Abstract
Azaspiracids (AZAs) are marine biotoxins produced by the dinoflagellate Azadinium spinosum that accumulate in several shellfish species. Azaspiracid poisoning (AZP) episodes have been described in humans due to ingestion of AZA-contaminated seafood. Therefore, the contents of AZA-1, AZA-2 and AZA-3, the best-known analogs of the group, in shellfish destined to human consumption have been regulated by food safety authorities of many countries to protect human health. In vivo and in vitro toxicological studies have described effects of AZAs at different cellular levels and on several organs, however, AZA target remains unknown. Very recently AZAs have been demonstrated to block the hERG cardiac potassium channel. In this study we explored the potential cardiotoxicity of AZA-2 in vivo. The effects of AZA-2 on rat electrocardiogram (ECG) and cardiac biomarkers were evaluated for cardiotoxicity signs besides corroborating the hERG blocking activity of AZA-2. Our results demonstrated that AZA-2 does not induce QT interval prolongation on rat ECGs in vivo, in spite of being an in vitro blocker of the hERG cardiac potassium channel. However, AZA-2 alters the heart electrical activity causing prolongation of PR intervals and the appearance of arrhythmias. More studies will be needed to clarify the mechanism by which AZA-2 causes these ECG alterations, however, the potential cardiotoxicity of AZAs demonstrated in this in vivo study should be taken into consideration when evaluating the possible threat that these toxins pose to human health, mainly for individuals with pre-existing cardiovascular disease when regulated toxin limits are exceeded.
Keywords: Azaspiracid, cardiotoxicity, hERG, ECG, cardiac biomarkers
INTRODUCTION
Azaspiracids (AZAs) are marine biotoxins discovered in 1995 due to ingestion of contaminated mussels from Killary Harbor, Ireland (MCMahon 1996; Satake et al. 1998). Since that first human intoxication episode, AZAs have appeared in multiple locations around the world (Furey et al. 2010). AZAs are produced by the dinoflagellate Azadinium spinosum and accumulate in shellfish through the tropic chain (Furey et al. 2010). Humans exposed to AZAs suffer azaspiracid poisoning (AZP), consisting predominantly of gastrointestinal symptoms (James et al. 2004). Currently, more than 20 analogues have been described (Furey et al. 2010), with AZA-1, AZA-2 and AZA-3 being the best-known compounds. Due to the threat that these toxins pose to human health, the contents of AZA-1, AZA-2 and AZA-3 in shellfish destined to human consumption have been regulated by food safety authorities in many countries (EC 2004).
In vivo toxicological data revealed that AZA injures several organs, including small intestine, liver, lung, pancreas, thymus and spleen (Ito et al. 2000; Ito et al. 2002). Moreover, AZA-1 has been related to the appearance of lung tumors (Ito et al. 2002). The mechanism of action of AZAs is still unknown. In vitro studies have shown that AZAs reduce cell viability, damage the cytoskeleton, activate apoptosis and modulate the calcium influx pathway, among others (Alfonso et al. 2005; Twiner et al. 2005; Vilariño et al. 2007). Additionally, studies with fish embryos point to a possible teratogenicity (Colman et al. 2005). Very recently, an open-state hERG (human ether-a-go-go-related gene) blocking activity has been reported for AZA-1, AZA-2 and AZA-3 (Twiner et al. 2012). Considering the generalized effects at cellular and organ levels and the recently described blockage of cardiac hERG potassium channel, the effects of AZAs on heart function should be explored. Drug cardiotoxicity studies have been guided by the recommendations of the EMEA (EMEA 2005), which lay out the key tests required to evaluate potential cardiotoxicity in the pharmaceutical industry. In recent years experts on heart toxicity have suggested additional studies (Guth 2007; Stummann et al. 2009). Cardiotoxicity evaluation should comprise in vitro and in vivo experiments. In vitro analysis of hERG function by patch clamp has been used for years for preliminary assessment of cardiac safety (Hancox et al. 2008). The hERG channel (Kv11.1) is responsible for the rapid delayed rectifier K+ current (Ikr), which is critical in cardiac action potential (AP) repolarization (Sanguinetti et al. 1995). Alterations of hERG function have been related to electrophysiological changes at the cellular and tissue levels that can trigger arrhythmia appearance. Specifically, a common manifestation of hERG inhibition is QT interval prolongation on the electrocardiogram (ECG), which has been linked to a type of arrhythmia known as “Torsades de Pointes” (TdP) (Redfern et al. 2003; Gintant et al. 2006). TdP can degenerate into ventricular fibrillation and sudden death (Hoffmann et al. 2006). Among cardiac channels and electrogenic transporters, the unique hERG channel gating, the structure of its inner cavity and the implications of hERG conductivity for heart function make this channel the first choice for in vitro evaluation of compound-related cardiotoxicity (Hancox et al. 2008; Priest et al. 2008; Perry et al. 2010).
A thorough evaluation of heart toxicity requires also in vivo studies. Functional and structural damage to the heart, although pathophysiologically related, are usually considered separately. For functional alterations, electrocardiography is the technique of choice (Guth 2007; Stummann et al. 2009). ECG is a record of the electrical charge changes of the heart chambers during each beat. The ECG may be affected by modifications of metabolic processes, cardiac channel function, cardiomyocyte membrane properties and intracellular signaling or structural injuries (Farraj et al. 2011). Regarding structural damage, histopathological studies help to reveal alterations of cardiac tissue. However, quantification of cardiotoxicity biomarkers to evaluate compound-induced structural injury has been included recently among the in vivo experiments recommended to complete the cardiotoxicity tests array (Kettenhofen et al. 2008). Cardiac troponin I (cTnI), cardiac troponin T (cTnT) and brain natriuretic peptide (BNP) are accepted biomarkers for assessing cardiac toxicity (Kettenhofen et al. 2008).
