Abstract
Drug-induced liver injury (DILI) is considered to be a diagnosis of exclusion. Liver biopsy may contribute to diagnostic accuracy, but the histological features of DILI and their relationship to biochemical parameters and outcomes are not well defined. We have classified the pathological pattern of liver injury and systematically evaluated histological changes in liver biopsies obtained from 249 patients with suspected DILI enrolled in the prospective, observational study conducted by the Drug Induced Liver Injury Network. Histological features were analyzed for their frequency within different clinical phenotypes of liver injury and to identify associations between clinical and laboratory findings and histological features. The most common histological patterns were acute (21%) and chronic hepatitis (14%), acute (9%) and chronic cholestasis (10%), and cholestatic hepatitis (29%). Liver histology from 128 patients presenting with hepatocellular injury had more severe inflammation, necrosis, and apoptosis and more frequently demonstrated lobular disarray, rosette formation, and hemorrhage than those with cholestasis. Conversely, histology of the 73 patients with cholestatic injury more often demonstrated bile plugs and duct paucity. Severe or fatal hepatic injury in 46 patients was associated with higher degrees of necrosis, fibrosis stage, microvesicular steatosis, and ductular reaction among other findings, whereas eosinophils and granulomas were found more often in those with milder injury. Conclusion: We describe an approach for evaluating liver histology in DILI and demonstrate numerous associations between pathological findings and clinical presentations that may serve as a foundation for future studies correlating DILI pathology with its causality and outcome. (Hepatology 2014;59:661–670)
Drug-induced liver injury (DILI) is an important cause of liver disease with significant morbidity and mortality.1,2 Accurate and early diagnosis is important, but challenging.3,4 There are no specific markers or diagnostic tests for DILI, and the diagnosis is by exclusion.5 In many instances, liver histology is considered helpful in strengthening the diagnosis or excluding other potential causes of liver injury. A key aspect of this evaluation is careful classification of the pattern of injury and a detailed description of the histological findings,6,7 yet there are limited examples of the application of this method across the spectrum of histological injury found in DILI. The Drug-Induced Liver Injury Network (DILIN) is an ongoing, multicenter observational study of consecutive cases of DILI enrolled at eight geographically distributed academic medical centers in the United States.8 The central aims of the DILIN are to more fully characterize the clinical syndromes of liver injury caused by prescription and nonprescription medications, and herbals and dietary supplements (HDS), standardize terminology and grading systems, and provide resources for mechanistic studies of DILI. For these purposes, carefully accrued clinical cases of suspected DILI are enrolled and followed longitudinally. Each case is reviewed by a causality committee using a formal adjudication process.9
In this study, liver biopsies taken at the time of presentation as part of a diagnostic assessment provided an opportunity to systematically assess the patterns of injury across this diverse patient population. In this article, we present this standardized method of evaluation and classification of histological features and demonstrate the most frequent associations among histological findings, laboratory data, clinical phenotype, and injury severity at presentation.
Patients and Methods
Patients with suspected DILI were enrolled in the DILIN Prospective Study, and data were collected as previously described.5 Patients or their next of kin provided written informed consent, which included pathology review. If available, up to 10 unstained recuts of liver tissue (needle or wedge biopsies, explanted native livers, and autopsies) obtained at investigator discretion were sent to the central pathology core laboratory (National Cancer Institute, Bethesda, MD) for repeat staining and storage. Slides were stained with hematoxylin and eosin, Masson's trichrome, reticulin, iron, copper, and periodic acid-Schiff with diastase. Biopsies were reviewed by the central hepatic pathologist (D.E.K.), who was blinded to all clinical information including the name(s) of the implicated drugs. All liver tissues received the same predefined structured histological evaluation. This systematic evaluation of 48 separate histologic features was divided into seven broad categories: inflammation; necrosis/cell injury; fibrosis; steatosis; cholestasis; vascular injury; and other findings, including evaluation of special stains (Supporting Table 1).10 The number of portal areas (complete and partial) was recorded as a measure of biopsy adequacy. The diagnostic classification used (Supporting Table 2) was based on published descriptions of pathologic changes in DILI.11,12 Standard hepatopathological diagnostic criteria13 were used to define patterns of injury. Overall injury pattern was classified into 1 of 18 patterns: acute hepatitis; chronic hepatitis; acute cholestasis; chronic cholestasis; cholestatic hepatitis (mixed hepatocellular and cholestatic injury); granulomatous changes; steatosis (macrovesicular or microvesicular); steatohepatitis; coagulative/confluent necrosis (zonal or nonzonal); massive/submassive necrosis; vascular injury; hepatocellular alteration; nodular regenerative hyperplasia; mixed or otherwise unclassifiable injury; minimal nonspecific changes, and absolutely normal.
For the purposes of the current analysis, a biopsy was eligible for inclusion if it was obtained within 6 months of the protocol-defined DILI onset date and was adequate, in the pathologist's opinion, to assign a pattern of injury. Tissues from explants or autopsies were excluded. If two biopsies qualified from the same patient, the larger biopsy was used. Once the biopsy data from blinded review were recorded, additional information was abstracted from the DILIN prospective database, including patient age, sex, and laboratory data (alanine aminotransferase [ALT], alkaline phosphatase [ALP], and total bilirubin) at the time of DILI onset and at or around the time of liver biopsy (within 7 days). The biochemical injury pattern (hepatocellular, mixed, or cholestatic) was calculated as the ratio (R) of ALT to ALP normalized by their respective upper limits of normal (ULN) from laboratory data at the time of onset. If a suspected case had undergone causality determination,9 then the causality score, severity score, and implicated medications were also obtained from the database.
