Abstract
Rodents frequently exhibit rotational bias associated with asymmetry in lesions and neurotransmitters in the striatum. However, in humans, turning preference is inconsistent across studies, and its neural correlates are unclear. We examined turning bias in 140 right-handed healthy adults (18 to 77 years old), who navigated a virtual Morris Water Maze. On magnetic resonance images, we measured volumes of brain regions relevant to spatial navigation. We classified turns that occurred during virtual navigation as veering (less than 10°), true turns (between 10° and 90°) and course reversals (over 90°). The results showed that performance (time of platform search and distance travelled) was negatively related to age. The distance travelled was positively associated with volume of the orbito-frontal cortex but not with the volumes of the cerebellum, the hippocampus or the primary visual cortex. Examination of turning behavior showed that all participants veered to the right. In turns and reversals, although on average there was no consistent direction preference, we observed significant individual biases. Virtual turning preference correlated with volumetric asymmetry in the striatum, cerebellum, and hippocampus but not in the prefrontal cortex. Participants preferred to turn towards the hemisphere with larger putamen, cerebellum and (in younger adults only) hippocampus. Advanced age was associated with greater rightward turning preference. Men showed greater leftward preference whereas women exhibited stronger rightward bias.
Keywords: aging, brain volume, brain asymmetry, MRI, Morris Water Maze
1. Introduction
Changing direction is an integral part of search and navigation in complex and dynamic environments encountered in daily experience. Surprisingly, in the extant literature, this essential skill received substantially lesser attention than other component processes and skills related to spatial navigation (see Moffat, 2009 for a review). It is unclear, for example, how changes of search direction affect overall success of navigation, and whether the decision to alter the search path is based solely on the incoming information or is influenced by individual biases of the navigators and their individual characteristics such as age and sex.
Investigation of rotational asymmetry has a long history in studies of animal behavior. These studies show that ambulating rats exhibit a significant degree of turning bias that depends on neurochemical characteristics of the neostriatum and other brain structures that are associated with execution and planning of movement. Rats tend to turn in the direction of the brain hemisphere with lower striatal dopamine content (Zimmerberg, 1974), animals trained in circling show increased dopamine content in the caudate nucleus contralateral to the circling direction (Yamamoto and Freed, 1982; Yamamoto, Lane et al., 1982), but unilateral lesions in the caudate nucleus cause transient contralateral rotation bias (Glick, 1978). Notably, in contrast to multiple findings pertaining to neurochemical basis of turning behavior, there is no information about the relationship between structural (e.g., volumetric) asymmetry of the neostriatum and other brain structures and turning bias in intact animals.
In healthy humans, behavioral studies that measured veering and spontaneous turning in straight-line walking tasks produced inconsistent results. Some reported a leftward turning preference (Lenoir et al., 2006; Toussaint and Fagard, 2008; Mohr, Brugger et al., 2004), whereas others found a rightward bias (Mead and Hampson, 1997; Lyon and Satz, 1991) or no preference at all (Bracha, 1987). Several factors have been proposed to explain the predilection for turning in a specific direction, including handedness (Mohr and Bracha, 2004; Mohr et al., 2003; Bradshaw and Bradshaw, 1988), positional constraints on the starting point (Lenoir et al., 2006), hormonal fluctuations (Mead and Hampson, 1997), and differences in task demands (Mohr, Brugger et al., 2004; Bradshaw and Bradshaw, 1988). None of these studies evaluated the relationship between turning preference and structural or functional properties of the brain.
Information about turning bias in humans comes mostly from naturalistic studies, in which the investigators observed changes in whole-body position in real world environments during activities such as running, stepping, forward-backward walking, or long-term spontaneous ambulation (for a summary see Mohr and Lievesley, 2007). Thus, it is unclear whether the observed turning preference reflects asymmetry in gross motor activity or stems from differences in cognitive processing. To the best of our knowledge, none of the extant studies assessed rotation and turning preferences in a goal oriented navigation task, real or virtual. Thus, it is important to examine turning preference in a navigation task that is independent of gross motor function. Such disengagement of navigation-related cognition and gross motor activity is possible in a virtual environment exemplified by a virtual Morris Water Maze task (vMWM, Moffat and Resnick, 2002).
In this computerized adaptation of one of the most popular tasks for studying rodent navigation and memory, Morris Water Maze (Morris, 1984; Morris et al., 1982), a search for a hidden goal platform happens in a circular open field environment that does not enforce one direction over another, as do naturalistic environments that include barriers and structured paths. Unlike the animal MWM that requires whole–body gross-motor behavior, the vMWM yields the data that are largely independent of the whole-body responses and changes therein as the participants “swim” to the platform by controlling a joystick without altering their body position.
The neural basis of turning preference is poorly understood, but it is plausible that asymmetry in several brain regions that are involved in navigation may constitute its neuroanatomical substrate. These regions include hippocampus, neostriatum, frontal cortex, and cerebellum. Most studies of rodents and humans support the critical role of the hippocampus in spatial navigation (Morris et al., 1982; Maguire et al., 1998; Maguire et al., 2000; Kaplan et al., 2012). However, this may not always be the case (Moffat et al., 2007). Notably, most in vivo studies have not assessed the contribution of subcortical structures to individual differences in navigational skills and the one that did revealed that age-related differences in the caudate nucleus, rather than hippocampal, volume correlated with success in finding the goal in a virtual environment (Moffat et al., 2007). A complex skill, such as navigation, relies on multiple brain circuits and interactions among them. A recent study shows that during virtual navigation, both the hippocampus and the caudate are involved in managing contextual information via the orbital frontal cortical hub (Brown et al., 2012). Thus, at least three major brain structures contribute to one aspect of navigational behavior. Interestingly, the extant literature on spatial navigation pays little if any attention to the cerebellum, a functionally lateralized major neural system involved in tasks that call for coordination of visual input and motor response, as well as in more complex cognitive undertakings that engage working memory (Cabeza and Nyberg, 2000; Holmes, 1939; Botez et al., 1989). In one study, a non-significant trend for association between larger cerebellar hemispheres and better virtual navigation was noted (Moffat et al., 2007).
All of the studies of neural foundations of navigation in space, real or virtual, have focused on the time and distance that was required to reach the goal as the primary outcome and none considered the components of the search such as turns and reversals of course. Moreover, none of these studies examined the relationship between navigation and structural volumetric asymmetry. Although the majority of studies of structural hemispheric asymmetry revealed left-sided specialization in the areas associated with language, reports of volumetric asymmetries of brain regions beyond language areas have produced inconsistent results (Zilles et al., 1996; Good et al., 2001; Raz et al., 2004). It possible that regional volumetric asymmetry is unreliable at the population level, although it may be stable in individual brains (Raz et al., 2004).
To summarize, the extant literature indicates that rodents and humans exhibit turning bias in free movement conditions. Animal turning preference may be related to neurochemical asymmetry in the neostriatum. Although neural substrates of turning preference have not been studied in humans, human brains exhibit significant gross asymmetry and regional brain volumes are associated with proficiency in spatial navigation. Thus, in this study, our goal was threefold. First, we sought to establish if indeed healthy adults exhibited turning preference while navigating a virtual environment. Second, we inquired whether turning asymmetry, if found, would be related, like other aspects of navigational performance, to age and sex. Third, we examined the possible neuroanatomical correlates of turning behavior and turning asymmetry in human virtual navigation, based on rodent studies, we hypothesized that volume asymmetry of the neostriatum but not the hippocampus or prefrontal, orbito-frontal or primary visual cortices would be related to contralateral rotational preference. We also hypothesized that because of its importance in perceptual motor tasks that require continuous tracking of visual targets, hemispheric asymmetry in cerebellar volume would be also associated with turning preference.
2. Materials and Methods
2.1. Participants
The sample was part of a larger ongoing study of neural correlates of cognitive aging. The participants were recruited from the metropolitan Detroit area through advertisement in the local media and screened by telephone interview and a health questionnaire. The participants included in the current study attained a minimum of high school education, were native English speakers and were strongly right-handed (75% and above on the Edinburgh Handedness Questionnaire; Oldfield, 1971). Persons who reported a history of cardiovascular disease, neurological or psychiatric conditions, diabetes, head trauma with a loss of consciousness for more than 5 min, hypo- or hyperthyroidism, or drug and alcohol problems were excluded from participation in the study. Individuals who were taking anti-seizure medication, anxiolytics, or antidepressants were also excluded. Mini Mental State Examination (MMSE; Folstein et al., 1975) and Geriatric Depression Questionnaire (CES-D; Radloff, 1977) were used to screen for dementia and depression with only individuals who scored at least 26 on the MMSE and below 16 on the CES-D admitted in the study. Participants, who had a diagnosis of hypertension, were taking antihypertensive medications, or had a measured blood pressure (averaged over three testing days) in excess of 140 mm Hg (systolic) or 90 mm Hg (diastolic) were classified as hypertensive and excluded from the analyses. All participants provided informed consent for participation in this study, which was approved by university human investigations committee. Of 241 persons who completed the navigation study, 18 were excluded because they were not strongly right-handed, 45 because of diagnosed hypertension and additional 15 because of observed hypertension. Furthermore, 23 subjects of did not have MRI scans. Thus, the sample consisted of 140 persons (93 women), who were 18 to 77 years old (M = 48.5 years, SD = 15.8 years). There were no significant sex difference in age (M = 48.8, sd = 14.9 in females; M = 48.0, sd = 17.5 in males, t (138) = 0.277, p = 0.782), education, MMSE or CESD (all p > .1).
