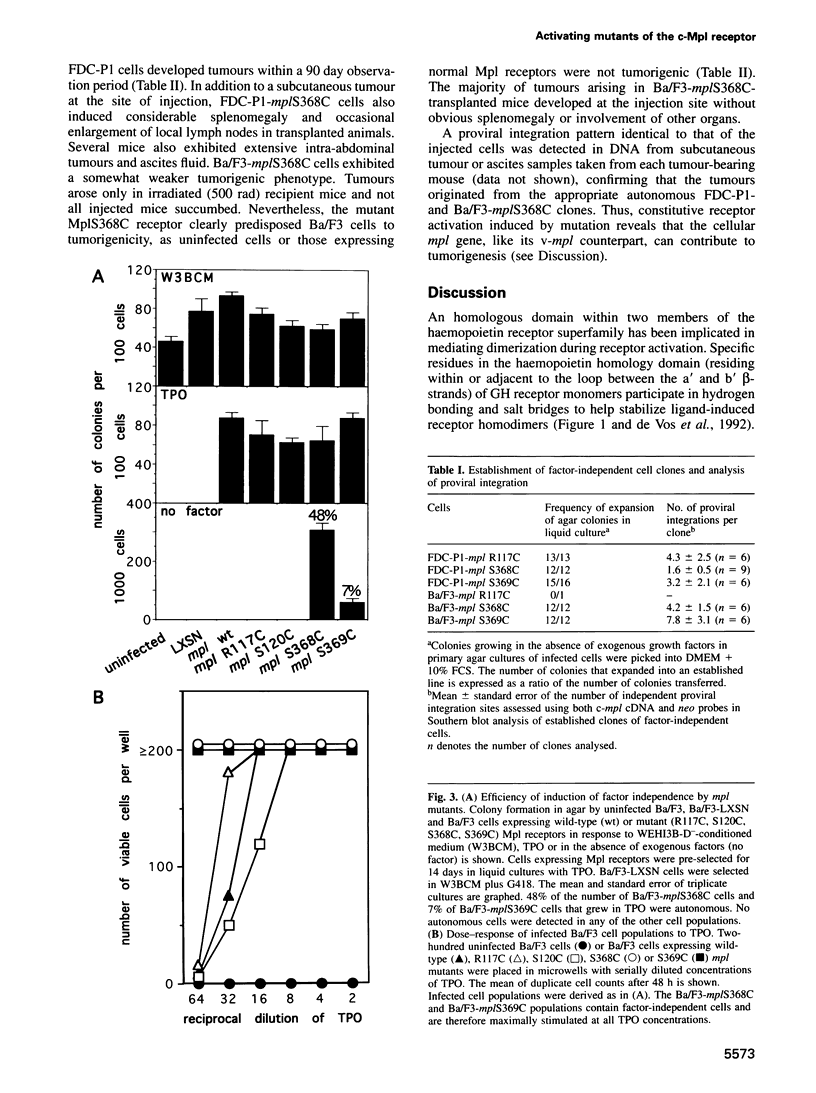
Abstract
c-Mpl, a receptor for thrombopoietin (TPO), belongs to the haemopoietin/cytokine receptor superfamily, a group of cell surface molecules characterized by conserved sequence motifs within their ligand binding domains. A recurring mechanism for the activation of haemopoietin receptors is the formation of functional complexes by receptor subunit oligomerization. Within the growth hormone receptor, a cluster of extracellular amino acids forms a dimer interface domain that stabilizes ligand-induced homodimers. This domain appears to be functionally conserved in the erythropoietin (EPO) receptor because substitution of cysteines for residues in the analogous region causes EPO-independent receptor activation via disulfide-linked homodimerization. This report identifies an homologous domain within the c-Mpl receptor. The substitution of cysteine residues for specific amino acids in the dimer interface homology regions of c-Mpl induced constitutive receptor activity. Factor-dependent FDC-P1 and Ba/F3 cells expressing the active receptor mutants no longer required exogenous factors and proliferated autonomously. The results imply that the normal process of TPO-stimulated Mpl activation occurs through receptor homodimerization and is mediated by a conserved haemopoietin receptor dimer interface domain. Moreover, cells expressing activated mutant Mpl receptors were tumorigenic in transplanted mice. Thus, like v-mpl, its viral counterpart, mutated forms of the cellular mpl gene also have oncogenic potential.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander W. S., Dunn A. R. Structure and transcription of the genomic locus encoding murine c-Mpl, a receptor for thrombopoietin. Oncogene. 1995 Feb 16;10(4):795–803. [PubMed] [Google Scholar]
- Bartley T. D., Bogenberger J., Hunt P., Li Y. S., Lu H. S., Martin F., Chang M. S., Samal B., Nichol J. L., Swift S. Identification and cloning of a megakaryocyte growth and development factor that is a ligand for the cytokine receptor Mpl. Cell. 1994 Jul 1;77(7):1117–1124. doi: 10.1016/0092-8674(94)90450-2. [DOI] [PubMed] [Google Scholar]
- Baumann H., Gearing D., Ziegler S. F. Signaling by the cytoplasmic domain of hematopoietin receptors involves two distinguishable mechanisms in hepatic cells. J Biol Chem. 1994 Jun 10;269(23):16297–16304. [PubMed] [Google Scholar]
- Bazan J. F. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broudy V. C., Lin N. L., Kaushansky K. Thrombopoietin (c-mpl ligand) acts synergistically with erythropoietin, stem cell factor, and interleukin-11 to enhance murine megakaryocyte colony growth and increases megakaryocyte ploidy in vitro. Blood. 1995 Apr 1;85(7):1719–1726. [PubMed] [Google Scholar]
- Cosman D., Lyman S. D., Idzerda R. L., Beckmann M. P., Park L. S., Goodwin R. G., March C. J. A new cytokine receptor superfamily. Trends Biochem Sci. 1990 Jul;15(7):265–270. doi: 10.1016/0968-0004(90)90051-c. [DOI] [PubMed] [Google Scholar]
- Courtois G., Bénit L., Mikaeloff Y., Pauchard M., Charon M., Varlet P., Gisselbrecht S. Constitutive activation of a variant of the env-mpl oncogene product by disulfide-linked homodimerization. J Virol. 1995 May;69(5):2794–2800. doi: 10.1128/jvi.69.5.2794-2800.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham B. C., Ultsch M., De Vos A. M., Mulkerrin M. G., Clauser K. R., Wells J. A. Dimerization of the extracellular domain of the human growth hormone receptor by a single hormone molecule. Science. 1991 Nov 8;254(5033):821–825. doi: 10.1126/science.1948064. [DOI] [PubMed] [Google Scholar]
