Abstract
Acetaminophen (APAP) is a widely used analgesic. However, APAP overdose is hepatotoxic and is the primary cause of acute liver failure in the developed world. The mechanism of APAP-induced liver injury begins with protein binding and involves mitochondrial dysfunction and oxidative stress. Recent efforts to discover blood biomarkers of mitochondrial damage have identified increased plasma glutamate dehydrogenase activity and mitochondrial DNA concentration in APAP overdose patients. However, a problem with these markers is that they are too large to be released from cells without cell death or loss of membrane integrity. Metabolomic studies are more likely to reveal biomarkers that are useful at early time points, before injury begins. Similar to earlier work, our metabolomic studies revealed that acylcarnitines are elevated in serum from mice after treatment with toxic doses of APAP. Importantly, a comparison with furosemide demonstrated that increased serum acylcarnitines are specific for mitochondrial dysfunction. However, when we measured these compounds in plasma from humans with liver injury after APAP overdose, we could not detect any significant differences from control groups. Further experiments with mice showed that N-acetylcysteine, the antidote for APAP overdose in humans, can reduce acylcarnitine levels in serum. Altogether, our data do not support the clinical measurement of acylcarnitines in blood after APAP overdose due to the standard N-acetylcysteine treatment in patients, but strongly suggest that acylcarnitines would be useful mechanistic biomarkers in other forms of liver injury involving mitochondrial dysfunction.
Keywords: Acetaminophen Toxicity, Biomarkers, Mitochondria, Acylcarnitines
INTRODUCTION
Acetaminophen (APAP) is one of the most widely used drugs (Kaufman et al. 2002). However, large doses can be hepatotoxic to both rodents and humans (McGill et al., 2012). In mice, the mechanism of APAP-induced liver injury involves protein binding, mitochondrial dysfunction, and oxidative stress. APAP is converted to the reactive electrophile N-acetyl-p-benzoquinone imine (NAPQI) (Dahlin et al. 1984), which depletes hepatic glutathione (GSH) and binds to proteins (Mitchell et al. 1973). Covalent binding to proteins in mitochondria causes dysfunction and mitochondrial oxidative stress (Jaeschke 1990; Cover et al. 2005). The oxidative stress triggers phosphorylation of the c-Jun N-terminal kinase (JNK) (Gunawan et al. 2006; Saito et al. 2010) through activation of the upstream kinases apoptosis signal-regulating kinase 1 (ASK1) (Nakagawa et al. 2008) and mixed lineage kinase 3 (MLK3) (Sharma et al. 2012). Phospho-JNK then translocates into mitochondria and enhances the mitochondrial dysfunction and oxidative stress (Saito et al. 2010a; Hanawa et al. 2008; Win et al. 2011; Ramachandran et al. 2011). Along with movement of Bax into the outer mitochondrial membrane (Bajt et al. 2008), the mitochondrial membrane permeability transition (Kon et al. 2004) and mitochondrial lysis result in release of endonucleases into the cytosol which then enter the nucleus and fragment nuclear DNA (Bajt et al. 2006; 2011). Ultimately, the hepatocyte dies by oncotic necrosis. While the mechanisms of APAP hepatotoxicity are well established in mice, less work has been done in humans. Recently, efforts have been made to develop blood biomarkers of mitochondrial damage during APAP hepatotoxicity in overdose patients. This work has identified the mitochondrial matrix enzyme glutamate dehydrogenase (GDH) and mitochondrial DNA (mtDNA) as candidate markers (McGill et al. 2012). Because nuclear DNA fragmentation during APAP toxicity in mice requires release of endonucleases from damaged mitochondria, this may also be indicative of mitochondrial dysfunction, and nuclear DNA fragments have been measured in overdose patient plasma as well (McGill et al. 2012). Unfortunately, although plasma GDH at the time of hospital admission may be somewhat predictive of later ALT levels (Antoine et al. 2013), a disadvantage of these biomarkers is that they do not achieve very high levels in plasma until after the onset of injury. In most APAP overdose patients, the plasma values for these parameters appear to mirror ALT (McGill et al. 2012). A promising approach to the identification of biomarkers of injury that are useful at earlier time points may involve metabolomics. In general, metabolic intermediates are much smaller than proteins and nucleic acids and more likely to cross cell membranes and enter plasma before the development of injury.
In metabolomic studies, Chen et al. (2009) measured increased levels of acylcarnitines in serum from APAP-treated mice. Acylcarnitines are derivatives of long chain fatty acids which are required for transport of these fatty acids into mitochondria for β-oxidation. First, a coenzyme A (CoA) group is attached in a reaction catalyzed by acyl-CoA synthetase. The CoA group is then displaced by carnitine through the action of carnitine palmitoyl transferase I (CPT I), forming an acylcarnitine that can enter the mitochondrial matrix through facilitated diffusion with the help of a carnitine-acylcarnitine translocase (CACT). Because acylcarnitines are broken down within mitochondria by carnitine palmitoyl transferase II (CPT II) and beta-oxidation, mitochondrial dysfunction may result in accumulation. We hypothesized that these fatty acid-carnitine conjugates can be measured in serum as additional mechanistic biomarkers of mitochondrial damage after APAP overdose. To test this, we treated mice with the drug furosemide, which has been shown to cause liver injury without primarily affecting mitochondrial function (Wong et al. 2000), as a negative control for mitochondrial damage. We then measured acylcarnitines in plasma from APAP overdose patients. Our results demonstrate that circulating acylcarnitines are specific biomarkers of mitochondrial dysfunction. While they were not elevated in our samples from APAP overdose patients, they may be very useful for mechanistic studies of other forms of liver injury. We provide evidence that the low values in human blood after APAP overdose may be due in part to the standard-of-care treatment N-acetylcysteine (NAC).
