Abstract
Rationale
Inactivating dopamine (DA) receptors in the caudate-putamen (CPu) attenuates basal and DA agonist-induced behaviors of adult rats, while paradoxically increasing the locomotor activity of preweanling rats.
Objective
The purpose of this study was to determine (a) whether D1 or D2 receptor inactivation is responsible for the elevated locomotion shown by preweanling rats and (b) whether DA receptor inactivation produces a general state in which any locomotor-activating drug will cause a potentiated behavioral response.
Methods
DMSO or N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ) was bilaterally infused into the CPu on postnatal day (PD) 17. In Experiment 1, DA receptors were selectively protected from EEDQ-induced alkylation by pretreating rats with D1 and/or D2 antagonists. On PD 18, rats received bilateral microinjections of the DA agonist R(–)-propylnorapomorphine into the dorsal CPu and locomotor activity was measured for 40 min. In subsequent experiments, the locomotion of DMSO- and EEDQ-pretreated rats was assessed after intraCPu infusions of the selective DA agonists SKF82958 and quinpirole, the partial agonist terguride, or after systemic administration of nonDAergic compounds.
Results
Experiment 1 showed that EEDQ's ability to enhance the locomotor activity of preweanling rats was primarily due to the inactivation of D2 receptors. Consistent with this finding, only drugs that directly or indirectly stimulated D2 receptors produced a potentiated locomotor response in EEDQ-treated rats.
Conclusions
These results show that DA receptor inactivation causes dramatically different behavioral effects in preweanling and adult rats, thus providing additional evidence that the D2 receptor system is not functionally mature by the end of the preweanling period.
Keywords: Caudate-putamen; N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ); Behavior; Ontogeny; Dopamine receptors
Introduction
In regard to adult rats and mice, microinjection studies typically report that stimulating dopamine (DA) receptors in the nucleus accumbens has pronounced locomotor activating effects (Plaznik et al. 1989; Delfs et al. 1990), whereas DA receptors in the caudate-putamen (CPu) are critically involved in stereotypic responding (Kelley et al. 1988; Bordi et al. 1989). Although this dichotomy is far from absolute even in adult rats (Dickson et al. 1994; Dias et al. 2006), the few microinjection studies done in young animals suggest that the CPu is an important structure mediating both locomotor activity and stereotypy during the preweanling period (Charntikov et al. 2011).
As these results imply, DA systems often exhibit ontogenetic changes that can impact both behavioral and neural functioning (Andersen 2003). In terms of behavioral responsiveness, for example, preweanling and adult rats respond in a nearly opposite manner after pharmacologically-induced DA receptor inactivation. More specifically, microinjecting the irreversible receptor antagonist N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ) into the CPu depresses the basal locomotor activity of adult rats, while increasing the locomotion of preweanling rats (Der-Ghazarian et al. 2012). This unusual ontogenetic effect is even more prominent after treatment with a nonselective DA receptor agonist, because EEDQ-treated preweanling rats given R-propylnorapomorphine (NPA) infusions into the CPu exhibit significantly more locomotor activity than rats treated with NPA alone (Der-Ghazarian et al. 2012). In contrast, DA receptor inactivation fully attenuates the NPA- and quinpirole-induced behaviors of adult rats (Bordi et al. 1989; Giorgi and Biggio 1990a,b). Surprisingly, EEDQ's ability to enhance the NPA-induced locomotor activity of preweanling rats is due to the inactivation of DA receptors, and not some other receptor type, because behavioral potentiation was not evident if D1 and D2 receptors were selectively protected from EEDQ-induced alkylation (McDougall et al. 1993; Der-Ghazarian et al. 2012). Thus, only when D1 and D2 receptors were inactivated by EEDQ did NPA produce a potentiated locomotor response.
Taken together, these results suggest that the neural systems mediating locomotion, especially those involving DA receptors, differ in meaningful ways across ontogeny. Previous research has frequently shown that systemic and intracerebral administration of DA-acting drugs can cause quantitative behavioral differences in young and adult rats (Sobrian et al. 2003; Charntikov et al. 2011). In most cases, the potency of DAergic drugs varies according to age, with older and younger animals exhibiting relatively greater or lesser behavioral responsiveness at a given dose of the drug. Occasionally, DA agonists induce qualitatively different behavioral effects depending on age, however these ontogenetic differences usually involve the emergence of age-specific responses (Moody and Spear 1992). EEDQ, on the other hand, affects an already established behavior (i.e., locomotor activity) in a qualitatively different manner depending upon the age of the rat. The neural basis of this unusual ontogenetic effect remains uncertain.
The goals of this study were four-fold: First, to determine which DA receptor subtype (D1 or D2) is responsible for the paradoxical locomotor activating effects of EEDQ in preweanling rats; Second, to examine whether DA agonists are uniquely able to potentiate the locomotor activity of EEDQ-treated preweanling rats or if DA receptor inactivation produces a state in which any locomotor-activating drug will cause a potentiated behavioral response; Third, to determine whether bilateral infusion of a partial DA agonist is also able to increase the locomotor activity of EEDQ-treated preweanling rats. This question is of interest because partial agonists (e.g., terguride) function as antagonists during periods of high DAergic tone, but they act as agonists during periods of low DAergic tone (Arnt and Hyttel 1990; Svensson et al. 1991). A fourth goal was to use autoradiography to assess the pattern of D1 and D2 receptor inactivation in EEDQ-treated preweanling rats. To accomplish these goals, EEDQ or DMSO was bilaterally infused into the CPu on postnatal day (PD) 17. One day later, distance traveled scores were measured after administration of various classes of DA agonists (NPA, SKF82958, quinpirole, terguride, and cocaine) as well as nonDAergic locomotor activating compounds (U50488 and MK801). It was predicted that D2 receptor inactivation underlies EEDQ's paradoxical behavioral effects, and that only drugs capable of directly or indirectly stimulating D2 receptors (i.e., NPA, quinpirole, terguride, and cocaine) would produce a potentiated locomotor response in preweanling rats.
Materials and methods
Subjects
Subjects were 354 male and female rats of Sprague-Dawley descent (Charles River, Hollister, CA), born and raised at California State University, San Bernardino (CSUSB). Litters were culled to ten pups on PD 3. Rat pups were kept with the dam and littermates in large maternity cages. Food and water were freely available. The colony room was maintained at 22−24°C and kept under a 12 L:12 D cycle. Testing was done in a separate experimental room and was conducted during the light phase of the cycle. Subjects were cared for according to the “Guide for the Care and Use of Laboratory Animals” (National Research Council, 2010) under a research protocol approved by the Institutional Animal Care and Use Committee of CSUSB.
Drugs
(+)-MK801 hydrogen maleate, (−)-cocaine hydrochloride, and (±)-trans-U50488 methanesulfonate salt were dissolved in saline, while (–)-quinpirole hydrochloride was dissolved in distilled water (DW). R(–)-propylnorapomorphine hydrochloride (NPA) was dissolved in DW containing 0.1% metabisulfite (an antioxidant). R(+)-SCH 23390 hydrochloride and (−)-sulpiride were dissolved in a minimal amount of glacial acetic acid and diluted with saline; whereas, haloperidol and R(+)-terguride were dissolved in 0.2% glacial acetic acid in DW. (±)-SKF82958 hydrobromide was dissolved in 10% dimethyl sulfoxide (DMSO) in DW. EEDQ was dissolved in DMSO. Systemically administered drugs were injected intraperitoneally (IP) at a volume of 5 ml/kg. EEDQ was microinjected at a volume of 0.75 μl per side, while NPA, quinpirole, SKF82958, terguride, and haloperidol were infused at a volume of 0.5 μl per side. Nonlabeled ligands were purchased from Sigma (St. Louis, MO), whereas [3H]-SCH23390 (84.9 Ci/mmol) and [3H]-spiperone (83.9 Ci/mmol) were purchased from PerkinElmer (Boston, MA).