2.2. MRI Processing
Imaging was performed on a Bruker-Varian 4-Tesla MRI system with an 8-channel radio frequency coil. Magnetization-prepared rapid gradient echo (MPRAGE) T1-weighted images were acquired in the coronal plane with the following parameters: TE=4.38 ms, TR=1600 ms, TI=800 ms, FOV=256 mm, resolution=.67×.67×1.34 mm3, matrix size=384×384, and flip angle=8°.
Images were rotated to adjust for variation in head pitch (forward or backward), tilt (to the left or right) and rotation (to the right or to the left), so that the potential asymmetrical positioning in the scanner was corrected. The images were reformatted in native space to correct for undesired head tilt, pitch, and rotation, with Analyze 10.0 software (Biomedical Imaging Resource, Mayo Clinic College of Medicine). All images were processed by the same group of expert operators who attained reliability of at least .90 as measured by the intraclass correlation formula ICC(2) that assumes random raters (Shrout and Fleiss, 1979).
2.3. Volumetry
The volumes of the caudate nucleus (Cd), putamen (Pt), hippocampus (Hc), cerebellum (Cb), orbito-frontal (OFC) and dorsal lateral prefrontal cortex (DLPFC) in the left and right hemispheres were measured following the rules that were described in detail in previous publications (e.g., Raz et al., 2005; Raz et al., 2010). The volumes of all six brain regions were adjusted for individual differences in intracranial volume (ICV), using the ANOCVA approach described in previous publications (Jack et al., 1989; Raz et al., 2004). The volume asymmetries of these regions of interest (ROIs) were defined as asymmetry = (volume right - volume left)/ (volume right + volume left). The asymmetry indices ranged between −1 and 1. Positive asymmetry scores indicated larger ROI volume in the right hemisphere than in the left hemisphere and negative scores indicated larger ROI volume in the left than in the right hemisphere. The rules of region demarcation, previously described in detail (see Raz et al., 2004; Raz et al., 2005, for rules and illustrations) are summarized below.
Caudate nucleus (Cd)
The volume of the Cd was measured from the slice on which the Cd first appeared, usually lateral to the lateral ventricles, until it was no longer identifiable. The Cd was bordered medially by the lateral ventricle, laterally by the internal capsule, dorsally by the subcortical white matter, and ventrally by stria terminalis (rostral part) and septal nucleus (caudal part). The ROI included the head and the body of Cd but not the tail.
Putamen (Pt)
The volume of the Pt was measured from the slice on which the Pt first appeared until it was no longer identifiable. The Pt was bordered laterally by the external capsule, medially by the internal capsule (anterior to the anterior commissure) or globus pallidus (posterior to the anterior commissure), dorsally by the subcortical white matter, and ventrally by the temporal stem, optic radiations, amygdala, temporal horn, and anterior commissure.
Hippocampus (Hc)
Hc volume was measured on slices aligned perpendicular to the long axis of the right Hc between the mammillary bodies and the slice showing the fornices rising from the fimbria. The Hc included CA1-CA4, dentate gyrus and the subiculum. The medial border of the Hc was defined by tracing the subiculum to its most medial position and drawing a horizontal line at the medial curve. The dorsal medial boundary was the ambient cistern above the Hc. The lateral border of Hc was the lateral ventricle or the temporal lobe white matter.
Dorsal lateral prefrontal cortex (DLPFC)
The volume of the DLPFC was measured on the coronal slices located within the posterior 40% of the distance between the genu of the corpus callosum and the tip of the frontal pole. The described ROI included superior, middle, and inferior frontal gyri. The DLPFC was defined as the gray matter located between the most dorsomedial point of the cortex and the orbital sulcus.
Orbito-frontal cortex (OFC)
The range of the OFC was identical to DLPFC. The most lateral branch of the orbital sulcus that breaches the external aspect of the brain defines the lateral boundary of the OFC, which also served as the lower boundary for the DLPFC. The medial boundary was defined by the olfactory sulcus. The OFC was defined as the gray matter located between the orbital sulcus and olfactory sulcus.
Cerebellum (Cb)
The cerebellar hemispheres were measured on the coronal slices from the first slice on which it appeared to the last slice that it was visible. The hemispheric gray matter, the cerebellar tonsils, the vellum, and the corpus medullare were included, whereas the vermis, cerebellar peduncles, and the fourth ventricle were excluded in the tracing of each cerebellar hemisphere.
Primary visual cortex (VC)
This region was defined as the cortex located along the banks of the calcarine sulcus. It was measured on the anterior 50% coronal slices located between the mid-vermis slice and the occipital pole. This ROI was a part of area 17, although it does not constitute the entirety of the primary visual cortex.
2.4. vMWM Apparatus and Procedures
Apparatus
The vMWM task (Moffat and Resnick, 2002) was administered on a Dell desktop computer, with a 17-inch TFT LCD SONY monitor. Participants were seated in a chair and their heads were approximately 50 cm from the screen. Participants viewed the virtual environment from a first person perspective (Figure 1) and guided their movements with the use of a Thrustmaster Top Gun Fox 2 pro joystick.
Figure 1.
A typical view from within the virtual environment (left) and overhead diagram of the vMWM map (right). Participants viewed the environment from a first person perspective.
Pretest training
Pretest training familiarized participants with the virtual environment and movement in the virtual environment with a joystick. After the experimenter’s instruction, there was a period of free exploration of virtual environment using the joystick. Participants practiced until they were comfortable with the joystick and could control movement in the virtual environment while navigating to targets designated by experimenter. To demonstrate their abilities to use the joystick, participants completed two tasks in pretest training: moving onto top of small squares in the center of platforms; moving as quickly as possible to reach a goal along a hallway with long straight parts as well as turns in it. In addition, the participants practiced vMWM with a virtual circular pool filled with water and surrounded by prominent objects. Participants were instructed to move to a platform that alternated between being visible and invisible in five successive trials. The position of the platform remained constant across trials. Each participant completed the pretest training tasks, though the training time could be different among participants. Participants were instructed to move only forward and were informed that backward movement was against the rules. Although the software did not preclude participants from moving backwards in the virtual environment, if they attempted such backwards movement, it was disallowed by experimenter, who was present throughout the experiment. Movement speed varied and was individually controlled by changing the force applied to the joystick. Participants were able to change the direction of virtually movement either during forward motion or after stopping in the vMWM.
vMWM learning trials
The vMWM was represented on a computer screen as a circular pool filled with water (Figure 1). The diameter of the virtual pool was 4415 virtual units. The pool was presented with an image of an irregularly shaped room that contained several objects that could serve as visual cues: a lamp, a group of trees, a flag, a picture, and a tree. A rectangular platform, with side length of 883 virtual units, was hidden beneath the surface of water and participants were instructed to find the hidden platform as quickly as possible. When participants passed over the platform, it would emerge from the water, thus terminating the trial. There was a 12-second interval between two successive trials. During the inter-trial interval, participants were able to move on the platform, although they were not explicitly instructed to do so. Participants were informed that the platform remained in the same location on each trial and that the environment did not change. On each of the six trials, participants were placed into one of the three quadrants of the pool not containing the platform, facing a different direction at the beginning of each trial with the platform behind their field of view. Thus, participants were compelled to make turns before they were able to orient towards the platform. Although the start locations and directions differed between trials, the settings were identical for each participant. There was no time limit on the learning trials.
Probe trial and visible platform trial
Immediately after 6 vMWM learning trials, the participants performed a 1-minute probe trial, in which the environment was identical to the learning trials, except that the platform was removed. After the probe trial, there was a visible platform trial, in which the water maze was the same as the learning trials, but the platform was not submerged, and participants were instructed to move onto the visible platform as fast as possible.
2.5. Turning Angle
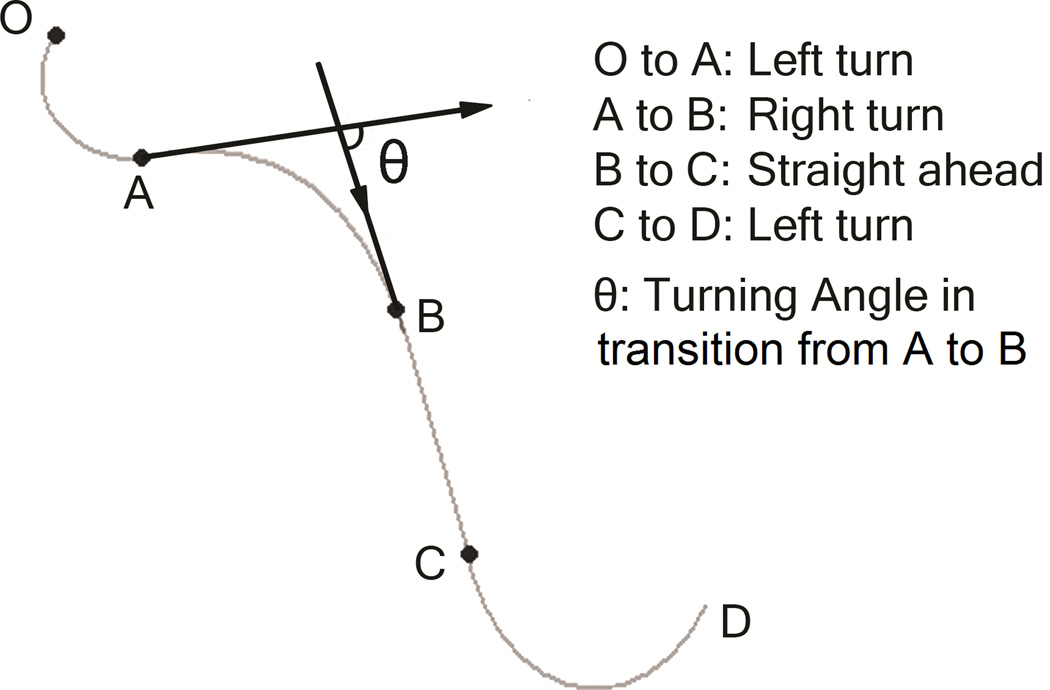
Cursor position and the azimuth of movement to the platform were continuously sampled at a rate of 85 Hz. Moment-by-moment change in azimuth was an index of moving segment. Three types of moving segments were defined: continuous clockwise turning (right rotation, Figure 2, from A to B), continuous anti-clockwise turning (left rotation, Figure 2, from C to D and from O to A), and no change of azimuth (straight segment, Figure 2, from B to C).
