Abstract
The endothelium transcends all clinical disciplines and is key to the function of every organ system. A crucial, but poorly understood role of the endothelium is its ability to control the transport of energy supply according to organ needs. Fatty acids (FAs) in particular represent a key energy source that is utilized by a number of tissues, but whose utilization must be tightly regulated to avoid potentially deleterious consequences of excess accumulation, including insulin resistance. Recent studies have identified key endothelial signaling mechanisms involving vascular endothelial growth factor B, peroxisome proliferator-activated receptor-γ, and the peptide ligand apelin, that are critical to endothelial regulation of FA transport. Here we discuss the mechanisms by which these signaling pathways regulate this key endothelial function.
Keywords: VEGF-B, PPAR-γ, Apelin, Endothelium, Fatty acid transport
Endothelium as a key energy barrier
Our understanding of the endothelial layer has progressed significantly since its historical view as an inert layer of cells that serve as the inner lining of “plumbing” for the circulatory system [1]. Now more than ever, the endothelium is implicated in regulation of both physiologic and pathologic processes via its signals and cues to the encasing organs in the context of development and function. The heterogeneity of the endothelial layer is also remarkable, from differences that exist based on the location of the conduit vessels (arteries vs. veins) to the functional heterogeneity in the context of specific organs, such as the heart, liver, and adipose tissue. Such diversity of the endothelium is key to specialized roles of its function, including permeability, leukocyte trafficking, and ability to achieve hemostasis marking some key aspects of endothelial function that varies depending on the location and the type of blood vessel. The dense network of endothelial capillaries is especially important for function of multiple organ systems, and indispensable for regulating transport of fluid and nutrient from the circulation to the target tissues. Endothelial cells (ECs) outnumber the tissue specific cells in the majority of the cases, with minimal distances (as close as 15 μm) in organs such as the heart [2]. Such architecture provides optimal context for the diffusion and transport of nutrients and oxygen to the target tissues, including the heart and the skeletal muscles. The vascular network needs to readily adapt to significant fluctuations in metabolic changes. Periods of fasting are associated with increased release of FAs from the white adipose tissue (WAT) into the circulation, to be utilized by highly metabolic tissues such as the heart and skeletal muscles. This release, as well as the endothelial mediated uptake, needs to be closely regulated. The concept of endothelial mediated FA transport has existed for many years, but has transitioned from a viewpoint of passive, diffusive transfer to a highly regulated, active process that involves multiple, complex signaling pathways [3]. This metabolic function of the endothelium, which likely has adapted evolutionarily to accommodate periods of fasting and regular diet, has been challenged by the emergence of obesity as a worldwide public health problem. Deleterious consequences of excess FA uptake in tissues including the skeletal muscle and the heart, and the resulting metabolic derangements such as impaired glucose uptake and insulin resistance [4, 5], continue to exponentially rise.
Obesity, metabolic syndrome, and endothelial dysfunction
Global changes, including trade liberalization, economic growth, and urbanization, have promoted lifestyle changes that have resulted in a net positive energy balance, with greater sedentary lifestyles and transitions to increased consumption of animal products, refined grains and sugar [6]. The worldwide obesity pandemic associated with these changes has brought to the forefront the need to better understand the endothelial mechanisms that can be targeted as novel therapeutic strategies to protect against the metabolic derangements in such context. These changes in metabolic state, in turn, have been widely associated with endothelial dysfunction. Endothelial dysfunction refers to the abnormal behavior of ECs under conditions that are associated with metabolic disturbances, including diabetes and insulin resistance. Signaling perturbations such as excess of pro-inflammatory cytokines (interleukin 6 (IL-6) [7, 8] and tumor necrosis factor α (TNF-α) [9] being two of those that are well-recognized) likely have a significant impact on promoting endothelial conversion from a quiescent phenotype towards an activated one. Signaling consequences include disruption of nitric oxide (NO) synthesis and its homeostatic effects [10], and exacerbation of reactive oxygen species (ROS) mediated effects [11]. These pro-inflammatory milieus likely place a significant burden on the endothelial signaling mechanisms that mediate the normal uptake of circulating FAs, leading to accumulation in non-adipose tissues such as the heart and skeletal muscles. Excess FAs in these organs are widely associated with impairment of glucose uptake and insulin signaling, leading to insulin resistance [4, 5]. What regulates the endothelial function as a regulator of FA transport and uptake by the target tissues have remained poorly defined until recently; emerging evidence make it evident that there is a clear orchestration of endothelial FA transport by molecules including vascular endothelial growth factor-B (VEGF-B), PPAR-γ, and apelin, that directly target the endothelium and determine its ability to maintain a balance of energy transfer and storage.
VEGF-B targeting of endothelial fatty acid transport proteins in regulation of FA transport
The VEGF family of growth factors has been studied extensively as key regulators of angiogenesis [12]. There are at least five members in this family, including VEGF-A, VEGF-B, VEGF-C, VEGF-D, and PlGF (placental growth factor). All members of the VEGF family bind to the VEGF receptors (VEGFRs), which are tyrosine kinase receptors expressed by ECs and by some leukocytes [13]. Of these factors, VEGF-B has been found to be less potent than the other class members in promoting angiogenesis, but does promote coronary arterialization in rats [14], with both pro-angiogenic (in ocular angiogenesis [15]) and anti-angiogenic (in tumor angiogenesis [16]) effects. A transgenic mouse line with cardiomyocyte specific overexpression of VEGF-B was found to induce cardiac hypertrophy with preservation of cardiac contractility, but also found to have evidence of mitochondrial lipotoxicity [17]. Based on the predominant expression of its receptors, namely VEGFR1 and neuropillin 1 (NRP1), being in the ECs, these observed changes in the cardiomyocytes are thought to be predominantly mediated via the endothelium.
