Abstract
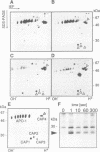
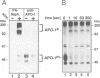
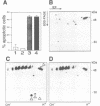
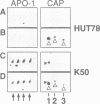
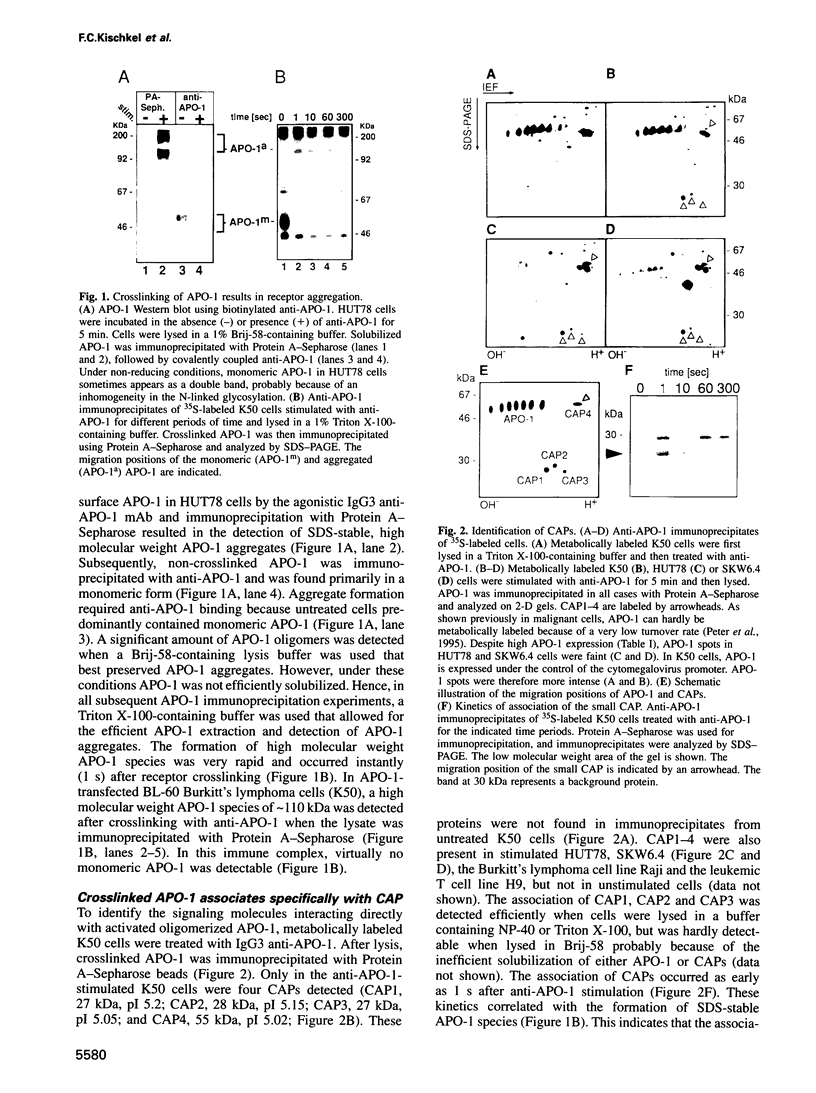
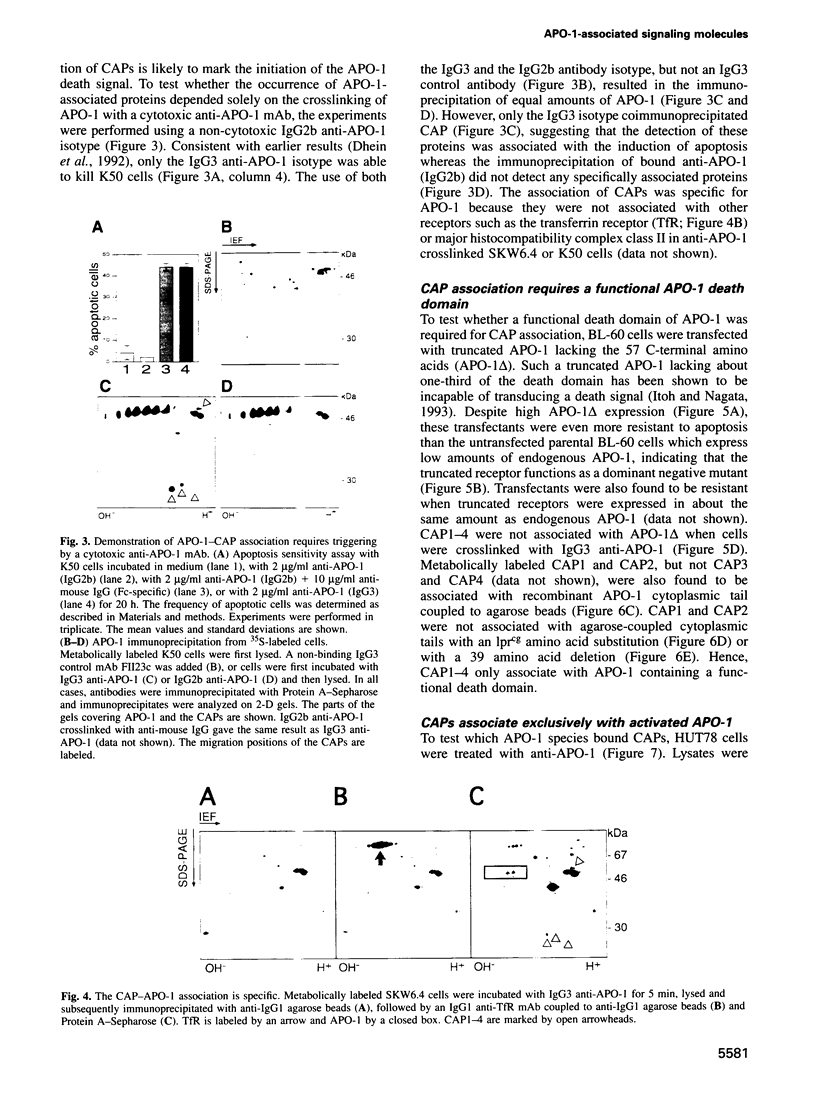
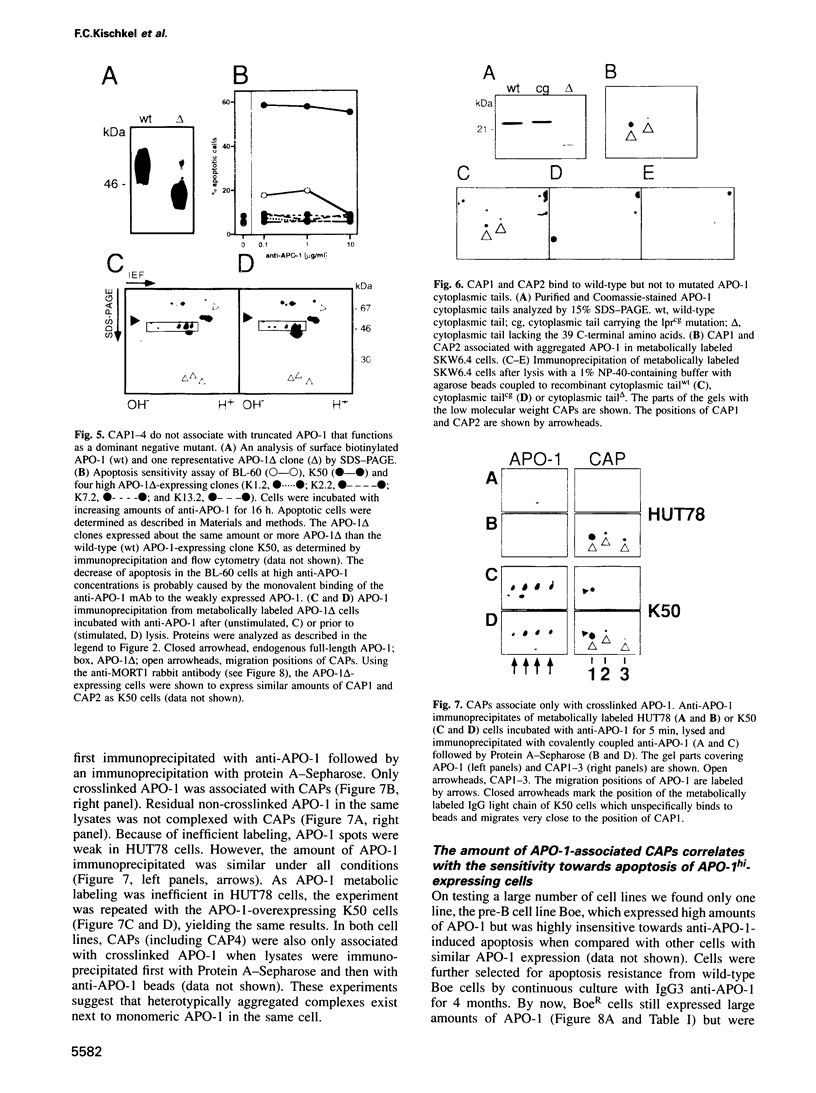
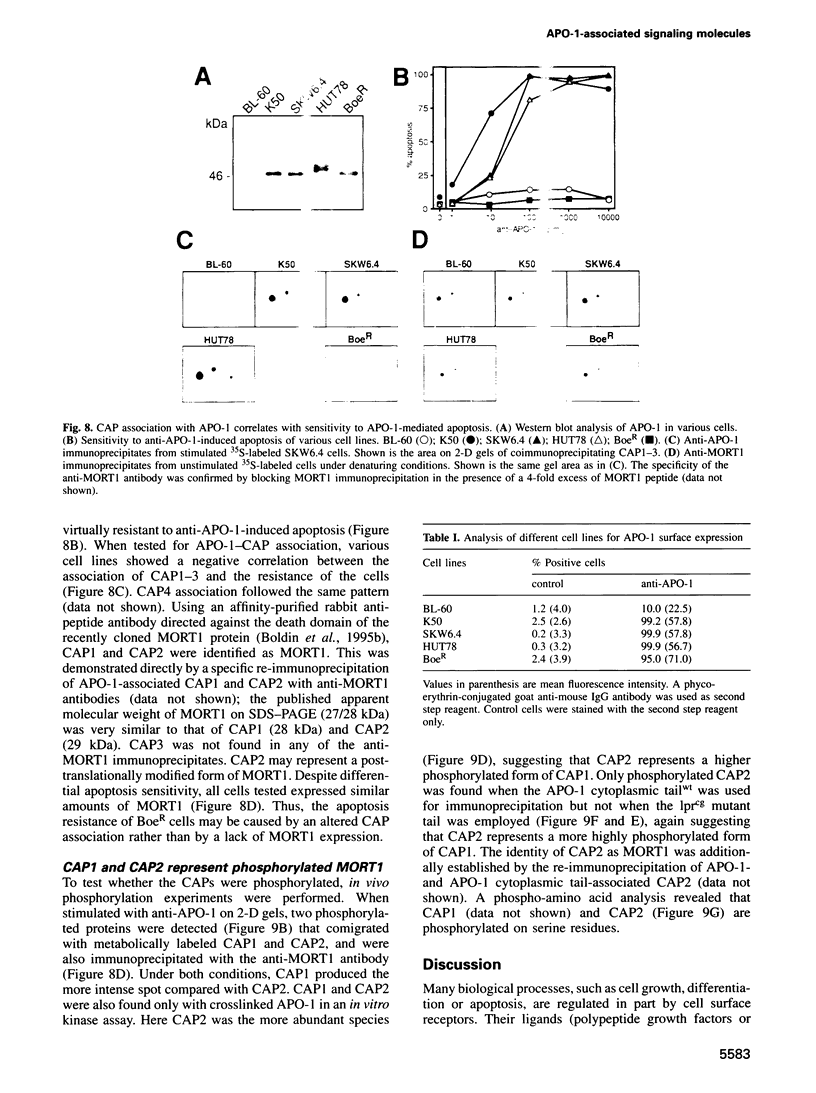
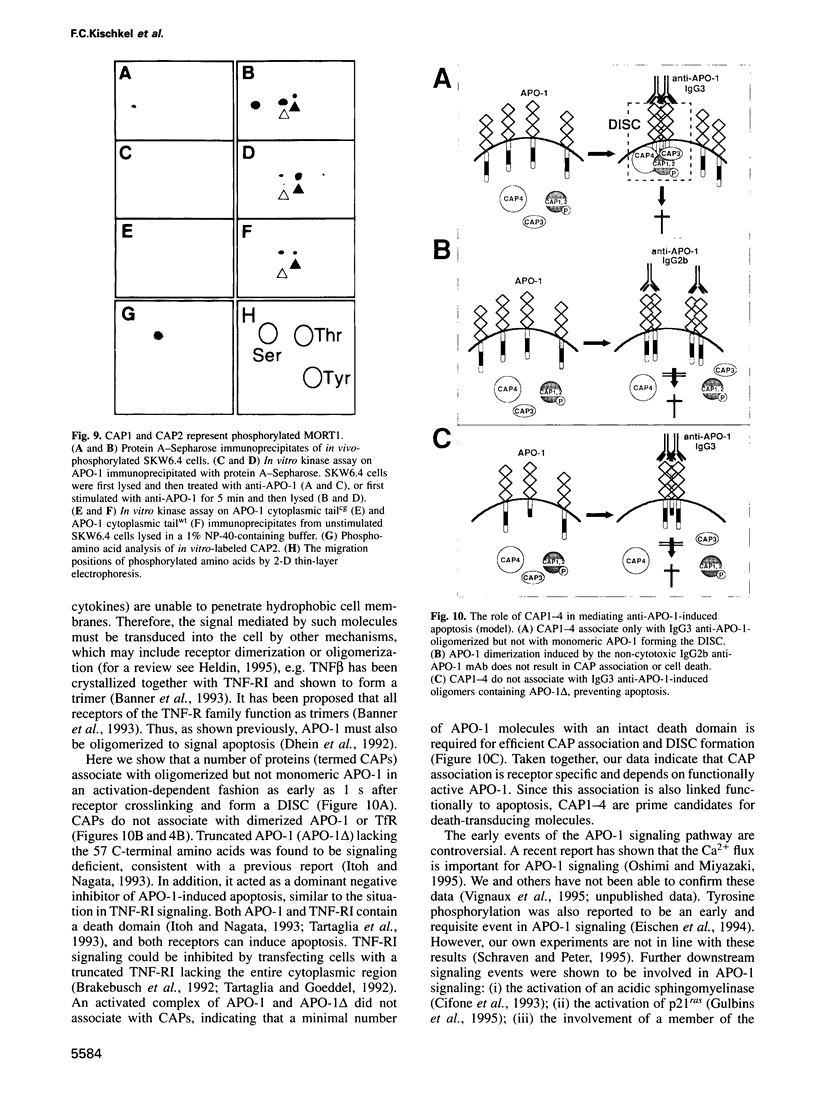
APO-1 (Fas/CD95), a member of the tumor necrosis factor receptor superfamily, induces apoptosis upon receptor oligomerization. In a search to identify intracellular signaling molecules coupling to oligomerized APO-1, several cytotoxicity-dependent APO-1-associated proteins (CAP) were immunoprecipitated from the apoptosis-sensitive human leukemic T cell line HUT78 and the lymphoblastoid B cell line SKW6.4. CAP1-3 (27-29 kDa) and CAP4 (55 kDa), instantly detectable after the crosslinking of APO-1, were associated only with aggregated (the signaling form of APO-1) and not with monomeric APO-1. CAP1 and CAP2 were identified as serine phosphorylated MORT1/FADD. The association of CAP1-4 with APO-1 was not observed with C-terminally truncated non-signaling APO-1. In addition, CAP1 and CAP2 did not associate with an APO-1 cytoplasmic tail carrying the lprcg amino acid replacement. Moreover, no APO-1-CAP association was found in the APO-1+, anti-APO-1-resistant pre-B cell line Boe. Our data suggest that in vivo CAP1-4 are the APO-1 apoptosis-transducing molecules.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argetsinger L. S., Campbell G. S., Yang X., Witthuhn B. A., Silvennoinen O., Ihle J. N., Carter-Su C. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell. 1993 Jul 30;74(2):237–244. doi: 10.1016/0092-8674(93)90415-m. [DOI] [PubMed] [Google Scholar]