Figure 2.
Examples of turn typology.
2.6. Laterality Indices
A regular turn was defined as a continuous change of movement direction by 10° to 90°; small deviations (veers) were defined as continuous changes of direction of no more than 10°; and a reversal was defined as continuous change of direction by at least 90°. To investigate rotation preference, we computed a laterality index by subtracting the number of left turns (or deviations, reversals) from the number of right turns (or deviations, reversals), and dividing this difference by their sum. This laterality index ranged between −1 and 1. A positive laterality index score indicated right rotation bias and negative scores indicated left rotation bias.
2.7. Data analyses
First, we examined age and sex differences in traditional indices of virtual navigation performance: search distance and time of search. After establishing a typical pattern of age and sex differences and the course of navigation skill acquisition, we examined the pattern of direction changes exhibited in the process of virtual search. Age was centered at the sample mean. The other continuous variables, when served as independent variables, were also centered at the sample mean. The performance in visible platform session served as control for possible age-related difference in visual and motor skill. All first-order interactions would be dropped from models if found non-significant (p > .1). Benjamini–Hochberg procedure (Benjamini and Hochberg, 1995) was applied to control false discovery rate (FDR) in multiple hypothesis testing.
To examine age and sex differences in vMWM performance, we fitted a general linear model (GLM) to the data. In this GLM, the travel distance or time was the dependent variable, age, sex and performance in visible platform trial served as between-subject independent variables, and trial was a six-level within-subject variable. To test the associations between regional brain volumes and vMWM performance, we evaluated separate GLMs for each of the seven regions, with the travel distance as the dependent variable, age, sex and regional volume as independent variables and trial as a repeated measure. The distance traveled in visible platform trial was also included in models. In order to verify the relationship between regional brain volumes and vMWM skill in the previous report (Moffat et al., 2007), in which participants were drawn from two extreme age groups and age was treated as a dichotomous variable, we also analyzed our data using a median split of age, in addition to treating age as a continuous variable.
To examine age and sex differences in virtual turning preference and the association between turning bias and regional hemispheric asymmetry, we analyzed laterality scores of all three types of turns in a GLM framework. In this model, the turn laterality index was the dependent variable, the turn type (veering, turns, and reversals) was a three-level repeated measure, whereas age, sex and volumetric asymmetry of the Hc, Cb, Cd, Pt, DLPFC, OFC and the VC served as between-subject independent variables. The performance in visible platform trial was included as a covariate as well.
3. Results
3.1. Behavioral Findings
3.1.1. Virtual navigation performance: Search distance
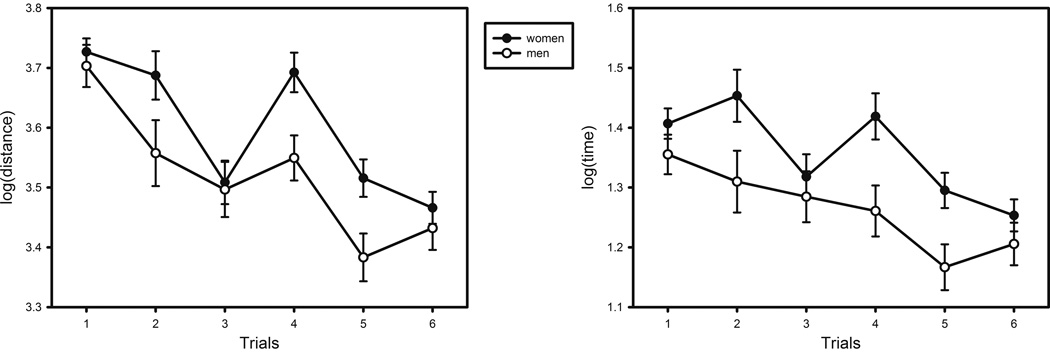
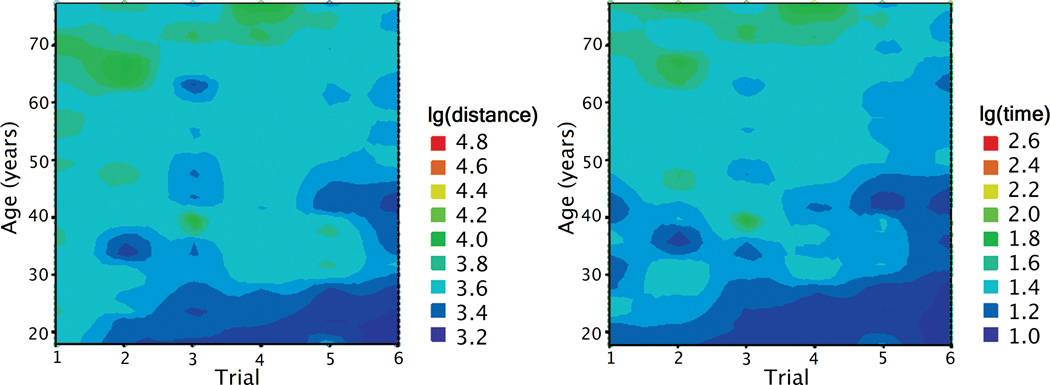
The mean distance traveled in the learning 6 trials was 31769 (sd = 21684) virtual units. The main effects of age (F (1, 136) = 33.859, p < .001) and sex (F (1, 136) = 4.819, p = .030) were significant, with women and older participants traveling longer distance in search of the platform. There was also a main effect of trial (Figure 3, F (5, 680) = 21.257, p < .001), indicating improvement in performance across trials. The interaction effects of trial × age (Figure 4, F (5, 680) = 3.252, p = .007), trial × sex (F (5, 680) = 2.342, p = .040) and trial × performance in visible platform trial (F (5, 680) = 2.704, p = .020) were also significant. The linear component of the trial effect was significant (F (1, 136) = 81.766, p < .001), as well as the quadratic component for trial × age interaction (F (1, 136) = 5.528, p = .020). Although performance improved with practice, age differences increased after the first trial (Table 1). The correlation between age and travel distance was smaller on trial 1 than that on trial 2: Steiger's Z* = −1.970, p < .05.
Figure 3.
Sex differences in vMWM performance: Distance and time of search by trial.
Figure 4.
Distance traveled and time spent in search of a hidden platform in the vMWM as a function of age and trial number. The color codes the magnitude of the performance index, distance and time.
Table 1.
Correlations between age and vMWM performance in each trial.
| Trial 1 | Trial 2 | Trial 3 | Trial 4 | Trial 5 | Trial 6 | |
|---|---|---|---|---|---|---|
| Age - Distance traveled | .145 | .362** | .270** | .338** | .293** | .320** |
| Age - Time of search | .340** | .412** | .351** | .421** | .352** | .394** |
Note: Travel distance and time were log-transformed to reduce skewness. Higher scores reflect poorer performance.
p < .05;
p < .01.
3.1.2. Virtual navigation performance: Time of search
The mean time to complete six vMWM trials was 186 (sd = 153) seconds. We examined age and sex differences in the time of search by using an identical GLM approach. Similar as in the search distance analyses, there were significant effects of age (F (1, 136) = 44.421, p < .001) and sex (F (1, 136) = 6.490, p = .012), with older participants and women evidencing longer search times. There was a significant main effect of trial (Figure 3, F (5, 680) = 9.218, p < .001) and a trend for trial × age interaction (Figure 4, F (5, 680) = 2.140, p = .059). The linear component of the trial effect (F (1, 136) = 40.867, p < .001) and the quadratic component for trial × age interaction (F (1, 136) = 5.250, p = .024) were also significant. The positive correlations between age and search time were significant across all six trials (Table 1).
3.1.3. Side preference in turning behavior
We observed a clear rightward bias in small deviations (veering) from the search course. The values of the laterality index ranged from .400 to 1.000 (M = .794, SD = .106). However, there was no bias in course reversals; the laterality score did not differ from zero: M = .063, SD = .458, median = .060. Although the mean laterality index for regular turns differed from zero (M = −.080, SD = .413, t (139) = −2.298, p < .05), the median of 0.000 indicated the apparent mean bias attributable to the influence of a few extreme values.
Older age was associated with a greater number of reversals: r = .333 and turns: r = .361; both log transformed to reduce skewness, for both p < .001. Number of reversals and turns positively correlated with time and distance of search across trials: all r > .6, p < .001. There were significantly more turns than reversals: t (139) = 3.952, p < .001. Although the asymmetry in turns and reversals correlated modestly (r = .202, p = .017), neither correlated with the asymmetry of veering (r = .156, p = .065 for turns and r = .022, p =.799 for reversals).