Although AZAs have been described to block hERG channels (Twiner et al. 2012), hERG blockage has not always been related to an alteration of heart functionality (Hoffmann et al. 2006; Hancox et al. 2008). Therefore, the aim of this study was to explore the cardiotoxic potential of AZAs in vivo. In order to evaluate the functional implications of AZA-induced hERG blockage and other cardiotoxic effects not related to hERG, AZA-2 was selected due to its higher toxicity (Ofuji et al. 1999) and higher hERG-blocking potency (Twiner et al. 2012).
METHODS
Materials
AZA-2 (Figure 1) was synthesized by Nicolaou and co-workers as previously described (Nicolaou et al. 2006). Nut Mix F-12 W/GLUTAMAX-I medium, fetal bovine serum (FBS), geneticin, Trypsin/EDTA and Dulbecco’s Phosphate-Buffered Saline (DPBS) were purchased from Invitrogen® (Madrid, Spain). IPatch™ Xcellerator Extracellular-Seal solution (EC-Seal), IPatch™ Xcellerator Intracellular-Kv solution (IC-Kv), IPatch™ Xcellerator Extracellular-Kv solution (EC-Kv) and Patch tips™ were from Flyion® ion channels solutions (Tubingen, Germany). Trypan blue, dimethyl sulfoxide (DMSO) and cisapride monohydrate were from Sigma-Aldrich Química S.A. (Madrid, Spain), Isoflurane (Isoba®vet) from Schering-Plough S.A. (Madrid, Spain), claritrhomycin (Klacid IV) from Abbott Laboratories (Abbott Park, Illinois) and sodium chloride solution 0.9% from Grifols Engineering, S.A. (Barcelona, Spain). CVD Milliplex® Map KIT was from Millipore® Iberica S.A. (Madrid, Spain). General Health Profile chemistry panel was obtained from IDEXX Laboratories (Barcelona, Spain).
Fig. 1.
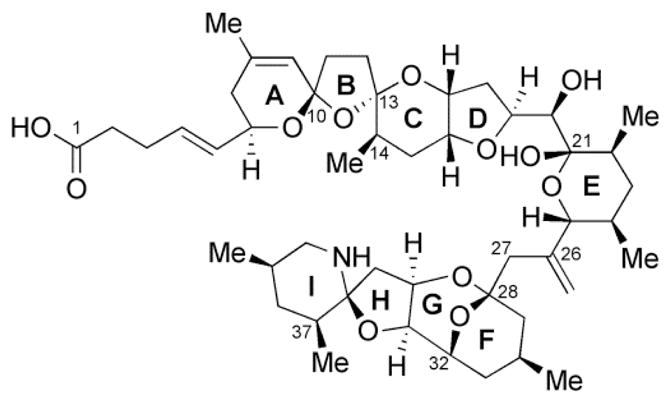
AZA-2 chemical structure.
Cell line
Precision™ hERG CHO (Chinese hamster ovary) Recombinant cell line (Millipore® Iberica S.A. Madrid, Spain) was grown as specified by the provider. Prior to patch-clamp assays, cell cultures were placed for at least one day at 30 °C in a humidified 5% CO2 incubator. For patch clamp experiments culture confluence was always 60–80%. The cells were dissociated with warm Trypsin/EDTA for 3 minutes at 37 °C. Then the cells were resuspended with 5 mL of culture media and centrifuged at 800 g and 19 °C for 5 min. Cell count and viability was determined using Trypan Blue. Viability was always higher than 99%. The cell pellet was suspended in EC seal buffer at 0.5.106 cells/mL immediately before the experiment.
Automated patch clamp
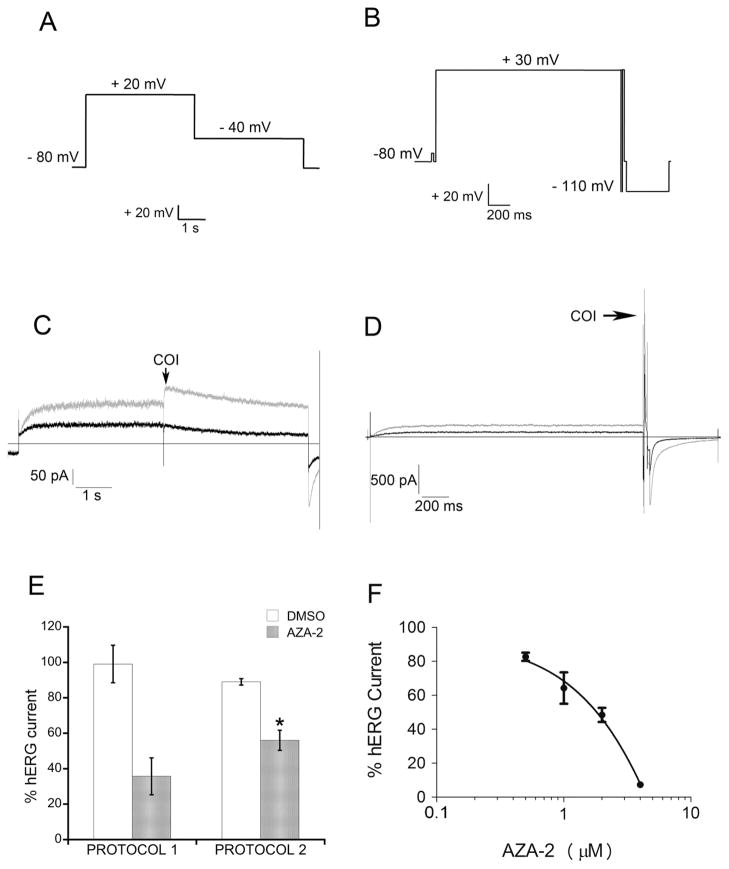
The effect of AZA-2 on hERG channel activity was tested using a Flyscreen® 8500 automated patch clamp (Flyion®, Tubingen, Germany) with an EPC-10 amplifier (HEKA Electronik, Lambrecht/Pfalz, Germany) and Pulse 8.67 software (HEKA Electronik, Lambrecht/Pfalz, Germany). All experiments were carried out at room temperature. After whole-cell patch-clamp configuration (WHC) was achieved (seal and WHC pressures of 15 mbar and 200 mbar respectively, pipette resistance of 1–4.5 MΩ and seal resistance ≥ 1GΩ), EC-seal solution was replaced with EC-Kv. Two voltage protocols were used to evoke the IKr. Protocol 1 (P1) is a conventional voltage step protocol used in cardiotoxicity studies (Hancox et al. 1998; Su et al. 2009) and protocol 2 (P2) is recommended by the instrument manufacturer to maximize the current generated by this channel. P1 (Fig. 2A) consisted of a voltage protocol stepping from holding potential (Vhold, − 80 mV) to + 20 mV for 4 s, and down to − 40 mV for 4 s. Peak tail current amplitude at − 40 mV was the current of interest (COI). hERG current was recorded for 240 s before toxin addition for this voltage protocol (control current). P2 (Fig. 2B) consisted of a voltage protocol that stepped from Vhold to + 30 mV for 2 s followed by repolarization to − 110 mV for 6 ms and a depolarization ramp of 2 ms to + 30 mV. Voltage was then maintained at + 30 mV for 20 ms and the tail outward current at this point was the COI. Finally, a succession of hiperpolarization steps (− 80 mV to − 110 mV to − 80 mV) ensured proper hERG deactivation and return to Vhold. Control current was recorded for 360 s. The current was measured for 360 s after compound addition in both voltage protocols. All toxin dilutions were in EC-Kv. Repeated compound additions were performed as previously recommended for automated patch clamp techniques (Guo et al. 2005). Carrier-control currents were obtained by adding only DMSO.