- Banner D. W., D'Arcy A., Janes W., Gentz R., Schoenfeld H. J., Broger C., Loetscher H., Lesslauer W. Crystal structure of the soluble human 55 kd TNF receptor-human TNF beta complex: implications for TNF receptor activation. Cell. 1993 May 7;73(3):431–445. doi: 10.1016/0092-8674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- Benhamou M., Ryba N. J., Kihara H., Nishikata H., Siraganian R. P. Protein-tyrosine kinase p72syk in high affinity IgE receptor signaling. Identification as a component of pp72 and association with the receptor gamma chain after receptor aggregation. J Biol Chem. 1993 Nov 5;268(31):23318–23324. [PubMed] [Google Scholar]
- Boldin M. P., Mett I. L., Varfolomeev E. E., Chumakov I., Shemer-Avni Y., Camonis J. H., Wallach D. Self-association of the "death domains" of the p55 tumor necrosis factor (TNF) receptor and Fas/APO1 prompts signaling for TNF and Fas/APO1 effects. J Biol Chem. 1995 Jan 6;270(1):387–391. doi: 10.1074/jbc.270.1.387. [DOI] [PubMed] [Google Scholar]
- Boldin M. P., Varfolomeev E. E., Pancer Z., Mett I. L., Camonis J. H., Wallach D. A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J Biol Chem. 1995 Apr 7;270(14):7795–7798. doi: 10.1074/jbc.270.14.7795. [DOI] [PubMed] [Google Scholar]
- Brakebusch C., Nophar Y., Kemper O., Engelmann H., Wallach D. Cytoplasmic truncation of the p55 tumour necrosis factor (TNF) receptor abolishes signalling, but not induced shedding of the receptor. EMBO J. 1992 Mar;11(3):943–950. doi: 10.1002/j.1460-2075.1992.tb05133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko G. W., Duchemin A. M., Coggeshall K. M., Osborne J. M., Brandt J. T., Anderson C. L. Clustering of the platelet Fc gamma receptor induces noncovalent association with the tyrosine kinase p72syk. J Biol Chem. 1994 Dec 23;269(51):32435–32440. [PubMed] [Google Scholar]
- Chan A. C., Desai D. M., Weiss A. The role of protein tyrosine kinases and protein tyrosine phosphatases in T cell antigen receptor signal transduction. Annu Rev Immunol. 1994;12:555–592. doi: 10.1146/annurev.iy.12.040194.003011. [DOI] [PubMed] [Google Scholar]