- Davis S., Aldrich T. H., Stahl N., Pan L., Taga T., Kishimoto T., Ip N. Y., Yancopoulos G. D. LIFR beta and gp130 as heterodimerizing signal transducers of the tripartite CNTF receptor. Science. 1993 Jun 18;260(5115):1805–1808. doi: 10.1126/science.8390097. [DOI] [PubMed] [Google Scholar]
- Debili N., Wendling F., Cosman D., Titeux M., Florindo C., Dusanter-Fourt I., Schooley K., Methia N., Charon M., Nador R. The Mpl receptor is expressed in the megakaryocytic lineage from late progenitors to platelets. Blood. 1995 Jan 15;85(2):391–401. [PubMed] [Google Scholar]
- Dexter T. M., Garland J., Scott D., Scolnick E., Metcalf D. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. 1980 Oct 1;152(4):1036–1047. doi: 10.1084/jem.152.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duquesnoy P., Sobrier M. L., Duriez B., Dastot F., Buchanan C. R., Savage M. O., Preece M. A., Craescu C. T., Blouquit Y., Goossens M. A single amino acid substitution in the exoplasmic domain of the human growth hormone (GH) receptor confers familial GH resistance (Laron syndrome) with positive GH-binding activity by abolishing receptor homodimerization. EMBO J. 1994 Mar 15;13(6):1386–1395. doi: 10.1002/j.1460-2075.1994.tb06392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elberg G., Kelly P. A., Djiane J., Binder L., Gertler A. Mitogenic and binding properties of monoclonal antibodies to the prolactin receptor in Nb2 rat lymphoma cells. Selective enhancement by anti-mouse IgG. J Biol Chem. 1990 Sep 5;265(25):14770–14776. [PubMed] [Google Scholar]
- Fuh G., Cunningham B. C., Fukunaga R., Nagata S., Goeddel D. V., Wells J. A. Rational design of potent antagonists to the human growth hormone receptor. Science. 1992 Jun 19;256(5064):1677–1680. doi: 10.1126/science.256.5064.1677. [DOI] [PubMed] [Google Scholar]
- Fukunaga R., Ishizaka-Ikeda E., Nagata S. Purification and characterization of the receptor for murine granulocyte colony-stimulating factor. J Biol Chem. 1990 Aug 15;265(23):14008–14015. [PubMed] [Google Scholar]
- Fukunaga R., Ishizaka-Ikeda E., Pan C. X., Seto Y., Nagata S. Functional domains of the granulocyte colony-stimulating factor receptor. EMBO J. 1991 Oct;10(10):2855–2865. doi: 10.1002/j.1460-2075.1991.tb07835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearing D. P., Comeau M. R., Friend D. J., Gimpel S. D., Thut C. J., McGourty J., Brasher K. K., King J. A., Gillis S., Mosley B. The IL-6 signal transducer, gp130: an oncostatin M receptor and affinity converter for the LIF receptor. Science. 1992 Mar 13;255(5050):1434–1437. doi: 10.1126/science.1542794. [DOI] [PubMed] [Google Scholar]
- Gearing D. P., King J. A., Gough N. M., Nicola N. A. Expression cloning of a receptor for human granulocyte-macrophage colony-stimulating factor. EMBO J. 1989 Dec 1;8(12):3667–3676. doi: 10.1002/j.1460-2075.1989.tb08541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney A. L., Carver-Moore K., de Sauvage F. J., Moore M. W. Thrombocytopenia in c-mpl-deficient mice. Science. 1994 Sep 2;265(5177):1445–1447. doi: 10.1126/science.8073287. [DOI] [PubMed] [Google Scholar]
- Hibi M., Murakami M., Saito M., Hirano T., Taga T., Kishimoto T. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990 Dec 21;63(6):1149–1157. doi: 10.1016/0092-8674(90)90411-7. [DOI] [PubMed] [Google Scholar]