Statistical Analysis
Chi-square and Fisher's exact tests were used to compare nominal variables. Mann-Whitney's U test was used to evaluate continuous variables stratified by a nominal variable.
Results
Description of Population
From September 2004 through October 2010, 249 patients with suspected DILI underwent a liver biopsy that was made available, reviewed centrally, and entered into the DILIN Prospective Study database (Table1). Mean age of patients in this cohort was 48 years (range, 7-87), and 144 (58%) were female. Biopsies contained a mean of 16.6 portal areas (median, 13) and generally were adequate to perform histological evaluations. Mean time from DILI onset to liver biopsy was 25 days (median, 14; interquartile range: 29). Causality evaluation was completed on 212 (84%) cases, with a final assessment of definite, highly likely, or probable in 174 (70%).9 Of these cases, 117 (67%) had a single agent implicated, whereas the rest had multiple implicated drugs or HDS products. Comparison of histological findings between cases adjudicated as probable to definite and those adjudicated as unlikely or possible showed few differences. Biopsies from the probable to definite group were more likely to have increased eosinophils (45% vs. 26%; P = 0.04), less likely to show ductular reaction (33% vs. 53%; P = 0.04), and had less hepatocellular iron accumulation (P = 0.0008).
Table 1.
Characteristics of 249 Patients With Suspected DILI Undergoing Biopsy
| N (%) | |
|---|---|
| Female | 144 (57.8) |
| Age, mean (range) | 48 (7-87) |
| Agents implicated | |
| Single agent | 172 (69.1) |
| Two agents | 56 (22.5) |
| ≥Three agents | 21 (8.4) |
| Most common agents implicated | |
| Amoxicillin/clavulanate | 19 (7.6) |
| Nitrofurantoin | 11 (4.4) |
| Sulfamethoxazole/trimethoprim | 9 (3.6) |
| Minocycline | 8 (3.2) |
| Ciprofloxacin | 7 (2.8) |
| Anabolic agents | 6 (2.4) |
| Azithromycin | 5 (2) |
| Levofloxacin | 5 (2) |
| Causality process completed | 208 (83.5) |
| Definite | 53 (21.3) |
| Very likely | 84 (33.7) |
| Probable | 33 (13.3) |
| Possible | 24 (9.6) |
| Unlikely | 14 (5.6) |
| DILIN severity score | |
| Mild | 40 (16.1) |
| Moderate | 39 (15.7) |
| Moderate-hospitalized | 83 (33.3) |
| Severe | 31 (12.5) |
| Fatal/transplanted | 15 (6.0) |
Frequency of individual histological findings is shown in Table2 and Supporting Table3. Inflammation and cholestasis were common. Interface hepatitis was noted in 91%, spotty lobular inflammation was noted in 99%, and some degree of cholestasis in 50%. Apoptosis and confluent necrosis were also frequently noted, with apoptotic bodies in 77% and areas of confluent necrosis in 25% of cases. Although almost all of the predefined characteristics were used, no case showed ductal cholestasis or peliosis. No cases were classified as having macrovesicular steatosis or nonzonal necrosis as the dominant pathology pattern of injury, although both were noted as components of other patterns of injury.
Table 2.
Distribution of Selected Histological Findings According to Biochemical Classification at Onset of Suspected DILI
| Biochemical Presentation |
|||||
|---|---|---|---|---|---|
| Feature | All Cases | Hepatocellular | Mixed | Cholestatic | P Value |
| N | 249 | 128 | 48 | 73 | |
| Interface hepatitis, mean | 2.21 | 2.63 | 2.17 | 1.5 | <0.0001 |
| Lobular inflammation, mean | 3.00 | 3.29 | 2.81 | 2.62 | <0.0001 |
| Portal inflammation, mean | 1.66 | 1.87 | 1.58 | 1.36 | 0.0080 |
| Confluent necrosis, mean | 1.11 | 1.71 | 0.67 | 0.36 | 0.0003 |
| Plasma cells | 56 (23) | 40 (32) | 7 (15) | 9 (12) | 0.0020 |
| Eosinophils | 94 (38) | 60 (48) | 17 (35) | 17 (23) | 0.0030 |
| Lipogranulomas | 47 (19) | 16 (13) | 16 (33) | 15 (21) | 0.0080 |
| Apoptosis | |||||
| None | 56 (23) | 15 (12) | 15 (31) | 26 (36) | <0.0001 |
| <1 per 40× HPF | 121 (49) | 52 (41) | 26 (54) | 43 (59) | |
| 1-3 per 40× HPF | 57 (23) | 47 (37) | 6 (13) | 4 (5) | |
| >3 per 40× HPF | 13 (5) | 12 (10) | 1 (2) | 0 (0) | |
| Degree of necrosis | |||||
| None | 186 (75) | 83 (65) | 41 (87) | 62 (85) | 0.0070 |
| <5% | 23 (9) | 14 (11) | 1 (2) | 8 (11) | |
| 5%-33% | 20 (8) | 16 (13) | 1 (2) | 3 (4) | |
| 33%-67% | 13 (5) | 10 (8) | 3 (6) | 0 (0) | |
| >67% | 5 (2) | 4 (3) | 1 (2) | 0 (0) | |
| Hepatocyte rosettes | 76 (31) | 57 (45) | 8 (17) | 11 (15) | <0.0001 |
| Lobular disarray | 46 (19) | 42 (33) | 2 (4) | 2 (3) | <0.0001 |
| Fibrosis stage, mean | 1.31 | 1.38 | 1.40 | 1.24 | 0.3500 |
| Cholestasis grade, mean | 1.71 | 0.68 | 1.08 | 1.53 | <0.0001 |
| Hepatocellular cholestasis | 112 (45) | 41 (32) | 23 (48) | 48 (66) | <0.0001 |
| Canalicular cholestasis | 120 (48) | 50 (39) | 23 (48) | 47 (64) | 0.0030 |
| Ductal paucity | |||||
| None | 231 (95) | 123 (98) | 45 (96) | 63 (89) | 0.0200 |
| Mild | 7 (3) | 0 (0) | 1 (2) | 6 (8) | |
| Moderate to marked | 6 (2) | 3 (2) | 1 (2) | 2 (3) | |
| Portal venopathy | 10 (4) | 1 (1) | 2 (5) | 7 (10) | 0.0080 |
| Hemorrhage | 25 (10) | 21 (17) | 2 (4) | 2 (3) | 0.0030 |
| Sinusoidal dilation (moderate to marked) | 12 (5) | 5 (4) | 0 (0) | 7 (10) | 0.0400 |
| Nodular transformation | 10 (4) | 2 (2) | 0 (0) | 8 (11.6) | 0.0010 |
Data are shown as N (%) or as mean values. Means of scores are shown only for simplicity. Chi-square analysis was used to determine significance of variation across the three biochemical presentations.