METHODS
Animals
Male C57Bl/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). The animals were housed in a temperature-controlled room with a 12 h light/dark cycle and had ad libitum access to food and water until the start of the experiments. All animal experiments were approved by the Animal Use and Care Committee of the University of Kansas Medical Center. APAP was dissolved in phosphate-buffered saline (PBS) and injected i.p. at 300 or 600 mg per kg body weight (mg/kg). Furosemide was dissolved in PBS at a final pH of 8.0 and injected i.p. at 500 mg/kg. All animals receiving the 300 mg/kg dose of APAP were deprived of food for 12–15 h before injection. The food was returned to these cages 6 h post-treatment, unless the animals were sacrificed before this time point. Where indicated, these mice were treated i.p. with 140 mg/kg NAC 1.5 h after APAP. All other animals were allowed food ad libitum throughout the study. At various time points post-treatment, mice were sacrificed by cervical dislocation under isoflurane anesthesia. Blood was drawn from the caudal vena cava and allowed to clot before centrifugation to obtain serum. Sections of each liver were cut and fixed in 10% phosphate-buffered formalin for histology. The remaining liver tissue was flash frozen in liquid nitrogen.
Patient enrollment and selection
Patients with evidence of APAP overdose were recruited at the University of Kansas Hospital in Kansas City, KS, USA or at the Banner Good Samaritan Health Center in Phoenix, AZ, USA. The study protocols were approved by the institutional review boards of both institutions. Physicians made the diagnosis of APAP overdose, and informed consent was obtained for all patients. Criteria for inclusion in the study included high serum APAP levels, evidence of liver injury (based on alanine aminotransferase (ALT), aspartate aminotransferase (AST), prothrombin time (PT), and bilirubin), and self-reported history of APAP overdose. All participants met at least two of these criteria. Patients with reasonable evidence of non-APAP-related liver injury (i.e. viral hepatitis, ischemic hepatitis, etc.) were excluded. All patients were given the standard-of-care treatment NAC. In all cases the first dose of NAC was received before the first blood draw for our study. Plasma was collected daily in heparin or EDTA-coated tubes. The patients were organized into three groups: APAP overdose patients with abnormal liver test results (“Abnormal LT”; peak ALT > 1,000 U/L and peak prothrombin time ≥ 18s), APAP overdose patients with normal liver test results (“Normal LT”; peak ALT < 100 U/L and peak prothrombin time < 18s), and healthy volunteers (“Vol.”; nurses and physicians recruited from the medical ICU at the University of Kansas Hospital).
Acylcarnitine measurement
Samples were prepared by mixing 25 µl of serum with 75 µl of methanol. After vortexing and centrifugation at 16,000 g for 10 min to remove protein, the supernatants were subjected to ultraperformance liquid chromatography quadrupole time-of-flight mass spectrometry (UPLC-QTOFMS) for analysis in positive and negative mode. Acylcarnitine standards were purchased from Sigma (St. Louis, MO) and IndoFine (Hillsborough, NJ). The data were acquired and processed using MassLynx and MarkerLynx (Waters, Milford, MA), respectively. Principal component analysis (PCA) and orthogonal projection to latent structures-discriminant analysis (OPLS-DA) were performed in SIMCAP+12 (Umetrics, Umeá, Sweden).
Clinical chemistry
Mouse ALT was measured in our laboratory with a kit from Pointe Scientific (Canton, MI), according to the manufacturer’s instructions. Patient ALT, AST, prothrombin time, and bilirubin were measured in the hospital using standard methods.
Histology
Liver sections were stained with hematoxylin and eosin (H&E) using a standard protocol to visualize necrosis, or with the TUNEL In Situ Cell Death Assay (Roche, Indianapolis, IN) according to the manufacturer’s instructions to assess nuclear DNA fragmentation.
Statistics
The Shapiro-Wilk test was used to assess normality. Normally distributed data were subjected to one-way analysis of variance (ANOVA) with post-hoc Student-Newman-Keul test. For non-normally distributed data, ANOVA on ranks was performed, followed by Dunn’s multiple comparisons test. P < 0.05 was considered significant.
RESULTS
Acetaminophen-induced liver injury in mice
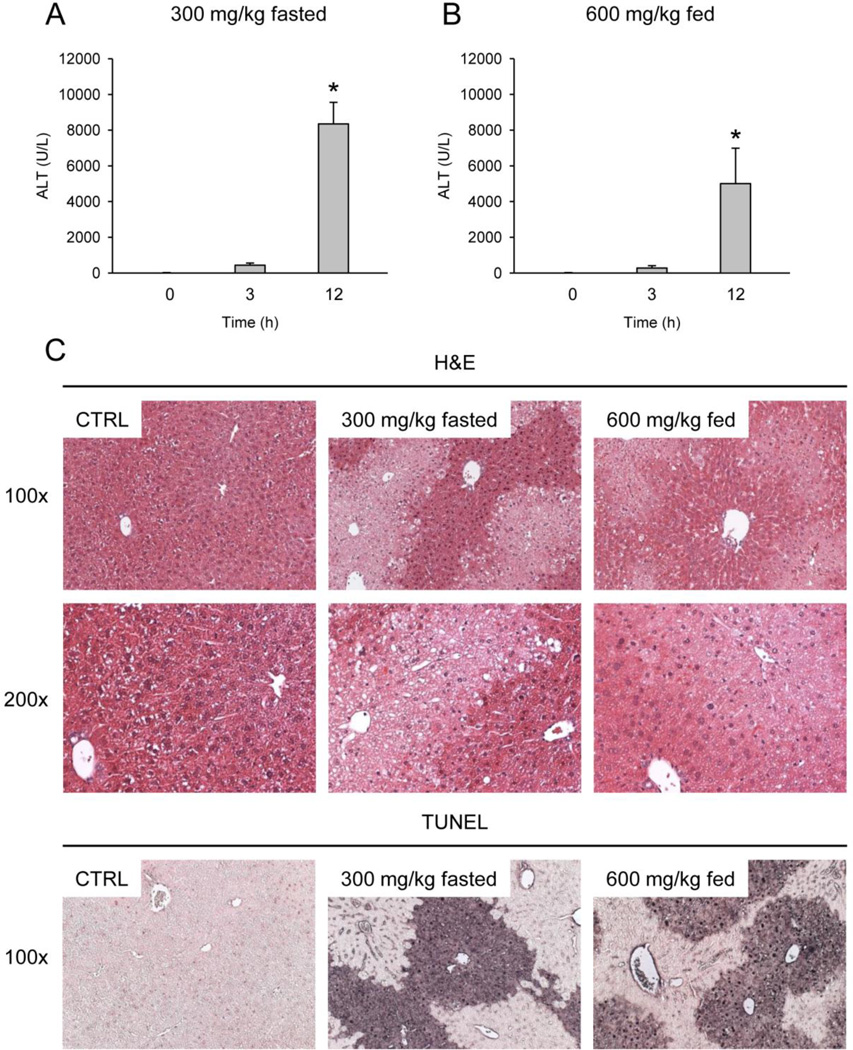
Starved mice developed severe liver injury by 12 h post-treatment with 300 mg/kg (ALT: 8,351 ± 1,209 U/L) (Fig. 1A). Consistent with this, large centrilobular areas of necrosis were visible by H&E, as well as large TUNEL-positive areas with both nuclear and cytosolic staining, which is characteristic of necrotic cell death (Fig. 1C). Similar results were obtained from fed mice treated with 600 mg/kg APAP for ALT (5,009 ± 1,980 U/L), histology, and TUNEL staining (Fig. 1B,C).