3.1.4. Performance in probe trial and visible platform trial
In probe trial, the proportion of distance and time spent in target quadrant were both significantly greater than 25% (distance: M = 0.347, sd = 0.199, t (139) = 5.797, p < .0001; time: M = 0.337, sd = 0.195, t (139) = 5.262, p < .0001). The association between age and proportion of time or distance in target quadrant was not significant (age-time percent: r = .129, p = .13; age-distance percent: r = .148, p = .08). Thus, older participants were not performing worse than the younger participants did in the probe trial.
In visible platform trial, the travel distance and time spent to complete visible platform session varied with age (distance: F (1, 137) = 10.558, p = .002; time: F (1, 137) = 34.994, p < .001) and sex (distance: F (1, 137) = 9.165, p = .003; time: F (1, 137) = 5.819, p = .017). However, they did not significantly affect the performance in learning trials when age and gender were controlled (both p > .3).
3.2. Neuroanatomical Findings
3.2.1. Regional brain volumes
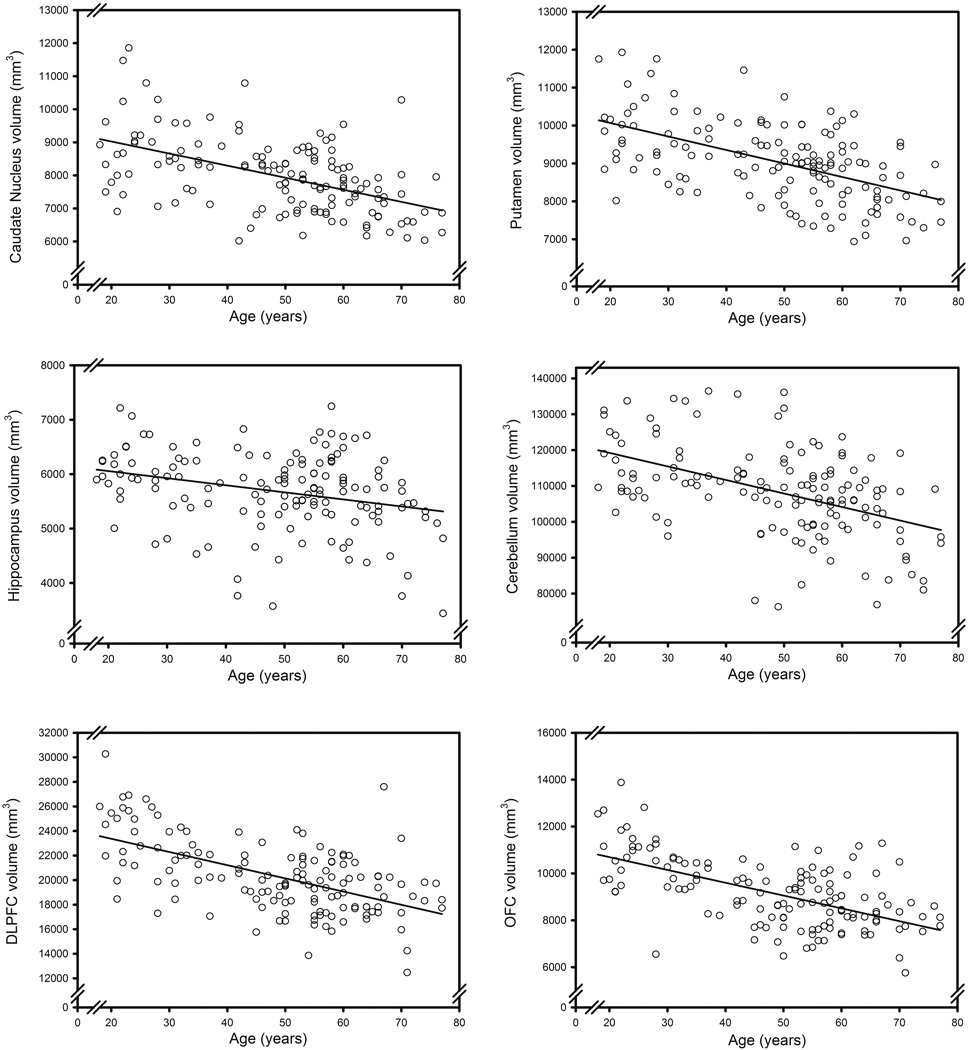
We fitted a GLM with the ICV-adjusted volume as a dependent measure, ROI as a seven-level repeated measure, and age and sex as independent variables to the data. The analysis revealed significant main effects of age (F (1, 136) = 79.978, p < .001) and region (F (6, 816) = 9912.976, p < .001), as well as interactions of age × region (F (6, 816) = 35.991, p < .001) and age × sex × region (F (6, 816) = 5.013, p < .001). The follow-up GLM analyses for each ROI revealed associations between advanced age and smaller volumes of all regions: Cd: F(1, 137) = 48.697, p < .001; Pt: F(1, 137) = 57.553, p < .001; Hc: F(1, 137) = 11.909, p < .001; OFC: F(1, 137) = 74.271, p < .001; DLPFC: F(1, 137) = 65.786, p < .001; Cb: F(1, 136) = 43.822, p < .001; VC: F(1, 136) = 6.043, p = .015; p values were adjusted for FDR; see the scatterplots of age differences in volume in Figure 5.
Figure 5.
Age differences in regional brain volumes.
3.2.2. Regional volumetric asymmetry
We observed anatomic asymmetry in the seven examined regions. The left hemisphere was larger than the right one in the putamen, the OFC and cerebellum. Examination of the confidence limits overlap among the regional indices of asymmetry (Table 2) revealed that the asymmetry was greater in the Pt than in the OFC, which in turn showed greater asymmetry than Cb did. In contrast, the right hemispheres were larger than the left ones in the caudate, the hippocampus, the DLPFC and the VC. The magnitude of right-sided asymmetry was greater in the VC than in caudate and hippocampus (Table 2).
Table 2.
Descriptive and inferential statistics of volume asymmetry indices.
| Region | Mean | SD | 95% confidence interval | t value |
|---|---|---|---|---|
| Putamen | −.032 | .028 | −.037 to −.028 | −13.741 |
| OFC | −.018 | .048 | −.026 to −.010 | −4.384 |
| Cerebellum | −.005 | .011 | −.007 to −.003 | −5.101 |
| Caudate Nucleus | .014 | .023 | .010 to .018 | 7.201 |
| Hippocampus | .015 | .041 | .008 to .021 | 4.171 |
| DLPFC | .023 | .036 | .017 to .029 | 7.591 |
| Primary Visual Cortex | .037 | .067 | .026 to .048 | 6.539 |
Note: Asymmetry direction: R > L positive; t values present the result of one-sample t-tests for volume asymmetry index of each ROI against zero, all p < .001.
A GLM with the asymmetry index as the dependent variable, age and sex as independent variables and the ROI as repeated measures revealed a significant main effect of region (F (6, 822) = 46.168, p < .001), that reflected the differences in direction and magnitude of asymmetry reported above. The effects of age and sex were not significant.
3.3. Neuroanatomical Correlates of Navigational Performance
3.3.1. Performance in vMWM and regional brain volumes
Except the age and sex effects, reported above, the associations between the indices of vMWM performance and regional brain volumes were not significant in any region (all p > .2), which is in contrast to the findings in the previous report (Moffat et al., 2007). However, once age was dichotomized, we replicated some of the results of the previous study. Consistent with Moffat et al. (2007) findings, there was a main effect of OFC volume on the travel distance, F (1, 135) = 7.572, p = .049. Larger Pt and DLPFC volumes were also not associated with better vMWM performance, although non-significant trends were observed: Pt: F (1, 135) = 4.853, p = .068; DLPFC: F (1, 135) = 5.752, p = .063. All p values were adjusted for FDR. The effects of VC, Cd, Hc and Cb volumes were not significant.
3.3.2. Turning preference and neuroanatomical asymmetry
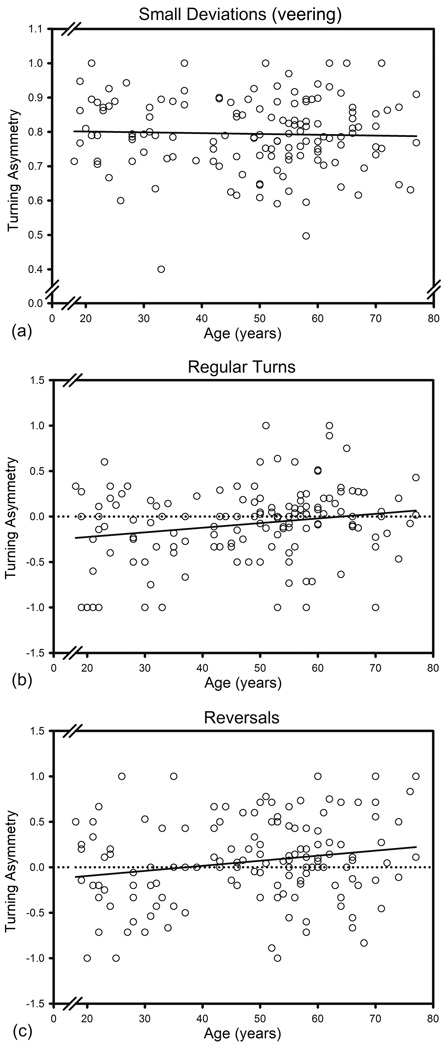
Age (F (1, 128) = 12.515, p < .001), sex (F (1, 128) = 7.970, p = .006), Pt asymmetry (F (1, 128) = 8.309, p = .005), Cb asymmetry (F (1, 128) = 11.133, p = .001), as well as the interaction between age and Hc asymmetry (F (1, 128) = 4.420, p = .038) were associated with turning preference. Within-subjects analysis revealed a significant main effect of rotation type (F (2, 256) = 280.812, p < .001) and several significant interactions: rotation type × age × Hc asymmetry (F (2, 256) = 3.107, p = .046), rotation type × age (F (2, 256) = 5.259, p = .006), rotation type × sex (F (2, 256) = 3.706, p = .026) and rotation type × Cb asymmetry (F (2, 256) = 3.142, p = .045). In addition, there were two nonsignificant trends for interactions: rotation type × Cd asymmetry (F (2, 256) = 2.500, p = .084) and rotation type × Pt asymmetry (F (2, 256) = 2.212, p = .112). The correlations among relevant variables are presented in Table 3.The asymmetry of veering (small deviations) was unrelated to age, sex or volume asymmetry: all p > .05, as depicted in Figure 6a. The interaction of turn types with age reflected greater counter-clockwise (left) preference in turns among younger participants (F (1, 128) = 9.277, p = .008, Figure 6b) and greater clockwise (right) preference in reversals among the older adults (F (1, 128) = 7.353, p = .011, Figure 6c). Both p values were adjusted for FDR.
