Abstract
Mesenchymal stem cells (MSCs) have potential therapeutic applications for musculoskeletal injuries due to their ability to differentiate into several tissue cell types and modulate immune and inflammatory responses. These immune-modulatory properties were examined in vivo during early stage rat medial collateral ligament healing. Two different cell doses (low dose 1×106 or high dose 4×106 MSCs) were administered at the time of injury and compared with normal ligament healing at days 5 and 14 post-injury. At both times, the high dose MSC group demonstrated a significant decrease in M2 macrophages compared to controls. At day 14, fewer M1 macrophages were detected in the low dose group compared to the high dose group. These results, along with significant changes in procollagen I, proliferating cells, and endothelialization suggest that MSCs can alter the cellular response during healing in a dose-dependent manner. The higher dose ligaments also had increased expression of several pro-inflammatory cytokines at day 5 (IL-1β, IFNγ, IL-2) and increased expression of IL-12 at day 14. Mechanical testing at day 14 revealed increased failure strength and stiffness in low dose ligaments compared to controls. Based on these improved mechanical properties, MSCs enhanced functional healing when applied at a lower dose. Different doses of MSCs uniquely affected the cellular response and cytokine expression in healing ligaments. Interestingly, the lower dose of cells proved to be most effective in improving functional properties.
Keywords: mesenchymal stem cells, ligament, healing, immunohistochemistry, immune modulation, cytokines, mechanical testing
Introduction
Ligament injuries are common musculoskeletal injuries that affect all age groups and often require an intervention for healing, depending on the location and healing environment. The healing process fills the void, yet the ligament portrays more scar-like extracellular matrix architecture and biology from the native tissue. This tissue can become problematic in ligaments because it consists of less organized, smaller collagen fibrils (1–3) that are associated with decreased tissue strength and compromised joint function. Subsequently, the normal healing cascade places the ligament at risk for re-injury. Thus, there is a growing interest in finding therapeutic interventions to minimize scar-like tissue and increase the tissue’s regenerative potential.
Mesenchymal stem cell (MSC) research continues to expand due to its relatively untapped potential as a therapeutic agent. MSCs are generally used for two reasons: 1) the ability for MSCs to differentiate into several different connective tissues such as cartilage, bone, muscle and fat (4–6), and 2) the capacity for MSCs to modulate immune and inflammatory responses that affect various healing environments (7,8). This paradigm shift from differentiation to immune modulation is studied in different areas of the body (9). Several reports suggest MSCs decrease inflammation by reducing pro-inflammatory cytokines and changing the macrophage phenotype from type 1 (M1, classically-activated) to type 2 (M2, alternatively-activated) (10–13). The M1 phenotype is classified as pro-inflammatory, while the M2 phenotype is more reparative and anti-inflammatory (14–16). A shift in macrophage phenotype is thought to be associated with improved healing (more regeneration of native tissue with less fibrotic scarring). Many of these studies have been performed in vitro but there is a lack of inquiry regarding the modulatory effects occurring within injured ligament. MSCs can behave differently depending on the tissue and healing environment they are exposed to, which leads to our specific interest in MSC’s immune modulatory effects in healing ligaments.
Several researchers have demonstrated improved mechanics in tendons and ligaments using MSCs (17–20). Kanaya et al. found that MSC injections into a rat knee joint improved both histological scores and ultimate failure loads 4 weeks post- anterior cruciate ligament partial tear (17). Chong et al. reported that administration of MSCs via fibrin glue in a rabbit Achilles injury model led to improved collagen organization and mechanical properties during early stage healing (18). These functional improvements show great promise for the way MSCs can modulate the healing cascade. However, not all studies utilizing MSCs have been successful (21). A better understanding of MSC regenerative mechanisms is necessary and could elucidate more effective regeneration strategies.
In the current study, we examined the specific influence MSCs had on an injured ligament by measuring spatial and temporal cellular responses. Second, we explored if there was a dose response, ultimately determining an optimal healing effect depending on the amount of MSCs administered. Finally we examined production of common pro-inflammatory and anti-inflammatory cytokines in relation to treatment groups and normal healing ligaments. We hypothesized that both doses of MSCs would result in a less inflammatory environment leading to improved mechanical properties, with the higher dose of MSCs yielding more optimal results.
Materials and Methods
Experimental Design
A medial collateral ligament (MCL) injury model was used since the normal healing cascade in this model is well characterized (22) and provides an appropriate comparison when perturbing the healing cascade. Results from this model can be applied to extra-articular ligament healing, whereas other models exist to study intra-articular healing (i.e. anterior cruciate ligament). MCLs were transected and MSCs were injected at the time of injury in the transected region without the use of a scaffold. Healing was analyzed at day 5 to examine a time point when macrophages peak (22), and day 14 to adequately assess mechanical properties. Two doses of MSCs were used: a low dose consisting of 1×106 cells and a high dose of 4×106 cells. Doses were selected based on animal model size and highest number of cells soluble in 50µl of fluid without becoming too viscous. Forty-seven adult male Wistar rats (275–299g) underwent bilateral MCL transections (right MCL=treatment, left MCL=control) with 15 rats (n=3 each dose and time point, 3 extras) used for immunohistochemistry, immunofluorescence, and hematoxylin and eosin (H&E) staining, 20 rats (n=5 each dose and time point) used for cytokine analysis, and 12 rats (n=6 each dose, day 14 only) used for mechanical testing.
Surgical Procedure
All procedures were approved by the University of Wisconsin Institutional Animal Care and Use Committee. Rats were anesthetized using isoflurane and prepared for surgery using sterile technique (day 0). A 1 cm longitudinal skin incision was made at the femoral-tibial junction. The subcutaneous tissue and gracilis muscle were dissected in order to expose the MCL. A scalpel blade was used to create a complete, uniform transection of the MCL just distal to the joint line. MCLs were transected instead of torn in order to improve reproducibility. Each rat underwent bilateral MCL transections with the right MCL being administered MSCs and the left serving as a control by receiving Hanks Balanced Saline Solution (HBSS; Hyclone Laboratories Inc, Logan UT). Transected ligaments were not repaired with suture. Once cells or HBSS were injected, the 3 tissue layers were closed using a 5-0 vicryl suture. The animals were allowed unrestricted cage mobility post-operatively and were euthanized at days 5 and 14 for ligament analysis.