- Chinnaiyan A. M., O'Rourke K., Tewari M., Dixit V. M. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995 May 19;81(4):505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- Cleveland J. L., Ihle J. N. Contenders in FasL/TNF death signaling. Cell. 1995 May 19;81(4):479–482. doi: 10.1016/0092-8674(95)90068-3. [DOI] [PubMed] [Google Scholar]
- Davidson W. F., Dumont F. J., Bedigian H. G., Fowlkes B. J., Morse H. C., 3rd Phenotypic, functional, and molecular genetic comparisons of the abnormal lymphoid cells of C3H-lpr/lpr and C3H-gld/gld mice. J Immunol. 1986 Jun 1;136(11):4075–4084. [PubMed] [Google Scholar]
- Dhein J., Daniel P. T., Trauth B. C., Oehm A., Möller P., Krammer P. H. Induction of apoptosis by monoclonal antibody anti-APO-1 class switch variants is dependent on cross-linking of APO-1 cell surface antigens. J Immunol. 1992 Nov 15;149(10):3166–3173. [PubMed] [Google Scholar]
- Dürkop H., Latza U., Hummel M., Eitelbach F., Seed B., Stein H. Molecular cloning and expression of a new member of the nerve growth factor receptor family that is characteristic for Hodgkin's disease. Cell. 1992 Feb 7;68(3):421–427. doi: 10.1016/0092-8674(92)90180-k. [DOI] [PubMed] [Google Scholar]
- Eischen C. M., Dick C. J., Leibson P. J. Tyrosine kinase activation provides an early and requisite signal for Fas-induced apoptosis. J Immunol. 1994 Sep 1;153(5):1947–1954. [PubMed] [Google Scholar]
- Gulbins E., Bissonnette R., Mahboubi A., Martin S., Nishioka W., Brunner T., Baier G., Baier-Bitterlich G., Byrd C., Lang F. FAS-induced apoptosis is mediated via a ceramide-initiated RAS signaling pathway. Immunity. 1995 Apr;2(4):341–351. doi: 10.1016/1074-7613(95)90142-6. [DOI] [PubMed] [Google Scholar]
