
- HR
Heart rate
- MAF
Minor Allele Frequency
- miR, miRNA
Micro-RNA
- MZ
Monozygotic (twin)
- 3’-UTR
3’ (downstream of the open reading frame) untranslated region (of the human CYB561 mRNA)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None to disclose.
REFERENCES
- 1.Greenland P, Daviglus ML, Dyer AR, et al. Resting heart rate is a risk factor for cardiovascular and noncardiovascular mortality: the Chicago Heart Association Detection Project in Industry. American journal of epidemiology. 1999;149:853–62. doi: 10.1093/oxfordjournals.aje.a009901. [DOI] [PubMed] [Google Scholar]
- 2.Zhang GQ, Zhang W. Heart rate, lifespan, and mortality risk. Ageing research reviews. 2009;8:52–60. doi: 10.1016/j.arr.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Palatini P, Julius S. Elevated heart rate: a major risk factor for cardiovascular disease. Clin Exp Hypertens. 2004;26:637–44. doi: 10.1081/ceh-200031959. [DOI] [PubMed] [Google Scholar]
- 4.Fung MM, Nguyen C, Mehtani P, et al. Genetic variation within adrenergic pathways determines in vivo effects of presynaptic stimulation in humans. Circulation. 2008;117:517–25. doi: 10.1161/CIRCULATIONAHA.107.706317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L, Rao F, Zhang K, et al. Discovery of common human genetic variants of GTP cyclohydrolase 1 (GCH1) governing nitric oxide, autonomic activity, and cardiovascular risk. J Clin Invest. 2007;117:2658–71. doi: 10.1172/JCI31093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L, Rao F, Wessel J, et al. Functional allelic heterogeneity and pleiotropy of a repeat polymorphism in tyrosine hydroxylase: prediction of catecholamines and response to stress in twins. Physiol Genomics. 2004;19:277–91. doi: 10.1152/physiolgenomics.00151.2004. [DOI] [PubMed] [Google Scholar]
- 7.Garland EM, Black BK, Harris PA, Robertson D. Dopamine-beta-hydroxylase in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2007;293:H684–90. doi: 10.1152/ajpheart.01389.2006. [DOI] [PubMed] [Google Scholar]
- 8.Jirout ML, Friese RS, Mahapatra NR, et al. Genetic regulation of catecholamine synthesis, storage and secretion in the spontaneously hypertensive rat. Hum Mol Genet. 2010;19:2567–80. doi: 10.1093/hmg/ddq135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilk JB, Myers RH, Pankow JS, et al. Adrenergic receptor polymorphisms associated with resting heart rate: the HyperGEN Study. Ann Hum Genet. 2006;70:566–73. doi: 10.1111/j.1469-1809.2005.00258.x. [DOI] [PubMed] [Google Scholar]
- 10.Duong LT, Fleming PJ. Isolation and properties of cytochrome b561 from bovine adrenal chromaffin granules. J Biol Chem. 1982;257:8561–4. [PubMed] [Google Scholar]
- 11.Pruss RM, Shepard EA. Cytochrome b561 can be detected in many neuroendocrine tissues using a specific monoclonal antibody. Neuroscience. 1987;22:149–57. doi: 10.1016/0306-4522(87)90205-3. [DOI] [PubMed] [Google Scholar]
- 12.Apps DK, Pryde JG, Phillips JH. Cytochrome b561 is identical with chromomembrin B, a major polypeptide of chromaffin granule membranes. Neuroscience. 1980;5:2279–87. doi: 10.1016/0306-4522(80)90143-8. [DOI] [PubMed] [Google Scholar]
- 13.Srivastava M, Duong LT, Fleming PJ. Cytochrome b561 catalyzes transmembrane electron transfer. J Biol Chem. 1984;259:8072–5. [PubMed] [Google Scholar]
- 14.Wu T, Treiber FA, Snieder H. Genetic influence on blood pressure and underlying hemodynamics measured at rest and during stress. Psychosom Med. 2013;75:404–12. doi: 10.1097/PSY.0b013e31828d3cb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen K, Rajewsky N. The evolution of gene regulation by transcription factors and microRNAs. Nat Rev Genet. 2007;8:93–103. doi: 10.1038/nrg1990. [DOI] [PubMed] [Google Scholar]
- 16.Rao F, Zhang L, Wessel J, et al. Tyrosine hydroxylase, the rate-limiting enzyme in catecholamine biosynthesis: discovery of common human genetic variants governing transcription, autonomic activity, and blood pressure in vivo. Circulation. 2007;116:993–1006. doi: 10.1161/CIRCULATIONAHA.106.682302. [DOI] [PubMed] [Google Scholar]