Fig. 2. AZA-2 effect on hERG channel activity.
Automated patch clamp experiments were performed with CHO cells stably expressing hERG. Two voltage protocols were used to elicit hERG currents. For all cells, control current was recorded before toxin addition. Current was monitored for 6 min after AZA-2 addition. (A) Voltage clamp protocol 1. (B) Voltage clamp protocol 2. Representative current traces obtained using (C) protocol 1 and (D) protocol 2 for cells treated with 2 μM AZA-2 (black line) and carrier controls (grey line). Arrow indicates current of interest (COI). (E) Current magnitude in 2 μM AZA-2-treated cells and carrier controls with voltage protocols 1 and 2 (mean ± SEM; P1, n=4; P2, n=5). (F) Concentration-dependence of hERG channel block caused by AZA-2 after 6 min of exposure using protocol 2 (mean ± SEM, n=5, * statistically different versus DMSO control). In (E) and (F) current magnitude was expressed as percentage of pre-treatment current that remained after 6 min of exposure to AZA-2.
Animals and in vivo experimental design
In vivo studies were performed with Sprague Dawley female rats aged 8–16 weeks (180–260 g). The rats were housed in a temperature- and humidity-controlled room (21±2 °C, 50±5% relative humidity) and mantained on a 12h/12h light/dark cycle. They were caged in pairs with free access to food and water,. For the experimental procedures, the rats were anesthetized with isoflurane (FIISO 1.5–2%) and two catheters were placed in the jugular veins, for compound administration and sample collection, and for fluid therapy maintenance (10 mL/Kg/h). ECG recordings were obtained using lead II. After surgery, a period of 15 minutes lapsed before ECG recordings to ensure stable vital signs. Then, ECG was recorded for 10 min and AZA-2 was administered by intravenous injection. Eight rats received a dose of 11 μg/kg and two received 55 μg/kg. For injection the AZA-2 stock solution solvent (methanol) was evaporated and the toxin was reconstituted with DMSO. Saline was added subsequently. Final concentrations were 2.9 μg/ml or 14.7 μg/ml AZA-2 (depending dosing level) and 4% DMSO. Control rats were injected with 4% DMSO in saline. Blood samples (400 μl) were collected in EDTA tubes before and every hour after administration for the cardiac damage biomarker detection. Blood samples (500 μl) were also collected with heparin for biochemical analysis. All experiments lasted for 240 min after toxin administration, except when the animals died during the experiment (animals were euthanized by exsanguination). Animal procedures were approved by the Institutional Animal Care Committee of Universidad de Santiago de Compostela.
Electrocardiography
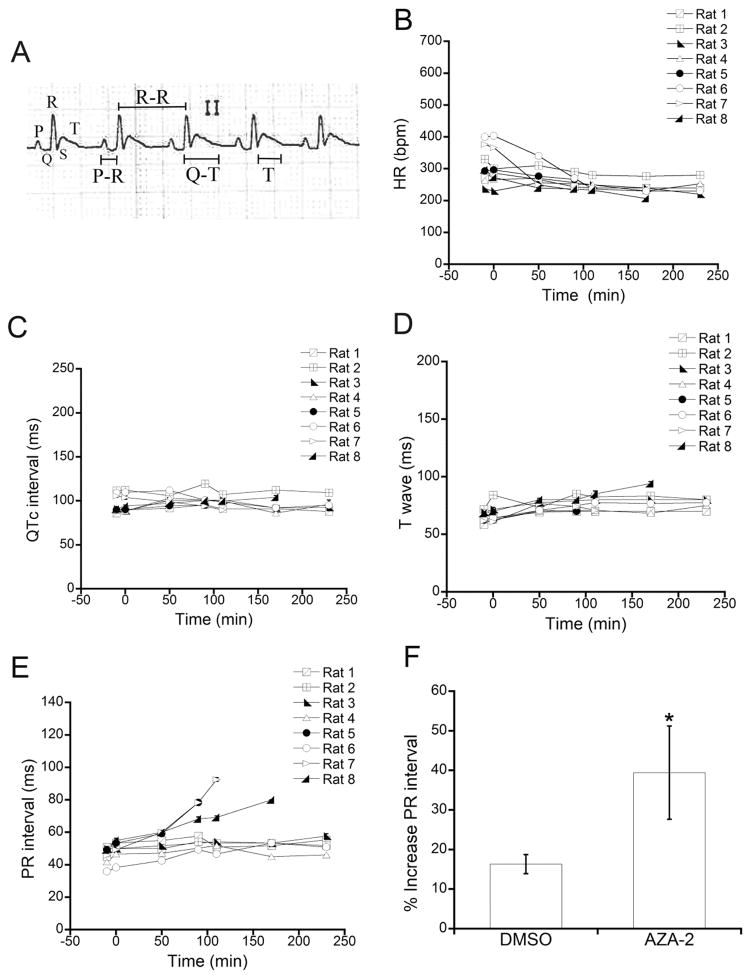
Lead II ECG was recorded at several times during the experiment before and after toxin administration and every recording period lasted for at least 10 min. ECG abnormalities detected before administration prompted experiment interruption. ECG recording speed was 25 mm/s or 50 mm/s. Heart rate (HR) was evaluated by counting QRS complexes per min. PR interval was measured from P wave onset to QRS complex onset. QT interval duration was determined from QRS onset complex to end of T wave. The base of R wave is used in rodents as a surrogate for Q wave, normally absent in rodent ECG (Farraj et al. 2011). Length and morphology of T waves were evaluated when possible. All intervals were measured at 50 mm/s (Figure 3A). For PR interval, QT interval and T wave length evaluations, six consecutive measurements were done at three nonconsecutive, randomly chosen points in every 10 min recording. The results are reported as mean ± standard error of the mean (SEM) of the three randomly selected segments. QT interval was corrected using normalized Bazett’s equation: QTc=QT/(RR/f½) (Kmecova et al. 2010); where QTc is corrected QT, QT is the QT interval measured on the ECG, RR is the RR interval corresponding to the QT intervals of the same ECG segment and f is a normalization factor equal to basal RR. Basal RR was obtained by measuring RR interval in 9 carrier-treated rats, yielding an average value of 205 ± 3 ms (300 ± 3 bpm HR). Consequently, f value was 205 ms. Increases of QT or PR were calculated as follows (e.g. for PR): Increase (%) = (PREnd − PR−10)/PR−10 × 100, where PREnd is PR interval at the end of the experiment and PR−10 is PR duration before administration. The same procedure was followed in carrier-control rats injected with DMSO.