- Heldin C. H. Dimerization of cell surface receptors in signal transduction. Cell. 1995 Jan 27;80(2):213–223. doi: 10.1016/0092-8674(95)90404-2. [DOI] [PubMed] [Google Scholar]
- Higuchi R., Krummel B., Saiki R. K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988 Aug 11;16(15):7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber L. A., Peter M. E. Mapping small GTP-binding proteins on high-resolution two-dimensional gels by a combination of GTP binding and labeling with in situ periodate-oxidized GTP. Electrophoresis. 1994 Feb;15(2):283–288. doi: 10.1002/elps.1150150148. [DOI] [PubMed] [Google Scholar]
- Itoh N., Nagata S. A novel protein domain required for apoptosis. Mutational analysis of human Fas antigen. J Biol Chem. 1993 May 25;268(15):10932–10937. [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Ishii A., Yonehara M., Mizushima S., Sameshima M., Hase A., Seto Y., Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991 Jul 26;66(2):233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Johnson D., Lanahan A., Buck C. R., Sehgal A., Morgan C., Mercer E., Bothwell M., Chao M. Expression and structure of the human NGF receptor. Cell. 1986 Nov 21;47(4):545–554. doi: 10.1016/0092-8674(86)90619-7. [DOI] [PubMed] [Google Scholar]
- Krammer P. H., Dhein J., Walczak H., Behrmann I., Mariani S., Matiba B., Fath M., Daniel P. T., Knipping E., Westendorp M. O. The role of APO-1-mediated apoptosis in the immune system. Immunol Rev. 1994 Dec;142:175–191. doi: 10.1111/j.1600-065x.1994.tb00889.x. [DOI] [PubMed] [Google Scholar]
- Kwon B. S., Weissman S. M. cDNA sequences of two inducible T-cell genes. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1963–1967. doi: 10.1073/pnas.86.6.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti J. M., Xiang J., Heath L. S., Campana D., Kidd V. J. PITSLRE protein kinase activity is associated with apoptosis. Mol Cell Biol. 1995 Jan;15(1):1–11. doi: 10.1128/mcb.15.