- 17.O'Connor DT, Kailasam MT, Kennedy BP, Ziegler MG, Yanaihara N, Parmer RJ. Early decline in the catecholamine release-inhibitory peptide catestatin in humans at genetic risk of hypertension. J Hypertens. 2002;20:1335–45. doi: 10.1097/00004872-200207000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Waalen J, Felitti V, Gelbart T, Ho NJ, Beutler E. Prevalence of coronary heart disease associated with HFE mutations in adults attending a health appraisal center. Am J Med. 2002;113:472–9. doi: 10.1016/s0002-9343(02)01249-4. [DOI] [PubMed] [Google Scholar]
- 19.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Libiger O, Nievergelt CM, Schork NJ. Comparison of genetic distance measures using human SNP genotype data. Hum Biol. 2009;81:389–406. doi: 10.3378/027.081.0401. [DOI] [PubMed] [Google Scholar]
- 22.Nievergelt CM, Libiger O, Schork NJ. Generalized analysis of molecular variance. PLoS Genet. 2007;3:e51. doi: 10.1371/journal.pgen.0030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Do KA, Broom BM, Kuhnert P, et al. Genetic analysis of the age at menopause by using estimating equations and Bayesian random effects models. Stat Med. 2000;19:1217–35. doi: 10.1002/(sici)1097-0258(20000515)19:9<1217::aid-sim421>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 24.Gibbs JR, van der Brug MP, Hernandez DG, et al. Abundant quantitative trait loci exist for DNA methylation and gene expression in human brain. PLoS Genet. 2010;6:e1000952. doi: 10.1371/journal.pgen.1000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahapatra NR, Mahata M, Datta AK, et al. Neuroendocrine cell type-specific and inducible expression of the chromogranin B gene: crucial role of the proximal promoter. Endocrinology. 2000;141:3668–78. doi: 10.1210/endo.141.10.7725. [DOI] [PubMed] [Google Scholar]
- 26.Mahapatra NR, Mahata M, Ghosh S, Gayen JR, O'Connor DT, Mahata SK. Molecular basis of neuroendocrine cell type-specific expression of the chromogranin B gene: Crucial role of the transcription factors CREB, AP-2, Egr-1 and Sp1. J Neurochem. 2006;99:119–33. doi: 10.1111/j.1471-4159.2006.04128.x. [DOI] [PubMed] [Google Scholar]
- 27.Shaw G, Morse S, Ararat M, Graham FL. Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. FASEB J. 2002;16:869–71. doi: 10.1096/fj.01-0995fje. [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Huang HY, Chien CH, Jen KH, Huang HD. RegRNA: an integrated web server for identifying regulatory RNA motifs and elements. Nucleic Acids Res. 2006;34:W429–34. doi: 10.1093/nar/gkl333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kruger J, Rehmsmeier M. RNAhybrid: microRNA target prediction easy, fast and flexible. Nucleic Acids Res. 2006;34:W451–4. doi: 10.1093/nar/gkl243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang L, Soonpaa MH, Adler ED, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–8. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 32.den Hoed M, Eijgelsheim M, Esko T, et al. Identification of heart rate-associated loci and their effects on cardiac conduction and rhythm disorders. Nat Genet. 2013;45:621–31. doi: 10.1038/ng.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manolio TA, Collins FS, Cox NJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–53. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McBride OW, Yi HF, Srivastava M. The human cytochrome b561 gene (CYB561) is located at 17q11-qter. Genomics. 1994;21:662–3. doi: 10.1006/geno.1994.1332. [DOI] [PubMed] [Google Scholar]
- 35.Eipper BA, Mains RE. Peptide alpha-amidation. Annual review of physiology. 1988;50:333–44. doi: 10.1146/annurev.ph.50.030188.002001. [DOI] [PubMed] [Google Scholar]
- 36.Zhang DL, Su D, Berczi A, Vargas A, Asard H. An ascorbate-reducible cytochrome b561 is localized in macrophage lysosomes. Biochimica et biophysica acta. 2006;1760:1903–13. doi: 10.1016/j.bbagen.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 37.Bashtovyy D, Berczi A, Asard H, Pali T. Structure prediction for the di-heme cytochrome b561 protein family. Protoplasma. 2003;221:31–40. doi: 10.1007/s00709-002-0065-0. [DOI] [PubMed] [Google Scholar]
- 38.Tsubaki M, Takeuchi F, Nakanishi N. Cytochrome b561 protein family: expanding roles and versatile transmembrane electron transfer abilities as predicted by a new classification system and protein sequence motif analyses. Biochimica et biophysica acta. 2005;1753:174–90. doi: 10.1016/j.bbapap.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 39.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.