Mesenchymal Stem Cell Culture
Rat mesenchymal stem cells were purchased from a commercial vendor (Trevigen, Gaithersburg, MD) at passage 3. Cells were isolated from adult, male Fisher 344 rat bone marrow by adherence to plastic and purified through passaging. This was confirmed by the vendor through flow cytometry which demonstrated less than 1% contamination by hematopoietic cells. Cells were positive for CD90 and CD29 and negative for CD11b and CD45 (hematopoietic markers). Using the vendor’s differentiation kits, these cells exhibit osteogenic and adipogenic phenotypes. Cells remain in an undifferentiated state and are guaranteed up to 10 population doublings using the vendor’s Qualified RMSC medium. Once the cells were received from the vendor, they were seeded in T175 flasks and administered Cultrex Qualified RMSC medium containing 10% fetal bovine serum (Trevigen, Gaithersburg, MD) and 1% antibiotic-antimycotic (Cellgro, Manassas, VA). Cells were incubated at 37° C and 5% CO2 and passaged at 70% confluency. Media was changed every 3–4 days and cell morphology was monitored throughout expansion to ensure MSCs maintained a spindle-like appearance. Cells were collected at passages 7 through 10 for in vivo application.
MSCs were collected the morning of surgery using Trypsin EDTA (Cellgro, Manassas, VA). Cells were fluorescently-labeled with Celltracker CM-DiI (Life Technologies, Grand Island, NY) in order to visualize their spatial distribution in the healing ligaments. Once fluorescently tagged, either 1×106 or 4×106 MSCs were suspended in 50µl of HBSS.
Immunohistochemistry/Immunofluorescence
At day 5 and day 14 post-injury, ligaments were collected, frozen in optimal cutting temperature (OCT), and longitudinally sectioned (5µm). Sections were mounted on Colorfrost Plus microscope slides and stored at −80° C. Mouse and rabbit monoclonal antibodies were used to detect the cell type of interest. Standard procedure for staining consisted of acetone fixation followed by 3% hydrogen peroxide to prevent endogenous peroxidase activity. The samples were then treated with Background Buster (Innovex Biosciences, Richmond, CA) to protect against non-specific antibody-protein interactions. Primary antibody was applied (2 hours) followed by a biotin-linked secondary (10 minutes) and streptavidin conjugated to horseradish peroxidase tertiary antibody (10 minutes) using a Stat Q staining kit (Innovex Biosciences, Richmond, CA). Diaminobenzidine (DAB) was used to detect the antibody-antigen complex of interest. Light microscopy allowed for spatial localization and cell counting of all IHC stains.
Mouse monoclonal antibodies were utilized to detect type 1 macrophages (CD68; 1:100; AbDSertoc, Raleigh, NC), type 2 macrophages (CD163; 1:100; AbDSertoc, Raleigh, NC), endothelial cells (CD31; 1:100; AbDSertoc, Raleigh, NC), proliferating cells (Ki-67; 1:25; Dako, Carpinteria, CA), procollagen I (straight; SP1.D8; Developmental Hybridoma, Iowa City, Iowa) and collagen III (1:8000; Sigma-Aldrich, St. Louis, MO). A rabbit monoclonal antibody was used to detect T cells (CD3; 1:100, Abcam, Cambridge, MA). 4',6-diamidino-2-phenylindole (DAPI) and Celltracker CM-DiI (Life Technologies, Grand Island, NY) were used for fluorescent detection of total cells and MSCs, respectively.
Histology
Hematoxylin and Eosin (H&E) staining allowed healing region size measurements and morphological observation of the ligament. The healing region area was measured using Image J (National Institutes of Health, Bethesda, MD) and expressed as a percentage of total ligament area within a sagittal section.
Cell Quantification
Images were taken using a camera-assisted microscope (Nikon Eclipse microscope, model E6000 with Olympus camera, model DP79). Five areas of the healing ligament were imaged at 400× in order to measure spatial distribution of the various cell types. The areas included the healing region, distal healing region edge, proximal healing region edge, distal ligament and proximal ligament. All 5 areas were combined for analysis of the entire MCL providing a 6th measure for comparison. Two or three sections from each ligament were counted and averaged for comparison. Cells were manually counted for each stain. Density of collagen III was measured using Image J (National Institutes of Health, Bethesda, MD).
Mechanical Testing
At day 14, rats were euthanized and frozen (−80°C) until dissections could take place. Right and left MCLs were carefully dissected by removing all surrounding tissue. The MCL tibial and femoral insertions were kept intact for mechanical testing. Once dissected, phosphate buffered saline (PBS) was applied to maintain ligament hydration.
Testing was performed in a custom-designed load frame that held the tibia and femur in the anatomical position for uniform loading. MCLs started in a slack position (without tension) and were not preconditioned to avoid damaging the healing region prior to failure testing. Pulling at a rate of 4.0 mm/sec, each ligament was stretched until it failed. Load and displacement data were collected to determine maximum load before failure, along with stiffness in the most linear region of the load-displacement curve.
Cytokine Analysis
Ligaments were collected at day 5 and day 14 post-operatively to assess the influence of MSC dosage on cytokine expression. Five MCLs were collected and pooled for each time point and group. MCLs were washed in Cell Wash Buffer (Bio-Rad, Hercules, CA) placed in Navy Bead Lysis Kit tubes (Next Advance, Averill Park, NY) containing Lysing Solution (Bio-Rad, Hercules, CA). A Bullet Blender (Next Advance, Averill Park, NY) was used to homogenize the MCLs and separate soluble from insoluble proteins. Supernatant was collected and frozen for total protein measurement (Pierce BCA Protein Assay, Rockford, IL) and cytokine multiplex analysis.
A rat cytokine 10-plex kit (Life Technologies, Grand Island, NY) was used to measure 10 pro-inflammatory and anti-inflammatory proteins. The proteins measured included GM-CSF, IFNγ, IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12 and TNFα. Samples were run in triplicate and incubated with primary antibodies overnight at 4° C on a plate shaker. The following day samples were treated with a biotinylated secondary antibody and streptavidin-RPE tertiary antibody for detection. Serial dilutions of standards, along with spleen (positive control) and lysis solution (negative control), were used for accurate and repeatable measurements. Samples were read using a Luminex Magpix (Life Technologies, Grand Island, NY) system. A standard curve was established and verified to ensure 80–120% recovery and detection a minimum of 2 standard deviations above background. Protein concentrations were normalized to total protein measurements and expressed as a percentage for analysis.
Statistics
A 2-tailed, paired student t-test was used to detect differences between MSC treated ligaments compared to controls since the comparison is based on ligaments within the same animal. A 2-tailed, unpaired student t-test was used to make comparisons between high and low dose MSC groups since the doses were administered in different animals. A p-value less than .05 was considered significant.