Figure 1. Acetaminophen-induced liver injury in mice.
Fasted or fed mice were treated with 300 or 600 mg/kg APAP, respectively, for various times. (A–B) ALT activity was measured in serum. Data are expressed as mean ± SEM of n = 3. *P<0.05 (compared to t=0 h) (C) Hematoxylin and eosin (H&E) staining and TUNEL labeling were performed with liver sections from these animals. Representative sections (×100 or ×200) are shown.
Metabolomics studies in mice
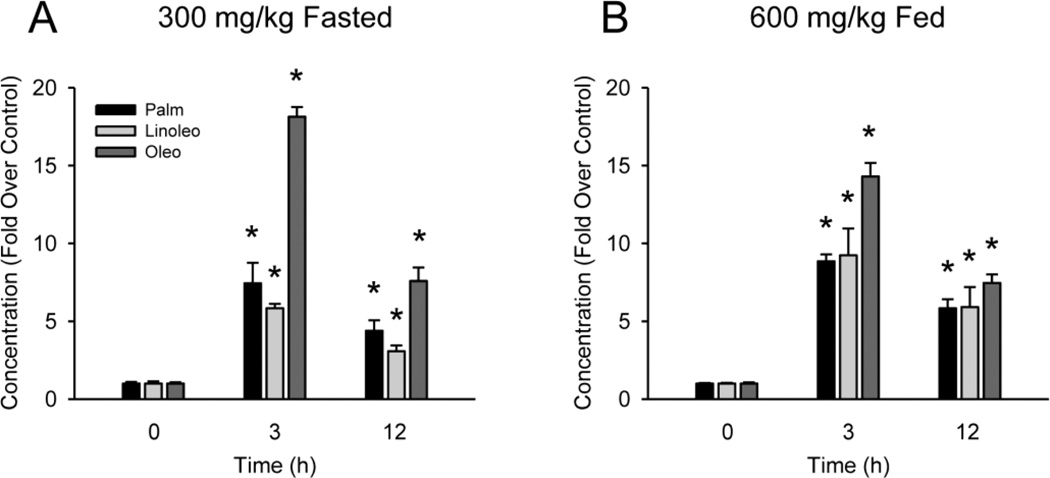
Serum samples from these mice were subjected to UPLC-QTOFMS for metabolomic analysis. For both the starved (300 mg/kg) and fed (600 mg/kg) mice, OPLS-DA analysis revealed clear separation of the APAP-treated animals from controls in the first component, with some differences between the 3 h and 12 h post-APAP groups in the second component (Fig. 2A,B). Further analyses revealed that three acylcarnitines were elevated in the serum from the APAP-treated mice (Fig. 2C,D). These compounds are labeled with their corresponding molecular weights: 400, 424, and 426 g/mol (Fig. 2C,D). Their structures were confirmed by comparing their fragmental patterns of MS/MS and retention times with those of standards. The 400 molecular weight acylcarnitine eluted with retention time of 7.19 min, having a mass of [M]+ = 400.3403 m/z. The major ions at m/z 341 (loss of NMe3), 239, 144, and 85 were interpreted in Online Resource 1, supplemental figure 1. This compound was identified as palmitoylcarnitine (Online Resource 1, figure 2), consistent with the earlier findings of Chen et al. (2009). The 424 acylcarnitine eluted with retention time of 6.97 min and mass of [M]+ = 424.3381 m/z. MS/MS analysis produced the major daughter ions at m/z 365 (loss of NCH3) 245, 144, and 85, which were interpreted Online Resource 1, supplemental figure 1. This compound was identified as linoleoylcarnitine (Online Resource 1, figure 3). The 426 acylcarnitine was observed at the retention time of 7.28 min, having a mass of [M]+ = 426.3542 m/z. The major fragmental ions at m/z 367 (loss of NMe3), 265, 247, 144, and 85 were interpreted in Online Resource 1, supplemental figure 1. This compound was identified as likely being oleoylcarnitine or its close analog (Online Resource 1, figure 4). We found that all three acylcarnitines were elevated in serum within 3 h of APAP treatment (Fig. 3A,B and Table 1), and this continued to at least 12 h (Fig. 3A,B). At the doses of APAP used, nutritional status did not affect the overall increase in acylcarnitine concentrations, although there were differences in specific compounds between the fasted and fed states at both time points (Fig. 3A,B).