Surgery
On PD 16, anesthesia was induced by isoflurane (2.5–5%) mixed with oxygen. For the behavioral experiments, a stainless steel 22 gauge double guide cannula was stereotaxically implanted in the dorsal CPu (A/P +5.3, M/L ±2.8, D/V +6.0 mm from the interaural line) as previously described (Der-Ghazarian et al. 2012). For the autoradiography experiment, two craniotomies were performed and needles attached to Hamilton microsyringes (2 μl) were bilaterally lowered into the dorsal CPu (Der-Ghazarian et al. 2012). Stereotaxic coordinates are from the developing rat brain atlas of Sherwood and Timiras (1970).
Behavioral procedures
Experiment 1. Impact of DA receptor protection on the NPA-induced locomotor activity of preweanling rats
On PD 17 (24 h after surgery), rats (N = 128) were randomly divided into one of four conditions: nonprotected, D1 protected, D2 protected, or D1/D2 protected. In the D1/D2 protected condition, preweanling rats were given an injection of sulpiride (100 mg/kg IP) followed, 30 min later, by an injection of SCH23390 (1 mg/kg IP). Using the same injection schedule, rats in the D1 protected condition were injected with saline followed by SCH23390, while rats in the D2 protected condition were injected with sulpiride followed by saline. Rats in the nonprotected condition received two saline injections. Thirty min after the second injection, the stainless steel stylets were removed and DMSO or EEDQ (100 μg) was microinjected into the CPu. Drugs were delivered at a constant rate over a 60 s period and the infusion cannulae were subsequently left in place for 2 min.
On PD 18, rats were habituated to photobeam chambers (41 × 41 × 41 cm, Coulbourn Instruments, Allentown, PA) for 40 min, after which NPA (10 μg) or DW was bilaterally infused into the CPu. Rats were then returned to the chambers for 40 min. Distance traveled was assessed across the 80-min session.
Experiment 2. Impact of EEDQ on the locomotor activating effects of selective D1 and D2 agonists
On PD 17, the stainless steel stylets were removed and DMSO or EEDQ (100 μg) was microinjected into the CPu. On PD 18, rats (N = 72) were placed in the testing chambers and habituated as described in Experiment 1. After 40 min, rats were removed from the chambers and SKF82958 (5 or 10 μg), quinpirole (10 μg), DW, or a 10% DMSO solution was bilaterally infused into the CPu. Rats were immediately returned to the testing chambers where distance traveled was measured for 40 min. Data from the two vehicle groups did not differ significantly, so these groups were combined for statistical and presentation purposes.
Experiment 3. Impact of EEDQ on the locomotor effects of a D2 partial agonist and a D2 antagonist
Surgical procedures and EEDQ/DMSO infusions were carried out as described in Experiment 1. After the 40-min habituation phase, rats (N = 81) were removed from the chambers and terguride (10 or 20 μg), haloperidol (5 μg), or vehicle (0.2% glacial acetic acid in DW) were bilaterally infused into the CPu. Distance traveled was measured for an additional 40 min.
Experiment 4. Impact of EEDQ on the locomotor activating effects of nonDAergic drugs and cocaine
Surgical procedures and EEDQ/DMSO infusions were carried out as described in Experiment 1. After the 40-min habituation phase, rats (N = 64) were removed from the chambers and given IP injections of the κ-opioid receptor agonist U50488 (5 mg/kg), the NMDA receptor antagonist MK801 (0.3 mg/kg), the indirect DA agonist cocaine (10 mg/kg), or saline. Rats were immediately returned to the testing chambers where distance traveled was measured for 60 min.
D1 and D2 autoradiography
On PD 17, 100 μg EEDQ (n = 6) or DMSO (n = 3) was infused (0.75 μl per side) into the dorsal CPu over a 2 min period. On PD 18, rats were decapitated and their brains were removed and frozen in liquid isopentane at –30° C. Brain sections (20 m) were cut, thaw mounted on electrostatically coated slides, air-dried under vacuum, and stored at –80° C until assay. D1 and D2 binding sites in the dorsal CPu, the ventral CPu, and the nucleus accumbens were assayed using [3H]-SCH23390 and [3H]-spiperone as previously described (Crawford et al. 2011; Der-Ghazarian et al. 2012).
Histology
Histological assessment of cannula placements was done as previously described (Charntikov et al. 2011; Der-Ghazarian et al. 2012). Overall, 90.3% of rats had proper cannula placements in the dorsal CPu. Data from animals with inappropriate guide cannula placements were not included in the statistical analyses and replacement rats were added as needed. A photomicrograph and schematic showing guide cannula placements can be seen in Fig. 1.
Fig. 1.
Schematic representations (a), as well as a typical photomicrograph (b), of cannula placements in the dorsal CPu of preweanling and adult rats from Experiments 1–4. In all cases, numbers on the right indicate distance (mm) from Bregma using coordinates from the rat brain atlas of Paxinos and Watson (1998).
Data analysis
Litter effects were minimized by assigning one subject from each litter to a particular group (Zorrilla 1997). In situations where this procedure was not possible (i.e., during the habituation phases), a single litter mean was calculated from multiple littermates assigned to the same group (Zorrilla 1997). For the behavioral experiments, separate repeated measures (5-min time blocks) analyses of variance (ANOVAs) were used for statistical analysis of time blocks 1–8 (habituation) and 9–16 or 9–20 (testing). When the assumption of sphericity was violated, as determined by Mauchly's test of sphericity, the Huynh-Feldt epsilon statistic was used to adjust degrees of freedom. Corrected degrees of freedom were rounded to the nearest whole number and are indicated by a superscripted “a”. For the autoradiography experiments, separate independent t-tests were used to analyze D1 and D2 specific binding. When required, significant higher order interactions were further analyzed using one- or two-way ANOVAs, while Tukey tests (P<0.05) were used for making post hoc comparisons.
Results
Experiment 1. Impact of DA receptor protection on the NPA-induced locomotor activity of preweanling rats
During time blocks 1–4 and 5–8 of the habituation phase, rats given EEDQ infusions had greater distance traveled scores than rats microinjected with DMSO (Fig. 2) [Pretreatment main effects, F1,112=17.45, P<0.001; F1,112=60.84, P<0.001]. These EEDQ-induced effects did not vary according to sex or protection condition (i.e., SCH23390 or sulpiride treatment).
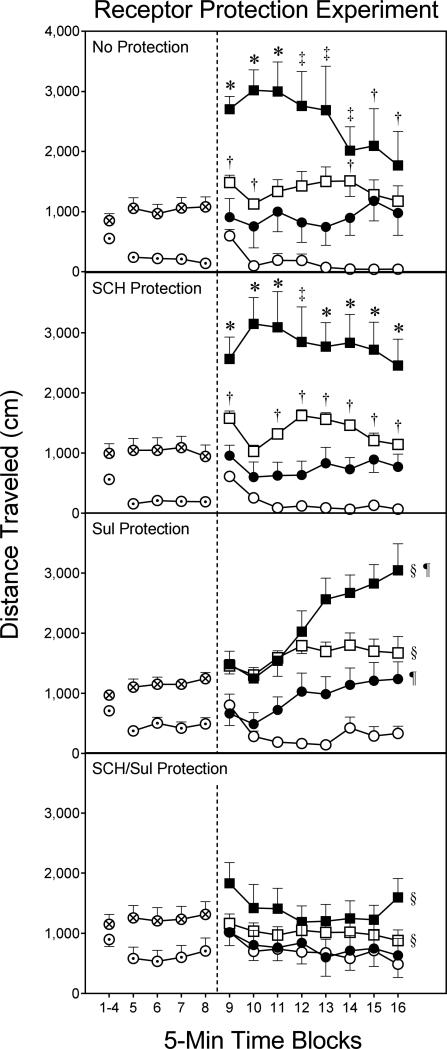
Fig. 2.