Fig. 3. AZA-2 effects on rat ECG. ECG parameters.
HR, QTc interval, T wave and PR interval were analyzed before and at different times after intravenous administration of 11 μg/kg of AZA-2 to 8 rats. (A) Representative rat ECG recording at 50 mm/s. The landmarks and the measurements of QT interval, R-R interval, T wave and PR interval are indicated. (B) HR, (C) QTc interval, (D) T wave and (E) PR interval measured in ECG recordings of 8 rats. (F) Increase of PR interval duration (%) in AZA-2-treated and carrier control rats. PR interval was measured in ECGs of 8 rats treated with 11 μg/kg AZA-2 and 9 carrier control rats. The increase of PR interval at the end of the experiment is expressed as percentage of pre-administration PR (mean ± SEM, * statistically different from DMSO).
Cardiac biomarkers
cTnI, cTnT and BNP were measured in plasma samples using a commercial assay based on the Luminex XMap® technology. Blood samples collected in vivo were centrifuged immediately after collection to separate the plasma fraction and stored at −80 °C until analysis. A panel of 3 immunoassays was used for the simultaneous quantification of cTnI, cTnT and BNP (rat cardiovascular disease panel 1, CVD Milliplex® Map KIT Millipore®) following the instructions provided by the manufacturer. All samples were assayed in duplicate and analyzed with a Luminex 200 Analyzer.
Biochemistry analysis
Eleven biochemistry parameters (albumin (ALB), alkaline phosphatase (ALKP) alanine aminotransferase (ALT), blood urea nitrogen (BUN), calcium (Ca), cholesterol (CHOL), creatine kinase (CK), creatinine (CREA), globulin (GLOB), phosphorus (PHOS), and total protein (TP)) were also analyzed in plasma samples collected in vivo and processed as described above. Samples were analyzed with a prepacked panel called General Health Profile (GHP) and an IDEXX VetTest® Chemistry Analyzer.
Histological damage evaluation
Samples of several organs were collected after euthanasia. After fixation by immersion in buffered 10% formalin and Bouin, all tissues were processed for paraffin embedding and sections (3 μm) were mounted on subbed slides. Tissue sections were stained with Mayer’s haematoxylin and eosin following standard procedures for routine histological evaluations.
Data analysis
Data were plotted as mean ± SEM. Statistical significance was determined by t test for unpaired data, ANOVA for multiple comparisons and one-sample t test for comparison with theoretical values. P < 0.05 was considered for significance. Sample size was calculated using the following equation: N = 2 × [((Zα + Zβ)2 × σ2)/Δ2)] where N is sample size, σ is the estimated value of standard deviation for population, Δ is the maximum difference between means and Zα and Zβ are constant values which depend on tail number (2), statistical significance level (5%) and potency (90%) of the analysis.
RESULTS
AZA-2 effects on hERG activity
The effect of AZA-2 on the activity of hERG was explored using a CHO cell line stably expressing hERG and patch clamp techniques. Two voltage protocols were used to activate hERG channels (Fig. 2A and 2B). Both protocols elicited the activation of the IKr (Fig. 2C and 2D, grey lines, arrow indicates COI). AZA-2 induced a reduction of the amplitude of the IKr triggered by both voltage protocols (Fig. 2C and 2D, black lines). Actually, IKr measured 6 min after the addition of 2 μM AZA-2 was only 36% of control current (same cell before toxin addition) when using protocol 1 or 56% of control with protocol 2 (Fig. 2E). Carrier controls showed no statistically significant difference of IKr before and after addition of DMSO (Fig. 2E). A dose-response curve of AZA-2-induced inhibition of IKr was obtained using protocol 2, which offered a lower variability, yielding an IC50 of 2.2 μM (Fig. 2F). Cisapride was included as a positive control for the inhibition of IKr. In our experimental conditions cisapride IC50 was 48.9 nM (data not shown).