Abbreviation: HPF, high power field.
Table 3.
Variation in Selected Histopathologic Features Between the Most Common Injury Patterns
| Acute Hepatitic | Chronic Hepatitic | Acute Cholestatic | Chronic Cholestatic | Cholestatic Hepatitic | |
|---|---|---|---|---|---|
| N | 51 | 35 | 23 | 25 | 74 |
| Feature | |||||
| Interface hepatitis, mean | 3.14 | 2.69 | 0.61 | 1.88 | 2.22 |
| Lobular inflammation, mean | 3.73 | 3.00 | 1.65 | 2.48 | 3.38 |
| Portal inflammation, mean | 2.14 | 2.14 | 0.74 | 1.52 | 1.60 |
| Confluent necrosis, mean | 2.08 | 1.17 | 0.09 | 0.36 | 1.00 |
| Plasma cells | 18 (35) | 13 (37) | 0 (0) | 3 (12) | 18 (25) |
| Neutrophils | 16 (31) | 2 (6) | 3 (13) | 6 (24) | 31 (43) |
| Lymphoid aggregates or germinal centers | 7 (14) | 11 (31) | 0 (0) | 2 (8) | 5 (7) |
| Apoptosis | 1.78 | 1.0 | 0.61 | 0.80 | 1.07 |
| Degree of necrosis, mean | 0.84 | 0.37 | 0.05 | 0.12 | 0.38 |
| Hepatocyte rosettes | 27 (54) | 12 (34) | 0 (0) | 5 (20) | 23 (32) |
| Lobular disarray | 29 (58) | 1 (3) | 0 (0) | 0 (0) | 16 (22) |
| Fibrosis stage, mean | 0.94 | 2.31 | 0.59 | 1.52 | 1.11 |
| Grade of cholestasis, mean | 0.04 | 0 | 2.09 | 0.84 | 2.07 |
| Hepatocellular cholestasis | 0 (0) | 0 (0) | 22 (96) | 8 (32) | 68 (92) |
| Canalicular cholestasis | 3 (6) | 0 (0) | 21 (91) | 8 (32) | 74 (100) |
| Cholate-stasis | 4 (8) | 2 (6) | 4 (17) | 23 (92) | 22 (30) |
| Duct injury, multiple ducts | 20 (40) | 8 (24) | 6 (27) | 18 (78) | 38 (52) |
| Ductal paucity, mild or worse | 0 (0) | 0 (0) | 3 (14) | 8 (32) | 2 (3) |
| Portal venopathy | 1 (2) | 0 (0) | 0 (0) | 4 (17) | 0 (0) |
| Copper | 0.10 | 0.12 | 0.09 | 0.74 | 0.13 |
| Patterns of DILI with particular agents (only one agent implicated) | |||||
| Amoxicillin/clavulanate | 0 | 0 | 3 | 3 | 9 |
| Nitrofurantoin | 2 | 4 | 0 | 2 | 1 |
| Minocycline | 3 | 2 | 0 | 0 | 2 |
| Sulfamethoxazole/trimethoprim | 1 | 0 | 1 | 0 | 4 |
Data are shown as N (%) or as mean values. Means of scores are shown only for simplicity.
The most common patterns of injury were those defined by primary features of either inflammation or cholestasis or both—the first five diagnostic patterns of injury, acute and chronic hepatitis, acute and chronic cholestasis, and cholestatic hepatitis, were used to classify 83% of cases (Figs. 1A and 2). Variation in common histological features among the different general patterns of injury is shown in Table3. Cases that were characterized as acute hepatitis had the most severe inflammation and were more likely to have confluent necrosis and apoptosis. In contrast, acute cholestatic cases showed the mildest degree of these features. Among the three cholestatic patterns, cholestatic hepatitis cases had a similar degree of bile stasis as the acute cholestatic cases, but more inflammation and hepatocellular injury, representing an overlap between the two distinct histologic patterns. Chronic cholestatic cases were more likely to have duct injury, duct paucity, cholate stasis, and copper accumulation. A mild degree of fibrosis was present in many cases, but most pronounced in those that were classified as chronic hepatitis. Table3 also shows patterns of injury observed for selected agents where only a single agent was identified as the cause of the injury.
Figure 1.