Results
IHC
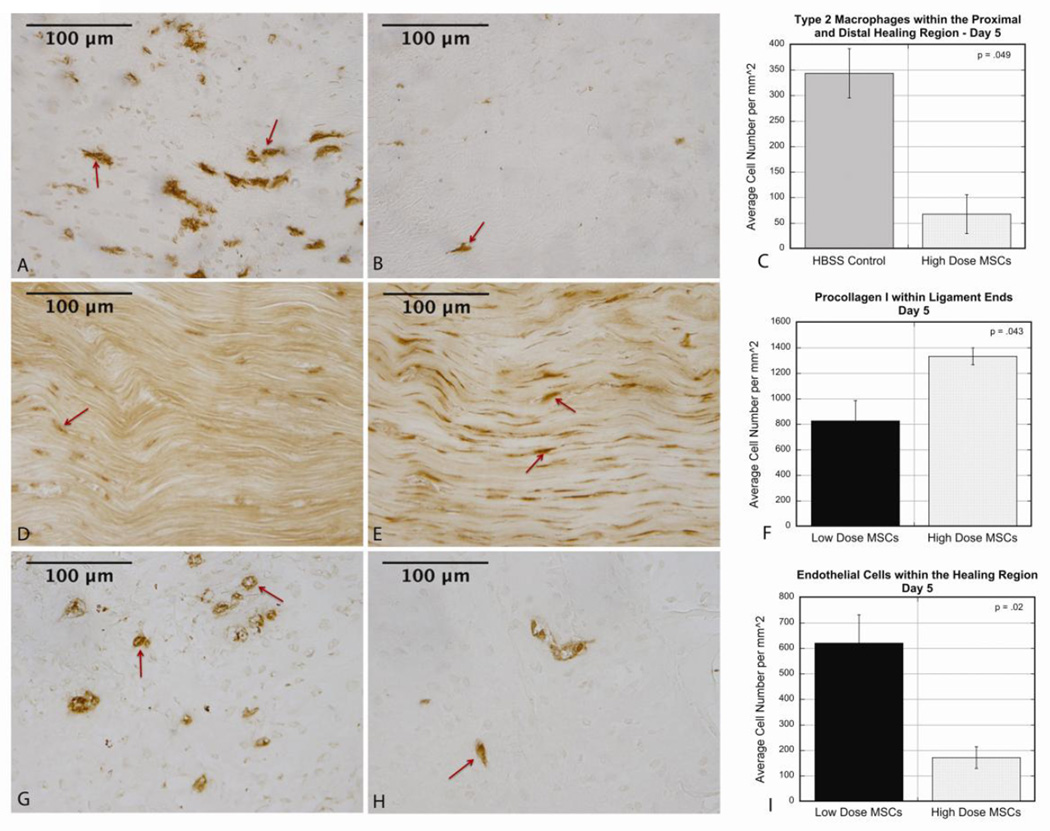
Comparisons were made between each MSC dose and animal matched controls along with comparisons between the 2 doses (Table 1). No significant changes in cellular distribution between HBSS control ligaments and low dose MSC (1×106) ligaments were noted at day 5 post-injury. However, treatment with the high dose (4×106) of cells demonstrated significant changes throughout the ligament in type 2 macrophages (M2s). Fewer M2s (p=.049) were present in the distal and proximal healing region of the high dose MSC group (Fig 1 A–C) compared to control ligaments. Comparing different doses, procollagen I (precursor to collagen I) was decreased (p=.042) throughout MCLs that received the low dose of MSCs compared to the high dose group at day 5 (Fig 1 D–F). Endothelial cell quantification showed that the low dose MSC group had more endothelial cells (p=.021) and lumen (p=.008) starting to form in the healing region compared to the high dose group (Fig 1 G–I).
Table 1.
Summary of Cellular Spatial Distribution within Ligaments at Days 5 and 14 Post-injury.
| Day 5 | Day 14 | |||||
|---|---|---|---|---|---|---|
|
Low dose MSCs vs. Control |
High Dose MSCs vs. Control |
Low dose vs. High Dose MSCs |
Low Dose MSCs vs. Control |
High Dose MSCs vs. Control |
Low dose vs. High Dose MSCs |
|
| M1 macrophages | ----- | ----- | ----- | ↓ (LD) Ends, MCL | ----- | ↓ (LD) Ends, MCL |
| M2 macrophages | ----- | ↓ (HD) D/P HR | ----- | ----- | ↓ (HD) Ends | ----- |
| Endothelial cells | ----- | ----- | ↑ (LD) HR | ↓ (LD) Ends | ----- | ↓ (LD) D/P HR, MCL |
| Blood vessel lumen | ----- | ----- | ↑ (LD) HR | ↓ (LD) Ends, MCL | ----- | ----- |
| Proliferating cells | ----- | ----- | ----- | ↓ (LD) HR | ----- | ----- |
| Procollagen I | ----- | ----- | ↓ (LD) MCL | ----- | ----- | ----- |
| T lymphocytes | ----- | ----- | ----- | ----- | ----- | ----- |
| Collagen III | ----- | ----- | ----- | NT | NT | NT |
Abbreviations: ↓ - decreased, ↑ - increased, HD – high dose MSC group, LD – low dose MSC group, HR – healing region, D/P HR – distal and proximal healing region edges, Ends – distal and proximal ligament ends, MCL – medial collateral ligament (Ends+D/P HR+HR), ----- no significance, NT – not tested.
Figure 1.
A–C: At day 5 healing, there were decreased type 2 macrophages in the proximal and distal healing region of MCLs that received a high dose of MSCs compared to control ligaments (controls 344.7±48.2, high dose 67.7±38.0, p=.049). A: Representative image of IHC in control ligament. B: Representative image of IHC in high dose ligament. C: Graph comparing average cell number for each condition. D–F: At day 5 healing, there was decreased procollagen I in the ligament ends of the low dose MSC group compared to the high dose group (low dose 827.6±158.6, high dose 1333.3±66.8, p=.043). D: Representative image of IHC in low dose ligament. E: Representative image of IHC in high dose ligament. F: Graph comparing average cell number for each dose. G–I: At day 5 healing there were increased endothelial cells in the healing region of the low dose group compared to the high dose group (low dose 620.7±111.1, high dose 172.4±42.7, p=.02). G: Representative image of IHC in low dose ligament. H: Representative image of IHC in high dose ligament. I: Graph comparing average cell number for each dose. Values are expressed as mean cell numbers ± S.E.M.