AZA-2 effect on rat ECG
ECG was used to evaluate the effects of AZA-2 on heart function in vivo. Several parameters of ECG recordings were analyzed before and at different times after intravenous administration of 11 μg/kg or 55 μg/kg AZA-2 to 8 and 2 rats respectively. HR (bpm), QT interval, T wave and PR interval durations (ms) were measured in ECG recordings at 50 mm/s (Fig. 3A). ECG was recorded at several time points along the experiment: −10 (before toxin administration), 0 (immediately after toxin administration), 50, 110, 170 and 230 min, and the duration of every ECG recording was at least 10 min continuously. The same exact protocol was performed with the administration of DMSO in the absence of toxin. AZA-2 (11 μg/kg) had no effect on HR, duration of QTc interval or T wave (Fig. 3B, 3C and 3D) at any time during the experiments. Additionally, due to the lack of effects on these parameters, a higher dose of AZA-2 (55 μg/kg) was injected to two rats. No change of HR, QTc interval or T wave was observed at 55 μg/kg (data not shown). The high amounts of toxin required to administer the higher dose precluded the inclusion of more rats at this dosing level.
Since hERG blockage has been associated to QTc prolongation, clarithromycin was used as a positive control for QTc interval prolongation. The administration of 2.2 mg/kg clarithromycin induced a 19 ± 3 % increase of QTc duration shortly after injection.
Regarding PR interval, there was a clear prolongation in 4 out of 8 (4/8) rats injected with 11 μg/kg AZA-2 (Fig. 3E) and in 1/2 rats injected with 55 μg/kg AZA-2 (data not shown). In 3 of the 4 rats injected with 11 μg/kg that showed PR prolongation, PR interval increase was followed by second and third degree atrioventricular (AV) blocks and death. No change of PR interval was observed in 9 rats that received carrier alone. The percentage of PR prolongation at the end of the experiment (last time point recorded before second degree AV block, apnea or experiment termination at 240 s, which ever occurred first) was calculated for the 8 rats administered with 11 μg/kg AZA-2, showing an AZA-2-induced increase of 39 % (Fig. 3F), statistically different from the data obtained for 9 carrier-control rats. The same calculation was done for 2 rats injected with 55 μg/kg AZA-2, and the average increase was 37% (72% increase in the rat that showed PR prolongation). Cardiac rhythm alterations were also evaluated considering type, total number of arrhythmia episodes, time of appearance, duration of arrhythmia sequences in ECG and survival until the experiment end (Table 1 and see Supplemental Material, Table S1). Interestingly, in rats receiving 11 μg/kg AZA-2 the appearance of arrhythmias, mainly ventricular extrasystoles (VES), was recorded in 3/8 animals. When the dose of AZA-2 was increased (55 μg/kg), VES appeared in 2/2 rats. The total number of arrhythmias detected in 5/10 AZA-2-treated rats was 50. Comparatively, in carrier-administered rats, only 2/9 rats showed arrhythmias and the total number was 3 (see Supplemental Material, Table S1). Additionally, 2 of the 5 AZA-2-treated rats that showed extrasystoles died before 240 min.
Table 1.
Cardiac rhythm alterations in AZA-2-treated rats. AZA-2 dose: 11 μg/kg unless indicated.
| Rat | Type | Total N° | Death before 240 min |
|---|---|---|---|
| 1 | 0 | no | |
| 2 | VES | 14 | no |
| 3 | VES SVES |
4 | yes |
| 4 | no | ||
| 5 | VES | 22 | yes |
| 6 | yes | ||
| 7 | yes | ||
| 8 | no | ||
| 9a | VES | 1 | no |
| 10a | VES | 9 | no |
55μl/ml AZA-2
VES: ventricular extrasystole
SVES: supraventricular extrasystole
Effect of AZA-2 on cardiac biomarkers
The levels of cTnI, cTnT and BNP were evaluated in plasma of rats injected with AZA-2 at 11 μg/kg (n=8) and 55 μg/kg (n=2). The quantification was performed in plasma samples collected before (−10 min) and at different times after (60, 120, 180 and 240 min) AZA-2 administration. The results showed that 11 μg/kg AZA-2 did not cause an increase of plasmatic cTnI or cTnT (see Supplemental Material, Fig. S1A and S1B). Actually, cTnT levels in plasma lowered at late time points. Similar results were obtained for 9 rats that received only carrier (see Supplemental Material, Fig. S1A and S1B) or 55 μg/kg AZA-2 (data not shown). Regarding BNP levels, 11 μg/kg AZA-2 seemed to cause a slight increase at 180 and 240 min (see Supplemental Material, Fig. S1C); however, this increase was not statistically significant. Additionally, BNP levels were higher in carrier controls than in AZA-2-administered rats (see Supplemental Material, Fig. S1C). Isoproterenol (4 mg/kg) was used as a positive control of cTnI release to plasma increasing 3 times cTnI levels versus pre-drug administration (data not shown).
Effect of AZA-2 on biochemistry parameters and organ histology
Alterations of the functionality and tissue structure of several organs were explored by analyzing biochemistry markers and histological preparations. Eleven markers were analyzed in plasma samples collected before (−10 min) and 90 or 240 min after toxin injection. ALKP, BUN, CREA, Ca and PHOS plasma levels were within normal physiological values (see Supplemental Material, Table S2). TP, ALB and GLOB diminished gradually along the experiment, both in AZA-2-treated and carrier-control rats. CHOL and CK plasma levels were respectively above and below normal values at all times (see Supplemental Material, Table S2). ALT plasma levels at 240 min (163 ± 88 U/L) were twice the value measured before toxin administration in AZA-2-treated rats (68 ± 9 U/L). Carrier controls did not show any increase of ALT levels (see Supplemental Material, Table S2).
At necropsy, no macroscopic alteration was observed. The histological analysis of heart, brain, lung, thymus, spleen, stomach, intestine (small and large), liver, pancreas and kidney showed no structural damage.