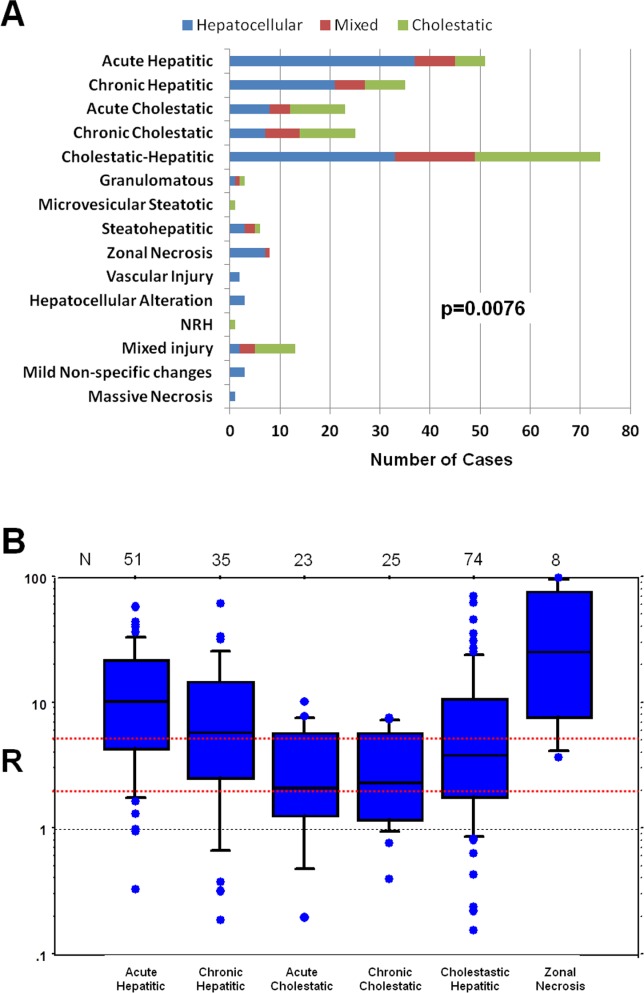
Relationship between pathological injury patterns and biochemical presentation. (A) The association of particular patterns with biochemical presentation was significant by chi-square analysis. (B) box plot showing range of “R” for selected histological patterns of injury. “R” is defined as the normalized ratio of ALT to ALP. The number of cases of each pattern of injury is shown above each box plot. Red dotted lines indicate the dividing point between hepatocellular (R > 5), mixed (R = 2-5), and cholestatic (R < 2) reactions.
Figure 2.
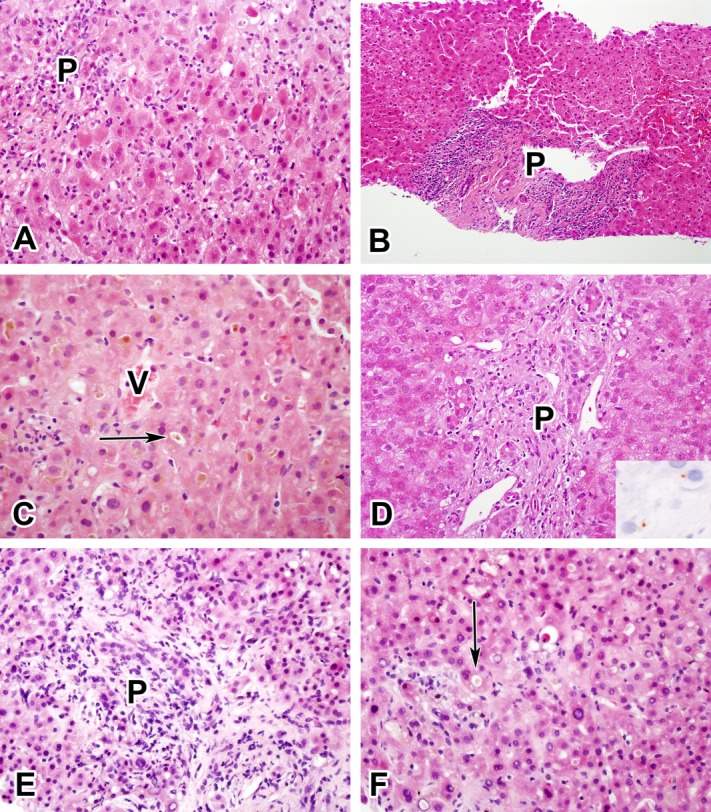
Examples of the five most common injury patterns. (A) Acute hepatitic injury resulting from ciprofloxacin. (B) Chronic hepatitic injury resulting from isoniazid. (C) Acute cholestatic injury resulting from an anabolic steroid. (D) Chronic cholestatic injury resulting from amoxicillin-clavulanate (inset shows positive copper stain). (E and F) Cholestatic hepatitic injury resulting from duloxetine. For orientation, P indicates portal area, V indicates central vein, and arrows indicate canalicular cholestasis.