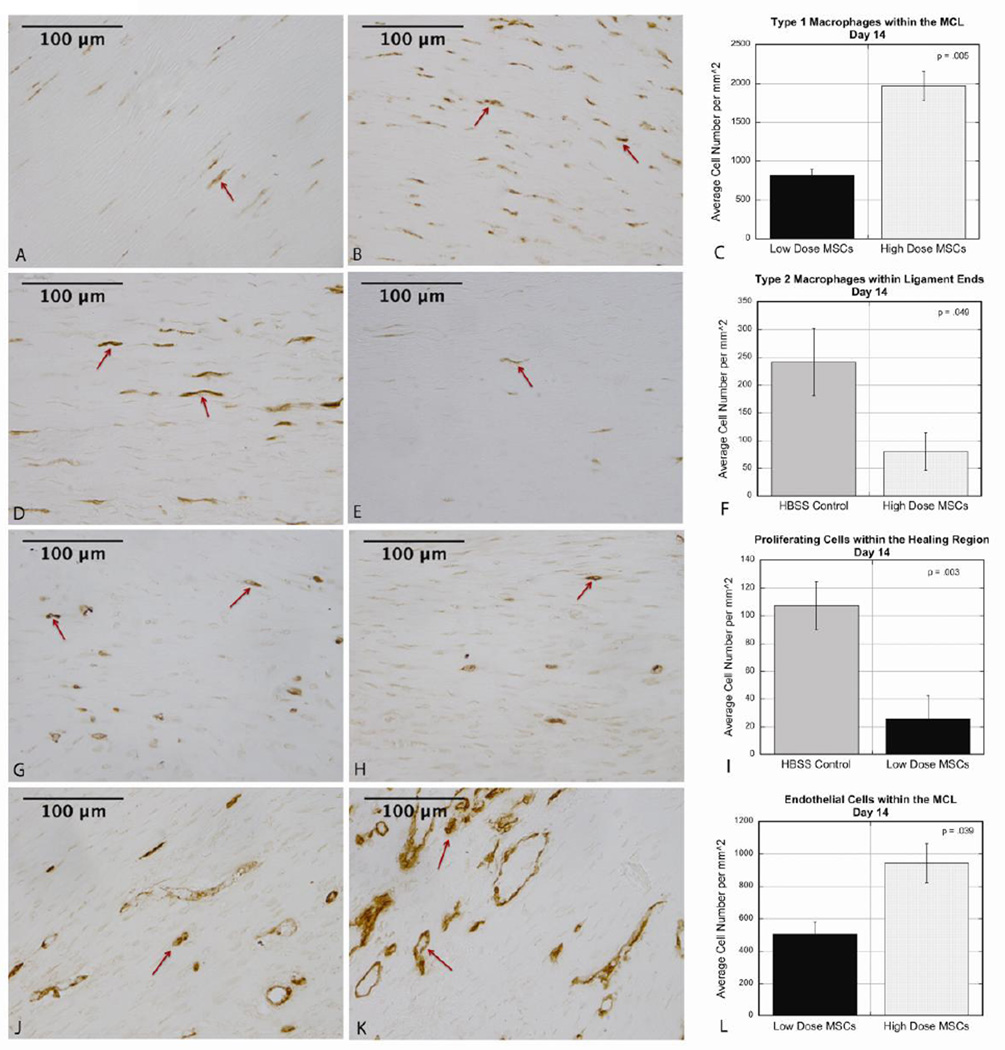
In contrast to day 5 healing, there were significant changes in both the low dose and high dose groups at day 14 compared to controls, along with significant changes between doses. Fewer type 1 macrophages (M1s) were found in the ends (p=.010) and throughout the MCL (p=.043) in the low dose ligaments compared to controls. Comparing doses showed a similar pattern with fewer M1s in low dose ligament ends (p=.002) and throughout the MCL (p=.005) compared to the high dose group (Fig 2 A–C). Similar to day 5, M2s were decreased in the proximal and distal ends (p=.049) in high dose ligaments compared to controls (Fig 2 D–F). Cellular proliferation was altered in the low dose MSC group marked by fewer proliferating cells in the healing region (p=.003) compared to the controls (Fig 2 G–I).
Figure 2.
A–C: At day 14 healing, there were fewer type 1 macrophages throughout the MCL in the low dose group compared to the high dose group (low dose 816.1±83.0, high dose 1965.5±186.0, p=.005). A: Representative image of IHC in low dose ligament. B: Representative image of IHC in high dose ligament. C: Graph comparing average cell number for each dose. D–F: At day 14 healing, there were fewer type 2 macrophages in the ligament ends of high dose ligaments compared to controls (controls 241.4±60.2, high dose 80.5±33.4, p=.049). D: Representative image of IHC in control ligament. E: Representative image of IHC in high dose ligament. F: Graph comparing average cell number for each condition. G–I: At day 14 healing, there were fewer proliferating cells in the healing region of low dose MCLs compared to controls (controls 107.2±17.3, low dose 25.5±16.9, p=.003). G: Representative image of IHC in control ligament. H: Representative image of IHC in low dose ligament. I: Graph comparing average cell number for each condition. J–L: At day 14 healing, there was decreased endothelialization throughout the MCL in the low dose group compared to the high dose group (low dose 505.7±73.4, high dose 942.5±121.0, p=.039). J: Representative image of IHC in low dose ligament. K: Representative image of IHC in high dose ligament. L: Graph comparing average cell number for each dose. Values are expressed as mean cell numbers ± S.E.M.
When comparing endothelial cells and lumen formation, there were fewer endothelial cells (p=.026) and blood vessel lumen (p=.044) in the ligament ends of the low dose MSC group compared to control ligaments. Significantly fewer lumen were observed throughout the low dose MCLs (p=.002) as a whole compared to controls. When comparing doses of MSCs at day 14, there were less endothelial cells in the distal and proximal healing region (p=.026) and throughout the MCL (p=.039) in the low dose group compared to the high dose group (Fig 2 J–L).
T-lymphocytes were analyzed at both time points due to the extensive research showing that MSCs modulate T lymphocyte proliferation and function (7,8,23–25). Regardless of treatment or time, there were few T cells found within the ligaments (data not shown), and therefore it was difficult to make meaningful comparisons based on such small numbers. Collagen III was analyzed at day 5 and yielded no difference between groups (data not shown).