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loetscher H., Pan Y. C., Lahm H. W., Gentz R., Brockhaus M., Tabuchi H., Lesslauer W. Molecular cloning and expression of the human 55 kd tumor necrosis factor receptor. Cell. 1990 Apr 20;61(2):351–359. doi: 10.1016/0092-8674(90)90815-v. [DOI] [PubMed] [Google Scholar]
- Los M., Van de Craen M., Penning L. C., Schenk H., Westendorp M., Baeuerle P. A., Dröge W., Krammer P. H., Fiers W., Schulze-Osthoff K. Requirement of an ICE/CED-3 protease for Fas/APO-1-mediated apoptosis. Nature. 1995 May 4;375(6526):81–83. doi: 10.1038/375081a0. [DOI] [PubMed] [Google Scholar]
- Mallett S., Fossum S., Barclay A. N. Characterization of the MRC OX40 antigen of activated CD4 positive T lymphocytes--a molecule related to nerve growth factor receptor. EMBO J. 1990 Apr;9(4):1063–1068. doi: 10.1002/j.1460-2075.1990.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani S. M., Matiba B., Armandola E. A., Krammer P. H. The APO-1/Fas (CD95) receptor is expressed in homozygous MRL/lpr mice. Eur J Immunol. 1994 Dec;24(12):3119–3123. doi: 10.1002/eji.1830241231. [DOI] [PubMed] [Google Scholar]
- Oehm A., Behrmann I., Falk W., Pawlita M., Maier G., Klas C., Li-Weber M., Richards S., Dhein J., Trauth B. C. Purification and molecular cloning of the APO-1 cell surface antigen, a member of the tumor necrosis factor/nerve growth factor receptor superfamily. Sequence identity with the Fas antigen. J Biol Chem. 1992 May 25;267(15):10709–10715. [PubMed] [Google Scholar]
- Oshimi Y., Miyazaki S. Fas antigen-mediated DNA fragmentation and apoptotic morphologic changes are regulated by elevated cytosolic Ca2+ level. J Immunol. 1995 Jan 15;154(2):599–609. [PubMed] [Google Scholar]
- Peter M. E., Hellbardt S., Schwartz-Albiez R., Westendorp M. O., Walczak H., Moldenhauer G., Grell M., Krammer P. H. Cell surface sialylation plays a role in modulating sensitivity towards APO-1-mediated apoptotic cell death. Cell Death Differ. 1995 Jul;2(3):163–171. [PubMed] [Google Scholar]