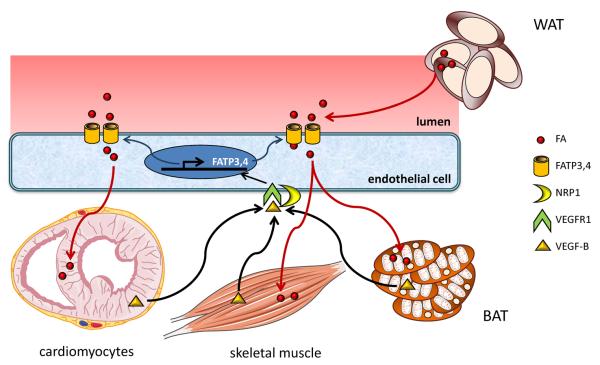
Two recent studies shed further insights into the endothelial based mechanisms by which VEGF-B regulates endothelial metabolic control (Figure 1). Hagberg et.al. made the initial observation that VEGF-B expression was closely correlated to expression of nuclear-encoded mitochondrial genes, suggesting that VEGF-B may be important in metabolism [18]. Evaluation of Vegfb−/− mice led to the observation that lipid uptake in the heart, skeletal muscle, and brown adipose tissue (BAT) was significantly decreased, while excess lipid uptake was observed in WAT. This was associated with a likely compensatory increase in glucose utilization and insulin sensitivity, which was in part attributed to increased expression of Glut4, a glucose transporter in heart and skeletal muscles.
Figure 1. Mechanism of VEGF-B mediated FA uptake.
Vascular endothelial growth factor B (VEGF-B) expressed in skeletal muscle, heart, and brown adipose tissue (BAT) binds to VEGF receptor-1 (VEGFR1) and neuropillin 1 (NRP1) on the endothelial cells to induce expression of fatty acid transport proteins 3 and 4 (FATP3/FATP4). Increased fatty acid (FA) uptake in these tissues in diabetic mice result in insulin resistance and increased blood glucose. WAT: white adipose tissue.
The endothelial based metabolic effects of VEGF-B were linked to increased expression of fatty acid transport proteins 3 and 4 (FATP3 and FATP4). FATPs (solute carrier family 27) are a family of integral transmembrane proteins that enhance cellular long chain FA uptake [19–21]. The mechanism by which FATPs mediate long chain FA uptake remains poorly defined, and may involve cooperation with other transmembrane proteins such as Cluster of Differentiation 36 (CD36) [22, 23]. Of the FATPs evaluated in response to VEGF-B stimulation, only FATP3 and FATP4 were induced in ECs [18]. FATP3 and FATP4 were increased in mouse hearts that overexpress VEGF-B, and conversely were reduced in Vegfb−/− mouse hearts. VEGF-B effect on FATP3 and FATP4 expression is mediated by the receptors VEGFR1 and NRP1, as their expression was significantly decreased in Flt1-TK−/− mice [24], lacking the tyrosine kinase domain of VEGFR1, and Nrp1-EC−/− mice [25], with EC specific deletion of the Nrp1 gene. These initial findings were further extended to the context of rodent diabetic models in a follow up study [26]. Again, invoking the primary mechanism of VEGF-B mediated tissue lipid deposition, Vegfb−/− mice on either high fat diet (HFD) or on the db/db diabetic background (carrying mutation in the leptin receptor [27]) were found to have normalization of glucose levels. This was in the context of comparable insulin levels with increased insulin sensitivity. Rather, the improved metabolic state was attributed to decreased lipid storage in the heart, skeletal muscle, and pancreas, rendering these tissues to be more efficient in their glucose uptake. Moreover, normalization of the high density lipoprotein-c (HDL-c) to low density lipoprotein-c (LDL-c) ratio, as well as decreased circulating levels of non-esterified fatty acids (NEFAs) and ketones were also seen in the Vegfb−/− mice, although the mechanism of these serum metabolic improvements remain unclear.
Advancing the potential therapeutic aspect of their mechanism, db/db mice were injected with an anti-VEGF-B antibody (2H10). Administration of 2H10 led to improved glucose levels, decreased lipid deposition in skeletal muscle and heart, and improved glucose tolerance. 2H10 administration also led to improved triglyceride levels, HDL-c to LDL-c ratio, NEFA and ketone levels.
Collectively, these observations demonstrate a promising role for antagonism of VEGF-B as a potential therapeutic target in type 2 diabetes (T2D). However, the path towards developing this as a clinically feasible anti-diabetic therapy would need to overcome a number of hurdles. First, although VEGF-B is not known as a crucial factor for normal angiogenesis, overexpression of VEGF-B in rats resulted in robust coronary arteriogenesis [14]. Abrogating such effect of endogenously expressed VEGF-B may be detrimental in patients with T2D, many of whom suffer from myocardial ischemia and coronary artery disease, and would potentially benefit from VEGF-B's coronary arteriogenic effects. Moreover, prominent neuroprotective and neurogenic effects of VEGF-B have been described, which would be important considerations given the high prevalence of diabetic neuropathy [28, 29].
PPAR-γ as a key regulator of endothelial FA transport
PPAR-γ, also known as the glitazone receptor, represents a class of nuclear receptors that belong to the nuclear receptor superfamily of ligand-inducible transcription factors [30]. Three PPARs exist in mammals: PPAR-α (NR1C1), PPAR-β/δ (NR1C2), and PPAR-γ (NR1C3). The ligands of PPARs include common dietary fats such as oleic, linoleic and linolenic acids, as well as other diverse group of lipid metabolites [31–33]. PPARs bind to PPAR-responsive transcriptional regulatory elements (PPREs) as heterodimers with retinoid X receptor (RXR), and regulate transcription of key target genes involved in biological processes such as adipogenesis, lipid storage and transport, inflammation, and metabolic homeostasis [34, 35]. Of the PPARs, PPAR-γ has been studied extensively, and targeted therapeutically by the class of anti-diabetic drugs known as thiazolidinediones (TZDs). Although an endogenous ligand for PPAR-γ remains elusive [31, 33], TZDs provide potent activation of PPAR-γ with robust insulin-sensitizing activities [36]. Unfortunately, the initial enthusiasm for these agents have been hampered by recent meta-analyses of clinical trials that have demonstrated increased risk of cardiovascular morbidities such as congestive heart failure and myocardial infarction and all-cause mortality with one of these agents, rosiglitazone [37]. A second agent currently available, pioglitazone, has also been associated with increased risk of congestive heart failure and bladder cancer, and have been taken off the market in a number of European and Asian countries [38–40]. These findings demonstrate a clear need to reconsider and improve upon the use of PPAR-γ agonists as a mainstay therapy for T2D, to achieve better outcomes that can minimize the adverse effects while maintaining the insulin-sensitizing efficacy.