MSC Localization and Morphological Measurements
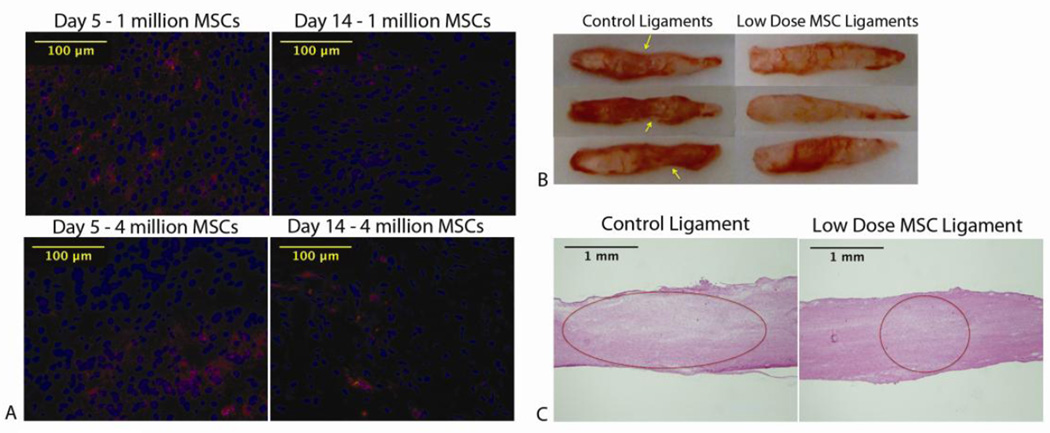
MSCs were detected in the healing region and healing region edges at days 5 and 14 in both dose groups using fluorescence microscopy (Fig 3 A).
Figure 3.
A: MSCs were detected at day 5 and day 14 in the healing region in both dose groups. MSCs were stained red using Celltracker CM-DiI. Dapi was used as a nuclear stain for total cells (blue). B: At day 14, there was increased narrowing in the healing region of control ligaments (indicated by arrows) compared to low dose ligaments. C: H+E stains at day 14 healing show a larger healing region in control ligaments compared to the low dose group (controls 10.4± .7%, low dose 8.4±1.2%, p=.049). Healing region measurements were taken using Image J and values expressed as mean percentage of total ligament area ± S.E.M.
At day 14, a noticeable decrease was noted in the length of the healing region in the low dose MSC group compared to the controls. The average length of the healing region in the MSC group was 1.4 ± .61mm compared to 2.5 ± .18mm (p=.003) in the control group. There was also narrowing in the healing region of control ligaments whereas the low dose MSC group’s granulation tissue looked congruent with the distal and proximal ligament ends (Fig 3 B).
H&E staining was performed to measure the area of the healing region for comparison. By calculating percentage of the healing region size, the low dose MSC group demonstrated a significantly smaller healing region (p=.049) when compared to the controls (Fig 3 C). This MSC group’s average healing region size was 8.4±1.2% of the total area of the ligament and the control averaged 10.4± .72%.
Mechanical Data
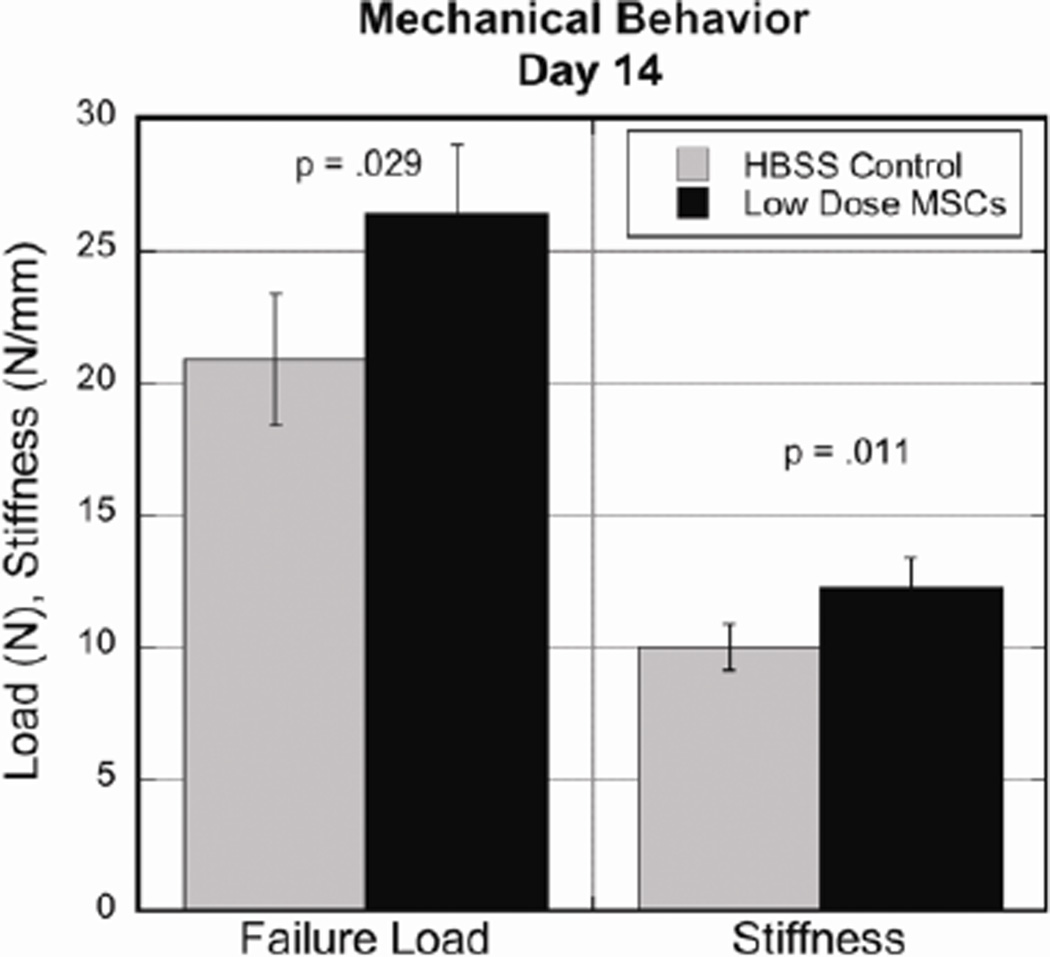
Ligament failure loads and stiffness were compared at day 14 of healing to assess functional improvements. Larger differences were seen in the low dose group compared to the control when analyzing these two properties. The low dose MSC group demonstrated increased strength with an average failure load of 26.41± 1.95N compared to 20.88± 2.64N in the control group (p=.029). Ligaments receiving the low dose of MSCs also showed increased stiffness with an average of 12.24± .95 N/mm compared to 10.01± 1.02 N/mm (p=.011) in the control ligaments (Fig 4).
Figure 4.
Day 14 comparison of mechanical properties showed low dose ligaments exhibiting increased failure load (controls 20.88± 2.64N, low dose 26.41± 1.95N, p=.029) and stiffness measurements (controls 10.01± 1.02 N/mm, low dose 12.24± .95 N/mm, p=.011). Values are expressed as mean ± S.E.M.