DISCUSSION
Data regarding AZAs toxicity to humans and animals are limited and their potential cardiotoxicity had not been explored. Recently AZAs have been characterized as open state hERG blockers (Twiner et al. 2012), which clearly points to a possible cardiotoxic effect. However, in vitro blockage of hERG is not sufficient evidence for cardiotoxicity, and most experts insist on the relevance of in vivo assessment (EMEA 2005; Guth 2007; Stummann et al. 2009). In our hands AZA-2 blocked hERG activity with an IC50 of 2.2 μM, which attending to hERG blocking drugs classification would place AZA-2 among low-potency blockers (IC50 >1 μM) (Katchman et al. 2006). The classification of a compound as a low potency hERG blocker reduces the probability of finding in vivo QT prolongation related to this channel impairment. The IC50 reported by Twiner et al. for AZA-2 blockade of hERG was 0.64 μM (moderate blockers IC50: 0.1–1 μM) (Twiner et al. 2012). High variations of IC50 values among publications are common for hERG blocking drugs (Polak et al. 2009). As an example, loratadine would be classified as low or moderate hERG blocker depending on the results obtained in different laboratories; however, it does not affect QT (Katchman et al. 2006).
Our in vivo experiments showed that AZA-2 does not prolong QT interval in rats. Usually, a 10 % increase is sufficient to consider that a drug induces QTc prolongation (Redfern et al. 2003). Therefore, our study, which had a sample size of 8 rats, was powered to detect a 10 % QTc prolongation with 95 % probability. Although the dose of AZA-2 tested in vivo might be considered not sufficient to induce QT prolongation, 4/8 rats injected with the lower AZA-2 dose died during the experiment, which indicates that AZA-2 is already inducing an important toxic effect. Our results indicate that the hERG blocking activity of AZA-2 does not translate into in vivo QT prolongation in rats. Considering that more than 70 % new compounds tested by pharmaceutical companies have hERG blocking activity (Shah 2005) and that AZA-2 would be a low potency blocker, as discussed above, these results are not surprising. Currently, reduction of IKr is not considered absolutely predictive of QT prolongation, because many factors modulate drug effects on heart function and they are not possibly mimicked under in vitro conditions (Hancox et al. 2008; Farraj et al. 2011; Gintant 2011).
Although AZA-2 did not affect QTc interval, ECG recordings evidenced that AZA-2 induced a statistically significant PR interval prolongation and an increase of arrhythmia appearance frequency. The lowest dose of AZA-2 caused second and third degree AV blocks and death in 3/4 rats that evidenced prolonged PR intervals. The highest dose showed PR prolonged in 1 of 2 rats, however the high increase (72 %) could suggest an eventual evolution into second/third degree block. In addition, a total of 5/10 AZA-2-treated rats showed arrhythmia episodes, mainly VES, with arrhythmia occurrence per rat clearly higher than in carrier controls. PR interval represents the time for atria depolarization, conduction of cardiac impulse from sinoatrial node to AV node and His Bundle and cardiac impulse delay at AV node before ventricular depolarization. AV conduction blocks may be caused by different mechanisms like enhanced vagal tone, ion flux disturbances, ischemia damage or toxic insults (Zeltser et al. 2004; Lorsheyd et al. 2005). On the other hand VES are often related to an abnormal pattern of depolarization with varied etiology (Keating et al. 2001). Overall, these results suggest that AZA-2 alters normal heart conduction and rhythm. However, more studies will be needed to clarify the mechanism by which AZA-2 causes these ECG alterations.
Cardiac biomarkers are included in cardiotoxicity studies to evaluate structural myocardium damage (Kettenhofen et al. 2008). AZA-2 caused no increase of cTnI and cTnT plasma levels so acute myocardial damage can be excluded. The duration of the experiment was enough to detect a possible increase of cTnI and cTnT levels, because in animals and humans release of these markers has been detected within minutes of myocardial injury (O’Brien 2008). Additionally, isoproterenol induced a detectable increase of cTnI levels 2 h after injection. Regarding the heart dysfunction marker, BNP, which is elevated in the presence of hemodynamic changes, ventricular damage or stress (Walker 2006), AZA-2 did not affect BNP plasma levels; actually, its concentration in AZA-2-treated rats was lower (but not statistically different) than in carrier controls. Accordingly, histopathological analysis did not show any alterations in heart tissue. Overall, these results indicate that AZA-2 did not cause pathological processes that acutely alter heart structure. Besides structural damage, heart function alteration might be secondary to other organ malfunction. A biochemistry analysis was performed on the same AZA-2-treated and control rats in order to detect toxicological damage to other organs. All parameters, except TP (ALB and GLOB), CHOL, ALT and CK were within normal values. The gradual reduction of TP values observed in AZA-2-treated and control rats was probably due to plasma dilution caused by prolonged fluid therapy. Cholesterol levels were high and CK levels were low before toxin/carrier administration and therefore are not related to toxin administration or experimental procedure. Only ALT plasma levels were elevated above physiological values in AZA-2-treated rats, being also statistically higher than in DMSO-treated rats. ALT is a liver injury biomarker and it is considered the clinical chemistry gold standard of hepatotoxicity (Ozer et al. 2008). High plasma levels of ALT are predictive of hepatic damage; however, they are usually accompanied with high levels of additional hepatic markers or histopathological alterations. Neither plasma concentrations of other hepatic markers (ALKP, TP, ALB) nor histopathological results were indicative of hepatic damage in our experiments. However, the elevated plasma concentration of ALT in AZA-2-treated rats might reflect incipient liver damage, which would be in agreement with the liver lesions observed in AZA-treated mice (i.p.) at longer times after toxin exposure (24 h) (Ito et al. 2000).