Mean distance traveled (±SEM) of DMSO- and EEDQ-pretreated rats during the habituation (i.e., time blocks 1–8) and testing (i.e., time blocks 9–16) phases on PD 18 (n = 8 per group). On PD 17, rats received no protection, SCH23390 (SCH), sulpiride (Sul), or SCH/Sul protection prior to EEDQ or DMSO infusions. DMSO pretreatment = ⊙; EEDQ pretreatment = ⊗; DMSO–Vehicle group = ○; DMSO–NPA group = □; EEDQ–Vehicle group = ●; EEDQ–NPA group = ■.
* Significantly different from all other groups in the same protection condition.
‡ Significantly different from the EEDQ-Vehicle and DMSO-Vehicle groups in the same protection condition.
† Significantly different from the DMSO-Vehicle group in the same protection condition.
§ Significantly different from vehicle-treated rats (circles) in the same protection condition (collapsed across time blocks 9–16).
¶ Significantly different from DMSO-pretreated rats (open symbols) in the same protection condition (collapsed across time blocks 9–16).
During the testing phase (i.e., time blocks 9–16), both EEDQ and NPA enhanced the locomotor activity of preweanling rats [Pretreatment main effect, F1,96=39.37, P<0.001; Agonist main effect, F1,96=117.18, P<0.001]; however, both of these variables interacted with protection condition and time block to affect performance [aPretreatment × Protection Condition × Agonist × Time Block interaction, F10,382=2.46, P<0.01]. The main effect and interactions involving the sex variable were not significant.
No protection condition (D1 and D2 receptor inactivation)
Among rats given no receptor protection (Fig. 2, top graph), both NPA and EEDQ increased distance traveled scores [Pretreatment main effect, F1,28=10.55, P<0.01; Agonist main effect, F1,28=22.80, P<0.001], with the DMSO-NPA group having greater distance traveled scores than the DMSO-Vehicle group on time blocks 9, 10, and 14 [aPretreatment × Agonist × Time Block interaction, F3,93=4.40, P<0.01]. EEDQ potentiated NPA's effects, because rats in the EEDQ-NPA group had greater distance traveled scores than the DMSO-NPA group on time blocks 9– 11. Moreover, the EEDQ-NPA group exhibited more locomotor activity than the EEDQ-Vehicle group on time blocks 9–14 and the DMSO-Vehicle group on time blocks 9–16.
SCH23390 protection (D2 receptor inactivation)
Protecting D1 receptors with SCH23390 (i.e., allowing D2 receptor inactivation), produced a similar pattern of NPA-induced locomotor activity to that just described (Fig. 2, second graph from the top). More specifically, the EEDQ-NPA group had greater distance traveled scores than all other groups on time blocks 9–11 and time blocks 13–16 [aPretreatment × Agonist × Time Block interaction, F4,118=3.80, P<0.01]. The DMSO-NPA group exhibited more locomotor activity than the DMSO-Vehicle controls on all time blocks except for time block 10.
Sulpiride protection condition (D1 receptor inactivation)
Protecting D2 receptors with sulpiride (i.e., allowing D1 receptor inactivation) produced a different pattern of effects, as the Pretreatment and Agonist independent variables did not interact. Rats pretreated with EEDQ exhibited more locomotor activity than DMSO-infused rats [Pretreatment main effect, F1, 28=10.23, P<0.001], while groups given NPA infusions had greater distance traveled scores than rats microinjected with vehicle (Fig. 2, second graph from the bottom) [Agonist main effect, F1,28=49.54, P<0.001].
SCH23390/sulpiride protection condition (D1 and D2 receptors are intact)
EEDQ was without behavioral impact if DA receptors were protected by SCH23390/sulpiride (Fig. 2, bottom graph). Even so, infusing NPA into the CPu enhanced distance traveled scores relative to vehicle-treated rats [Agonist main effect, F1,28=5.23, P<0.05].
Comparisons across protection conditions
Separate statistical analyses of only the protected conditions showed that the EEDQ-NPA group in the SCH23390 protection condition (Fig. 2, second graph from the top, filled squares) had significantly greater distance traveled scores than the EEDQ-NPA group in the SCH23390/sulpiride protection condition (bottom graph, filled squares) [F2,21=4.64, P<0.05]. The EEDQ-NPA group from the sulpiride protection condition (second graph from the bottom, filled squares) had distance traveled scores that were intermediate between, and significantly different from, the two aforementioned groups. Relative to the other protection conditions, SCH23390/sulpiride protection significantly enhanced the locomotor activity of the DMSO-Vehicle group (compare the open circles) [F3,28=6.97, P<0.001].
Experiment 2. Impact of EEDQ on the locomotor activating effects of selective D1 and D2 agonists
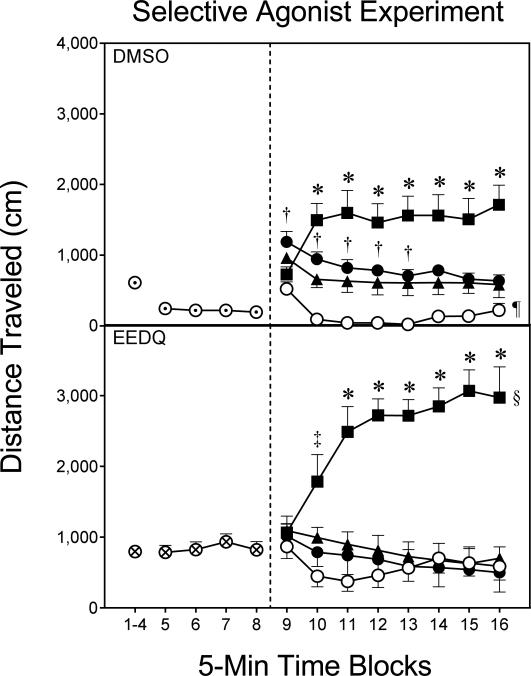
EEDQ pretreatment significantly enhanced distance traveled scores on time blocks 1–4 and time blocks 5–8 of the habituation phase (Fig. 3) [F1,68=5.25, P<0.05; F1,68=27.33, P<0.001]. During the testing phase, the pretreatment and agonist variables interacted to affect performance [Pretreatment × Agonist interaction, F3,56=4.63, P<0.01], although no sex differences were apparent. Among DMSO-pretreated rats (Fig. 3, upper graph), the DMSO-Quinpirole group had significantly greater distance traveled scores than the DMSO-Vehicle group, with SKF82958 (5 and 10 μg) stimulating an intermediate amount of locomotor activity that was significantly different from both [Agonist main effect, F3, 32=22.41, P<0.001]. This effect varied across the testing session, because on time blocks 10–16 the DMSO-Quinpirole group had greater distance traveled scores than all other groups in the DMSO condition [aAgonist × Time Block interaction, F13,138=5.24, P<0.001]. Preweanling rats given SKF82958 (5 or 10 μg) infusions exhibited more locomotor activity than the DMSO-Vehicle group on time blocks 9–13.
Fig. 3.
Mean distance traveled (±SEM) during the habituation (i.e., time blocks 1–8) and testing (i.e., time blocks 9–16) phases on PD 18 (n = 8–12 per group). DMSO pretreatment = ⊙; EEDQ pretreatment = ⊗; Vehicle = ○; 5 μg SKF82958 = ●; 10 μg SKF82958 = ▲; 10 μg Quinpirole = ■.
* Significantly different from all other groups in the same pretreatment condition.