Cytokine Analysis
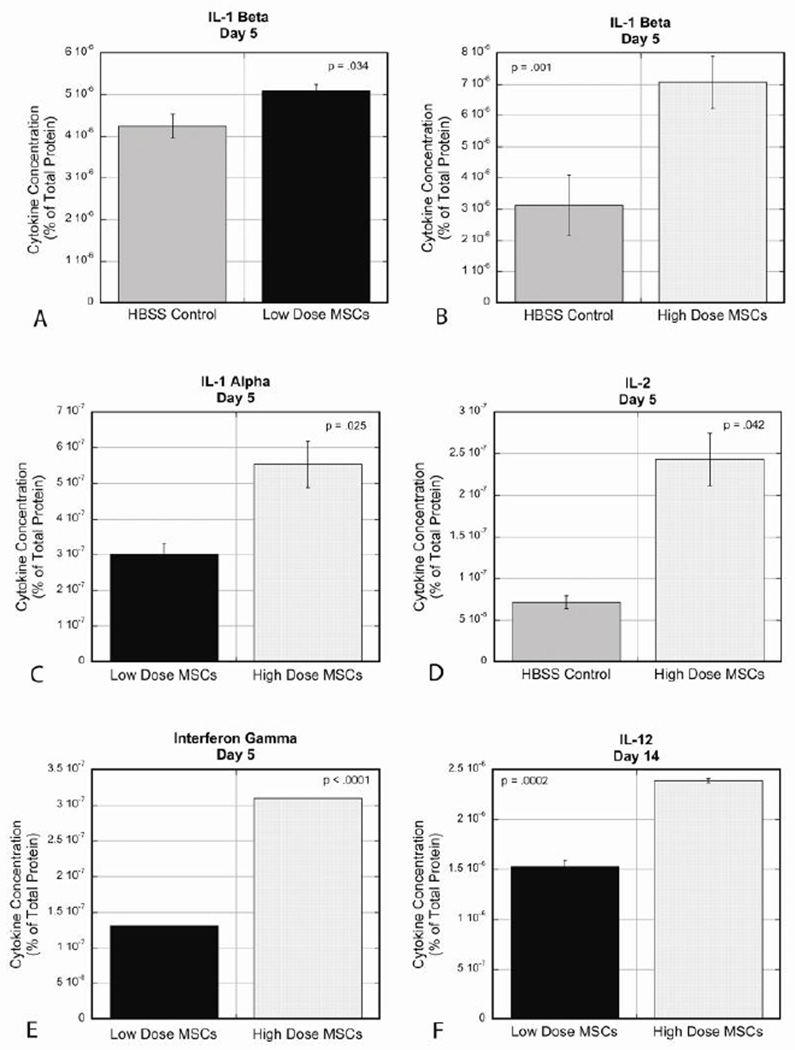
Significant changes in protein production were detected in 5 out of the 10 cytokines tested. Cytokine levels are expressed as a percentage of total protein within the ligament. At day 5, IL-1β (Fig 5A) was increased in the low dose group compared to the control (controls 4.25×10−06± 2.90×10−07% vs. low dose 5.10×10−06± 1.52×10−07 %, p=.034). The same pattern was seen in the high dose group compared to controls (Fig 5B) but demonstrated a larger magnitude of change (controls 3.13×10−06± 9.62×10−07% vs. high dose 7.07×10−06± 8.35×10−07%, p=.001). IL-1α (Fig 5C) was decreased in both MSC dose groups at day 5 compared to controls along with the low dose group expressing significantly less cytokine compared to the high dose (controls 1.99×10−06± 8.95×10−08% vs. low dose 3.02×10−07± 3.03×10−08%, p=.004; controls 8.52×10−07± 3.27×10−08% vs. high dose 5.53×10−07± 6.51×10−08%, p=.020, high dose vs. low dose, p=.025). IL-2 (Fig 5D) was increased in the high dose group compared to controls at day 5 (controls 7.13×10−08± 7.50×10−09% vs. high dose 2.43×10−07± 3.18×10−08%, p=.042). IFN-γ (Fig 5E) was also increased in the high dose group compared to controls, and when compared to the low dose group (controls below detection level = 0 vs. high dose 3.10×10−07%, p<.0001; high dose vs. low dose 1.31×10−07%, p<.0001). At day 14, the high dose had increased IL-12 (Fig 5F) production compared to controls and compared to the low dose group (controls 1.00×10−06± 2.88×10−08% vs. high dose 2.39×10−06± 2.00×10−08%, p=.0008; high dose vs. low dose 1.53×10−06± 5.95×10−08%, p=.0002). GM-CSF, IL-4, IL-6, IL-10, and TNFα were below detectable levels in all groups at both time points.
Figure 5.
Cytokine analysis at day 5 and day 14 showed significant changes in 5 cytokines: IL-1β, IL-1α, IL-2, IFNγ, and IL-12. No significant changes were found in levels of TNFα, GM-CSF, IL-4, IL-6, and IL-10. A: At day 5, IL-1β was increased in the low dose group compared to controls (controls 4.25×10−06± 2.90×10−07%, low dose 5.10×10−06± 1.52×10−07 %, p=.034). B: At day 5, IL-1β was also increased in the high dose group compared to controls (controls 3.13×10−06± 9.62×10−07%, high dose 7.07×10−06± 8.35×10−07%, p=.001). C: At day 5, IL-1α was decreased in both dose groups compared to controls, with the low dose having significantly less IL-1α compared to the high dose (low dose 3.02×10−07± 3.03×10−08%, high dose 5.53×10−07± 6.51×10−08%, p=.025). D: At day 5, IL-2 was increased in the high dose group compared to controls (controls 7.13×10−08± 7.50×10−09%, high dose 2.43×10−07± 3.18×10−08%, p=.042). E: At day 5, IFNγ was increased in both dose groups compared to controls, along with the high dose having significantly increased expression compared to the low dose (low dose 1.31×10−07%, high dose 3.10×10−07%, p<.0001). F: At day 14, there was increased expression of IL-12 in the high dose group compared to the low dose group (low dose 1.53×10−06± 5.95×10−08%, high dose 2.39×10−06± 2.00×10−08%, p=.0002).
Discussion
The inflammatory response to injury is a complicated cascade of interactions that vary temporally, spatially and in magnitude. It is a well-conserved process that ultimately results in more scar-like versus native tissue in ligaments. A better understanding of this process is necessary to identify therapeutic interventions that minimize scar formation and stimulate regeneration of native tissue.