Figure 2. Metabolomic analysis of serum from mice with acetaminophen-induced liver injury.
Score plots (A–B) and S-plots (C–D) for fasted mice treated with 300 mg/kg APAP (A,C) or fed mice treated with 600 mg/kg APAP (B,D). Ions from acylcarnitines are shown by the arrows, with their respective molecular weights. In the Score plots (A,B), the x-axis shows differences in total ion profiles between groups while the y-axis shows differences between individual mice. In the S-plots (C,D), the x-axis indicates changes in ion average concentration after APAP treatment, while the y-axis indicates variance confidence (changes in ion concentration farther from 0 have less variation within groups).
Figure 3. Serum acylcarnitines during acetaminophen-induced liver injury in mice.
Fasted or fed mice were treated with 300 or 600 mg/kg APAP, respectively, for various times. (A) Acylcarnitine levels in serum from fasted mice after APAP treatment. The control concentrations were 200 ± 20, 139 ± 19 and 467 ± 35 nM for palmitoylcarnitine (Palm), linoleoylcarnitine (Linoleo) and oleoylcarnitine (Oleo), respectively. (B) Acylcarnitine levels in serum from fed mice after APAP treatment. The control concentrations were 72 ± 2, 29 ± 1 and 166 ± 12 nM for Palm, Linoleo, and Oleo, respectively. Data are expressed as mean ± SEM of n = 3. *P < 0.05 (compared to t=0 h).
Table 1.
Serum acylcarnitine concentrations (nM) in mice 3 h after APAP treatment.
| Group | Palmitoylcarnitine | Linoleoylcarnitine | Oleoylcarnitine |
|---|---|---|---|
| Fasted, 300 mg/kg APAP | |||
| Control | 200 ± 20 | 139 ± 19 | 467 ± 35 |
| APAP | 1,488 ± 261* | 809 ± 39* | 8,464 ± 287* |
| Fed, 600 mg/kg APAP | |||
| Control | 72 ± 2 | 29 ± 1 | 166 ± 12 |
| APAP | 637 ± 31* | 271 ± 51* | 2,379 ± 145* |
Data are expressed as mean ± SEM of n = 3.
p < 0.05 vs. Control.
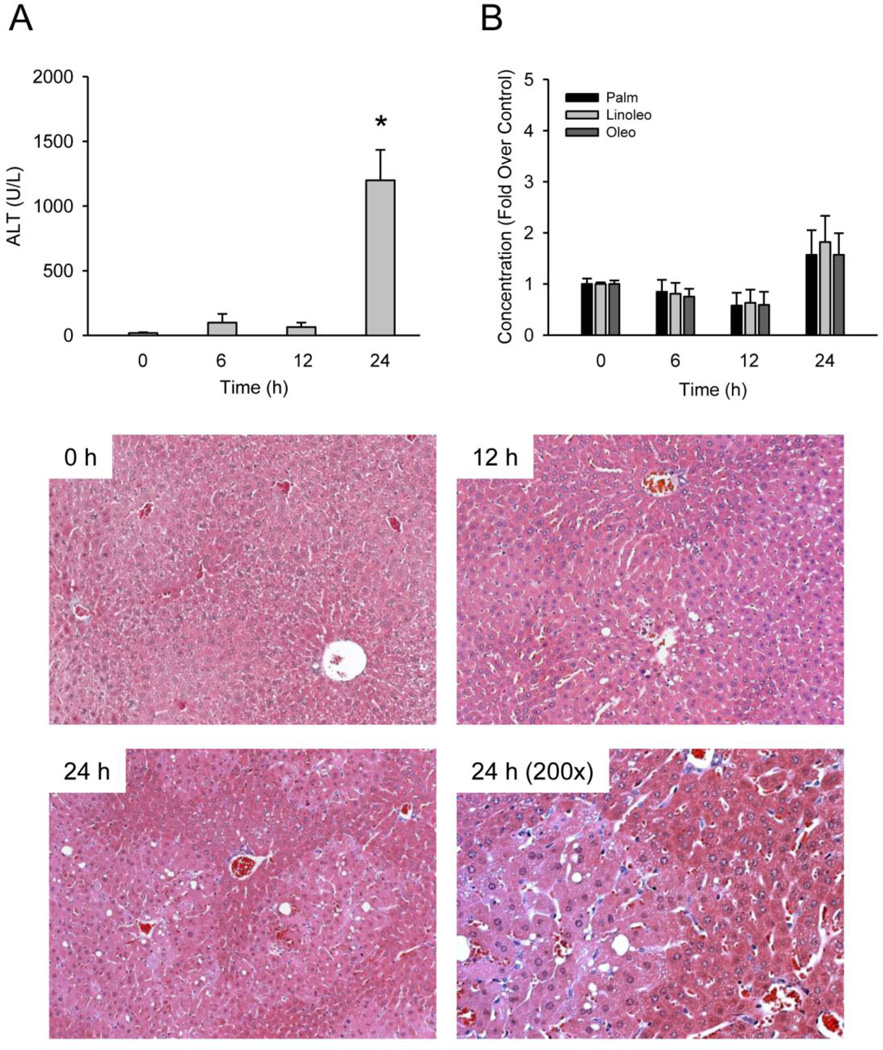
Because acylcarnitines are transported into mitochondria where the fatty acid chains are separated to undergo β-oxidation, we hypothesized that mitochondrial damage could lead to accumulation of these acylcarnitines and that they could be mechanistic biomarkers of mitochondrial dysfunction. To test this, we treated mice with a high dose of furosemide, a diuretic that is known to cause centrilobular necrosis similar to APAP but without primarily affecting mitochondria (Wong et al. 2000). Failure to find an increase could indicate that these acylcarnitines are in fact specific for mitochondrial dysfunction. This approach has been used in the past to identify GDH and mitochondrial DNA as circulating markers of mitochondrial damage (McGill et al. 2012). Treatment with 500 mg/kg furosemide caused significant liver injury at 24 h, as indicated by ALT and histology (Fig. 4A). Importantly, treatment with 500 mg/kg furosemide did not cause any major elevations in serum acylcarnitine levels (Fig. 4B). These data indicate that these acylcarnitines may be specific biomarkers of mitochondrial dysfunction in mice.