- Hilton D. J., Hilton A. A., Raicevic A., Rakar S., Harrison-Smith M., Gough N. M., Begley C. G., Metcalf D., Nicola N. A., Willson T. A. Cloning of a murine IL-11 receptor alpha-chain; requirement for gp130 for high affinity binding and signal transduction. EMBO J. 1994 Oct 17;13(20):4765–4775. doi: 10.1002/j.1460-2075.1994.tb06802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper K. P., Padmanabhan R., Ebner K. E. Expression of the extracellular domain of the rat liver prolactin receptor and its interaction with ovine prolactin. J Biol Chem. 1993 Oct 25;268(30):22347–22352. [PubMed] [Google Scholar]
- Ip N. Y., Nye S. H., Boulton T. G., Davis S., Taga T., Li Y., Birren S. J., Yasukawa K., Kishimoto T., Anderson D. J. CNTF and LIF act on neuronal cells via shared signaling pathways that involve the IL-6 signal transducing receptor component gp130. Cell. 1992 Jun 26;69(7):1121–1132. doi: 10.1016/0092-8674(92)90634-o. [DOI] [PubMed] [Google Scholar]
- Kaushansky K., Broudy V. C., Lin N., Jorgensen M. J., McCarty J., Fox N., Zucker-Franklin D., Lofton-Day C. Thrombopoietin, the Mp1 ligand, is essential for full megakaryocyte development. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3234–3238. doi: 10.1073/pnas.92.8.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushansky K., Lok S., Holly R. D., Broudy V. C., Lin N., Bailey M. C., Forstrom J. W., Buddle M. M., Oort P. J., Hagen F. S. Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature. 1994 Jun 16;369(6481):568–571. doi: 10.1038/369568a0. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Taga T., Akira S. Cytokine signal transduction. Cell. 1994 Jan 28;76(2):253–262. doi: 10.1016/0092-8674(94)90333-6. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok S., Kaushansky K., Holly R. D., Kuijper J. L., Lofton-Day C. E., Oort P. J., Grant F. J., Heipel M. D., Burkhead S. K., Kramer J. M. Cloning and expression of murine thrombopoietin cDNA and stimulation of platelet production in vivo. Nature. 1994 Jun 16;369(6481):565–568. doi: 10.1038/369565a0. [DOI] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Methia N., Louache F., Vainchenker W., Wendling F. Oligodeoxynucleotides antisense to the proto-oncogene c-mpl specifically inhibit in vitro megakaryocytopoiesis. Blood. 1993 Sep 1;82(5):1395–1401. [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Miyajima A., Mui A. L., Ogorochi T., Sakamaki K. Receptors for granulocyte-macrophage colony-stimulating factor, interleukin-3, and interleukin-5. Blood. 1993 Oct 1;82(7):1960–1974. [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D. Subunit promiscuity among hemopoietic growth factor receptors. Cell. 1991 Oct 4;67(1):1–4. doi: 10.1016/0092-8674(91)90564-f. [DOI] [PubMed] [Google Scholar]
- Palacios R., Steinmetz M. Il-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985 Jul;41(3):727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- Skoda R. C., Seldin D. C., Chiang M. K., Peichel C. L., Vogt T. F., Leder P. Murine c-mpl: a member of the hematopoietic growth factor receptor superfamily that transduces a proliferative signal. EMBO J. 1993 Jul;12(7):2645–2653. doi: 10.1002/j.1460-2075.1993.tb05925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin A., Lemmon M. A., Ullrich A., Schlessinger J. Stabilization of an active dimeric form of the epidermal growth factor receptor by introduction of an inter-receptor disulfide bond. J Biol Chem. 1994 Apr 1;269(13):9752–9759. [PubMed] [Google Scholar]
- Souyri M., Vigon I., Penciolelli J. F., Heard J. M., Tambourin P., Wendling F. A putative truncated cytokine receptor gene transduced by the myeloproliferative leukemia virus immortalizes hematopoietic progenitors. Cell. 1990 Dec 21;63(6):1137–1147. doi: 10.1016/0092-8674(90)90410-g. [DOI] [PubMed] [Google Scholar]