MSCs demonstrated a positive healing effect when applied at an appropriate dose. This was shown by a smaller wound size and improved mechanical properties at day 14. Interestingly, the lower dose of 1×106 cells proved more successful than the higher dose of 4×106 cells at day 14, indicating the importance of dosage in cell therapy. This is in contrast to our hypothesis where we expected the high dose to be more optimal. Cytokine production and cellular composition at the times examined portray the higher dose of MSCs as promoting inflammation. This is evidenced by increased production of pro-inflammatory cytokines (IL-1β, IL-2, IFNγ) and decreased anti-inflammatory M2 macrophages in the high dose group compared to controls. The reason for this response is unknown, however, evidence is emerging showing that allogeneic MSCs can trigger an immune response in the host (26–30). Zangi et al. showed that allogeneic MSC survival upon transplantation was significantly shortened compared to syngeneic MSCs (27). Schu and colleagues found that rats receiving an intravenous injection of allogeneic MSCs formed alloantibodies leading to complement-mediated lysis (28). These studies exploring the effects of allogeneic MSCs along with a review paper by Gebler et al. (31) detail circumstances where MSCs have pro-inflammatory effects under specific conditions. In our study, the low dose of MSCs expressing a certain amount of foreign antigen may have been insufficient to trigger an immune response and the MSCs modulated healing in a beneficial manner. The high dose of MSCs may have triggered an inflammatory reaction that negated the improved healing seen in the low dose group. Support for this theory is evidenced by the increased pro-inflammatory cytokines detected at day 5 healing in the high dose group ligaments. However, a more direct measure, such as immune cell presence and alloantibodies in the animal serum, is necessary to confirm this theory and could be incorporated into future studies using allogeneic MSCs. These findings suggest that when using allogeneic MSCs for cell therapy, using an appropriate number of MSCs is essential to minimize host immune detection yet still be able to positively modulate the healing environment.
Past research performed in our laboratory on ligament healing showed that the wound size continues to expand with time due to remodeling (22). Remodeling that creeps beyond the injury site and progresses into the healthy section of the ligament is thought to be one of the factors that contribute to inferior mechanical properties after injury. Therefore, our results in the low dose MSC group demonstrating a smaller wound size and less creeping holds promise for stronger ligaments. Inflammatory cytokines, such as IL-1β, are known to activate matrix metalloproteinases (MMPs) in tendon and ligament leading to remodeling activity (32,33). The increased pro-inflammatory cytokines at day 5, including IL-1β, seen in the high dose MSC ligaments correlated with a larger healing region and active tissue seen later during healing. At day 14, more pro-inflammatory M1 macrophages were present throughout the MCL in the high dose group compared to control ligaments and the low dose group. This was consistent with increased IL-12, a cytokine characteristic of M1s (14,15) found in the high dose group. A prolonged M1 macrophage response can be indicative of chronic inflammation, which represents increased cellular activity and results in more scar tissue. Collectively, these cytokine and macrophage profiles, along with increased proliferating cells in the healing region, suggest that the healing response in the high dose group was still active, whereas the low dose ligaments were becoming more quiescent.
Previous research has reported the ability of MSCs to alter macrophage phenotype to be more anti-inflammatory (9–12). Our day 5 cellular and cytokine data did not show this same trend. We hypothesized there would be increased M2s and anti-inflammatory cytokines in the MSC treatment groups, with the higher dose of MSCs having higher levels compared to the low dose. Instead we found that the high dose of MSCs had significantly fewer M2s and increased pro-inflammatory cytokines both at day 5 and day 14. The differing results between our findings and previous reports may be due to in vivo versus in vitro experimental models and a potential immune reaction to larger amounts of foreign antigen present in the higher dose of MSCs. Collagen I and collagen III are the predominant extracellular matrix proteins that make up ligaments and tendons. Healthy ligaments and tendons consist mainly of collagen I, whereas injured structures have increased collagen III (34,35). Increased procollagen I at day 5 in the high dose group appeared to predict that these ligaments were on the path to regenerative healing. Surprisingly, this increased production did not lead to better healing at day 14 based on mechanical properties. Blood vessel formation is another marker used to analyze healing in ligaments. Some level of vessel formation is necessary to promote healing, however, excessive formation can negatively impact mechanical properties (36,37). At day 14, there were fewer endothelial cells and blood vessel lumen in the low dose MSC ligaments compared to the controls. This decrease in lumen formation correlated with improved mechanical properties in the low dose group and may have contributed to these outcomes.
Consistent with other reports, this study supports the potential therapeutic value of MSCs to enhance ligament healing. However, the precise mechanisms remain unclear. Inflammatory cytokines were not all down-regulated with either of our MSC therapy groups. Our study emphasizes that more MSCs is not necessarily better when using allogeneic MSCs and therefore dosage should be closely examined for each application. The high MSC dose had a measureable impact on early healing (day 5) in cellular and cytokine changes, which altered the course of healing and led to poorer outcomes. The low dose had fewer detectable changes during early healing, but resulted in improved functional mechanical outcomes (day 14). Dosage needs to be considered in each injury model since MSCs have the ability to alter the progression and final outcomes both positively and negatively during healing. There will likely be unique contributions from MSCs depending on the source of MSCs (including auto- versus allo-MSCs), amount of MSCs, animal model and tissue of interest. In summary, dose can affect the cellular response and cytokine expression during healing when used as a therapeutic intervention for ligament tears. Optimizing the regenerative response to accelerate healing and minimize re-injuries may prolong independence and mobility for people who experience ligament tears in a cost-effective manner.
Acknowledgements
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number AR059916. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Cover Letter with Assurances:
This letter is to confirm that the manuscript “Enhanced Medial Collateral Ligament Healing using Mesenchymal Stem Cells: Dosage Effects on Cellular Response and Cytokine Profile” has not been previously published and that no other submission or publication of the original work has been or will be made. Furthermore, each author meets the qualifications for authorship and has had the opportunity to read and comment upon the submitted manuscript.
The authors declare no potential conflicts of interest.