Figure 4. Furosemide-induced liver injury and serum acylcarnitines.
Fed mice were treated with 500 mg/kg furosemide for various times. (A) ALT was measured in serum from these animals. (B) Acylcarnitine levels were also measured in serum. Control concentrations were 761 ± 82, 409 ± 13 and 2,805 ± 194 nM for palmitoylcarnitine, linoleoylcarnitine and oleoylcarnitine, respectively. (C) Hematoxylin and eosin-stained liver sections from these mice (100×, except where indicated). Data are expressed as mean ± SEM of n = 3. *P < 0.05 (compared to t=0 h).
Plasma acylcarnitines after acetaminophen overdose in humans
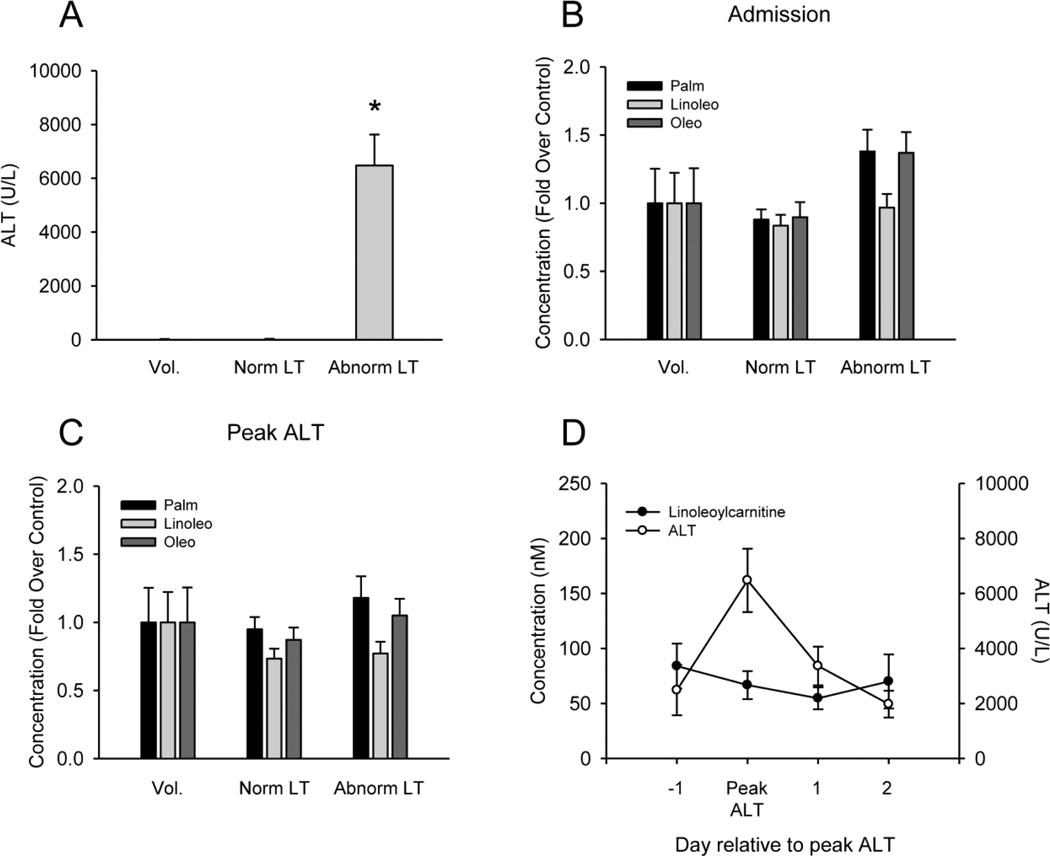
To determine whether or not plasma acylcarnitines are elevated after APAP overdose in humans, we measured these compounds in plasma from APAP overdose patients. Unfortunately, when we compared acylcarnitine levels at the time of admission or at the time of peak ALT in our abnormal LT group with those in the normal LT and healthy volunteer groups, we were unable to detect any significant increases despite a large difference in injury (Fig. 5A–C). Time course data revealed that the acylcarnitine levels did not change significantly over time after APAP overdose (Fig. 5D). Morevoer, the levels were much lower than in mice treated with APAP. These data show that these biomarkers are not clinically useful after APAP overdose. Nevertheless, our rodent data strongly suggest that circulating acylcarnitines are likely to be useful, specific markers of mitochondrial dysfunction in other forms of liver injury or disease.
Figure 5. Plasma ALT and acylcarnitines in samples from APAP overdose patients.
(A) Average peak ALT values for the patients. (B–C) Acylcarnitines were measured in plasma from APAP overdose patients at the first blood draw after admission and study enrollment and at the time of peak liver injury, as indicated by ALT. (D) Average ALT and lineoylcarnitine levels in plasma from 6 overdose patients in the abnormal LT group. In panels A–C, data are expressed as mean ± SEM for 6 healthy volunteers, 14 normal liver test result (LT) overdose patients, and 16 abnormal LT overdose patients. *P<0.05 (compared to Vol.).
Effect of N-acetylcysteine on serum acylcarnitines
A major difference between the mice and humans in these experiments is treatment with NAC. All patients enrolled in our study received NAC as part of their standard-of-care treatment. When given early after APAP overdose, the major function of this intervention is to serve as a precursor for the re-synthesis of GSH, which is depleted by NAPQI. The GSH can then scavenge any additional NAPQI that is formed, and act as an antioxidant to cope with the oxidative stress (Knight et al. 2002; Bajt et al. 2003; 2004). On the other hand, NAC has also been shown to enhance mitochondrial function when given late by providing substrates for the Krebs cycle (Saito et al. 2010b). We hypothesized that the lack of increase in acylcarnitines during APAP hepatotoxicity in humans was due in part to NAC. To test this possibility, we treated mice with 140 mg/kg NAC 1.5 h after treatment with APAP. Consistent with the idea that NAC supports mitochondrial function, acylcarnitine levels were lower in serum from these animals than from mice treated with APAP alone (Fig. 7).