PPAR-γ in the endothelium
PPAR-γ is expressed in both WAT and BAT and with highest expression in WAT, where it serves as a key regulator of adipogenesis, lipid metabolism, and insulin sensitivity [30, 41]. In addition to its specific role in adipocytes [42], cell type specific deletion of PPAR-γ have shed insights into its role in neurons [43, 44], myocytes [45, 46], and hepatocytes [47]. Most pertinent to the present topic, the metabolic phenotypes of EC specific deletion of PPAR-γ have been recently described [48, 49]. Prior endothelial-based studies of PPAR-γ function have predominantly described an anti-inflammatory effects of PPAR-γ, with mechanisms including decreased endothelial expression of chemokines [50], inhibition of NF-κB [50], and inhibition of pro-inflammatory adhesion molecules such as ICAM-1 and VCAM-1 [51, 52]. The key limitation of these findings is the reliance on cell culture systems and PPAR-γ agonists including TZDs and 15 deoxy-Δ-12,14-prostaglandin J2 (15-dPGJ2), providing a rather limited perspective of endothelial PPAR-γ function.
Two recent studies utilized the Cre recombinase driven by the Tie2 promoter to specifically delete PPAR-γ in the endothelial and hematopoietic cells (Pparg-EC−/−) (Figure 2) [48, 49]. The earlier study by Kanda and colleagues found Pparg-EC−/− mice to have significantly decreased adiposity, increased insulin sensitivity, and glucose tolerance when they were metabolically challenged with HFD [49]. These improved metabolic states were associated with significantly increased circulating free FA and triglyceride levels in the Pparg-EC−/− mice compared to the control mice, which also inversely correlated with decreased triglyceride content and increased insulin sensitivity in the skeletal muscles. Moreover, administration of the PPAR-γ agonist rosiglitazone led to significant increase in adipose tissue weight in wildtype mice; however, Pparg-EC−/− demonstrated an overall weaker response to rosiglitazone. Given that the Tie2 promoter can also lead to expression of the Cre recombinase in the hematopoietic cell lineage, elegant bone marrow transplantation studies to reconstitute the hematopoietic PPAR-γ established that the metabolic effects observed in these mice were primarily due to the endothelial PPAR-γ function. Lastly, despite the metabolic improvements in Pparg-EC−/− mice, these mice demonstrated impairment of vasodilation, suggesting distinct mechanisms that mediate the endothelial metabolic response from arterial vasoreactivity. The more recent study [48] also examined the role of endothelial PPAR-γ function using an independent line of Pparg-EC−/− mice, focusing on fatty acid binding protein 4 (FABP4, also known as AP2), and CD36 (fatty acid translocase, FAT), which had been found to be decreased in Pparg-EC−/− mice [49]. FABP4 is a member of the FABP family of proteins, which consist of at least 13 members that serve as intracellular lipid chaperones. These proteins reversibly bind hydrophobic ligands such as long chain FAs, eicosanoids and other lipids [53]. However, the exact biological function of these proteins remains poorly defined. FABPs vary in their expression profile; however, at least three FABPs, namely FABP3, FABP4, and FABP5 are known to be expressed in ECs [54–56]. CD36 is a membrane glycoprotein that is present on multiple cell types, including platelets, mononuclear phagocytes, adipocytes, myocytes, hepatocytes, and some epithelia [57]. In addition, CD36 is also expressed in microvascular ECs, where multiple studies have described its anti-angiogenic effects as a receptor for thrombospondin-1 [58, 59]. In the context of lipid metabolism, CD36 is a key membrane molecule involved in transport of long chain FA uptake [60, 61]. Cd36−/− mice were found to have decreased FA uptake in the heart, skeletal muscle, and adipose tissue, associated with concomitant increase in insulin sensitivity and glucose uptake [22, 62]. In human microvascular ECs, induction of FABP4 and CD36 by pioglitazone was found to be important for enhanced FA uptake by the ECs, as knockdown of FABP4 or CD36 abrogated the effects of pioglitazone. Moreover, uptake of the FA analog 15-[piodophenyl]-3-[R,S]-methyl pentadecanoic acid (125I-BMIPP) was significantly reduced in the heart, skeletal muscle, and adipose tissue of the Pparg-EC−/− mice after olive oil gavage, although this decrease was not evident in two other nutritional states (HFD and standard chow diet) [48].
Figure 2. Endothelial PPAR-γ regulates fatty acid transport.
PPAR-γ in endothelial cells regulates the transcription of fatty acid binding protein 4 (FABP4) and fatty acid translocase CD36, which increases endothelial transport of FAs to the surrounding tissues, including heart, skeletal muscle, and white adipose tissue (WAT). Disruption of endothelial PPAR-γ results in improved insulin sensitivity and glucose tolerance. PPRE: PPAR responsive element.