The dose of 11 μg/kg of AZA-2 used in this study seems to be close to the rat LD50 by i. v. administration. There are not many data available that would allow a comparison of AZA toxicity among different species and administration routes. If assume a similarity among rodent species regarding sensitivity to the toxin, this i.v. dose would be equivalent to an oral dose in mice of 250–600 μg/kg (there is a high variability of estimated LD50 in mice among different studies) (EFSA 2008). Also in mice, the lowest dose that has been described to have an acute effect by the oral route is 250 μg/kg (Ito et al. 2002; Aasen et al. 2010), which is quite close to the LD50 in these conditions. After an evaluation of the scarce toxicological data regarding AZP in humans, a panel of experts has estimated the lowest-observable-adverse-effect level in humans in 1.9 μg/kg (EFSA 2008), which might suggest a higher sensitivity of humans to this toxin. Current monitoring of shellfish destined to human consumption prevents exceeding exposures of 1 μg/kg (for a 60 kg person and a 400 g shellfish meat portion) with an estimated probability of 0.13 %. Therefore it might be suggested that the doses used in this study are not comparable to usual human exposure. Fortunately, no human deaths due to AZP have been reported. However, regarding human risk, it should also be noted that monitoring has already failed with an AZP episode occurring in 2008 (EFSA 2008), that AZA has been detected in the heart after oral administration in mice (Aasen et al. 2010) that lethal doses in mice seem fairly close to doses with no effect and that there may be a high variability of sensitivity among individuals of the same species.
In summary, AZA-2 blocks the hERG cardiac potassium channel in vitro and, although no QTc interval prolongation is observed in vivo, AZA-2 clearly causes prolongation of PR interval and the appearance of arrhythmias in rat ECGs, indicating an alteration of heart electrophysiology in vivo. This potential cardiotoxicity of AZAs should be taken into consideration when evaluating the threat that these toxins pose to human health. AZAs effects on heart function might not be a threat for the general population but the situation could be different for individuals with high cardiovascular risk.
Acknowledgments
This work was funded with the following FEDER cofunded-grants: From Ministerio de Ciencia y Tecnología, Spain: SAF2009-12581 (subprograma NEF), AGL2009 13581-CO2-01, TRA2009-0189, AGL2010-17875. From Xunta de Galicia, Spain: GRC 2010/10, and PGDIT 07MMA006261PR, PGIDIT (INCITE) 09MMA003261PR, PGDIT (INCITE) 09261080PR, 2009/XA044, and 10PXIB261254 PR. From EU VIIth Frame Program: 211326 – CP (CONffIDENCE), 265896 BAMMBO, 265409 μAQUA, and 262649 BEADS, 312184 PharmaSea, 315285 Ciguatools. From the Atlantic Area Programme (Interreg IVB Trans-national): 2009-1/117 Pharmatlantic. From the National Institutes of Health (USA) (grant ES013314, to K.C.N.). No competing financial interests.
Footnotes
Conflict of interests
The authors declare that they have no conflict of interest.
References
- Aasen JA, Espenes A, et al. Sub-lethal dosing of azaspiracid-1 in female NMRI mice. Toxicon. 2010;56(8):1419–1425. doi: 10.1016/j.toxicon.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Alfonso A, Román Y, et al. Azaspiracid-4 inhibits Ca2+ entry by stored operated channels in human T lymphocytes. Biochemical Pharmacology. 2005;69(11):1627–1636. doi: 10.1016/j.bcp.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Colman JR, Twiner MJ, et al. Teratogenic effects of azaspiracid-1 identified by microinjection of Japanese medaka (Oryzias latipes) embryos. Toxicon. 2005;45(7):881–890. doi: 10.1016/j.toxicon.2005.02.014. [DOI] [PubMed] [Google Scholar]
- EC. Regulation (EC) No. 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food animal origin. Off J Eur Commun L 134/55 2004 [Google Scholar]
- EFSA. Marine biotoxin in shellfish-Azaspiracid group. Scientific Opinion of the Panel on Contaminants in the Food chain. The EFSA Journal. 2008;723:1–52. [Google Scholar]
- EMEA. ICH Topic S 7 B. The nonclinical evaluation of the potential for delayed ventricular repolarization (QT interval prolongation) by human pharmaceuticals. 2005 http:/www.emea.europa.eu/pdfs/human/ich/042302en.pdf/
- Farraj AK, Hazari MS, et al. The utility of the small rodent electrocardiogram in toxicology. Toxicol Sci. 2011;121(1):11–30. doi: 10.1093/toxsci/kfr021. [DOI] [PubMed] [Google Scholar]
- Furey A, O’Doherty S, et al. Azaspiracid poisoning (AZP) toxins in shellfish: toxicological and health considerations. Toxicon. 2010;56(2):173–190. doi: 10.1016/j.toxicon.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Gintant G. An evaluation of hERG current assay performance: Translating preclinical safety studies to clinical QT prolongation. Pharmacol Ther. 2011;129(2):109–119. doi: 10.1016/j.pharmthera.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Gintant GA, Su Z, et al. Utility of hERG assays as surrogate markers of delayed cardiac repolarization and QT safety. Toxicol Pathol. 2006;34(1):81–90. doi: 10.1080/01926230500431376. [DOI] [PubMed] [Google Scholar]
- Guo L, Guthrie H. Automated electrophysiology in the preclinical evaluation of drugs for potential QT prolongation. Journal of Pharmacological and Toxicological Methods. 2005;52(1):123–135. doi: 10.1016/j.vascn.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Guth BD. Preclinical cardiovascular risk assessment in modern drug development. Toxicol Sci. 2007;97(1):4–20. doi: 10.1093/toxsci/kfm026. [DOI] [PubMed] [Google Scholar]
- Hancox JC, Levi AJ, et al. Time course and voltage dependence of expressed HERG current compared with native “rapid” delayed rectifier K current during the cardiac ventricular action potential.” Pflügers Archiv. European Journal of Physiology. 1998;436(6):843–853. doi: 10.1007/s004240050713. [DOI] [PubMed] [Google Scholar]