DISCUSSION
Acetaminophen hepatotoxicity in mice and humans
A considerable amount of work has been done using rodent models to elucidate the mechanisms of APAP toxicity in the liver. It is well-established that the injury is initiated by binding of NAPQI to proteins (McGill and Jaeschke 2013). Because NAPQI targets mitochondrial proteins more than the non-hepatotoxic isomer 3’-hydroxyacetanilide (Tirmenstein and Nelson 1989), it is thought that binding to proteins in mitochondria is especially important. The resulting mitochondrial protein damage leads to mitochondrial dysfunction and oxidative stress, with JNK activation, and nuclear DNA fragmentation (Jaeschke et al. 2012). While the mechanisms are clear in mice, much less work has been done to improve our understanding of APAP-induced liver injury in humans or with human models. Though there is strong evidence that GSH depletion and protein binding also occur in humans (Davis et al. 1975; Lauterburg et al. 1987; Davern et al. 2006), the role of mitochondria has not been well studied. In the unique human liver cell line HepaRG, loss of mitochondrial membrane potential and the development of oxidative stress preceded the onset of cell death (McGill et al. 2011). Furthermore, a comparison of mice treated with APAP or furosemide provided evidence that high levels of GDH and mtDNA in blood are specific for mitochondrial damage, and we were able to measure these novel mechanistic biomarkers in plasma from APAP overdose patients (McGill et al. 2012). We were also able to measure higher levels of nuclear DNA fragments in the plasma from APAP overdose patients (McGill et al. 2012). Together, these data suggest that mitochondrial damage may also be part of the mechanism of APAP hepatotoxicity in humans. We used the same approach with furosemide in the present work to demonstrate that an increase in acylcarnitine concentrations in blood may be specific for mitochondrial dysfunction. A major advantage of the measurement of metabolic intermediates such as acylcarnitines as biomarkers in acute liver injury is that they are more likely to cross intact cell membranes and become detectable in blood before cell death, making them potentially better candidates for the early prediction of patient outcome. Furthermore, because acylcarnitines are broken down within mitochondria, they would not be expected to be present at high levels in intact mitochondria released into blood from dying cells, possibly avoiding the potential issue of contributions from the intact organelle present in plasma (Jaeschke and McGill 2013).
Acylcarnitines as biomarkers of mitochondrial dysfunction
Acylcarnitines are conjugates of fatty acids with carnitine. While medium- and short-chain fatty acids can enter mitochondria unassisted, formation of long-chain acylcarnitines is necessary for movement of long-chain fatty acids into mitochondria for β-oxidation. Serum acylcarnitine concentrations are currently measured in the screening of neonates for mitochondrial fatty acid oxidation deficiency disorders (mFAODs). mFAODs can be caused by defects in carnitine and acylcarnitine transporters, and by defects in enzymes involved in β-oxidation (Rinaldo et al. 2002). The reason for the increased acylcarnitine levels in serum in APAP-induced liver injury in mice is not known, although there is evidence that PPARα activation can prevent it (Chen et al. 2009). Because severe mitochondrial dysfunction is central in the mechanism of APAP toxicity, it seems likely that multiple steps in acylcarnitine and fatty acid metabolism involving mitochondria are affected by APAP. It is possible that NAPQI binds to and disrupts the function of CACT or CPT II. This would be expected to result in accumulation of acylcarnitines within the cytosol, which could then be transported into the extracellular space. It is also possible that β-oxidation is affected, resulting in accumulation of acylcarnitines within the mitochondria which are then released when the mitochondria undergo the membrane permeability transition (MPT) or when they swell and lyse. In any case, although the correlation between acylcarnitines and ALT in plasma from some patients may support our hypothesis that mitochondrial dysfunction is also important in APAP hepatotoxicity in humans, overall we did not find a significant difference in circulating acylcarnitines in samples from overdose patients with liver injury compared to those with normal liver test results or even to healthy volunteers. The reason for this is not clear. It is possible that there is a transient increase in acylcarnitines early during APAP-induced liver injury in humans that is simply missed later on. This seems unlikely, as several of the patients in our abnormal LT group presented before the peak of ALT but did not have elevated levels in the first study sample after admission (Fig. 5). In mice the elevated acylcarnitine levels persisted until at least 12 h, which is the peak of injury in mice (McGill et al. 2013). Even if this could be true despite our data, most patients do not present to the hospital until later time points. Another possible explanation is the clinical use of NAC, discussed below. Although acylcarnitines are not clinically useful biomarkers after APAP overdose, the dramatic increases in their serum levels after APAP treatment in mice, coupled with the lack of an increase in serum from furosemidetreated mice, suggests that they may be useful biomarkers of mitochondrial dysfunction in other forms of liver injury. These data provide a strong foundation for future studies.