A number of key observations can be derived from these studies. First, it is clear that endothelial PPAR-γ is an important gatekeeper of FA transport, similar to the effects of VEGF-B. Second, FABP4 and CD36 are identified as important endothelial PPAR-γ transcriptional targets, although additional in vivo studies will be necessary to establish these molecules as the primary mediators of PPAR-γ effect in the endothelium. These findings also raise an intriguing question with respect to the ongoing debate on the use of TZDs to treat T2D. Despite multiple studies demonstrating significant improvement in both diabetic and cardiovascular parameters, recent studies have identified increased cardiovascular risk in some contexts [37]. One could hypothesize that such a difference in outcomes may in part be related to the cell types that may be preferentially targeted by specific TZDs, as a TZD that significantly activates endothelial PPAR-γ and increase FABP4 and CD36 expression may promote endothelial and cardiac dysfunction. Moreover, these findings support the potential therapeutic role of FABP4 or CD36 inhibition [63, 64], which could provide further protection against cardiovascular complications of T2D.
The metabolic effects of VEGF-B and endothelial PPAR-γ function also suggest key differences. Both VEGF-B and PPAR-γ seem to promote endothelial mediated FA transport into the encasing tissue. However, VEGF-B effects are predominantly restricted to those tissues that highly express the ligand—namely the skeletal muscle, heart, and BAT, with its inhibition leading to shunting of FA to the WAT; endothelial PPAR-γ, on the other hand, has a broader effect on enhancing FA tissue uptake, including its key role in uptake by the WAT promoting insulin sensitivity. These findings raise the possibility that the PPAR-γ regulated increase of endothelial FABP4 and CD36 may have secondary effects in heart and the ECs themselves, which may be contributing to the emerging cardiovascular adverse effects of TZDs.
Apelin and regulation of endothelial permeability: role in its effect as insulin sensitizer?
Emerging studies of apelin/APLNR signaling have identified a key role in insulin sensitivity. Since its original description as a peptide that binds to the orphan receptor APLNR (also known as APJ or AGTRL1), apelin has been studied extensively in the context of cardiac and vascular function [65]. Apelin can augment cardiac contractility, and induce vasorelaxation at least in part via NO dependent manner. These effects of apelin are attributed to its binding of the APLNR receptor, a seven transmembrane G protein coupled receptor that is thus far the only known receptor of the apelin ligand. Apelin and APLNR expression have been studied in many contexts. The most reliable expression data of apelin is from the apelin-lacZ reporter mouse, demonstrating that it is predominantly expressed in the microvascular ECs [66]. Adipocyte expression has also been demonstrated [67, 68], but whether its main cellular source of expression is from the adipocytes themselves or the encasing ECs remains to be determined. With respect to the APLNR receptor, the lack of a robust antibody has clouded determination of its tissue expression pattern. Existing studies suggest that it is expressed in ECs, cardiomyocytes, and vascular smooth muscle cells. In situ hybridizations, including our unpublished data, have demonstrated predominant endothelial expression in the developing cardiovascular system [69].
Apelin in diabetes
Emerging body of work has evaluated the role of apelin signaling as an insulin sensitizer [70, 71]. In obese, insulin-resistant, HFD fed mice, injection of apelin-13 (a cleaved product of apelin with highest affinity for the receptor) led to improved glucose tolerance via increased glucose uptake in skeletal muscle [70]. This effect of apelin was associated with increased endothelial nitric oxide synthase (eNOS) phosphorylation, as well as increased phosphorylation of AMPK and Akt in isolated soleus muscle. Providing perhaps even stronger genetic evidence, separate studies evaluated the metabolic phenotype of Apln−/− null mice [71, 72]. These mice on normal chow diet exhibited significantly increased insulin levels and developed glucose intolerance, associated with weight gain and increased abdominal fat. Subjecting Apln−/− mice to HFD and high sucrose water resulted in further exacerbation of the insulin resistant, glucose intolerant phenotype [71]. Overall, this line of evidence demonstrates an insulin sensitizing effect of apelin that can be explored as another strategy to improve metabolic and cardiovascular outcomes in T2D.
Apelin's role in maintenance of vascular integrity: indirect effect of FA uptake inhibition leading to improved insulin sensitivity?
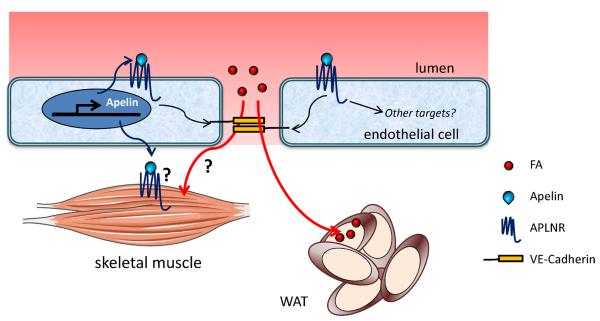
The recent data demonstrating a role for apelin signaling as an insulin sensitizer begs the question: what are the cells that are targeted by apelin? The short term, acute effects of apelin were tested in isolated skeletal muscles, which may suggest that this is a direct targeting of APLNR on the skeletal myocytes. Moreover, increased muscle FA oxidation in response to apelin stimulation has been described [73]. However, no definitive evidence of APLNR expression in skeletal myocytes currently exists. Moreover, as described above in cases of VEGF-B and PPAR-γ, endothelial-based metabolic regulation may also be a significant factor in apelin mediated metabolic response. Vascular effects of apelin on the mature endothelium include antagonism of angiotensin II induced atherosclerosis and aneurysm [74], inhibition of vascular permeability [75], and promotion of pleiotropic effects of statin agents (HMG-CoA reductase inhibitors) in ECs [76]. Moreover, roles in lymphatic vascular function and pulmonary vascular disease have also been described [77–80]. Recent work by Sawane and colleagues highlights how the role of apelin as a regulator of transendothelial FA transport may be involved in its metabolic effects (Figure 3) [77]. These authors suggest that the metabolic changes in Apln−/− mice, including weight gain and increased adipocyte mass, are related to increased vascular permeability that allows for greater FA uptake in the adipose tissue. Their hypothesis was further supported by demonstration that apelin transgenic mice are protected against obesity and decreased vascular permeability. Assuming that apelin's effects on regulation of vascular permeability and FA uptake into adipose tissue also functions in a similar manner in other organs such as heart and skeletal muscles, one could postulate that inhibition of FA transport to these organs may be a key mechanism of apelin mediated increase in insulin sensitivity. Such possibility remains to be tested. Moreover, further analysis of intrinsic endothelial transcriptional targets regulated by apelin/APLNR [81] may lead to identification of other mechanisms by which apelin affects endothelial FA transport, glucose utilization, and insulin sensitivity. Additional in vivo studies using conditional, tissue specific Aplnr null mice will provide further insights into the metabolic effects of apelin signaling.