- Hancox JC, McPate MJ, et al. The hERG potassium channel and hERG screening for drug-induced torsades de pointes. Pharmacol Ther. 2008;119(2):118–132. doi: 10.1016/j.pharmthera.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Hoffmann P, Warner B. Are hERG channel inhibition and QT interval prolongation all there is in drug-induced torsadogenesis? A review of emerging trends. Journal of Pharmacological and Toxicological Methods. 2006;53(2):87–105. doi: 10.1016/j.vascn.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Ito E, Satake M, et al. Chronic effects in mice caused by oral administration of sublethal doses of azaspiracid, a new marine toxin isolated from mussels. Toxicon. 2002;40(2):193–203. doi: 10.1016/s0041-0101(01)00226-4. [DOI] [PubMed] [Google Scholar]
- Ito E, Satake M, et al. Multiple organ damage caused by a new toxin azaspiracid, isolated from mussels produced in Ireland. Toxicon. 2000;38(7):917–930. doi: 10.1016/s0041-0101(99)00203-2. [DOI] [PubMed] [Google Scholar]
- James KJ, Fidalgo Saez MJ, et al. Azaspiracid poisoning, the food-borne illness associated with shellfish consumption. Food Addit Contam. 2004;21(9):879–892. doi: 10.1080/02652030400002105. [DOI] [PubMed] [Google Scholar]
- Katchman AN, Koerner J, et al. Comparative evaluation of HERG currents and QT intervals following challenge with suspected torsadogenic and nontorsadogenic drugs. J Pharmacol Exp Ther. 2006;316(3):1098–1106. doi: 10.1124/jpet.105.093393. [DOI] [PubMed] [Google Scholar]
- Keating MT, Sanguinetti MC. Molecular and cellular mechanisms of cardiac arrhythmias. Cell. 2001;104(4):569–580. doi: 10.1016/s0092-8674(01)00243-4. [DOI] [PubMed] [Google Scholar]
- Kettenhofen R, Bohlen H. Preclinical assessment of cardiac toxicity. Drug Discov Today. 2008;13(15–16):702–707. doi: 10.1016/j.drudis.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Kmecova J, Klimas J. Heart rate correction of the QT duration in rats. Eur J Pharmacol. 2010;641(2–3):187–192. doi: 10.1016/j.ejphar.2010.05.038. [DOI] [PubMed] [Google Scholar]
- Lorsheyd A, de Lange DW, et al. PR and OTc interval prolongation on the electrocardiogram after binge drinking in healthy individuals. Neth J Med. 2005;63(2):59–63. [PubMed] [Google Scholar]
- MCMahon TSJ. Winter toxicity of unknown aetiology in mussels. Harmful Algae. 1996;14:2. [Google Scholar]
- Nicolaou KC, Frederick MO, et al. Second-generation total synthesis of azaspiracids-1, -2, and -3. Chem Asian J. 2006;1(1–2):245–263. doi: 10.1002/asia.200600059. [DOI] [PubMed] [Google Scholar]
- O’Brien PJ. Cardiac troponin is the most effective translational safety biomarker for myocardial injury in cardiotoxicity. Toxicology. 2008;245(3):206–218. doi: 10.1016/j.tox.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Ofuji K, Satake M, et al. Two analogs of azaspiracid isolated from mussels, Mytilus edulis, involved in human intoxication in Ireland. Nat Toxins. 1999;7(3):99–102. doi: 10.1002/(sici)1522-7189(199905/06)7:3<99::aid-nt46>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Ozer J, Ratner M, et al. The current state of serum biomarkers of hepatotoxicity. Toxicology. 2008;245(3):194–205. doi: 10.1016/j.tox.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Perry M, Sanguinetti M, et al. Revealing the structural basis of action of hERG potassium channel activators and blockers. J Physiol. 2010;588(Pt 17):3157–3167. doi: 10.1113/jphysiol.2010.194670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak S, Wisniowska B, et al. Collation, assessment and analysis of literature in vitro data on hERG receptor blocking potency for subsequent modeling of drugs’ cardiotoxic properties. J Appl Toxicol. 2009;29(3):183–206. doi: 10.1002/jat.1395. [DOI] [PubMed] [Google Scholar]
- Priest BT, I, Bell M, et al. Role of hERG potassium channel assays in drug development. Channels (Austin) 2008;2(2):87–93. doi: 10.4161/chan.2.2.6004. [DOI] [PubMed] [Google Scholar]
- Redfern WS, Carlsson L, et al. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res. 2003;58(1):32–45. doi: 10.1016/s0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Jiang C, et al. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81(2):299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- Satake M, Ofuji K, et al. Azaspiracid, a new marine toxin having unique spiro ring assemblies, isolated from Irish mussels, Mytilus edulis. Journal of the American Chemical Society. 1998;120(38):9967–9968. [Google Scholar]
- Shah RR. Drug-induced QT interval prolongation--regulatory guidance and perspectives on hERG channel studies. Novartis Found Symp. 2005;266:251–280. [PubMed] [Google Scholar]
- Stummann TC, Beilmann M, et al. Report and recommendations of the workshop of the European Centre for the Validation of Alternative Methods for Drug-Induced Cardiotoxicity. Cardiovasc Toxicol. 2009;9(3):107–125. doi: 10.1007/s12012-009-9045-3. [DOI] [PubMed] [Google Scholar]
- Su Z, Limberis J, et al. Electrophysiologic characterization of a novel hERG channel activator. Biochemical Pharmacology. 2009;77(8):1383–1390. doi: 10.1016/j.bcp.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Twiner MJ, Doucette GJ, et al. Marine algal toxin azaspiracid is an open-state blocker of HERG potassium channels. Chem Res Toxicol. 2012;25(9):1975–1984. doi: 10.1021/tx300283t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twiner MJ, Hess P, et al. Cytotoxic and cytoskeletal effects of azaspiracid-1 on mammalian cell lines. Toxicon. 2005;45(7):891–900. doi: 10.1016/j.toxicon.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Vilariño N, Nicolaou KC, et al. Irreversible cytoskeletal disarrangement is independent of caspase activation during in vitro azaspiracid toxicity in human neuroblastoma cells. Biochemical Pharmacology. 2007;74(2):327–335. doi: 10.1016/j.bcp.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Walker DB. Serum chemical biomarkers of cardiac injury for nonclinical safety testing. Toxicol Pathol. 2006;34(1):94–104. doi: 10.1080/01926230500519816. [DOI] [PubMed] [Google Scholar]
- Zeltser D, Justo D, et al. Drug-induced atrioventricular block: prognosis after discontinuation of the culprit drug. J Am Coll Cardiol. 2004;44(1):105–108. doi: 10.1016/j.jacc.2004.03.057. [DOI] [PubMed] [Google Scholar]