References
- 1.Frank C, McDonald D, Shrive N. Collagen fibril diameters in the rabbit medial collateral ligament scar: a longer term assessment. Connect Tissue Res. 1997;36(3):261–269. doi: 10.3109/03008209709160226. [DOI] [PubMed] [Google Scholar]
- 2.Frank C, McDonald D, Bray R, Rangayyan R, Chimich D, Shrive N. Collagen fibril diameters in the healing adult rabbit medial collateral ligament. Connect Tissue Res. 1992;27(4):251–263. doi: 10.3109/03008209209007000. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura N, Hart D, Boorman R, et al. Decorin antisense gene therapy improves functional healing of early rabbit ligament scar with enhanced collagen fibrillogenesis in vivo. J Orthop Res. 2000;18(4):517–523. doi: 10.1002/jor.1100180402. [DOI] [PubMed] [Google Scholar]
- 4.Caplan A. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213(2):341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 5.Caplan A. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 6.Caplan A. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11(7–8):1198–1211. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- 7.Groh M, Maitra B, Szekely E, Koc O. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp Hematol. 2005;33(8):928–934. doi: 10.1016/j.exphem.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Singer N, Caplan A. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol. 2011;6:457–478. doi: 10.1146/annurev-pathol-011110-130230. [DOI] [PubMed] [Google Scholar]
- 9.Prockop D, Oh J. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20(1):14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nemeth K, Leelahavanichkul A, Yuen P, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15(1):42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009;37(12):1445–1453. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maggini J, Mirkin G, Bognanni I, et al. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 2010;5(2):e9252. doi: 10.1371/journal.pone.0009252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Q, Su W, Shi S, et al. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28(10):1856–1868. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mosser D. The many faces of macrophage activation. J Leukoc Biol. 2003;73(2):209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 15.Martinez F, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 16.Gordon S, Martinez F. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Kanaya A, Deie M, Adachi N, Nishimori M, Yanada S, Ochi M. Intra-articular injection of mesenchymal stromal cells in partially torn anterior cruciate ligaments in a rat model. Arthroscopy. 2007;23(6):610–617. doi: 10.1016/j.arthro.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Chong A, Ang A, Goh J, et al. Bone marrow-derived mesenchymal stem cells influence early tendon-healing in a rabbit achilles tendon model. J Bone Joint Surg Am. 2007;89(1):74–81. doi: 10.2106/JBJS.E.01396. [DOI] [PubMed] [Google Scholar]
- 19.Awad H, Butler D, Boivin G, et al. Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Eng. 1999;5(3):267–277. doi: 10.1089/ten.1999.5.267. [DOI] [PubMed] [Google Scholar]
- 20.Lim J, Hui J, Li L, Thambyah A, Goh J, Lee E. Enhancement of tendon graft osteointegration using mesenchymal stem cells in a rabbit model of anterior cruciate ligament reconstruction. Arthroscopy. 2004;20(9):899–910. doi: 10.1016/j.arthro.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 21.Gulotta L, Kovacevic D, Ehteshami J, Dagher E, Packer J, Rodeo S. Application of bone marrow-derived mesenchymal stem cells in a rotator cuff repair model. Am J Sports Med. 2009;37(11):2126–2133. doi: 10.1177/0363546509339582. [DOI] [PubMed] [Google Scholar]
- 22.Chamberlain C, Crowley E, Vanderby R. The spatio-temporal dynamics of ligament healing. Wound Repair Regen. 2009;17(2):206–215. doi: 10.1111/j.1524-475X.2009.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Augello A, Tasso R, Negrini S, et al. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35(5):1482–1490. doi: 10.1002/eji.200425405. [DOI] [PubMed] [Google Scholar]
- 24.Lanz T, Opitz C, Ho P, et al. Mouse mesenchymal stem cells suppress antigen-specific Th-cell immunity independent of indoleamine 2,3-dioxygenase 1 (IDO1) Stem Cells Dev. 2010;19(5):657–668. doi: 10.1089/scd.2009.0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karlsson H, Samarasinghe S, Ball L, et al. Mesenchymal stem cells exert differential effects on alloantigen- and virus-specific T-cell responses. Blood. 2008;112(3):532–541. doi: 10.1182/blood-2007-10-119370. [DOI] [PubMed] [Google Scholar]
- 26.Griffin M, Ryan A, Alagesan S, Lohan P, Treacy O, Ritter T. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells: what have we learned so far? Immunol Cell Biol. 2013;91(1):40–51. doi: 10.1038/icb.2012.67. [DOI] [PubMed] [Google Scholar]
- 27.Zangi L, Margalit R, Reich-Zeliger S, et al. Direct Imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells. 2009;27(11):2865–2874. doi: 10.1002/stem.217. [DOI] [PubMed] [Google Scholar]
- 28.Schu S, Nosov M, O’Flynn L, et al. Immunogenicity of allogeneic mesenchymal stem cells. J Cell Mol Med. 2012;16(9):2094–2103. doi: 10.1111/j.1582-4934.2011.01509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Camp D, Loeffler D, Farrah D, Borneman J, LeWitt P. Cellular immune response to intrastriatally implanted allogeneic bone marrow stromal cells in a rat model of Parkinson’s disease. J Neuroinflammation. 2009;6:17. doi: 10.1186/1742-2094-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eliopoulos N, Stagg J, Lejeune L, Pommey S, Galipeau J. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood. 2005;106(13):4057–4065. doi: 10.1182/blood-2005-03-1004. [DOI] [PubMed] [Google Scholar]
- 31.Gebler A, Zabel O, Seliger B. The immunomodulatory capacity of mesenchymal stem cells. Trends Mol Med. 2012;18(2):128–134. doi: 10.1016/j.molmed.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Xie J, Wang C, Yin L, Xu C, Zhang Y, Sung K. Interleukin-1 beta influences on lysyl oxidases and matrix metalloproteinases profile of injured anterior cruciate ligament and medial collateral ligament fibroblasts. Int Orthop. 2013;37(3):495–505. doi: 10.1007/s00264-012-1549-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Tang Z, Xue R. Combined effects of TNF-α, IL-1β, and HIF-1α on MMP-2 production in ACL fibroblasts under mechanical stretch: an in vitro study. J Orthop Res. 2011;29(7):1008–1014. doi: 10.1002/jor.21349. [DOI] [PubMed] [Google Scholar]
- 34.Riley G, Harrall R, Constant C, Chard M, Cawston T, Hazleman B. Tendon degeneration and chronic shoulder pain: changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Ann Rheum Dis. 1994;53(6):359–366. doi: 10.1136/ard.53.6.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am. 2005;87(1):187–202. doi: 10.2106/JBJS.D.01850. [DOI] [PubMed] [Google Scholar]
- 36.Sahin H, Tholema N, Petersen W, Raschke M, Stange R. Impaired biomechanical properties correlate with neoangiogenesis as well as VEGF and MMP-3 expression during rat patellar tendon healing. J Orthop Res. 2012;30(12):1952–1957. doi: 10.1002/jor.22147. [DOI] [PubMed] [Google Scholar]
- 37.Peterson W, Pufe T, Pfrommer S, Tillmann B. Overload damage to the achilles tendon: the importance of vascularization and angiogenesis. Orthopade. 2005;34(6):533–542. doi: 10.1007/s00132-005-0808-7. [DOI] [PubMed] [Google Scholar]