- Vigon I., Dreyfus F., Melle J., Viguié F., Ribrag V., Cocault L., Souyri M., Gisselbrecht S. Expression of the c-mpl proto-oncogene in human hematologic malignancies. Blood. 1993 Aug 1;82(3):877–883. [PubMed] [Google Scholar]
- Vigon I., Florindo C., Fichelson S., Guenet J. L., Mattei M. G., Souyri M., Cosman D., Gisselbrecht S. Characterization of the murine Mpl proto-oncogene, a member of the hematopoietic cytokine receptor family: molecular cloning, chromosomal location and evidence for a function in cell growth. Oncogene. 1993 Oct;8(10):2607–2615. [PubMed] [Google Scholar]
- Vigon I., Mornon J. P., Cocault L., Mitjavila M. T., Tambourin P., Gisselbrecht S., Souyri M. Molecular cloning and characterization of MPL, the human homolog of the v-mpl oncogene: identification of a member of the hematopoietic growth factor receptor superfamily. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5640–5644. doi: 10.1073/pnas.89.12.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watowich S. S., Hilton D. J., Lodish H. F. Activation and inhibition of erythropoietin receptor function: role of receptor dimerization. Mol Cell Biol. 1994 Jun;14(6):3535–3549. doi: 10.1128/mcb.14.6.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watowich S. S., Yoshimura A., Longmore G. D., Hilton D. J., Yoshimura Y., Lodish H. F. Homodimerization and constitutive activation of the erythropoietin receptor. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2140–2144. doi: 10.1073/pnas.89.6.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner D. B., Liu J., Cohen J. A., Williams W. V., Greene M. I. A point mutation in the neu oncogene mimics ligand induction of receptor aggregation. Nature. 1989 May 18;339(6221):230–231. doi: 10.1038/339230a0. [DOI] [PubMed] [Google Scholar]
- Wendling F., Maraskovsky E., Debili N., Florindo C., Teepe M., Titeux M., Methia N., Breton-Gorius J., Cosman D., Vainchenker W. cMpl ligand is a humoral regulator of megakaryocytopoiesis. Nature. 1994 Jun 16;369(6481):571–574. doi: 10.1038/369571a0. [DOI] [PubMed] [Google Scholar]
- Wendling F., Varlet P., Charon M., Tambourin P. MPLV: a retrovirus complex inducing an acute myeloproliferative leukemic disorder in adult mice. Virology. 1986 Mar;149(2):242–246. doi: 10.1016/0042-6822(86)90125-x. [DOI] [PubMed] [Google Scholar]
- Wendling F., Vigon I., Souyri M., Tambourin P. Myeloid progenitor cells transformed by the myeloproliferative leukemia virus proliferate and differentiate in vitro without the addition of growth factors. Leukemia. 1989 Jul;3(7):475–480. [PubMed] [Google Scholar]
- Yawata H., Yasukawa K., Natsuka S., Murakami M., Yamasaki K., Hibi M., Taga T., Kishimoto T. Structure-function analysis of human IL-6 receptor: dissociation of amino acid residues required for IL-6-binding and for IL-6 signal transduction through gp130. EMBO J. 1993 Apr;12(4):1705–1712. doi: 10.1002/j.1460-2075.1993.tb05815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A., Longmore G., Lodish H. F. Point mutation in the exoplasmic domain of the erythropoietin receptor resulting in hormone-independent activation and tumorigenicity. Nature. 1990 Dec 13;348(6302):647–649. doi: 10.1038/348647a0. [DOI] [PubMed] [Google Scholar]
- de Sauvage F. J., Hass P. E., Spencer S. D., Malloy B. E., Gurney A. L., Spencer S. A., Darbonne W. C., Henzel W. J., Wong S. C., Kuang W. J. Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature. 1994 Jun 16;369(6481):533–538. doi: 10.1038/369533a0. [DOI] [PubMed] [Google Scholar]
- de Vos A. M., Ultsch M., Kossiakoff A. A. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science. 1992 Jan 17;255(5042):306–312. doi: 10.1126/science.1549776. [DOI] [PubMed] [Google Scholar]