Table 3.
Correlations between age, vMWM performance, regional volumes, volume asymmetries and asymmetries in rotations.
| AGE | vMWM performance |
Regional volume (adjusted for ICV) | Asymmetry of regional volume | Turning Asymmetry |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Distance | Time | CDV | PTV | HCV | DLPFCV | OFC | CBV | VCV | CD | PT | HC | DLPFC | OFC | CB | VC | veer | turn | ||
| lgDistance | .47 | ||||||||||||||||||
| lgTime | .53 | .92 | |||||||||||||||||
| CDV | −.51 | −.26 | −.29 | ||||||||||||||||
| PTV | −.54 | −.34 | −.39 | .67 | |||||||||||||||
| HCV | −.28 | −.15 | −.19 | .36 | .42 | ||||||||||||||
| DLPFCV | −.57 | −.32 | −.35 | .48 | .46 | .35 | |||||||||||||
| OFCV | −.59 | −.34 | −.38 | .54 | .49 | .40 | .77 | ||||||||||||
| CBV | −.47 | −.26 | −.30 | .46 | .41 | .39 | .37 | .39 | |||||||||||
| VCV | −.18 | −.04 | −.05 | .28 | .27 | .22 | .22 | .28 | .28 | ||||||||||
| CD_Asym | −.07 | −.02 | .00 | −.01 | .01 | −.02 | .09 | .12 | −.05 | −.18 | |||||||||
| PT_Asym | −.15 | −.19 | −.15 | −.06 | −.01 | −.02 | .13 | .17 | .05 | .14 | −.05 | ||||||||
| HC_Asym | −.13 | −.12 | −.15 | .14 | .11 | .08 | .17 | .19 | .07 | −.08 | −.14 | .13 | |||||||
| DLPFC_Asym | .11 | −.02 | −.03 | −.04 | −.01 | −.02 | −.14 | −.13 | −.06 | .05 | −.01 | .02 | −.39 | ||||||
| OFC_Asym | −.01 | .01 | −.02 | −.01 | −.01 | −.15 | −.10 | −.05 | .01 | .06 | .14 | −.01 | −.21 | −.09 | |||||
| CB_Asym | .03 | −.06 | −.09 | −.03 | .10 | −.07 | −.04 | −.05 | .00 | −.08 | .01 | −.12 | .08 | .08 | −.01 | ||||
| VC_Asym | .14 | −.02 | −.00 | −.07 | −.15 | .01 | .04 | .10 | −.13 | −.05 | .04 | −.11 | .09 | −.05 | −.09 | .10 | |||
| Asym_veer | −.03 | −.02 | −.14 | −.06 | −.06 | −.01 | −.09 | −.01 | −.02 | .06 | .08 | .05 | .00 | .15 | .02 | .07 | .00 | ||
| Asym_turn | .19 | .26 | .25 | −.13 | −.06 | .02 | −.05 | .00 | −.05 | .01 | .00 | .10 | −.01 | −.02 | −.02 | .21 | .17 | .16 | |
| Asym_reversal | .19 | −.01 | .07 | −.09 | −.08 | −.14 | −.22 | −.25 | −.08 | .07 | −.20 | .15 | .07 | .04 | −.16 | .19 | .03 | .02 | .20 |
Note: CD_Asym, PT_Asym, HC_Asym, DLPFC_Asym, OFC_Asym, CB_Asym, VC_Asym: volumetric asymmetry indices of Cd, Pt, Hc, DLPFC, OFC, Cb, VC, respectively. Asym_veer, Asym_turn, Asym_reversal: asymmetry indices of small veering, regular turns and reversals, respectively. Asymmetry direction: R > L positive.
Figure 6.
Age differences in lateral bias for three types of turns. Asymmetry direction: R > L positive.
Men evidenced bias towards leftward (counter-clockwise) on normal turns (M = −.184, t (46) = −2.912, p = .006) but not on reversals, whereas women showed rightward (clockwise) preference on reversals (M = .111, t (92) = 2.274, p = .025) but not on regular turns. The sex differences were significant: F (1, 128) = 5.538 for turns, and F (1, 128) = 5.345 for reversals, both p = .034 after controlling for FDR.
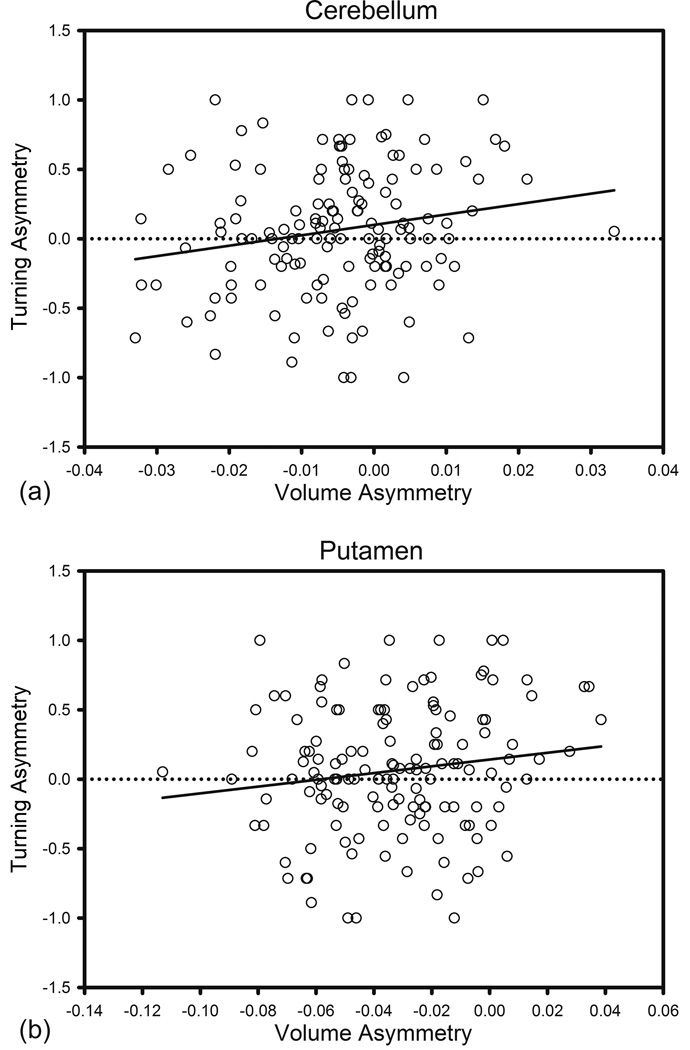
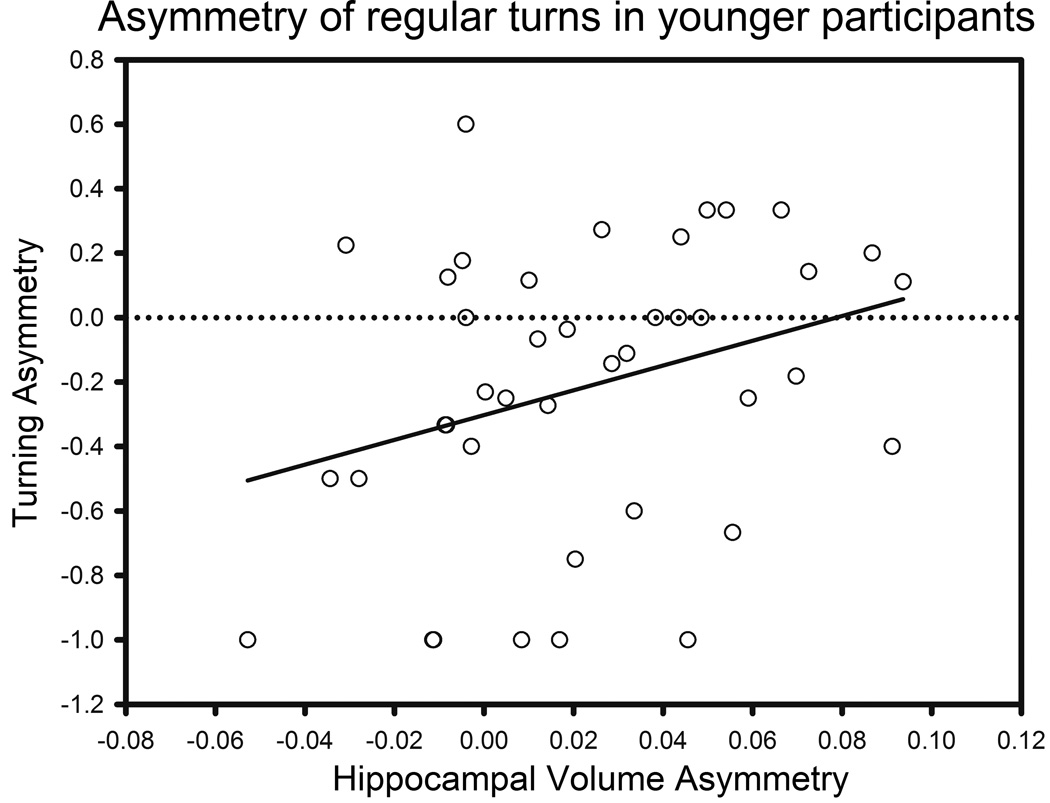
The asymmetry of Cb volume was associated with ipsilateral turning bias: F (1, 128) = 6.553, p = .021 for turns, Figure 7a; F (1, 128) = 6.198, p = .021 for reversals, Figure 8a; i.e., the participants rotated towards the larger hemisphere. Ipsilateral Pt asymmetry was also marginally related to lateral bias in reversals (F (1, 128) = 5.665, p = .056, Figure 8b) and turns (F (1, 128) = 3.366, p = .10, Figure 7b); i.e., course reversals were more likely in the direction of the larger hemisphere. In contrast, Cd asymmetry was not associated with turning bias (F (1, 128) = 3.881, p = .153). Although the main effect of Hc asymmetry was not significant, there was an interaction of age with Hc asymmetry (F (1, 128) = 7.950, p = .006) for regular turns. Hc asymmetry was related to ipsilateral turn preference in young (aged < 41, N = 41, r = .310, p = .049, Figure 9) but not in older participants. The asymmetry of the VC, DLPFC and OFC were unrelated to the turning bias for any type of turn.