‡ Significantly different from the SKF82958 (5 μg) and vehicle groups in the same pretreatment condition.
† Significantly different from the vehicle group in the same pretreatment condition.
§ Significantly different from the DMSO-Quinpirole group (collapsed across time blocks 9–16).
¶ Significantly different from the EEDQ-Vehicle group (collapsed across time blocks 9–16).
Among EEDQ-pretreated rats (Fig. 3, lower graph), quinpirole significantly enhanced distance traveled scores relative to SKF82958 (5 or 10 μg) and vehicle on time blocks 10–16 [aAgonist × Time Block interaction, F8,85=7.56, P<0.001]. Comparisons between the pretreatment conditions showed that EEDQ potentiated quinpirole-induced locomotor activity, because the EEDQ-Quinpirole group had significantly greater distance traveled scores than the DMSO-Quinpirole group [Pretreatment × Agonist interaction, F(3, 64) = 4.66, P<0.01]. EEDQ did not potentiate SKF82958-induced locomotion, although the EEDQ-Vehicle group did have greater distance traveled scores than the DMSO-Vehicle group.
Experiment 3. Impact of EEDQ on the locomotor effects of a D2 partial agonist and a D2 antagonist
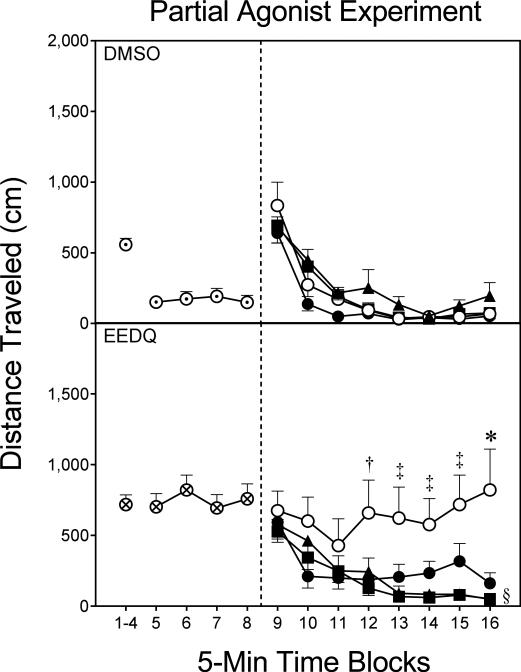
Although no group differences were apparent on time blocks 1–4, EEDQ did enhance distance traveled scores on time blocks 5–8 of the habituation phase (Fig. 4) [F1,77=28.93, P<0.001]. During the testing phase, EEDQ pretreatment significantly enhanced the distance traveled scores of preweanling rats [Pretreatment main effect, F1,65=5.90, P<0.05]. This EEDQ-induced increase in locomotion was restricted to rats given vehicle infusions, as the EEDQ-Vehicle group had greater distance traveled scores than the DMSO-Vehicle group [Pretreatment × Drug interaction, F3,65=4.10, P<0.01]. Among the EEDQ-pretreated groups (Fig. 4, lower graph), rats given terguride (10 or 20 μg) had smaller distance traveled scores than the EEDQ-Vehicle group [Drug main effect, F3,37=4.02, P<0.05], an effect that was apparent on time blocks 12–16 [aDrug × Time Block interaction, F11,137=2.72, P<0.01]. Only on time block 16 did haloperidol (2.5 μg) cause a statistically significant reduction in the locomotor activity of EEDQ-treated rats. Sex differences were not apparent during either the habituation or testing phases.
Fig. 4.
Mean distance traveled (±SEM) during the habituation (i.e., time blocks 1–8) and testing (i.e., time blocks 9–16) phases on PD 18 (n = 10–11 per group). DMSO pretreatment = ⊙; EEDQ pretreatment = ⊗; Vehicle = ○; 2.5 μg Haloperidol = ●; 10 μg Terguride = ▲; 20 μg Terguride = ■.
* Significantly different from all other groups given EEDQ.
‡ Significantly different from the 10 and 20 μg terguride groups given EEDQ.
† Significantly different from the 20 μg terguride group given EEDQ.
§ Significantly different from the vehicle group given EEDQ (collapsed across time blocks 9–16).
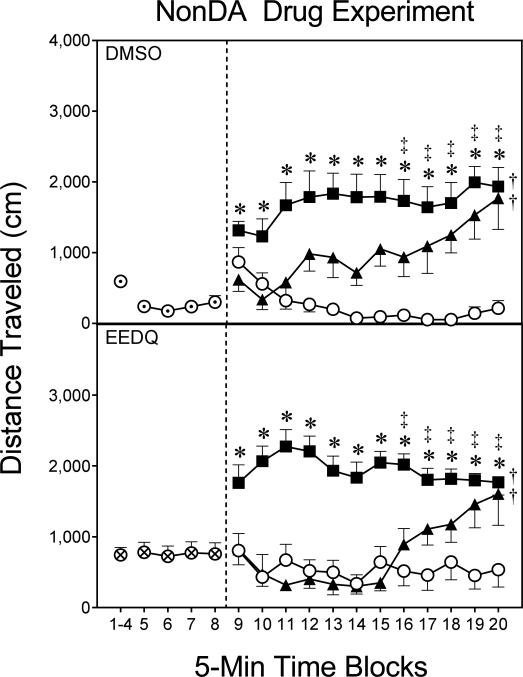
Experiment 4. Impact of EEDQ on the locomotor activating effects of nonDAergic drugs and cocaine
Effects of EEDQ on U50488- and MK801-induced locomotor activity
EEDQ-pretreated rats had greater distance traveled scores than DMSO controls on time blocks 5–8 (Fig. 5) [F1,44=10.27, P<0.01]. During the testing phase, neither the main effects nor interactions involving the pretreatment variable (i.e., EEDQ vs. DMSO) were statistically significant, thus indicating that EEDQ did not attenuate or potentiate U50488- or MK801-induced locomotor activity. Overall, both U50488 (5 mg/kg IP) and MK801 (0.3 mg/kg IP) increased the distance traveled scores of preweanling rats relative to vehicle-treated controls (Fig. 5) [Drug main effect, F2,36=44.70, P<0.001]. Differences between MK801- and vehicle-treated rats were statistically significant on time blocks 16–20, while U50488 stimulated more locomotor activity than vehicle on time blocks 9–20 [aDrug × Time Block interaction, F10,207=5.12, P<0.001]. Performance during the habituation and testing phases did not vary according to sex.
Fig. 5.
Mean distance traveled (±SEM) during the habituation (i.e., time blocks 1–8) and testing (i.e., time blocks 9–20) phases on PD 18 (n = 8 per group). DMSO pretreatment = ⊙; EEDQ pretreatment = ⊗; Saline = ○; 0.2 mg/kg MK801 = ▲; 5 mg/kg U50488 = ■.
* Significant difference between the U50488 and saline groups.
‡ Significant difference between the MK801 and saline groups.
† Significantly different from saline-treated rats (collapsed across time blocks 9–20).