Figure 3. Apelin regulates vascular permeability and fatty acid accumulation.
Apelin regulates endothelial permeability/integrity via stabilization of VE-cadherin, which inhibits fatty acid (FA) uptake into white adipose tissue (WAT). Unresolved questions are whether apelin acts directly on the skeletal myocytes to improve insulin sensitivity, and whether the apelin's effect on vascular integrity affects FA uptake in other tissues such as the heart and skeletal muscles.
Concluding Remarks and Future Perspectives
The signaling paradigms discussed here demonstrate the complexity of the endothelial layer as a key regulator of metabolic homeostasis. It is most likely that rather than working independently from one another, the mechanisms described here are integrated in ways yet to be fully understood. A recent study demonstrating regulation of apelin expression by PPAR-γ in pulmonary artery ECs is an example of such possibility [82]. In addition to apelin and VEGF-B, other adipokines and endothelial targeting growth factors also will likely have roles in regulation of endothelial regulation of FA transport. Moreover, recent studies on the role of glycosylphosphatidylinositol-anchored high density lipoprotein-binding protein 1 (GPIHBP1) have shed light on the role of this molecule in the hydrolysis of triglycerides to generate FAs (Box 1). Ultimately, combinatorial approaches targeting specific aspects of endothelial signaling may lead to more robust strategies to counter the metabolic imbalances caused by increasing prevalence of obesity and associated metabolic disorders.
From a clinical perspective, the roles VEGF-B and apelin in the clinical context of FA uptake regulation remain to be evaluated. With respect to PPAR-γ, although the use of TZDs in the clinical setting consistently enhance FA uptake in multiple tissues, their endothelial specific effects remain to be determined. Further translational studies to determine the clinical relevance of the endothelial signaling components discussed here will provide greater insights that may ultimately lead to therapeutic advances against the increasing burden of the obesity pandemic and associated metabolic derangements.
Box 1. Role of GPIHBP1 in regulation of lipolysis.
Triglyceride hydrolysis is an important aspect of lipid metabolism, providing fuel to myocytes and delivering lipids to adipocytes for storage. Lipoprotein lipase (LPL), that catalyses the hydrolysis, generates the fatty acids (FA) in the endothelial capillaries of skeletal muscle, heart and adipose tissue [83]. It is synthesized and secreted by the parenchymal cells, myocytes and adipocytes[84] and contains positively charged heparin-binding sequences essential for anchoring on EC heparan sulfate proteoglycans (HSPGs)[85]. However, LPL depends on glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 (GPIHBP1) for its transportation in the endothelial lumens, where lipolysis takes place[83]. GPIHBP1 is a lymphocyte antigen 6 (Ly6) protein expressed by capillary ECs[83, 86]. Recent electron microscopy studies have shown that both proteins localize in plasma membrane invaginations and transcytotic vesicles, while their trafficking is bidirectional, thus allowing GPIHBP1 recycling to the basolateral face of the cells[84]. In mice deficient for Gpihbp1, LPL mis-localizes in the interstitial spaces around parenchymal cells[83], resulting in severe hypertriglyceridemia even on low fat diet[86], phenocopying human mutations. To date one G56R substitution mutation[87] and seven missense mutations have been identified in patients with chylomicronemia[88–91]. Mutations in LPL that abolish binding to GPIHBP1 have also been identified in patients with chylomicronemia[92], underscoring the importance of LPL-GPIHBP1 interplay in lipid metabolism. This interaction is regulated on multiple levels, including LPL expression, GPIHBP1-controlled transport, recycling as well as the number of LPL binding sites within the endothelial capillaries. LPL requires apolipoprotein CII (apo-CII) to become catalytically active[93] while it is inhibited by angiopoietin-related protein 4 (ANGPTL4)[94]. Peroxisome proliferator-activated receptors (PPARs) are involved in the regulation of both LPL and GPIHBP1 expression. PPARα and PPARγ are known to increase Lpl expression in the liver and adipose tissue respectively[95]. PPARγ but not PPARα or PPARδ agonists increase Gpihbp1 transcripts in all expressing tissues apart from the liver[96]. Moreover, PPARγ deficient mice present with significantly lower levels of Gpihbp1 transcript in adipose tissue compared to littermate controls[96]. It was recently demonstrated that hypoxia could decrease Lpl and Pparγ transcription but not Gpihbp1, Cd36 or Angptl4 in adipose tissue[97]. Last, dietary elements present an additional level of control. Correlating with tissue requirements for fuel, fasting results in downregulation of Lpl in white adipose tissue and upregulation in skeletal muscle and heart, which is reversed after feeding[98]. Conversely, Gpihbp1 expression levels practically double during fasting in heart, brown and white adipose tissues, but return to normal after feeding[96]. Taken together, these results demonstrate that to ensure homeostasis, lipid metabolism in the endothelium must be tightly regulated on several levels and by different signaling pathways.
Box 2. Outstanding Questions
How does a strategy to inhibit VEGF-B in diabetes affect its function in other processes such as arteriogenesis and neurogenesis?
Can inhibition of endothelial PPAR-γ targets such as FABP4 or CD36 minimize the adverse effects of TZDs?