- Pleiman C. M., Abrams C., Gauen L. T., Bedzyk W., Jongstra J., Shaw A. S., Cambier J. C. Distinct p53/56lyn and p59fyn domains associate with nonphosphorylated and phosphorylated Ig-alpha. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4268–4272. doi: 10.1073/pnas.91.10.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe M. J., Coggeshall K. M., Newell M. K., Julius M. H. T cell receptor aggregation, but not dimerization, induces increased cytosolic calcium concentrations and reveals a lack of stable association between CD4 and the T cell receptor. J Immunol. 1992 Mar 15;148(6):1643–1651. [PubMed] [Google Scholar]
- Samelson L. E., Phillips A. F., Luong E. T., Klausner R. D. Association of the fyn protein-tyrosine kinase with the T-cell antigen receptor. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4358–4362. doi: 10.1073/pnas.87.11.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Irie S., Kitada S., Reed J. C. FAP-1: a protein tyrosine phosphatase that associates with Fas. Science. 1995 Apr 21;268(5209):411–415. doi: 10.1126/science.7536343. [DOI] [PubMed] [Google Scholar]
- Schall T. J., Lewis M., Koller K. J., Lee A., Rice G. C., Wong G. H., Gatanaga T., Granger G. A., Lentz R., Raab H. Molecular cloning and expression of a receptor for human tumor necrosis factor. Cell. 1990 Apr 20;61(2):361–370. doi: 10.1016/0092-8674(90)90816-w. [DOI] [PubMed] [Google Scholar]
- Schraven B., Kirchgessner H., Gaber B., Samstag Y., Meuer S. A functional complex is formed in human T lymphocytes between the protein tyrosine phosphatase CD45, the protein tyrosine kinase p56lck and pp32, a possible common substrate. Eur J Immunol. 1991 Oct;21(10):2469–2477. doi: 10.1002/eji.1830211025. [DOI] [PubMed] [Google Scholar]