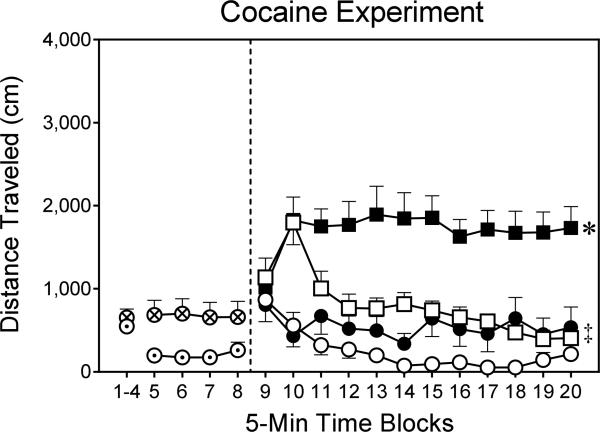
Effects of EEDQ on cocaine-induced locomotor activity
EEDQ increased the distance traveled scores of preweanling rats on time blocks 5–8 of the habituation phase (Fig. 6) [F1,28=6.35, P<0.05]. During the testing phase, both EEDQ [Pretreatment main effect, F1,24=20.11, P<0.001] and cocaine [Drug main effect, F1,24=40.83, P<0.001] increased the locomotor activity of preweanling rats (Fig. 6). Analysis of the significant Pretreatment × Drug interaction [F1,24=5.14, P<0.05] showed that the DMSO-Cocaine group had greater distance traveled scores than the DMSO-Saline group. EEDQ potentiated cocaine-induced locomotor activity, because the EEDQ-Cocaine group had significantly greater distance traveled scores than all other groups. The locomotor activity of male and female rats did not differ during the habituation or testing phases.
Fig. 6.
Mean distance traveled (±SEM) during the habituation (i.e., time blocks 1–8) and testing (i.e., time blocks 9–20) phases on PD 18 (n = 8 per group). DMSO pretreatment = ⊙; EEDQ pretreatment = ⊗; DMSO–Saline group = ○; DMSO–Cocaine group = □; EEDQ–Saline group = ●; EEDQ–Cocaine group = ■.
* Significantly different from all other groups (collapsed across time blocks 9–20).
‡ Significantly different from the DMSO-Saline group (collapsed across time blocks 9–20).
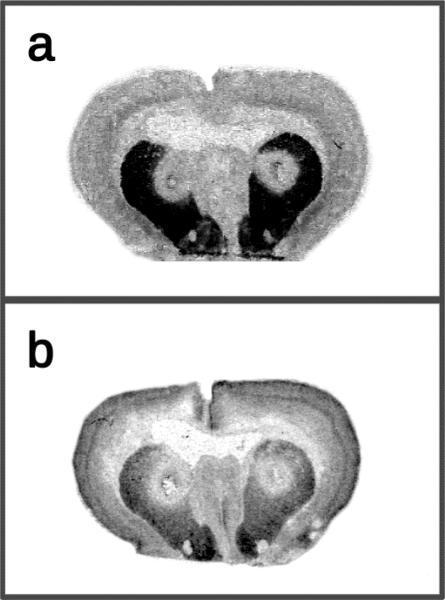
Effects of EEDQ on D1 and D2 binding sites
D2 receptor densities in the dorsal CPu of EEDQ-pretreated rats (x̄ = 5.17 fmol/mg tissue, ± 0.66 SEM) declined by 57% relative to DMSO controls (x̄ = 11.96 fmol/mg tissue, ± 1.57 SEM) [t7=4.81, P<0.01]. D1 receptor densities of EEDQ-pretreated rats (x̄ = 6.50 fmol/mg tissue, ± 0.88 SEM) showed an even greater reduction of 66% when compared to DMSO-pretreated rats (x̄ = 18.85 fmol/mg tissue, ± 0.88 SEM) [t7=8.75, P<0.001]. As a consequence of excess diffusion, the most dorsal portions of the ventral CPu, but not the nucleus accumbens, also exhibited a significant decline in D1 and D2 receptor densities. Representative autoradiograms of [3H]-SCH23390 and [3H]-spiperone binding in EEDQ-treated preweanling rats are shown in Fig. 7.
Fig. 7.
Representative autoradiograms of (a) [3H]-SCH23390 and (b) [3H]-spiperone binding after bilateral infusions of EEDQ into the dorsal CPu on PD 17.
Effects of EEDQ on body weight
An ANOVA assessing the effects of drug pretreatment (EEDQ vs. DMSO), route of EEDQ administration (IC vs. IP), protection condition, and sex, showed that drug pretreatment was the only variable that reliably affected the body weights of rats on PD 18 (Table 1). Male and female rats receiving EEDQ (IC or IP) on PD 17 showed a significant decline in body weight when measured on PD 18 [F1, 341=74.01, P<0.001]. The behavioral ramifications of these EEDQ-induced reductions in body weight are uncertain; however they were not responsible for EEDQ's paradoxical behavioral effects. For instance, the EEDQ-induced decline in body weight occurred regardless of protection condition, yet EEDQ-pretreated rats did not show elevated levels of NPA-induced locomotor activity if D2 receptors were protected from alkylation.
Table 1.
Mean (+SEM) body weights (g) of male and female PD 18 rats (N = 345) measured 24 h after DMSO or EEDQ administration.
| Pretreatment Group | Sex |
||
|---|---|---|---|
| Male | Female | (M,F) | |
| DMSO | 45.8 g (+0.67) | 44.6 g (+0.70) | 45.2 g (+0.48) |
| EEDQ | 40.2 g (+0.58) | 39.1 g (+0.64) | 39.6 g (+0.43)* |
Significantly different from the DMSO group (P < 0.05).
Discussion
As reported before, microinjecting EEDQ into the CPu both increased the basal locomotion of preweanling rats and potentiated the locomotor activity resulting from DA agonist treatment (Der-Ghazarian et al. 2012). These findings contrast with adult rat studies, in which EEDQ infusions significantly reduced basal as well as NPA- and quinpirole-induced locomotor activity (Bordi et al. 1989; Giorgi and Biggio 1990a,b). An important goal of the present study was to determine which DA receptor subtype was responsible for the paradoxical locomotor activating effects of EEDQ in preweanling rats. EEDQ alkylates a variety of different receptor types, including muscarinic M1 and M2, α2-adrenergic, GABAA, 5-HT1A and 5-HT2A receptors (for discussion, see Der-Ghazarian et al. 2012). Even so, the protection experiment clearly showed that EEDQ's ability to enhance NPA-induced locomotor activity was primarily due to the inactivation of D2 receptors, although it remains possible that the D1 receptor plays a secondary role. Specifically, when D1 and D2 receptors were inactivated (i.e., the no protection condition) or D2 receptors alone were inactivated (i.e., the SCH23390 protection conditions), NPA caused a dramatic increase in the locomotor activity of preweanling rats; however, if both receptor types were protected from EEDQ-induced inactivation then NPA did not produce a potentiated locomotor response. Thus, D2 receptor inactivation is critical for this EEDQ-induced phenomenon.
Unexpectedly, selectively inactivating D1 receptors (i.e., the sulpiride protection condition) resulted in an intermediate level of locomotor activity that was not as robust as when D2 receptors were inactivated. The pattern of behavior occurring after D1 receptor inactivation was curious, since NPA-induced locomotor potentiation only became evident during the last portion of the testing phase (Fig. 2, second graph from the bottom). This biphasic effect may indicate that only one component of the NPA response involved D1 receptors, thus resulting in a more gradual onset of action. Other DA-acting drugs can also have multiphasic behavioral effects, but these actions are most often attributed to time-dependent changes in the tissue levels of the drug or to a more prominent behavior temporarily masking a second behavior (Segal and Kuczenski 1987; Bordi et al. 1989). It is also possible that D1 receptor inactivation may have impacted NPA-induced locomotion by altering the synergism between D1 and D2 receptors (Giorgi and Biggio 1990b). It is well established that during both the preweanling period and in adulthood D1 receptors in the CPu play an “enabling” or “permissive” role for D2-mediated behaviors (Moody and Spear 1992; Dziedzicka-Wasylewska 2004), thus the loss of D1 “tone” would be expected to affect the expression of NPA-induced behaviors. Lastly, the apparent behavioral impact of D1 receptor inactivation may have been an artifact caused by sulpiride incompletely protecting D2 receptors. Although frequently used to protect D2 receptors from EEDQ-induced alkylation (Cameron and Crocker 1988; Goodale et al. 1988), Fuxe et al. (1986) found that the striatal D2 receptors of sulpiride-pretreated adult rats exhibited a nonsignificant decline of approximately 30% after systemic EEDQ treatment (see also Crawford et al. 1994). In the same study, EEDQ produced a 90% reduction of D2 receptors in nonprotected rats. Thus, it is possible that mild D2 receptor inactivation, rather than any D1 involvement, caused the intermediate level of locomotor activity exhibited by NPA-treated rats in the sulpiride protection condition.