What are the key cells that respond to apelin to mediate its insulin sensitizing effects?
Does apelin regulate FA uptake in hearts and skeletal muscles?
Highlights
Endothelium is a key regulator of fatty acid transport
Endothelial fatty acid transport is regulated by a complex integration of signaling paradigms, including VEGF-B, PPAR-γ, and apelin
These molecules can potentially be targeted as adjunct therapies to treat diabetes
Acknowledgements
We thank P. Yue for critical reading of this manuscript. This work was supported by the National Institutes of Health grants (HL095654 and HL113005 to H.J.C.) and the Howard Hughes Medical Institute Physician Scientist Early Career Award (H.J.C.). Some of the figures were generated using Servier Medical Art, available from www.servier.com/Powerpoint-image-bank.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Florey L. The endothelial cell. British medical journal. 1966;2:487–490. doi: 10.1136/bmj.2.5512.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsieh PC, et al. Endothelial-cardiomyocyte interactions in cardiac development and repair. Annual review of physiology. 2006;68:51–66. doi: 10.1146/annurev.physiol.68.040104.124629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van der Vusse GJ, et al. Transport of long-chain fatty acids across the muscular endothelium. Advances in experimental medicine and biology. 1998;441:181–191. doi: 10.1007/978-1-4899-1928-1_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roden M, et al. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996;97:2859–2865. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samuel VT, et al. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375:2267–2277. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malik VS, et al. Global obesity: trends, risk factors and policy implications. Nature reviews. Endocrinology. 2013;9:13–27. doi: 10.1038/nrendo.2012.199. [DOI] [PubMed] [Google Scholar]
- 7.Yudkin JS, et al. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, et al. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM, et al. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000;101:2149–2153. doi: 10.1161/01.cir.101.18.2149. [DOI] [PubMed] [Google Scholar]
- 10.Huang PL. eNOS, metabolic syndrome and cardiovascular disease. Trends in endocrinology and metabolism: TEM. 2009;20:295–302. doi: 10.1016/j.tem.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 12.Olsson AK, et al. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 13.Stuttfeld E, Ballmer-Hofer K. Structure and function of VEGF receptors. IUBMB life. 2009;61:915–922. doi: 10.1002/iub.234. [DOI] [PubMed] [Google Scholar]
- 14.Bry M, et al. Vascular endothelial growth factor-B acts as a coronary growth factor in transgenic rats without inducing angiogenesis, vascular leak, or inflammation. Circulation. 2010;122:1725–1733. doi: 10.1161/CIRCULATIONAHA.110.957332. [DOI] [PubMed] [Google Scholar]
- 15.Zhang F, et al. VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proc Natl Acad Sci U S A. 2009;106:6152–6157. doi: 10.1073/pnas.0813061106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albrecht I, et al. Suppressive effects of vascular endothelial growth factor-B on tumor growth in a mouse model of pancreatic neuroendocrine tumorigenesis. PloS one. 2010;5:e14109. doi: 10.1371/journal.pone.0014109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karpanen T, et al. Overexpression of vascular endothelial growth factor-B in mouse heart alters cardiac lipid metabolism and induces myocardial hypertrophy. Circ Res. 2008;103:1018–1026. doi: 10.1161/CIRCRESAHA.108.178459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagberg CE, et al. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature. 2010;464:917–921. doi: 10.1038/nature08945. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch D, et al. A family of fatty acid transporters conserved from mycobacterium to man. Proc Natl Acad Sci U S A. 1998;95:8625–8629. doi: 10.1073/pnas.95.15.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaffer JE, Lodish HF. Expression cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell. 1994;79:427–436. doi: 10.1016/0092-8674(94)90252-6. [DOI] [PubMed] [Google Scholar]
- 21.Kazantzis M, Stahl A. Fatty acid transport proteins, implications in physiology and disease. Biochim Biophys Acta. 2012;1821:852–857. doi: 10.1016/j.bbalip.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hajri T, et al. Defective fatty acid uptake modulates insulin responsiveness and metabolic responses to diet in CD36-null mice. J Clin Invest. 2002;109:1381–1389. doi: 10.1172/JCI14596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calvo D, et al. Human CD36 is a high affinity receptor for the native lipoproteins HDL, LDL, and VLDL. Journal of lipid research. 1998;39:777–788. [PubMed] [Google Scholar]
- 24.Hiratsuka S, et al. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci U S A. 1998;95:9349–9354. doi: 10.1073/pnas.95.16.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acevedo LM, et al. Semaphorin 3A suppresses VEGF-mediated angiogenesis yet acts as a vascular permeability factor. Blood. 2008;111:2674–2680. doi: 10.1182/blood-2007-08-110205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagberg CE, et al. Targeting VEGF-B as a novel treatment for insulin resistance and type 2 diabetes. Nature. 2012;490:426–430. doi: 10.1038/nature11464. [DOI] [PubMed] [Google Scholar]
- 27.Genuth SM, et al. Insulin resistance in genetically obese, hyperglycemic mice. Endocrinology. 1971;88:1230–1238. doi: 10.1210/endo-88-5-1230. [DOI] [PubMed] [Google Scholar]
- 28.Poesen K, et al. Novel role for vascular endothelial growth factor (VEGF) receptor-1 and its ligand VEGF-B in motor neuron degeneration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:10451–10459. doi: 10.1523/JNEUROSCI.1092-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, et al. VEGF-B inhibits apoptosis via VEGFR-1-mediated suppression of the expression of BH3-only protein genes in mice and rats. J Clin Invest. 2008;118:913–923. doi: 10.1172/JCI33673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans RM, et al. PPARs and the complex journey to obesity. Nat Med. 2004;10:355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- 31.Forman BM, et al. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 32.Forman BM, et al. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci U S A. 1997;94:4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forman BM, et al. The peroxisome proliferator-activated receptors: ligands and activators. Annals of the New York Academy of Sciences. 1996;804:266–275. doi: 10.1111/j.1749-6632.1996.tb18621.x. [DOI] [PubMed] [Google Scholar]