Relationship of Findings to Biochemical Injury Classification
Suspected DILI was classified based upon the value of the ratio (R) of ALT to ALP levels (expressed as multiples of the ULN) obtained at the onset of injury.14 Hepatocellular injury was defined as an R ratio >5, cholestatic injury an R <2, and “mixed” cholestatic-hepatocellular injury as an R ratio between 2 and 5. This terminology was used regardless of the presence of jaundice. The ability of the R ratio to predict histological findings and patterns of injury has not been rigorously analyzed. Distribution of histological findings stratified by R ratios calculated from initial laboratory results are shown in Fig. 1 and Table2. Many correlations were evident. Patients with hepatocellular injury tended to have more inflammation, more necrosis, and more apoptosis. Inflammation was assessed using the Ishak scale,15 because of its familiarity, and all four subscales (interface hepatitis, lobular inflammation, portal inflammation, and confluent necrosis) showed higher scores in hepatocellular injury cases. Plasma cells and eosinophils were usually located in portal areas and were more often increased in hepatocellular injury. When present, confluent necrosis usually involved zone 3, with more severe manifestations showing bridging or multiacinar necrosis. Other changes associated with hepatocellular injury included hepatocyte rosette formation, lobular disarray, and hemorrhage, all features observed in cases of severe parenchymal injury. In contrast, patients with cholestatic biochemical injury tended to have canalicular and hepatocellular cholestasis in zone 3 and the degree of cholestasis was more severe. Ductal paucity, portal venopathy, sinusoidal dilation, and nodular regenerative hyperplastic changes were also more common in cholestatic injury. Distribution of histological changes in mixed injury was more similar to that of cholestatic than hepatocellular injury. With respect to injury pattern, patients with hepatocellular injury were more likely to have acute or chronic hepatitic changes on biopsy and less likely to have acute or chronic cholestasis. Cases of cholestatic hepatitis were nearly evenly distributed between hepatocellular, mixed, and cholestatic injury, reflecting the wide variation in the degree of hepatitis and cholestasis in this category.
The range of R ratios at the onset of injury for selected histologic patterns is displayed in Fig. 1B. Although the range varied between these pathologic patterns of injury, there was also significant overlap.
Relationship of Histology to Laboratory Data at the Time of Biopsy
To better understand the relationship of histological findings to common serum biochemical markers, we compared the histological findings to laboratory data obtained at, or close to, the time of liver biopsy, limiting the comparison to biochemical data collected within 7 days of biopsy. To simplify the analysis, laboratory data were dichotomized, using 5 times the ULN as the breakpoint for ALT, 3 times the ULN for ALP, and 3 mg/dL for total bilirubin. The significant associations are shown in Supporting Table4; associations with P > 0.05 are not shown. Histological features associated with ALT levels >5 times the ULN were similar to those associated with hepatocellular injury. Elevated ALP was associated with some, but not all, of the cholestatic changes. Specifically, the degree of cholestasis was worse and there were more cases of cholate stasis, duct injury, and duct paucity. Almost all (38 of 40) of the cases in which microvesicular steatosis was observed had low ALP levels as did all of the cases with hepatocellular inclusions, which were mostly megamitochondria. Changes of inflammation and necrosis were not associated with ALP levels.
Table 4.
Range of ALT and ALP by Injury Pattern
| Labs at Biopsy |
Zimmerman† |
|||
|---|---|---|---|---|
| Injury Pattern | ALT/ULN* (×) | ALP/ULN* (×) | ALT/ULN (×) | ALP/ULN (×) |
| Acute hepatitic | 12.8-27.4 | 1.0-2.9 | 10-100 | 1-3 |
| Chronic hepatitic | 3.4-9.5 | 0.9-2.3 | 3-50 | 1-3 |
| Acute cholestatic | 2.1-10.0 | 1.3-3.8 | 1-5 | 1-3 |
| Chronic cholestatic | 2.5-11.5 | 2.2-8.4 | 1-5 | 3-20 |
| Cholestatic hepatitic | 1.7-13.1 | 1.2-3.2 | 1-10 | >3 |
| Necrosis (zonal) | 6.4-46.7 | 0.9-1.7 | 10-1,000 | 1-3 |
Ranges represent 25th to 75th percentile of the laboratory data obtained at the time of liver biopsy, with ALT and ALP normalized to the local laboratory ULN.
Ranges adapted from Tables 4.25 and 4.26 on p. 103 of a previous work.9
In Table4, these biochemical-histological associations were compared with the similar associations described by Hyman Zimmerman in his classic textbook on DILI,11 which were based upon his clinical experience reviewing large numbers of cases both clinically and histologically. There was good correspondence, although, for acute hepatitis and zonal necrosis, our range of ALT did not extend as high, probably because the DILIN's exclusion of patients with acetaminophen injury and relatively few patients with acute liver failure.
Relationship of Histological Findings to Clinical Severity
Each case in the DILIN prospective study was assigned a score ranging from 1 to 5 for clinical severity level based on clinical and laboratory findings.1 Mild cases (1+) were defined as serum enzyme elevations without jaundice (total bilirubin <2.5 mg/dL); moderate cases (2+) had jaundice (total bilirubin >2.5 mg/dL) and were not hospitalized for the liver injury; moderately severe cases (3+) had jaundice and were hospitalized; severe cases (4+) had jaundice and evidence of hepatic failure (any combination of prolongation of international normalized ratio, ascites, or encephalopathy) or other organ failure (renal, bone marrow, or lung); and fatal cases (5+) were those who died as a result of hepatic failure or underwent liver transplantation (LT) within 6 months of onset. Associations between severity scores and histological features are shown in Table5. Degree of necrosis, fibrosis stage, microvesicular steatosis, panacinar steatosis, cholangiolar cholestasis, ductular reaction, neutrophils, and portal venopathy were all associated with higher scores of severity (Fig. 3A-C). Granulomas and eosinophils were more likely to be noted in milder cases (Fig. 3D). Fatal outcome was more likely in patients with an injury pattern of zonal necrosis (13% vs. 3%) or with mixed injury (27% vs. 3%; P = 0.019).
Table 5.