Data gained using selective DA agonists also highlight the important role played by the D2 receptor for EEDQ's paradoxical behavioral effects. For example, preweanling rats given intraCPu infusions of quinpirole 24 h after EEDQ treatment showed a potentiated locomotor response when compared to control rats given the D2 agonist alone. In contrast, the D1 agonist SKF82958 (5 or 10 μg) did not increase the locomotor activity of EEDQ-treated rats, even though the identical doses of SKF82958 enhanced locomotion in control animals. Thus, locomotor potentiation was only apparent after D2 receptor stimulation. To determine whether EEDQ would potentiate the effects of other classes of locomotor activating drugs, U50488, MK801, and cocaine were injected 24 h after EEDQ or DMSO treatment. All three compounds significantly enhanced the locomotor activity of DMSO-pretreated rats; however, U50488-induced locomotor activity was not affected by DA receptor inactivation, while MK801-induced locomotion showed a nonsignificant decline after EEDQ pretreatment. Cocaine, on the other hand, caused a potentiated locomotor response after DA receptor inactivation. Presumably this effect was mediated by D2 receptor stimulation because SKF82958 does not enhance the locomotor activity of EEDQ-pretreated rats. When these results are considered together, it appears that DA receptor inactivation does not produce a state characterized by an excess of motoric activity; instead, only drugs capable of directly or indirectly stimulating D2 receptors (NPA, quinpirole, and cocaine) potentiate the locomotor activity of EEDQ-treated preweanling rats.
The only exception to this conclusion involved terguride, because this partial D2 agonist did not induce locomotion in preweanling rats. It has been established that partial agonists act as either agonists or antagonists depending on, among other things, the state of DAergic tone and the density of the targeted receptor (Kenakin 1997; Koener et al. 2012). More specifically, manipulations that reduce DAergic tone (e.g., 6-OHDA lesions or reserpine treatment) produce a condition in which partial agonists function as agonists (Arnt and Hyttel 1990; Svensson et al. 1991). Based on the present results, it appears that EEDQ does not produce a receptor state comparable to those induced by 6-OHDA or reserpine. In normosensitive rats, it is also the case that partial D2 agonists function as antagonists in the absence of a receptor reserve and as agonists in the presence of a receptor reserve (Meller et al. 1987). Postsynaptic D2 receptors in the CPu exhibit a minimal receptor reserve at best (Arnt et al. 1988; Meller et al. 1988), so drugs with low intrinsic activity should produce antagonist-like effects when microinjected into the dorsal CPu. For this reason, it is not surprising that terguride, like haloperidol, functioned like an antagonist after pharmacologically-induced receptor inactivation (Burris et al. 2002). However, the fact that terguride did act like an antagonist indicates that the locomotor potentiation exhibited by EEDQ-treated preweanling rats is not the result of a transient D2 receptor reserve. D2 receptor numbers increase progressively across the preweanling period and are over-produced during adolescence (Tarazi and Baldessarini 2000; Andersen 2003); yet, it does not appear that ontogenetic changes in D2 receptors, or a transient D2 receptor reserve, can account for the paradoxical actions of EEDQ in preweanling rats.
Autoradiographic analysis of DA receptor densities showed that EEDQ caused a substantial reduction of D1 and D2 receptors in the preweanling rat. More specifically, microinjecting EEDQ (100 μg) into the dorsal CPu caused a 66% and 57% decline, respectively, in D1 and D2 binding sites when measured 24 h later. Interestingly, systemic administration of 7.5 mg/kg EEDQ produces an almost identical reduction of D1 and D2 receptors (69% and 61%, respectively) in the CPu of PD 17 rats (Crawford et al. 1992). In the latter study, the D1 and D2 receptors of EEDQ-pretreated adult rats decreased by 86% and 80%, thus suggesting that EEDQ causes a more pronounced reduction of DA receptors in adults animals. Therefore, while intraCPu infusions of EEDQ inactivates a large proportion of D1 and D2 binding sites in the preweanling rat, the receptor inactivation is by no means complete and may not be as extensive as in the adult rat.
Although it is now clear that D2 receptors are primarily responsible for EEDQ's paradoxical effects, it remains uncertain why DA receptor inactivation causes a potentiated locomotor response in preweanling rats. A likely possibility is that a sufficient number of functional D2 receptors remain after EEDQ treatment to mediate locomotor activity and these receptors are supersensitive. A related idea is that DA receptors repopulate at a faster rate in EEDQ-treated preweanling rats than adults (Leff et al. 1984; Kula et al. 1992) and these newly synthesized receptors are supersensitive. Evidence in support of these hypotheses is sparse, although Trovero et al. (1992) did show that very low doses of EEDQ (0.8 mg/kg) caused D1 receptor supersensitivity in the prefrontal cortex of adult rats. In this regard, it is notable that EEDQ irreversibly antagonizes DA receptors, decreases DA levels in the CPu (Crawford et al. 1992, 1994), and enhances the basal locomotor activity of preweanling rats; thus, receptor inactivation produces a state often associated with the induction of receptor supersensitivity (see Arnt and Hyttel 1984; Carvalho et al. 2009). Autoreceptors may play a role in this effect, because inactivating D2 autoreceptors should increase DA release and provide a “corona” of enhanced DA neurotransmission that would potentially stimulate surviving supersensitive postsynaptic receptors. Of course, EEDQ attenuates, rather than potentiates, DA agonist-induced behaviors in adult rats. This age-dependent difference may result from EEDQ inactivating a greater percentage of receptors in adult rats (Crawford et al. 1992, 1994) or DA receptors may be more plastic during early ontogeny (i.e., the receptors may repopulate faster or they may be more prone to becoming supersensitive). Curiously, EEDQ caused substantial reductions of both D2 and D1 receptors in the CPu, yet there was no evidence of SKF82958-induced behavioral supersensitivity. D1-mediated supersensitivity is an established phenomenon (Kostrzewa et al. 2008), so it is uncertain why only D2 receptor inactivation produced supersensitive-like effects. The presence of a measurable D1 receptor reserve in the CPu may be an important factor (Hess et al. 1987; Zou et al. 1997).
As is typically reported in studies involving prepubescent rats, the locomotor activating effects of NPA (Der-Ghazarian et al. 2012), cocaine (Snyder et al. 1998), MK801 (Frantz and Van Hartesveldt 1999), and U50488 (Duke et al. 1997) did not vary according to sex. Moreover, EEDQ did not differentially affect the locomotor activity of male and female preweanling rats in any of the behavioral experiments. We previously found that EEDQ preferentially enhanced the baseline locomotor activity of male rats (Der-Ghazarian et al. 2012), although we stated that those particular results needed “to be confirmed by studies employing more male and female rats.” On the basis of the present data, it appears unlikely that EEDQ differentially affects the locomotor activity of male and female preweanling rats. The previously reported differences were probably a consequence of an insufficient number of male and female subjects being tested.