- 34.Chandra V, et al. Structure of the intact PPAR-gamma-RXR- nuclear receptor complex on DNA. Nature. 2008;456:350–356. doi: 10.1038/nature07413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chawla A, et al. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 36.Yki-Järvinen H. Thiazolidinediones. New England Journal of Medicine. 2004;351:1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 37.Nissen SE, Wolski K. Effect of Rosiglitazone on the Risk of Myocardial Infarction and Death from Cardiovascular Causes. New England Journal of Medicine. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 38.Lincoff AM, et al. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA : the journal of the American Medical Association. 2007;298:1180–1188. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 39.Azoulay L, et al. The use of pioglitazone and the risk of bladder cancer in people with type 2 diabetes: nested case-control study. BMJ. 2012;344 doi: 10.1136/bmj.e3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neumann A, et al. Pioglitazone and risk of bladder cancer among diabetic patients in France: a population-based cohort study. Diabetologia. 2012;55:1953–1962. doi: 10.1007/s00125-012-2538-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annual review of biochemistry. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 42.He W, et al. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A. 2003;100:15712–15717. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu M, et al. Brain PPAR-gamma promotes obesity and is required for the insulin-sensitizing effect of thiazolidinediones. Nat Med. 2011;17:618–622. doi: 10.1038/nm.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryan KK, et al. A role for central nervous system PPAR-gamma in the regulation of energy balance. Nat Med. 2011;17:623–626. doi: 10.1038/nm.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hevener AL, et al. Muscle-specific Pparg deletion causes insulin resistance. Nat Med. 2003;9:1491–1497. doi: 10.1038/nm956. [DOI] [PubMed] [Google Scholar]
- 46.Norris AW, et al. Muscle-specific PPARgamma-deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. J Clin Invest. 2003;112:608–618. doi: 10.1172/JCI17305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gavrilova O, et al. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem. 2003;278:34268–34276. doi: 10.1074/jbc.M300043200. [DOI] [PubMed] [Google Scholar]
- 48.Goto K, et al. Peroxisome proliferator-activated receptor-gamma in capillary endothelia promotes fatty acid uptake by heart during long-term fasting. Journal of the American Heart Association. 2013;2:e004861. doi: 10.1161/JAHA.112.004861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanda T, et al. PPARgamma in the endothelium regulates metabolic responses to high-fat diet in mice. J Clin Invest. 2009;119:110–124. doi: 10.1172/JCI36233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marx N, et al. Peroxisome proliferator-activated receptor-gamma activators inhibit IFN-gamma-induced expression of the T cell-active CXC chemokines IP-10, Mig, and I-TAC in human endothelial cells. Journal of immunology. 2000;164:6503–6508. doi: 10.4049/jimmunol.164.12.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pasceri V, et al. Modulation of vascular inflammation in vitro and in vivo by peroxisome proliferator-activated receptor-gamma activators. Circulation. 2000;101:235–238. doi: 10.1161/01.cir.101.3.235. [DOI] [PubMed] [Google Scholar]
- 52.Jackson SM, et al. Peroxisome proliferator-activated receptor activators target human endothelial cells to inhibit leukocyte-endothelial cell interaction. Arterioscler Thromb Vasc Biol. 1999;19:2094–2104. doi: 10.1161/01.atv.19.9.2094. [DOI] [PubMed] [Google Scholar]
- 53.Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov. 2008;7:489–503. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Antohe F, et al. Heart microvessels and aortic endothelial cells express the 15 kDa heart-type fatty acid-binding proteins. European journal of cell biology. 1998;76:102–109. doi: 10.1016/S0171-9335(98)80022-8. [DOI] [PubMed] [Google Scholar]
- 55.Elmasri H, et al. Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2009;23:3865–3873. doi: 10.1096/fj.09-134882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Masouye I, et al. Endothelial cells of the human microvasculature express epidermal fatty acid-binding protein. Circ Res. 1997;81:297–303. doi: 10.1161/01.res.81.3.297. [DOI] [PubMed] [Google Scholar]
- 57.Febbraio M, et al. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest. 2001;108:785–791. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chu LY, et al. Thrombospondin-1 modulates VEGF signaling via CD36 by recruiting SHP-1 to VEGFR2 complex in microvascular endothelial cells. Blood. 2013;122:1822–1832. doi: 10.1182/blood-2013-01-482315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dawson DW, et al. CD36 mediates the In vitro inhibitory effects of thrombospondin-1 on endothelial cells. The Journal of cell biology. 1997;138:707–717. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ibrahimi A, et al. Expression of the CD36 homolog (FAT) in fibroblast cells: effects on fatty acid transport. Proc Natl Acad Sci U S A. 1996;93:2646–2651. doi: 10.1073/pnas.93.7.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nickerson JG, et al. Greater transport efficiencies of the membrane fatty acid transporters FAT/CD36 and FATP4 compared with FABPpm and FATP1 and differential effects on fatty acid esterification and oxidation in rat skeletal muscle. J Biol Chem. 2009;284:16522–16530. doi: 10.1074/jbc.M109.004788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coburn CT, et al. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem. 2000;275:32523–32529. doi: 10.1074/jbc.M003826200. [DOI] [PubMed] [Google Scholar]
- 63.Geloen A, et al. CD36 inhibitors reduce postprandial hypertriglyceridemia and protect against diabetic dyslipidemia and atherosclerosis. PloS one. 2012;7:e37633. doi: 10.1371/journal.pone.0037633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Furuhashi M, et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature. 2007;447:959–965. doi: 10.1038/nature05844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barnes G, et al. Translational promise of the apelin--APJ system. Heart. 2010;96:1011–1016. doi: 10.1136/hrt.2009.191122. [DOI] [PubMed] [Google Scholar]