Significant Associations of Histological Findings With Outcome
| Severe or Fatal |
Fatal Outcome |
|||||
|---|---|---|---|---|---|---|
| Feature | No | Yes | P Value* | No | Yes | P Value* |
| Total N | 162 | 46 | 193 | 15 | ||
| Granulomas | ||||||
| None | 57 (35) | 28 (62) | 0.0010 | 74 (39) | 11 (73) | 0.0300 |
| Microgranulomas | 97 (60) | 13 (29) | 106 (55) | 4 (27) | ||
| Epith granulomas | 8 (5) | 4 (9) | 12 (6) | 0 (0) | ||
| Eosinophils | 76 (47) | 10 (22) | 0.0030 | 84 (44) | 2 (13) | 0.0300 |
| Neutrophils | 43 (27) | 22 (49) | 0.0060 | |||
| Degree of necrosis, mean | 0.36 | 0.89 | 0.0100 | 0.43 | 1.13 | 0.0200 |
| Fibrosis stage, mean | 1.15 | 1.73 | 0.0030 | 1.18 | 2.53 | <0.0001 |
| Type of steatosis | ||||||
| Macrovesicular | 84 (82) | 12 (39) | <0.0001 | 95 (77) | 1 (9) | <0.0001 |
| Mixed | 9 (9) | 10 (32) | 14 (11) | 5 (46) | ||
| Microvesicular | 10 (10) | 9 (29) | 14 (11) | 5 (46) | ||
| Cholangiolar cholestasis | 2 (1) | 6 (13) | 0.0020 | 5 (3) | 3 (20) | 0.0100 |
| Ductular reaction | 52 (32) | 24 (53) | 0.0100 | 65 (34) | 15 (73) | 0.0040 |
| Portal venopathy | 4 (2) | 2 (17) | 0.0400 | |||
Data are shown as either mean score or N (%). Means of scores are shown only for simplicity.
Statistical significance of differences was evaluated using chi-square test or Fisher's exact test for 2 × 2 comparisons.
Figure 3.
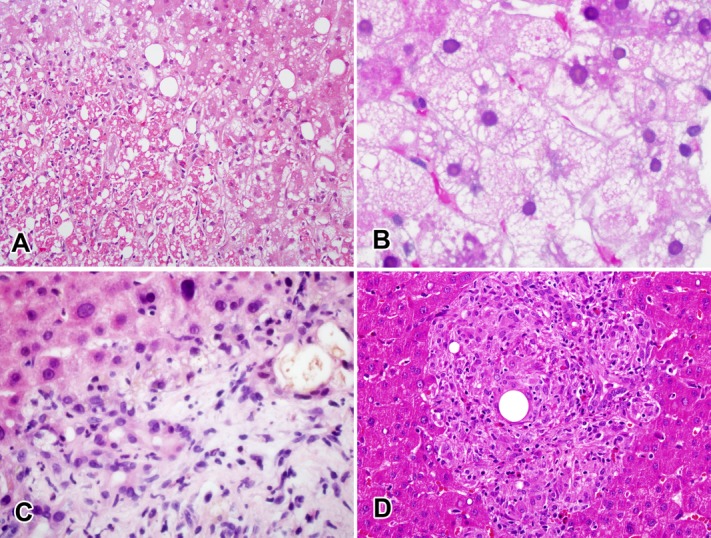
Histological features associated with outcome. (A) Zone 3 coagulative necrosis in a patient with severe injury resulting from duloxetine. (B) Microvesicular steatosis in a patient with fatal injury probably resulting from erythromycin. (C) Ductular reaction, cholangiolar cholestasis, and neutrophilic infiltration in a patient with severe injury resulting from duloxetine. (D) Granulomatous and eosinophilic inflammation in a patient with moderate (but hospitalized) injury probably resulting from atenolol.
Discussion
We and others6,7 have argued that when pathologists review and report on DILI, they should classify the changes as a particular pattern of pathological injury. Pattern classification is critical to define the pathological differential diagnosis, which can be used in conjunction with the clinical evaluation to establish the etiology of the injury. For the purposes of DILIN, we predefined 18 histological patterns that were likely to be encountered given the kinds of patients and drug etiologies that we expected to accrue. We did not include tumors in the classification, despite well-described associations between drugs and tumors. Although the clinical cases of DILI in this cohort had a great diversity of findings, nearly 83% of our 249 cases could be classified into one of five patterns: acute hepatitis; chronic hepatitis; acute cholestasis; chronic cholestasis; and cholestatic hepatitis. This distribution reflects both the character of our patient population and the injury patterns associated with the most commonly implicated drugs, particularly antibiotics, psychoactive agents, and drugs used to treat musculoskeletal disorders.5 Other populations with different types of drug injury may display different frequencies of the various injury patterns, and so we would recommend that pathologists use the full spectrum of possible patterns when reporting on DILI. Despite the variety of injury patterns observed, there was good correlation between biochemical tests at the time of biopsy and individual histological findings. These associations serve to validate the use of systematic histological scoring methods across the range of pathology that is observed in suspected DILI.
One of the standard definitions used in the study of DILI is the division of cases cholestatic, mixed, and hepatocellular injury using results of commonly used biochemical tests, based upon the normalized ratio of ALT to ALP, sometimes referred to as the “R” value. These definitions are used based upon the R ratio determined at or near the time of presentation, although the ratio may change markedly over time as enzyme levels rise and fall. The current analysis showed that there was a limited correlation between the biochemical categorization and the pathological pattern of injury. For example, a patient with hepatocellular injury might show any of a number of histological patterns on biopsy, including acute cholestasis. Likewise, an injury that would be classified as cholestatic on biochemical grounds could show acute or chronic hepatitis without any evidence of bile accumulation. It is possible that other methods of classifying biochemical injury, such as examining the profile over time or including changes in bilirubin levels would provide clearer correlation with histological findings, but, for now, it is clear that it is inappropriate to draw conclusions about the precise nature of histological findings based solely on the biochemical classification determined at or near the time of onset of injury.