Mechanisms of protection against acetaminophen hepatotoxicity by N-acetylcysteine
NAC protects against APAP-induced liver injury in several ways. Although the primary mechanism by which this intervention protects is replenishment of the NAPQI scavenger GSH, there is evidence for GSH-independent effects. The duodenum is the primary site of absorption of APAP from the GI tract and limited data suggest that if oral NAC is co-administered or given very soon after APAP overdose, it may delay gastric emptying and reduce absorption (Whitehouse et al. 1981). Unfortunately, such early patient presentation is rare in the clinic. GSH is also well known to be an effective antioxidant, and there is strong evidence that it scavenges peroxynitrite in mitochondria during APAP hepatotoxicity (Knight et al. 2002; Bajt et al. 2003; 2004). Importantly, patients presenting late may benefit from the metabolic effects of NAC. Saito et al. found that treatment with excess NAC after APAP not only replenishes hepatic GSH, but supplies mitochondrial energy substrates in the Krebs cycle and restores hepatic ATP levels (Saito et al. 2010b). In our study, we performed a similar post-treatment with 140 mg/kg NAC, a standard loading dose for humans. Consistent with the work showing enhanced mitochondrial function due to NAC, we found that this treatment reduced serum acylcarnitine levels in mice. Although we cannot rule out NAPQI or ROS scavenging as a contributing protective effect in these experiments, our previous studies have shown that binding of NAPQI to proteins in the liver reaches a plateau by 1.5 h after APAP treatment (McGill et al. 2013), suggesting that much of the protein adduction in the liver had already occurred by the time the mice were treated with NAC. We think that enhancement of mitochondrial function likely played a role in the reduction of serum acylcarnitines in these experiments. Interestingly, it was recently proposed that inhibition of fatty acid oxidation and accumulation of acylcarnitines may contribute to or enhance the toxicity of APAP (Bi et al. 2013). If correct, prevention of this effect may be another way in which NAC protects against APAP. It is perplexing that we did not find an increase in acylcarnitines in APAP overdose patients even though we have found evidence for mitochondrial dysfunction in APAP overdose patients in the past (McGill et al. 2012). This could be due to the fact that our previous biomarkers of mitochondrial damage require the mitochondrial permeability transition (MPT) and/or mitochondrial membrane swelling and lysis. It is possible that less-damaged surrounding mitochondria may experience dysfunction leading to increased acylcarnitines in mice, but NAC prevents this in humans. In any case, it seems likely from these data that NAC can reduce acylcarnitine levels by supporting mitochondria.
Conclusions
Our data confirm that acylcarnitine levels are increased in serum from mice after APAP treatment. Furthermore, we have shown that this is likely specific for mitochondrial dysfunction, based on our comparison of APAP and furosemide. Unfortunately, there were no significant increases in plasma acylcarnitine concentrations in our patients with abnormal liver test results compared to control subjects. Mouse experiments suggested that this may have been due in part to treatment with NAC. Altogether, we believe that serum acylcarnitines are useful as novel mechanistic markers of mitochondrial damage. These biomarkers may be helpful in the study of conditions affecting mitochondria dysfunction in which NAC is not a common treatment.
Supplementary Material
Figure 6. Effect of N-acetylcysteine on serum acylcarnitines during APAP-induced liver injury.
Mice were treated with 140 mg/kg NAC 1.5 h after 300 mg/kg APAP. Acylcarnitines were measured 3 h post-APAP. Control concentrations were 387 ± 69, 244 ± 40 and 1,855 ± 258 nM for palmitoylcarnitine (Palm), linoleoylcarnitine (Linoleo) and oleoylcarnitine (Oleo), respectively. Data are expressed as mean ± SEM of n = 3. *P < 0.05 (compared to CTRL); #P < 0.05 (compared to 3 h APAP).
ACKNOWLEDGEMENTS
This work was supported in part by grants from McNeil Consumer Health, Inc. (to H.J. and S.C.C.), by the University of Kansas Medical Center Liver Center (to H.J.), by the National Institutes of Health grants R01 DK070195 and R01 AA12916 (to H.J.), and by grants from the National Center for Research Resources (5P20RR021940-07) and the National Institute of General Medical Sciences (8 P20 GM103549-07) of the National Institutes of Health. Additional support came from an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20 GM12345 and, from the “Training Program in Environmental Toxicology” T32 ES007079-26A2 (to M.R.M. and C.D.W.) from the National Institute of Environmental Health Sciences.
ABBREVIATIONS
- ALT
Alanine aminotransferase
- APAP
Acetaminophen
- CACT
Carnitine-acylcarnitine translocase
- CPT
Carnitine palmitoyltransferase
- GDH
Glutamate dehydrogenase
- JNK
c-Jun N-terminal kinase
- MLK3
Mixed lineage kinase 3
- MPT
Mitochondrial membrane permeability transition
- mtDNA
Mitochondrial DNA
- NAC
N-acetylcysteine
- NAPQI
N-acetyl-p-benzoquinone imine
- OPLS-DA
Orthogonal projection to latent structures-discriminant analysis
- PCA
Principal component analysis
- ROS
Reactive oxygen species
Footnotes
CONFLICT OF INTEREST DISCLOSURE
The authors declare no competing financial interest.