Figure 7.
Lateral bias for regular turns vs. volume asymmetry. Asymmetry direction: R > L positive.
Figure 8.
Lateral bias in reversals vs. volume asymmetry. Asymmetry direction: R > L positive.
Figure 9.
Lateral bias in regular turns vs. hippocampal volume asymmetry in younger participants (less than 41 years of age). Asymmetry direction: R > L positive.
4. Discussion
We found that in a manually executed desk-top virtual navigation task, healthy adults exhibited a wide range of individual differences in turning preference. The direction and magnitude of turning bias differed according to participants’ age and sex. Moreover, the turning asymmetry correlated with volume asymmetry of the neostriatum, cerebellum, and (among younger people) hippocampus but not of the prefrontal cortex.
4.1. Age differences in turning asymmetry
In their deviation from the search path, the participants displayed a wide range of deviation angles, and the magnitude of deviation mattered. Right-side preference was clear for small deviations from the course – veering. We can only speculate that such deviations are likely non-volitional and reflect participants’ strong right-handedness.
In contrast to veering, when participants decided to change the course or reverse the direction of the virtual search, they showed, as a group, no directional preference, while exhibiting a wide range of individual differences in turning bias. The latter were related to age and the type of turns that were executed during vMWM navigation. The younger participants evidenced counter-clockwise (leftward) preferences in true turns (10° to 90°), but with increasing age of the participants, progressively smaller preference was observed, with the older adults showed no consistent turning bias at all. A contrary pattern was noticed for course reversals: the younger participants showed no directional bias, whereas the older adults evidenced clockwise (right-side) preference.
In as much as the virtual manual navigation corresponds to a full body movement in a naturalistic setting, the leftward turning bias of the younger adults is in accord with the reported counter-clockwise bias in running within an arena configured similarly to a vMWM (Toussaint and Fagard, 2008). It is unclear why such bias is absent in the older adults and why younger participants exhibited no bias in course reversals. Increases in turns and reversals were associated with advanced age, regardless of the direction, and the number of turns and reversals correlated with longer time of search (i.e., worse performance). Thus, both types of deviations from course indicated maladaptive search behavior. However, reversals were less frequent than turns and it is possible that the young participants just did not produce a sufficient number of reversals to yield valid indices of asymmetry.
Because the indices of turning and reversals asymmetry correlated weakly among themselves and did not show significant correlations with veering asymmetry, it is unclear whether turning represents a coherent construct. Veering asymmetry may reflect a bias in unilateral joystick use by strongly right-handed participants. Indeed, veering preference for the right side was almost universal and unrelated to age, sex or brain asymmetry. In contrast, individual asymmetry in true turns and course reversals may reflect a combination of automatized movement programs and deliberate data-driven decisions. Course reversals, in addition may reflect significant disorientation in the search space. Which of those play a greater role in spatial navigation is unclear, but the observed relationships between turning bias and brain asymmetries provide some clues.
4.2. Sex differences in turning asymmetry
In true turns, men showed stronger leftward bias relative to women, whereas in reversals, men shifted towards no clear directional preference, whereas women exhibited propensity to turn rightward. This sex difference is consistent with the previous experimental (Mead and Hampson, 1996) and questionnaire (Stochl and Croudace, 2012) studies that reported greater rightward bias in women compared to men. However, our findings are in contrast with an observational study that reported rightward turning bias in right-handed males and leftward bias in right-handed females (Bracha, Seitz et al., 1987). This discrepancy may arise from the difference in measures of rotational preference. The first study (Mead and Hampson, 1996) tested turning preference in a limited and structured environment: the participants were instructed to turn 180° and walk directly to a tape recorder located behind them. The second study (Bracha, Seitz et al., 1987) investigated human turning preference during long-term spontaneous activity. Different tasks may evoke different rotational preference even in the same participants (Mohr, Brugger et al., 2004). Besides the difference in the context and structure of navigation tasks, the definition of a turn also varies among the studies. For example, in the assessment of bias in long-term spontaneous turning, investigators (Bracha, Seitz et al., 1987) counted right and left full turns of at least 360°, whereas Mead (Mead and Hampson, 1996) counted 180° turns. In the current study, these types of turns would be viewed as course reversals. These discrepancies hamper the comparison of results.
4.3. Neuroanatomical correlates of navigation and turning bias
In accord with the results of a previous study on the same task (Moffat et al., 2007), better performance in vMWM was associated with larger OFC and (marginally) larger DLPFC volumes. There was also a trend that better navigational performance correlated with larger volumes of brain regions in a cortico-striatal system, which plays an important role in supporting computational and strategic aspects of the search. However, neither the volume of the hippocampus, a putative neural substrate of spatial mapping, nor the volume of the cerebellum, which supports various cognitive and perceptual-motor operations were related to speed and efficiency of navigation. These results are in accord with the previous report (Moffat et al., 2007), in which the effect of hippocampus volume was nonsignificant, whereas the effect of cerebellum volume was only marginally significant on vMWM performance. Future studies measuring the volume of hippocampal subfields may clarify further the association between hippocampal volume and navigational performance.
To the best of our knowledge, this is the first study to examine the structural brain correlates of turning preference in healthy humans. We observed that the lateral, age and sex differences in turning preference and its brain correlates varied across three types of turning behavior. Asymmetry in turning behavior, especially in course reversals, was associated with ipsilateral volume asymmetry in the cerebellum and putamen. Hippocampal volume asymmetry was related to ipsilateral turn preference, but only in younger (under the age of 41) participants. The asymmetry of the cortical regions, VC, DLPFC and OFC was unrelated to the turning bias. Notably, veering, the activity with consistent lateral bias was unrelated to anatomical asymmetry in any of the examined brain regions.
The absence of associations between prefrontal asymmetry and any sort of turning bias suggests that the latter was unlikely to reflect bias in higher cognitive processes that rely on the DLPFC and OFC. In contrast, the observed correlations between hemispheric asymmetry in motor structures (Cb and Pt) and rotational bias indicate that asymmetric structure of the motor, primarily extrapyramidal, system may be responsible for at least some of the observed bias.
What aspect or property of these motor structures supports the observed behavioral asymmetry is unknown and in the absence of neurochemical measures we can only speculate about the possible underlying mechanisms. Given the association of dopaminergic activity with rotational bias in rodents (Zimmerberg, 1974), hemispheric asymmetry in dopaminergic activity may be a good candidate for a neurochemical substrate of the observed bias. The literature suggests that asymmetry of spontaneous rotation may depend on asymmetric striatal dopaminergic activity (Bracha, Shults et al., 1987; Lyon and Satz, 1991). However, in the absence of measurement of DA activity, the link between the observed turning asymmetry and striatal DA remains a speculation.
To the best of our knowledge, in contrast to the relative abundance of studies on neostriatal dopamine, hemispheric asymmetry of this neurotransmitter in the Cb remains unexplored. Although the Cb controls ipsilateral finger movements (Solodkin et al., 2001; Wiestler et al., 2011), the association of volumetric Cb asymmetry with turning preference is unlikely to reflect exclusively asymmetry of hand movement. The Cb is involved in spatial navigation (Rondi-Reig and Burguiere, 2005; Rondi-Reig et al., 2002) as it shapes hippocampal spatial representation and therefore affects navigation that is based on self-motion cues (Rochefort et al., 2011). The Cb may affect the turning asymmetry by influencing the processing of optic flow cues in the hippocampus, and this hypothesis merits further investigation.