In conclusion, selectively inactivating D2 receptors in the CPu increases the basal and DA agonist-induced locomotor activity of preweanling rats. A potentiated locomotor response was only apparent after D2 receptor stimulation, but not after D1 receptor stimulation or when various nonDAergic locomotor-enhancing compounds were administered systemically. Adult rats respond in a qualitatively different manner after systemic or intracerebral EEDQ treatment, since irreversible DA receptor inactivation attenuates, rather than potentiates, NPA- and quinpirole-induced locomotion in older animals. We believe that these age-dependent behavioral differences, which only become evident after DA receptor inactivation, are indicative of important ontogenetic changes in the neurobiological mechanisms underlying the functioning of the CPu. It remains possible that these EEDQ-induced behavioral effects result from maturational changes in the size of D1 and/or D2 receptor reserves; however, we think it more likely that DA receptors exhibit enhanced plasticity during early ontogeny and are more prone to becoming supersensitive. Manipulations that produce DA supersensitivity are almost always associated with increased levels of the D2High receptor (Seeman et al. 2005); therefore, it is possible that EEDQ differentially triggers the shift from D2Low to D2High states in preweanling and adult rats. In addition to assessing this possibility, future research should examine the age-dependent behavioral effects of microinjecting EEDQ into the nucleus accumbens, a brain area critically involved in the mediation of locomotor activity and reward processes (Delfs et al. 1990).
Acknowledgements
We thank Fausto Varela for his surgical expertise. This research was supported by NIMH grant MH102930 (SAM), NIGMS training grant GM083883 (AEG), and NIDA training grant DA025319 (TD).
Funding sources:
This research was supported by NIMH grant MH102930 (SAM), NIGMS training grant GM083883 (AEG), and NIDA training grant DA025319 (TD).
Footnotes
Current address: T. Der-Ghazarian, School of Life Sciences, Arizona State University, 427 East Tyler Mall, Tempe, AZ 85287, USA
A. Gutierrez, College of Medicine, University of Cincinnati, 231 Albert Sabin Way, Cincinnati, OH 45267, USA
L.R. Amodeo, Department of Psychology, University of Illinois at Chicago, 1007 West Harrison Street, Chicago, IL 60607, USA
Conflict of Interest
All authors declare no conflict of interest.
References
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Arnt J, Hyttel J. Postsynaptic dopamine agonistic effects of 3-PPP enantiomers revealed by bilateral 6-hydroxy-dopamine lesions and by chronic reserpine treatment in rats. J Neural Transm. 1984;60:205–223. doi: 10.1007/BF01249094. [DOI] [PubMed] [Google Scholar]
- Arnt J, Hyttel J. Dopamine D-2 agonists with high and low efficacies: differentiation by behavioural techniques. J Neural Transm. 1990;80:33–50. doi: 10.1007/BF01245021. [DOI] [PubMed] [Google Scholar]
- Arnt J, Hyttel J, Meier E. Inactivation of dopamine D-1 or D-2 receptors differentially inhibits stereotypies induced by dopamine agonists in rats. Eur J Pharmacol. 1988;155:37–47. doi: 10.1016/0014-2999(88)90400-1. [DOI] [PubMed] [Google Scholar]
- Bordi F, Carr KD, Meller E. Stereotypies elicited by injection of N-propylnorapomorphine into striatal subregions and nucleus accumbens. Brain Res. 1989;489:205–215. doi: 10.1016/0006-8993(89)90852-4. [DOI] [PubMed] [Google Scholar]
- Burris KD, Molski TF, Xu C, Ryan E, Tottori K, Kikuchi T, Yocca FD, Molinoff PB. Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther. 2002;302:381–389. doi: 10.1124/jpet.102.033175. [DOI] [PubMed] [Google Scholar]
- Cameron DL, Crocker AD. Alkylation of striatal dopamine receptors abolishes stereotyped behavior but has no effect on dopamine adenylate cyclase activity. Neurosci Lett. 1988;90:165–171. doi: 10.1016/0304-3940(88)90805-1. [DOI] [PubMed] [Google Scholar]
- Carvalho RC, Fukushiro DF, Helfer DC, Callegaro-Filho D, Trombin TF, Zanlorenci LH, Sanday L, Silva RH, Frussa-Filho R. Long-term haloperidol treatment (but not risperidone) enhances addiction-related behaviors in mice: role of dopamine D2 receptors. Addict Biol. 2009;14:283–293. doi: 10.1111/j.1369-1600.2008.00145.x. [DOI] [PubMed] [Google Scholar]
- Charntikov S, Der-Ghazarian T, Herbert MS, Horn LR, Widarma CB, Gutierrez A, Varela FA, McDougall SA. Importance of D1 and D2 receptors in the dorsal caudate-putamen for the locomotor activity and stereotyped behaviors of preweanling rats. Neuroscience. 2011;183:121–133. doi: 10.1016/j.neuroscience.2011.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford CA, Akopian G, Ring J, Jakowec MW, Petzinger GM, Andersen JK, Vittozzi-Wong P, Wang K, Farley CM, Charntikov S, Mitroi D, Beal MF, Chow R, Walsh JP. Acute and long-term response of dopamine nigrostriatal synapses to a single, low-dose episode of 3-nitropropionic acid-mediated chemical hypoxia. Synapse. 2011;65:339–350. doi: 10.1002/syn.20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford CA, McDougall SA, Rowlett JK, Bardo MT. Depletion of dopamine binding sites and dopamine levels and changes in dihydroxyphenylacetic acid levels in the 17- and 90-day-old rat striatum after irreversible receptor antagonism. Neurosci Lett. 1992;137:265–269. doi: 10.1016/0304-3940(92)90419-8. [DOI] [PubMed] [Google Scholar]
- Crawford CA, Rowlett JK, McDougall SA, Bardo MT. Age-dependent differences in the rate of recovery of striatal dopamine D1 and D2 receptors after inactivation with EEDQ. Eur J Pharmacol. 1994;252:225–231. doi: 10.1016/0014-2999(94)90601-7. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Kelley AE. The role of D1 and D2 dopamine receptors in oral stereotypy induced by dopaminergic stimulation of the ventrolateral striatum. Neuroscience. 1990;39:59–67. doi: 10.1016/0306-4522(90)90221-o. [DOI] [PubMed] [Google Scholar]
- Der-Ghazarian T, Gutierrez A, Varela FA, Herbert MS, Amodeo LR, Charntikov S, Crawford CA, McDougall SA. Dopamine receptor inactivation in the caudate-putamen differentially affects the behavior of preweanling and adult rats. Neuroscience. 2012;226:427–440. doi: 10.1016/j.neuroscience.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias FR, Carey RJ, Carrera MP. Conditioned locomotion induced by unilateral intrastriatal administration of apomorphine: D2 receptor activation is critical but not the expression of the unconditioned response. Brain Res. 2006;1083:85–95. doi: 10.1016/j.brainres.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Dickson PR, Lang CG, Hinton SC, Kelley AE. Oral stereotypy induced by amphetamine microinjection into striatum: an anatomical mapping study. Neuroscience. 1994;61:81–91. doi: 10.1016/0306-4522(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Duke MA, Meier TL, Bolanos CA, Crawford CA, McDougall SA. Paradoxical effects of kappa opioid stimulation on the locomotor activity and Fos immunoreactivity of the preweanling rat: role of dopamine receptors. Behav Neurosci. 1997;111:1114–1122. doi: 10.1037//0735-7044.111.5.1114. [DOI] [PubMed] [Google Scholar]
- Dziedzicka-Wasylewska M. Brain dopamine receptors – research perspectives and potential sites of regulation. Pol J Pharmacol. 2004;56:659–671. [PubMed] [Google Scholar]