Another area of interest is the potential relationship between specific histological features and clinical severity of liver injury. Studies of acute liver failure have demonstrated that both the degree of necrosis and the presence of ductular reaction on liver biopsy correlate with eventual LT and death.16 Although our patient population included many patients who did not have liver failure, extensive necrosis and ductular reaction were found more frequently in those with severe or fatal injury, consistent with the previous study. Similarly, a large meta-analysis of case reports of DILI demonstrated that necrosis was associated with poor outcome, whereas eosinophils were associated with a good outcome.17 Both observations were confirmed in our prospective, blinded study. In addition to these findings, we observed that fibrosis, microvesicular steatosis, cholangiolar cholestasis, neutrophils, and portal venopathy were associated with either severe or fatal injury, whereas granulomas were associated with mild or moderate injury. There may be multiple explanations for these findings, some of which may be related to true drug injury, whereas others may be related to underlying liver disease or other comorbidities. Advanced fibrosis can be caused by drugs such as amiodarone18 and nitrofurantoin19,20 or may be the result of an underlying chronic liver disease. In either case, advanced fibrosis may limit the ability of the liver to respond to acute injury. Only one case was diagnosed with the overall pattern of microvesicular steatosis, whereas 27% showed either microvesicular steatosis alone or in combination with macrovesicular steatosis as part of another histological injury pattern. Microvesicular steatosis has often been related to mitochondrial injury, and it is possible that this mechanism may be occurring alongside other mechanisms of liver injury in DILI.21 Cholangiolar cholestasis is a characteristic finding in sepsis and may be acting as a marker of this comorbidity. Neutrophils are commonly observed associated with ductular reaction and cholangiolar cholestasis and therefore may not be an independent marker of poor outcome. Granulomas, both large epithelioid granulomas and microgranulomas, were a common finding, noted in 62% of cases. Eosinophils and granulomas were the only findings that were inversely associated with severe injury. Both are thought be histological evidence of immunoallergic reactions that generally carry a better prognosis than other kinds of DILI.17 The diversity of findings associated with clinical severity suggests that multiple mechanisms may be responsible for the severity of injury in any particular case.
There were a number of strengths and limitations to this study. This systematic histological assessment of a large number of liver biopsies in patients with detailed clinical and laboratory data represented a unique opportunity to combine these facets in well-characterized DILIN study patients. Despite the diverse nature of the patient population and the large number of agents implicated, a number of common observations could be made based on these data: Liver histology can be categorized according to stereotypical patterns of injury, and these patterns of injury, though generally correlated with biochemical patterns, do not match perfectly. Histological evaluation allows the distinction of different tissue injury patterns that may all present with similar biochemical patterns. Finally, there are a variety of histological changes and patterns of injury that are associated with clinical severity and, indirectly, with outcome. The histological review for DILIN was done by a single pathologist, which represents both a strength and a limitation. For DILIN, it permitted a fresh, internally consistent reexamination of the histological findings. However, it precluded a consensus approach to biopsy evaluation or an interobserver validation study. Nevertheless, our standardized approach used patterns and individual histological features that are well described in textbooks of hepatic pathology, so these methods should be readily accessible to experienced hepatic pathologists. In addition, the study was limited, in that biopsies were done only when considered clinically indicated and were not performed in all patients enrolled in DILIN. The liver biopsy is one of several diagnostic tools available and was not considered clinically necessary for all cases, and the criteria used to perform a biopsy may have varied among the eight enrolling centers. It is not possible to know how biases in biopsy collection might have affected the data, but it is likely that the incidence of certain histological patterns of injury might be different if every patient had undergone liver biopsy. However, it is unlikely that these biases would affect the correlations observed between the biopsies and the corresponding clinical data.
This study was not designed to address the diagnostic utility of liver biopsy in DILI. Although biopsy may be a useful diagnostic and management tool in DILI, we are unable to delineate specific advantages in the context of the current DILIN study. This study was also not designed to address whether there are particular biopsy features that should suggest DILI, as opposed to other diagnoses, although we did observe a few differences in biopsies later confirmed to be DILI. However, to demonstrate differences in DILI cases, additional control cases with similar clinical presentations and/or patterns of injury would need to be matched to authentic cases of DILI, as was done in the recent comparison of autoimmune hepatitis cases to DILI.22
The liver biopsy remains a valuable tool in the evaluation of patients suspected to have DILI. As a clinical test, it provides more information about the state of the liver than any other single assay. Pathologists should make use of the full spectrum of hepatic injury patterns when assessing and reporting on DILI,6 either in practice or in publications. Analysis of the pattern of injury provides a pathological differential diagnosis that can be evaluated against the clinical differential diagnosis and matched to published patterns of injury caused by suspect agents. The pattern classification should be accompanied by a detailed description of the severity and distribution of individual pathological findings. Histological observations can provide a direct, unambiguous assessment of the severity of liver injury that can help guide clinical management. Our study provides extensive data on the relationship between histological and clinical observations and provides a standardized framework for systematic review and classification of pathological changes in liver biopsies. Adoption of a standardized, systematic approach to describe the histology of DILI will allow for comparison of findings across studies and will help in standardizing management and providing insights into pathogenesis as well as approaches to therapy.