In the current study, the association between turning preference and volumetric asymmetry of the Hc was significant among the younger participants only. A similar observation of the association between the Hc and navigational performance as specific to younger participants has been reported by others (Moffat et al., 2007). Moreover, the extant findings linking hippocampal size and navigational process (e.g. Maguire et al., 1998; Maguire et al., 2000) are limited to samples of younger and middle-aged participants. It is possible that younger adults adopted an allocentric (place-oriented) strategy that relies on the Hc, whereas older individuals tend to adhere to an egocentric (self-referential) strategy that makes lesser use of the Hc circuitry. Indeed, older adults are less likely than their younger counterparts to adopt an allocentric strategy in virtual navigation (Rodgers et al., 2012) and are less likely to abandon an egocentric strategy even in the presence of corrective feedback (Harris et al., 2012). Because a desktop vMWM task allows for subject’s head movements, it may invoke activity in the hippocampal place cells that is greatly reduced by the absence of vestibular cues in head-restrained animals (Chen et al., 2013). In this context, lack of association between turning bias and hippocampal asymmetry in older participants may be related to lesser dependence on place cell activity in older adults.
Advanced age was associated with weaker leftward turning bias in true turns (10° to 90°) and greater rightward bias in reversals (90° and above). The observed age-related turning preference might be related to age-related differences in asymmetry of striatal dopamine availability. Rightward asymmetry of dopamine D2 and D3 receptors availability in the caudate nucleus decreases with age (Vernaleken, 2007), and thus, can attenuate leftward or exaggerate rightward turning bias.
4.4. Limitations of the study
Although it may seem obvious, it is important to emphasize that virtual and real navigations are not identical, and interpretation of the extant virtual navigation experiments conducted at the desktop or in an MRI scanner must proceed with caution. Navigation in a virtual reality environment executed by a person sitting in front of a computer screen shares many characteristics with navigation in real space: reliance on visual cues to solve a spatial problem, root planning, and use of working memory for spatial locations and management of search history. However, the view field afforded by the 17-inch monitor used in this study was limited, and using larger screen or goggles would make the virtual environment more similar to the real world. In addition, unlike its real counterpart, virtual navigation at the desktop gives little opportunity for use of motor and proprioceptive information, i.e. idiothetic cues (Taube et al., 2013). Nonetheless, unlike executing virtual spatial search from supine position with constrained head movements in fMRI experiments, conducting a search in virtual environment while being deprived of whole-body motor cues may be not of a considerable detriment to navigation if at least some head position cues are available (see Taube et al., 2013 for a review), as is the case in the desktop vMWM task used in our experiment.
Because virtual environments lack proprioceptive cues and visual flow that are inherent to real world navigation, spatial information acquired from the virtual environment is less accurate than that from a real environment (Wilson, 1997). However, humans can transfer spatial knowledge acquired from virtual environments to the real world (Wilson, 1997), and the spatial knowledge in a virtual environment is highly predictive of performance in a similar real-world maze (Waller, 2000). Thus, although it remains unclear how the results of two-dimensional joystick-powered virtual navigation are related to true body movement in space, the observed associations between turning laterality and regional volume asymmetry contribute to the understanding of the neural basis of spatial cognition. Systematic manipulation of availability of vestibular and motor cues in virtual navigation is necessary for clarification of conditions, under which a task such as vMWM can be treated as a window into real navigational skills.
Several limitations of the current study reflect selection of subjects and task parameters. Relative rarity of left-handers in the population (Tan, 1988) prompted us to limit our sample to strong right-handed participants, who, with very rare exceptions, operated the joystick exclusively with their right hand. None of the participants consistently used left hand, although in 3 – 4 cases they briefly switched to non-dominant hand or used both hands for unknown reasons. Thus, our findings are limited to persons who exhibit strong right-hand preference. Moreover, with age, the proportion of right-handers in the population steadily increases (Coren and Halpern, 1991; Hugdahl et al., 1993; Porac and Friesen, 2000). As a result of that age-related variation at the population level, the representativeness of participants with a particular hand preference might vary with age.
Because of its cross-sectional design, this study cannot inform about age-related change in turning preference, cerebral asymmetry and the relationship between them. In addition, generalizability of our findings is limited by selection of participants with no reported health problems.
Finally, currently available structural MRI methods do not allow for a precise measurement of volumes in the regions that are dimmed critical of utilization of head direction cues in rodents - the lateral mammillary nuclei, anterior dorsal thalamus and post-subiculum (Taube et al., 2013). Future improvements in MRI instrumentation and refinement of currently available sequences are needed for closing this gap between animal studies and human virtual navigation experiments. In future research it is also important to explore the roles of hippocampus sub-regions (CA1, CA3, the dentate gyrus) in navigation. Precise measure of sub-region volumes would help to reveal their relation to turning behavior during navigation. Unfortunately, the sequence that would allow such precision of Hc parcellation (see Bender et al., 2013) was unavailable to us at the time of this study. Use of semi-automatic measurement tools, such as Freesurfer, has its own share of problem when applied to aging, For example, Hc volume is overestimated by about 20% in Freesurfer and the correlations between Freesurfer and manually traced volumes are around .75 at best (Cherbuin et al., 2009; Hasan & Pedraza, 2009; Pardoe et al., 2009; Shen et al., 2010). Thus, clarification of the role played by specific Hc regions in various aspects of navigation would require further development of neuroanatomical measurement techniques.
In summary, turning preference observed during goal-directed navigation in a virtual environment correlates with age, sex and hemispheric asymmetry of the putamen, cerebellum, and hippocampus. The observed differences in associations between cortical and subcortical structural asymmetries and turning behavior point to a previously undeclared source of variance in virtual navigation. Although landmark orientation based on spatial cues is the crucial part of navigation in mammals (Taube et al., 2013; Wang and Spelke, 2002), the observed bias in turning could introduce an unconscious component that might impair course correction based on noisy visual-spatial information. When under conditions of uncertainty that are especially likely in a search for a hidden platform, the cortex commands to make a left, cerebellum and striatum can give an unwanted nudge to the right.
Rotational bias is associated with striatal asymmetry in rodents.
We studied rotational bias in humans navigating a virtual Morris Water Maze.
Virtual turning preference correlated with volumetric asymmetry in the putamen, cerebellum, and hippocampus.
The turning bias was towards larger putamen, cerebellum and (in younger adults only) hippocampus.
Acknowledgments
The study was supported in part by a grant from the National Institute on Aging (R37 AG011230) to NR. We are grateful to Cheryl Dahle, Andrew Bender, and Yiqin Yang for help in data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bender AR, Daugherty AM, Raz N. Vascular risk moderates associations between hippocampal subfield volumes and memory. Journal of Cognitive Neuroscience. 2013 Jun 14; doi: 10.1162/jocn_a_00435. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B. 1995;57:289–300. [Google Scholar]
- Botez MI, Botez T, Elie R, Attig E. Role of the cerebellum in complex human-behavior. Italian Journal of Neurological Sciences. 1989;10:291–300. doi: 10.1007/BF02333774. [DOI] [PubMed] [Google Scholar]
- Bracha HS. Asymmetric rotational (circling) behavior, a dopamine-related asymmetry: Preliminary findings in unmedicated and never-medicated schizophrenic patients. Biological psychiatry (1969) 1987;22:995–1003. doi: 10.1016/0006-3223(87)90009-6. [DOI] [PubMed] [Google Scholar]
- Bracha HS, Seitz DJ, Otemaa J, Glick SD. Rotational movement (circling) in normal humans: Sex difference and relationship to hand, foot and eye preference. Brain Research. 1987;411:231–235. doi: 10.1016/0006-8993(87)91074-2. [DOI] [PubMed] [Google Scholar]
- Bracha HS, Shults C, Glick SD, Kleinman JE. Spontaneous asymmetric circling behavior in hemi-parkinsonism; a human equivalent of the lesioned-circling rodent behavior. Life Sciences. 1987;40:1127–1130. doi: 10.1016/0024-3205(87)90576-5. [DOI] [PubMed] [Google Scholar]
- Bradshaw JL, Bradshaw JA. Rotational and turning tendencies in humans: An analog of lateral biases in rats? International Journal of Neuroscience. 1988;39:229–232. doi: 10.3109/00207458808985708. [DOI] [PubMed] [Google Scholar]