- Schraven B., Peter M. E. APO-1(CD95)-mediated apoptosis in Jurkat cells does not involve src kinases or CD45. FEBS Lett. 1995 Jul 24;368(3):491–494. doi: 10.1016/0014-5793(95)00720-t. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Davis T., Anderson D., Solam L., Beckmann M. P., Jerzy R., Dower S. K., Cosman D., Goodwin R. G. A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science. 1990 May 25;248(4958):1019–1023. doi: 10.1126/science.2160731. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Clark E. A., Seed B. A B-lymphocyte activation molecule related to the nerve growth factor receptor and induced by cytokines in carcinomas. EMBO J. 1989 May;8(5):1403–1410. doi: 10.1002/j.1460-2075.1989.tb03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger B. Z., Leder P., Lee T. H., Kim E., Seed B. RIP: a novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell. 1995 May 19;81(4):513–523. doi: 10.1016/0092-8674(95)90072-1. [DOI] [PubMed] [Google Scholar]
- Tartaglia L. A., Ayres T. M., Wong G. H., Goeddel D. V. A novel domain within the 55 kd TNF receptor signals cell death. Cell. 1993 Sep 10;74(5):845–853. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- Tartaglia L. A., Goeddel D. V. Tumor necrosis factor receptor signaling. A dominant negative mutation suppresses the activation of the 55-kDa tumor necrosis factor receptor. J Biol Chem. 1992 Mar 5;267(7):4304–4307. [PubMed] [Google Scholar]
- Tartaglia L. A., Weber R. F., Figari I. S., Reynolds C., Palladino M. A., Jr, Goeddel D. V. The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9292–9296. doi: 10.1073/pnas.88.20.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin J. L., Tian Q., Kedersha N., Robertson M., Anderson P. The RNA-binding protein TIAR is translocated from the nucleus to the cytoplasm during Fas-mediated apoptotic cell death. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1629–1633. doi: 10.1073/pnas.92.