- Frantz K, Van Hartesveldt C. Locomotion elicited by MK801 in developing and adult rats: temporal, environmental, and gender effects. Eur J Pharmacol. 1999;369:145–157. doi: 10.1016/s0014-2999(99)00070-9. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Meller E, Goldstein M, Benfenati F, Agnati LF. Analysis of [3H]spiperone binding sites in the rat striatum and frontoparietal cortex by means of quantitative receptor autoradiography after inactivation of dopamine receptors by N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline in vivo: selective protection by sulpiride in the striatum. Neurosci Lett. 1986;64:163–168. doi: 10.1016/0304-3940(86)90093-5. [DOI] [PubMed] [Google Scholar]
- Giorgi O, Biggio G. Selective unilateral inactivation of striatal D1 and D2 dopamine receptor subtypes by EEDQ: turning behavior elicited by D2 dopamine receptor agonists. Brain Res. 1990a;533:53–59. doi: 10.1016/0006-8993(90)91794-h. [DOI] [PubMed] [Google Scholar]
- Giorgi O, Biggio G. Unilateral inactivation of dopamine receptors after intrastriatal injection of N-ethoxy-carbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ): a novel rotational model to investigate dopamine receptor interactions. Pharmacol Biochem Behav. 1990b;35:877–884. doi: 10.1016/0091-3057(90)90374-q. [DOI] [PubMed] [Google Scholar]
- Goodale DB, Jacobi AG, Seyfried DM, Weiss B. Selective protection from the inhibition by EEDQ of D1 and D2 dopamine agonist-induced rotational behavior in mice. Pharmacol Biochem Behav. 1988;30:457–462. doi: 10.1016/0091-3057(88)90480-7. [DOI] [PubMed] [Google Scholar]
- Hess EJ, Battaglia G, Norman AB, Creese I. Differential modification of striatal D1 dopamine receptors and effector moieties by N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline in vivo and in vitro. Mol Pharmacol. 1987;31:50–57. [PubMed] [Google Scholar]
- Kelley AE, Lang CG, Gauthier AM. Induction of oral stereotypy following amphetamine microinjection into a discrete subregion of the striatum. Psychopharmacology (Berl) 1988;95:556–559. doi: 10.1007/BF00172976. [DOI] [PubMed] [Google Scholar]
- Kenakin TP. Pharmacologic analysis of drug-receptor interactions. Lippincott, Williams & Wilkins; Philadelphia: 1997. [Google Scholar]
- Koener B, Focant MC, Bosier B, Maloteaux J–M, Hermans E. Increasing the density of the D2L receptor and manipulating the receptor environment are required to evidence the partial agonist properties of aripiprazole. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36:60–70. doi: 10.1016/j.pnpbp.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Kostrzewa RM, Kostrzewa JP, Brown RW, Nowak P, Brus R. Dopamine receptor supersensitivity: development, mechanisms, presentation, and clinical applicability. Neurotox Res. 2008;14:121–128. doi: 10.1007/BF03033804. [DOI] [PubMed] [Google Scholar]
- Kula NS, George T, Baldessarini RJ. Rate of recovery of D1 and D2 dopaminergic receptors in young vs. adult rat striatal tissue following alkylation with ethoxycarbonyl-ethoxy-dihydroquinoline (EEDQ). Dev Brain Res. 1992;66:286–289. doi: 10.1016/0165-3806(92)90095-e. [DOI] [PubMed] [Google Scholar]
- Leff SE, Gariano R, Creese I. Dopamine receptor turnover rates in rat striatum are age-dependent. Proc Natl Acad Sci USA. 1984;81:3910–3914. doi: 10.1073/pnas.81.12.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall SA, Crawford CA, Nonneman AJ. Behavioral effects of selective and nonselective dopamine agonists on young rats after irreversible antagonism of D1 and/or D2 receptors. Psychopharmacology (Berl) 1993;111:225–232. doi: 10.1007/BF02245528. [DOI] [PubMed] [Google Scholar]
- Meller E, Enz A, Goldstein M. Absence of receptor reserve at striatal dopamine receptors regulating cholinergic neuronal activity. Eur J Pharmacol. 1988;155:151–154. doi: 10.1016/0014-2999(88)90413-x. [DOI] [PubMed] [Google Scholar]
- Meller E, Bohmaker K, Namba Y, Friedhoff AJ, Goldstein M. Relationship between receptor occupancy and response at striatal autoreceptors. Mol Pharmacol. 1987;31:592–598. [PubMed] [Google Scholar]
- Moody CA, Spear LP. Ontogenetic differences in the psychopharmacological responses to separate and combined stimulation of D1 and D2 dopamine receptors during the neonatal to weanling age period. Psychopharmacology. 1992;106:161–168. doi: 10.1007/BF02801967. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guide for the Care and Use of Laboratory Animals. 8th ed. National Academies Press; Washington: 2010. [Google Scholar]
- Paxinos G, Watson C. The rat brain: in stereotaxic coordinates. 4th ed. Academic Press; San Diego: 1998. [Google Scholar]
- Plaznik A, Stefanski R, Kostowski W. Interaction between accumbens D1 and D2 receptors regulating rat locomotor activity. Psychopharmacology (Berl) 1989;99:558–562. doi: 10.1007/BF00589908. [DOI] [PubMed] [Google Scholar]
- Seeman P, Weinshenker D, Quirion R, Srivastava LK, Bhardwaj SK, Grandy DK, Premont RT, Sotnikova TD, Boksa P, El-Ghundi M, O'Dowd BF, George SR, Perreault ML, Männistö PT, Robinson S, Palmiter RD, Tallerico T. Dopamine supersensitivity correlates with D2High states, implying many paths to psychosis. Proc Natl Acad Sci USA. 2005;102:3513–3518. doi: 10.1073/pnas.0409766102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. Individual differences in responsiveness to single and repeated amphetamine administration: behavioral characteristics and neurochemical correlates. J Pharmacol Exp Ther. 1987;242:917–926. [PubMed] [Google Scholar]
- Sherwood NM, Timiras P. A stereotaxic atlas of the developing rat brain. University of California Press; Berkeley: 1970. [Google Scholar]
- Snyder KJ, Katovic NM, Spear LP. Longevity of the expression of behavioral sensitization to cocaine in preweanling rats. Pharmacol Biochem Behav. 1998;60:909–914. doi: 10.1016/s0091-3057(98)00078-1. [DOI] [PubMed] [Google Scholar]
- Sobrian SK, Jones BL, Varghese S, Holson RR. Behavioral response profiles following drug challenge with dopamine receptor subtype agonists and antagonists in developing rat. Neurotoxicol Teratol. 2003;25:311–328. doi: 10.1016/s0892-0362(03)00009-6. [DOI] [PubMed] [Google Scholar]
- Svensson K, Ekman A, Piercey MF, Hoffmann WE, Lum JT, Carlsson A. Effects of the partial dopamine receptor agonists SDZ 208-911, SDZ 208-912 and terguride on central monoamine receptors. A behavioral, biochemical and electrophysiological study. Naunyn-Schmiedebergs Arch Pharmacol. 1991;344:263–274. doi: 10.1007/BF00182999. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Baldessarini RJ. Comparative postnatal development of dopamine D1, D2 and D4 receptors in rat forebrain. Int J Dev Neurosci. 2000;18:29–37. doi: 10.1016/s0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Trovero F, Hervé D, Blanc G, Glowinski J, Tassin JP. In vivo partial inactivation of dopamine D1 receptors induces hypersensitivity of cortical dopamine-sensitive adenylate cyclase: permissive role of α1-adrenergic receptors. J Neurochem. 1992;59:331–337. doi: 10.1111/j.1471-4159.1992.tb08908.x. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol. 1997;30:141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Zou LL, Liu J, Jin GZ. Involvement of receptor reserve in D1 agonistic action of (–)-stepholidine in lesioned rats. Biochem Pharmacol. 1997;54:233–240. doi: 10.1016/s0006-2952(97)00153-6. [DOI] [PubMed] [Google Scholar]