- Brown TI, Ross RS, Tobyne SM, Stern CE. Cooperative interactions between hippocampal and striatal systems support flexible navigation. Neuroimage. 2012;60:1316–1330. doi: 10.1016/j.neuroimage.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition ii: An empirical review of 275 pet and fmri studies. Journal of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Chen G, King JA, Burgess N, O'Keefe J. How vision and movement combine in the hippocampal place code. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:378–383. doi: 10.1073/pnas.1215834110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbuin N, Anstey KJ, Réglade-Meslin C, Sachdev PS. In vivo hippocampal measurement and memory: a comparison of manual tracing and automated segmentation in a large community-based sample. PLoS One. 2009;4:e5265. doi: 10.1371/journal.pone.0005265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coren S, Halpern DF. Left-handedness: A marker for decreased survival fitness. Psychological Bulletin. 1991;109:90–106. doi: 10.1037/0033-2909.109.1.90. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Glick SD. Nocturnal rotation in normal rats: Correlation with amphetamine-induced rotation and effects of nigro-striatal lesions. Brain Research. 1978;150:149–161. doi: 10.1016/0006-8993(78)90659-5. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: A voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Harris MA, Wiener JM, Wolbers T. Aging specifically impairs switching to an allocentric navigational strategy. Frontiers in Aging Neuroscience. 2012;4:29. doi: 10.3389/fnagi.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan KM, Pedraza O. Improving the reliability of manual and automated methods for hippocampal and amygdala volume measurements. Neuroimage. 2009;48:497–498. doi: 10.1016/j.neuroimage.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes G. The cerebellum of man. Brain. 1939;62:1–30. [Google Scholar]
- Hugdahl K, Satz P, Mitrushina M, Miller EN. Left-handedness and old age: Do left-handers die earlier? Neuropsychologia. 1993;31:325–333. doi: 10.1016/0028-3932(93)90156-t. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Twomey CK, Zinsmeister AR, Sharbrough FW, Petersen RC, Cascino GD. Anterior temporal lobes and hippocampal formations: Normative volumetric measurements from mr images in young adults. Radiology. 1989;172:549–554. doi: 10.1148/radiology.172.2.2748838. [DOI] [PubMed] [Google Scholar]
- Kaplan R, Doeller CF, Barnes GR, Litvak V, Duzel E, Bandettini PA, Burgess N. Movement-related theta rhythm in humans: Coordinating self-directed hippocampal learning. Plos Biology. 2012;10:e1001267. doi: 10.1371/journal.pbio.1001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Van Overschelde S, De Rycke M, Musch E. Intrinsic and extrinsic factors of turning preferences in humans. Neuroscience Letters. 2006;393:179–183. doi: 10.1016/j.neulet.2005.09.061. [DOI] [PubMed] [Google Scholar]
- Lyon N, Satz P. Left turning (swivel) in medicated chronic schizophrenic patients. Schizophrenia Research. 1991;4:53–58. doi: 10.1016/0920-9964(91)90010-o. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Burgess N, Donnett JG, Frackowiak RS, Frith CD, O'Keefe J. Knowing where and getting there: A human navigation network. Science. 1998;280:921–924. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD. Navigation-related structural change in the hippocampi of taxi drivers. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead LA, Hampson E. A sex difference in turning bias in humans. Behavioural Brain Research. 1996;78:73–79. doi: 10.1016/0166-4328(95)00233-2. [DOI] [PubMed] [Google Scholar]
- Mead LA, Hampson E. Turning bias in humans is influenced by phase of the menstrual cycle. Hormones and Behavior. 1997;31:65–74. doi: 10.1006/hbeh.1997.1363. [DOI] [PubMed] [Google Scholar]
- Moffat SD. Aging and spatial navigation: What do we know and where do we go? Neuropsychology Review. 2009;19:478–489. doi: 10.1007/s11065-009-9120-3. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Kennedy KM, Rodrigue KM, Raz N. Extra hippocampal contributions to age differences in human spatial navigation. Cerebral Cortex. 2007;17:1274–1282. doi: 10.1093/cercor/bhl036. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Resnick SM. Effects of age on virtual environment place navigation and allocentric cognitive mapping. Behavioral Neuroscience. 2002;116:851–859. doi: 10.1037//0735-7044.116.5.851. [DOI] [PubMed] [Google Scholar]
- Mohr C, Bracha HS. Compound measure of hand-foot-eye preference masked opposite turning behavior in healthy right-handers and non-right-handers: Technical comment on mohr et al. (2003) Behavioral Neuroscience. 2004;118:1145–1146. doi: 10.1037/0735-7044.118.5.1145. [DOI] [PubMed] [Google Scholar]
- Mohr C, Brugger R, Bracha HS, Landis T, Viaud-Delmon I. Human side preferences in three different whole-body movement tasks. Behavioural Brain Research. 2004;151:321–326. doi: 10.1016/j.bbr.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Mohr C, Landis T, Bracha HS, Brugger P. Opposite turning behavior in right-handers and non-right-handers suggests a link between handedness and cerebral dopamine asymmetries. Behavioral Neuroscience. 2003;117:1448–1452. doi: 10.1037/0735-7044.117.6.1448. [DOI] [PubMed] [Google Scholar]
- Mohr C, Lievesley A. Test-retest stability of an experimental measure of human turning behaviour in right-handers, mixed-handers, and left-handers. Laterality. 2007;12:172–190. doi: 10.1080/13576500601051580. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. Journal of neuroscience methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pardoe HR, Pell GS, Abbott DF, Jackson GD. Hippocampal volume assessment in temporal lobe epilepsy: How good is automated segmentation? Epilepsia. 2009;50:2586–2592. doi: 10.1111/j.1528-1167.2009.02243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porac C, Friesen IC. Hand preference side and its relation to hand preference switch history among old and oldest-old adults. Developmental Neuropsychology. 2000;17:225–239. doi: 10.1207/S15326942DN1702_05. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The ces-d scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U. Trajectories of brain aging in middle-aged and older adults: Regional and individual differences. Neuroimage. 2010;51:501–511. doi: 10.1016/j.neuroimage.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: Replicability of regional differences in volume. Neurobiology of Aging. 2004;25:377–396. doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cerebral Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Rochefort C, Arabo A, Andre M, Poucet B, Save E, Rondi-Reig L. Cerebellum shapes hippocampal spatial code. Science. 2011;334:385–389. doi: 10.1126/science.1207403. [DOI] [PubMed] [Google Scholar]
- Rodgers MK, Sindone JA, 3RD, Moffat SD. Effects of age on navigation strategy. Neurobiology of aging. 2012;33:e15–e22. doi: 10.1016/j.neurobiolaging.2010.07.021. 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondi-Reig L, Burguiere E. Is the cerebellum ready for navigation? Progress in Brain Research. 2005;148:199–212. doi: 10.1016/S0079-6123(04)48017-0. [DOI] [PubMed] [Google Scholar]
- Rondi-Reig L, Le Marec N, Caston J, Mariani J. The role of climbing and parallel fibers inputs to cerebellar cortex in navigation. Behavioural Brain Research. 2002;132:11–18. doi: 10.1016/s0166-4328(01)00381-3. [DOI] [PubMed] [Google Scholar]
- Shen L, Saykin AJ, Kim S, Firpi HA, West JD, Risacher SL, McDonald BC, McHugh TL, Wishart HA, Flashman LA. Comparison of manual and automated determination of hippocampal volumes in MCI and early AD. Brain Imaging Behav. 2010;4:86–95. doi: 10.1007/s11682-010-9088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychological Bulletin. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Solodkin A, Hlustik P, Noll DC, Small SL. Lateralization of motor circuits and handedness during finger movements. European Journal of Neurology. 2001;8:425–434. doi: 10.1046/j.1468-1331.2001.00242.x. [DOI] [PubMed] [Google Scholar]
- Stochl J, Croudace T. Predictors of human rotation. Laterality. 2012 doi: 10.1080/1357650X.2012.662233. [DOI] [PubMed] [Google Scholar]
- Tan U. The distribution of hand preference in normal men and women. International Journal of Neuroscience. 1988;41:35–55. doi: 10.3109/00207458808985740. [DOI] [PubMed] [Google Scholar]
- Taube JS, Valerio S, Yoder RM. Is navigation in virtual reality with fmri really navigation? Journal of Cognitive Neuroscience. 2013;25:1008–1019. doi: 10.1162/jocn_a_00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussaint Y, Fagard J. A counterclockwise bias in running. Neuroscience Letters. 2008;442:59–62. doi: 10.1016/j.neulet.2008.06.056. [DOI] [PubMed] [Google Scholar]
- Vernaleken I. Asymmetry in dopamine d2/3 receptors of caudate nucleus is lost with age. NeuroImage. 2007;34:870. doi: 10.1016/j.neuroimage.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Waller D. Individual differences in spatial learning from computer-simulated environments. Journal of Experimental Psychology: Applied. 2000;6:307–321. doi: 10.1037//1076-898x.6.4.307. [DOI] [PubMed] [Google Scholar]
- Wang R, Spelke E. Human spatial representation: Insights from animals. Trends in Cognitive Sciences. 2002;6:376. doi: 10.1016/s1364-6613(02)01961-7. [DOI] [PubMed] [Google Scholar]
- Wiestler T, McGonigle DJ, Diedrichsen J. Integration of sensory and motor representations of single fingers in the human cerebellum. Journal of Neurophysiology. 2011;105:3042–3053. doi: 10.1152/jn.00106.2011. [DOI] [PubMed] [Google Scholar]
- Wilson PN. Transfer of spatial information from a virtual to a real environment. Human Factors. 1997;39:526–531. [Google Scholar]
- Yamamoto BK, Freed CR. The trained circling rat: A model for inducing unilateral caudate dopamine metabolism. Nature. 1982;298:467–468. doi: 10.1038/298467a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Lane RF, Freed CR. Normal rats trained to circle show asymmetric caudate dopamine release. Life Sciences. 1982;30:2155–2162. doi: 10.1016/0024-3205(82)90289-2. [DOI] [PubMed] [Google Scholar]
- Zilles K, Dabringhaus A, Geyer S, Amunts K, Qu M, Schleicher A, Gilissen E, Schlaug G, Steinmetz H. Structural asymmetries in the human forebrain and the forebrain of non-human primates and rats. Neuroscience and Biobehavioral Reviews. 1996;20:593–605. doi: 10.1016/0149-7634(95)00072-0. [DOI] [PubMed] [Google Scholar]
- Zimmerberg B. Neurochemical correlate of a spatial preference in rats. Science. 1974;185:623–625. doi: 10.1126/science.185.4151.623. [DOI] [PubMed] [Google Scholar]