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauth B. C., Klas C., Peters A. M., Matzku S., Möller P., Falk W., Debatin K. M., Krammer P. H. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989 Jul 21;245(4915):301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- Upton C., DeLange A. M., McFadden G. Tumorigenic poxviruses: genomic organization and DNA sequence of the telomeric region of the Shope fibroma virus genome. Virology. 1987 Sep;160(1):20–30. doi: 10.1016/0042-6822(87)90039-0. [DOI] [PubMed] [Google Scholar]
- Van Endert P. M., Moldenhauer G. Inhibitory and stimulatory signaling via immunoglobulin receptors: dichotomous responses elicited in clonal B cell populations. Eur J Immunol. 1992 May;22(5):1229–1235. doi: 10.1002/eji.1830220518. [DOI] [PubMed] [Google Scholar]
- Vignaux F., Vivier E., Malissen B., Depraetere V., Nagata S., Golstein P. TCR/CD3 coupling to Fas-based cytotoxicity. J Exp Med. 1995 Feb 1;181(2):781–786. doi: 10.1084/jem.181.2.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Copeland N. G., Jenkins N. A., Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992 Mar 26;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Wittinghofer A., Guariguata R., Leberman R. Bacterial elongation factor Ts: isolation and reactivity with elongation factor Tu. J Bacteriol. 1983 Mar;153(3):1266–1271. doi: 10.1128/jb.153.3.1266-1271.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin T., Tsang M. L., Yang Y. C. JAK1 kinase forms complexes with interleukin-4 receptor and 4PS/insulin receptor substrate-1-like protein and is activated by interleukin-4 and interleukin-9 in T lymphocytes. J Biol Chem. 1994 Oct 28;269(43):26614–26617. [PubMed] [Google Scholar]
- Yonehara S., Ishii A., Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989 May 1;169(5):1747–1756. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]