REFERENCES
- Antoine DJ, Dear JW, Starkey-Lewis P, Platt V, Coyle J, Masson M, Thanacoody RH, Gray AJ, Webb DJ, Moggs JG, Bateman DN, Goldring CE, Park BK. Mechanistic biomarkers provide early and sensitive detection of acetaminophen-induced acute liver injury at first presentation to hospital. Hepatology. 2013;58:777–787. doi: 10.1002/hep.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajt ML, Cover C, Lemasters JJ, Jaeschke H. Nuclear translocation of endonuclease G and apoptotis-inducing factor during acetaminophen-induced liver cell injury. Toxicol Sci. 2006;94:217–225. doi: 10.1093/toxsci/kfl077. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Farhood A, Lemasters JJ, Jaeschke H. Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2008;324:8–14. doi: 10.1124/jpet.107.129445. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Knight TR, Farhood A, Jaeschke H. Scavenging peroxynitrite with glutathione promotes regeneration and enhances survival during acetaminophen-induced liver injury in mice. J Pharmacol Exp Ther. 2003;307:67–73. doi: 10.1124/jpet.103.052506. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Knight TR, Lemasters JJ, Jaeschke H. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatotocytes: protection by N-acetylcysteine. Toxicol Sci. 2004;80:343–349. doi: 10.1093/toxsci/kfh151. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Ramachandran A, Yan HM, Lebofsky M, Farhood A, Lemasters JJ, Jaeschke H. Apoptosis-inducing factor modulates mitochondrial oxidant stress in acetaminophen hepatotoxicity. Toxicol Sci. 2011;122:598–605. doi: 10.1093/toxsci/kfr116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi H, Li F, Krausz KW, Qu A, Johnson CH, Gonzalez FJ. Targeted metabolomics of serum acylcarnitines evaluates hepatoprotective effect of wuzhi tablet (Schisandra sphenanthera extract) against acute acetaminophen toxicity. Evid Based Complement Alternat Med. 2013;2013:985257. doi: 10.1155/2013/985257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Krausz KW, Shah YM, Idle JR, Gonzalez FJ. Serum metabolomics reveals irreversible inhibition of fatty acid beta-oxidation through the suppression of PPARalpha activation as a contributing mechanism of acetaminophen-induced hepatotoxicity. Chem Res Toxicol. 2009;22:699–707. doi: 10.1021/tx800464q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2005;315:879–887. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- Dahlin DC, Miwa GT, Lu AY, Nelson SD. N-acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophen. Proc Natl Acad Sci USA. 1984;81:1327–1331. doi: 10.1073/pnas.81.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davern TJ, 2nd, James LP, Hinson JA, Polson J, Larson AM, Fontana RJ, Lalani E, Munoz S, Shakil AO, Lee WM Acute Liver Failure Study Group. Measurement of serum acetaminophen-protein adducts in patients with acute liver failure. Gastroenterology. 2006;130:687–694. doi: 10.1053/j.gastro.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Davis M, Ideo G, Harrison NG, Williams R. Hepatic glutathione depletion and impaired bromosulphthalein clearance early after paracetamol overdose in man and the rat. Clin Sci Mol Med. 1975;49:495–502. doi: 10.1042/cs0490495. [DOI] [PubMed] [Google Scholar]
- Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–178. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H. Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J Pharmacol Exp Ther. 1990;255:935–941. [PubMed] [Google Scholar]
- Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev. 2012;44:88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, McGill MR. Serum glutamate dehydrogenase – biomarker for liver cell death or mitochondrial dysfunction? Toxicol Sci. 2013;134:221–222. doi: 10.1093/toxsci/kft087. [DOI] [PubMed] [Google Scholar]
- Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA. 2002;287:337–344. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- Knight TR, Ho YS, Farhood A, Jaeschke H. Peroxynitrite is a critical mediator of acetaminophen hepatotoxicity in murine livers: protection by glutathione. J Pharmacol Exp Ther. 2002;303:468–475. doi: 10.1124/jpet.102.038968. [DOI] [PubMed] [Google Scholar]
- Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- Lauterburg BH, Mitchell JR. Therapeutic doses of acetaminophen stimulate turnover of cysteine and glutathione in man. J Hepatol. 1987;4:206–211. doi: 10.1016/s0168-8278(87)80081-8. [DOI] [PubMed] [Google Scholar]
- McGill MR, Jaeschke H. Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm Res. 2013 Mar 6; doi: 10.1007/s11095-013-1007-6. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Lebofsky M, Norris HK, Slawson MH, Bajt ML, Xie Y, Williams CD, Wilkins DG, Rollins DE, Jaeschke H. Plasma and liver acetaminophen-protein adduct levels in mice after acetaminophen treatment: dose-response, mechanisms, and clinical implications. Toxicol Appl Pharmacol. 2013;269:240–249. doi: 10.1016/j.taap.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122:1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Yan HM, Ramachandran A, Murray GJ, Rollins DE, Jaeschke H. HepaRG cells: a human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology. 2011;53:974–982. doi: 10.1002/hep.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther. 1973;187:211–217. [PubMed] [Google Scholar]
- Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai A, Sakamoto K, Ogura K, Noguchi T, Karin M, Ichijo H, Omata M. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibition c-Jun N-terminal kinase activation. Gastroenterology. 2008;135:1311–1321. doi: 10.1053/j.gastro.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Ramachandran A, Lebofsky M, Weinman SA, Jaeschke H. The impact of partial manganese superoxide dismutase (SOD2)-deficiency on mitochondrial oxidant stress, DNA fragmentation and liver injury during acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2011;251:226–233. doi: 10.1016/j.taap.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldo P, Matern D, Bennett MJ. Fatty acid oxidation disorders. Annu Rev Physiol. 2002;64:477–502. doi: 10.1146/annurev.physiol.64.082201.154705. [DOI] [PubMed] [Google Scholar]
- Saito C, Lemasters JJ, Jaeschke H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2010a;246:8–17. doi: 10.1016/j.taap.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito C, Zwingmann C, Jaeschke H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology. 2010b;51:246–254. doi: 10.1002/hep.23267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Gadang V, Jaeschke A. Critical role for mixed-lineage kinase 3 in acetaminophen-induced hepatotoxicity. Mol Pharmacol. 2012;82:1001–1007. doi: 10.1124/mol.112.079863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirmenstein MA, Nelson SD. Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxic regioisomer, 3’-hydroxyacetanilide, in mouse liver. J Biol Chem. 1989;264:9814–9819. [PubMed] [Google Scholar]
- Whitehouse LW, Wong LT, Solomonraj G, Paul CJ, Thomas BH. N-acetylcysteineinduced inhibition of gastric emptying: a mechanism affording protection to mice from the hepatotoxicity of concomitantly administered acetaminophen. Toxicology. 1981;19:113–125. doi: 10.1016/0300-483x(81)90093-7. [DOI] [PubMed] [Google Scholar]
- Win S, Than TA, Han D, Petrovic LM, Kaplowitz N. c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. J Biol Chem. 2011;286:35071–35078. doi: 10.1074/jbc.M111.276089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SG, Card JW, Racz WJ. The role of mitochondrial injury in bromobenzene and furosemide induced hepatotoxicity. Toxicol Lett. 2000;116:171–181. doi: 10.1016/s0378-4274(00)00218-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.