Glossary
- ALT
alanine aminotransferase
- ALP
alkaline phosphatase
- DILI
drug-induced liver injury
- DILIN
Drug-Induced Liver Injury Network
- HDS
herbals and dietary supplements
- LT
liver transplantation
- ULN
upper limit of the normal range
Additional Supporting Information may be found in the online version of this article.
References
- 1.Fontana RJ, Seeff LB, Andrade RJ, Bjornsson E, Day CP, Serrano J, Hoofnagle JH. Standardization of nomenclature and causality assessment in drug-induced liver injury: summary of a clinical research workshop. Hepatology. 2010;52:730–742. doi: 10.1002/hep.23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. et al. [DOI] [PubMed] [Google Scholar]
- 3.Lee WM, Senior JR. Recognizing drug-induced liver injury: current problems, possible solutions. Toxicol Pathol. 2005;33:155–164. doi: 10.1080/01926230590522356. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro MA, Lewis JH. Causality assessment of drug-induced hepatotoxicity: promises and pitfalls. Clin Liver Dis. 2007;11:477–505. doi: 10.1016/j.cld.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–1934. doi: 10.1053/j.gastro.2008.09.011. et al., 1934.e1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleiner DE. The pathology of drug-induced liver injury. Semin Liver Dis. 2009;29:364–372. doi: 10.1055/s-0029-1240005. [DOI] [PubMed] [Google Scholar]
- 7.Ramachandran R, Kakar S. Histological patterns in drug-induced liver disease. J Clin Pathol. 2009;62:481–492. doi: 10.1136/jcp.2008.058248. [DOI] [PubMed] [Google Scholar]
- 8.Fontana RJ, Watkins PB, Bonkovsky HL, Chalasani N, Davern T, Serrano J. Drug-Induced Liver Injury Network (DILIN) prospective study: rationale, design and conduct. Drug Saf. 2009;32:55–68. doi: 10.2165/00002018-200932010-00005. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rockey DC, Seeff LB, Rochon J, Freston J, Chalasani N, Bonacini M. US Drug-Induced Liver Injury Network. Causality assessment in drug-induced liver injury using a structured expert opinion process: comparison to the Roussel-Uclaf causality assessment method. Hepatology. 2010;51:2117–2126. doi: 10.1002/hep.23577. et al.; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleiner DE, Chalasani NP, Conjeevaram HS, Bonkovsky HL, Russo MW, Davern TJ. Relationship of biochemical to histologic findings and the pathological pattern of injury among cases identified in the NIH Drug-Induced Liver Injury Network (DILIN) Study. Gastroenterology. 2007;132:A773. et al. [Google Scholar]
- 11.Zimmerman HJ. Hepatotoxicity: The Adverse Effects of Drugs and Other Chemicals on the Liver. 2nd ed. Philadelphia. PA: Lippincott: Williams & Wilkins; 1999. [Google Scholar]
- 12.Zimmerman HJ, Ishak KG. Hepatic injury due to drugs and toxins. In: MacSween RNM, Burt AD, Portmann BC, Ishak KG, Scheuer PJ, Anthony PP, editors. Pathology of the Liver. London: Churchill Livingstone; 2002. pp. 621–710. In:, eds. [Google Scholar]
- 13.MacSween RNM, Burt AD, Portmann BC, Ishak KG, Scheuer PJ, Anthony PP. Pathology of the Liver. London: Churchill Livingstone; 2002. [Google Scholar]
- 14.Danan G, Benichou C. Causality assessment of adverse reactions to drugs—I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–1330. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- 15.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. et al. [DOI] [PubMed] [Google Scholar]
- 16.Katoonizadeh A, Nevens F, Verslype C, Pirenne J, Roskams T. Liver regeneration in acute severe liver impairment: a clinicopathological correlation study. Liver Int. 2006;26:1225–1233. doi: 10.1111/j.1478-3231.2006.01377.x. [DOI] [PubMed] [Google Scholar]
- 17.Bjornsson E, Kalaitzakis E, Olsson R. The impact of eosinophilia and hepatic necrosis on prognosis in patients with drug-induced liver injury. Aliment Pharmacol Ther. 2007;25:1411–1421. doi: 10.1111/j.1365-2036.2007.03330.x. [DOI] [PubMed] [Google Scholar]
- 18.Babany G, Mallat A, Zafrani ES, Saint-Marc Girardin MF, Carcone B, Dhumeaux D. Chronic liver disease after low daily doses of amiodarone. Report of three cases. J Hepatol. 1986;3:228–232. doi: 10.1016/s0168-8278(86)80031-9. [DOI] [PubMed] [Google Scholar]
- 19.Holmberg L, Boman G, Bottiger LE, Eriksson B, Spross R, Wessling A. Adverse reactions to nitrofurantoin. Analysis of 921 reports. Am J Med. 1980;69:733–738. doi: 10.1016/0002-9343(80)90443-x. [DOI] [PubMed] [Google Scholar]
- 20.Iwarson S, Lindberg J, Lundin P. Nitrofurantoin-induced chronic liver disease. Clinical course and outcome of five cases. Scand J Gastroenterol. 1979;14:497–502. [PubMed] [Google Scholar]
- 21.Pessayre D, Mansouri A, Berson A, Fromenty B. Mitochondrial involvement in drug-induced liver injury. Handb Exp Pharmacol. 2010:311–365. doi: 10.1007/978-3-642-00663-0_11. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki A, Brunt EM, Kleiner DE, Miquel R, Smyrk TC, Andrade RJ. The use of liver biopsy evaluation in discrimination of idiopathic autoimmune hepatitis versus drug-induced liver injury. Hepatology. 2011;54:931–939. doi: 10.1002/hep.24481. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