- 66.Sheikh AY, et al. In vivo genetic profiling and cellular localization of apelin reveals a hypoxia-sensitive, endothelial-centered pathway activated in ischemic heart failure. Am J Physiol Heart Circ Physiol. 2008;294:H88–98. doi: 10.1152/ajpheart.00935.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boucher J, et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology. 2005;146:1764–1771. doi: 10.1210/en.2004-1427. [DOI] [PubMed] [Google Scholar]
- 68.Daviaud D, et al. TNFalpha up-regulates apelin expression in human and mouse adipose tissue. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20:1528–1530. doi: 10.1096/fj.05-5243fje. [DOI] [PubMed] [Google Scholar]
- 69.Saint-Geniez M, et al. The msr/apj gene encoding the apelin receptor is an early and specific marker of the venous phenotype in the retinal vasculature. Gene Expression Patterns. 2003;3:467–472. doi: 10.1016/s1567-133x(03)00062-0. [DOI] [PubMed] [Google Scholar]
- 70.Dray C, et al. Apelin stimulates glucose utilization in normal and obese insulin-resistant mice. Cell metabolism. 2008;8:437–445. doi: 10.1016/j.cmet.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 71.Yue P, et al. Apelin is necessary for the maintenance of insulin sensitivity. American journal of physiology. Endocrinology and metabolism. 2010;298:E59–67. doi: 10.1152/ajpendo.00385.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yue P, et al. Apelin decreases lipolysis via G(q), G(i), and AMPK-Dependent Mechanisms. Endocrinology. 2011;152:59–68. doi: 10.1210/en.2010-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Attane C, et al. Apelin treatment increases complete Fatty Acid oxidation, mitochondrial oxidative capacity, and biogenesis in muscle of insulin-resistant mice. Diabetes. 2012;61:310–320. doi: 10.2337/db11-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chun HJ, et al. Apelin signaling antagonizes Ang II effects in mouse models of atherosclerosis. The Journal of clinical investigation. 2008;118:3343–3354. doi: 10.1172/JCI34871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kidoya H, et al. Apelin induces enlarged and nonleaky blood vessels for functional recovery from ischemia. Blood. 2010;115:3166–3174. doi: 10.1182/blood-2009-07-232306. [DOI] [PubMed] [Google Scholar]
- 76.McLean DL, et al. Apelin/APJ signaling is a critical regulator of statin effects in vascular endothelial cells--brief report. Arterioscler Thromb Vasc Biol. 2012;32:2640–2643. doi: 10.1161/ATVBAHA.112.300317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sawane M, et al. Apelin inhibits diet-induced obesity by enhancing lymphatic and blood vessel integrity. Diabetes. 2013;62:1970–1980. doi: 10.2337/db12-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sawane M, et al. Apelin attenuates UVB-induced edema and inflammation by promoting vessel function. The American journal of pathology. 2011;179:2691–2697. doi: 10.1016/j.ajpath.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim J, et al. An endothelial apelin-FGF link mediated by miR-424 and miR-503 is disrupted in pulmonary arterial hypertension. Nature medicine. 2013;19:74–82. doi: 10.1038/nm.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chandra SM, et al. Disruption of the apelin-APJ system worsens hypoxia-induced pulmonary hypertension. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:814–820. doi: 10.1161/ATVBAHA.110.219980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kang Y, et al. Apelin-APJ signaling is a critical regulator of endothelial MEF2 activation in cardiovascular development. Circulation research. 2013;113:22–31. doi: 10.1161/CIRCRESAHA.113.301324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alastalo TP, et al. Disruption of PPARgamma/beta-catenin-mediated regulation of apelin impairs BMP-induced mouse and human pulmonary arterial EC survival. J Clin Invest. 2011;121:3735–3746. doi: 10.1172/JCI43382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Davies BS, et al. GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell metabolism. 2010;12:42–52. doi: 10.1016/j.cmet.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Davies BS, et al. Assessing mechanisms of GPIHBP1 and lipoprotein lipase movement across endothelial cells. Journal of lipid research. 2012;53:2690–2697. doi: 10.1194/jlr.M031559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Young SG, Zechner R. Biochemistry and pathophysiology of intravascular and intracellular lipolysis. Genes & development. 2013;27:459–484. doi: 10.1101/gad.209296.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Beigneux AP, et al. Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell metabolism. 2007;5:279–291. doi: 10.1016/j.cmet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang J, Hegele RA. Homozygous missense mutation (G56R) in glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 (GPI-HBP1) in two siblings with fasting chylomicronemia (MIM 144650) Lipids in health and disease. 2007;6:23. doi: 10.1186/1476-511X-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Franssen R, et al. Chylomicronemia with low postheparin lipoprotein lipase levels in the setting of GPIHBP1 defects. Circulation. Cardiovascular genetics. 2010;3:169–178. doi: 10.1161/CIRCGENETICS.109.908905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Olivecrona G, et al. Mutation of conserved cysteines in the Ly6 domain of GPIHBP1 in familial chylomicronemia. Journal of lipid research. 2010;51:1535–1545. doi: 10.1194/jlr.M002717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Beigneux AP, et al. Chylomicronemia with a mutant GPIHBP1 (Q115P) that cannot bind lipoprotein lipase. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:956–962. doi: 10.1161/ATVBAHA.109.186577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Charriere S, et al. GPIHBP1 C89F neomutation and hydrophobic C-terminal domain G175R mutation in two pedigrees with severe hyperchylomicronemia. The Journal of clinical endocrinology and metabolism. 2011;96:E1675–1679. doi: 10.1210/jc.2011-1444. [DOI] [PubMed] [Google Scholar]
- 92.Voss CV, et al. Mutations in lipoprotein lipase that block binding to the endothelial cell transporter GPIHBP1. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7980–7984. doi: 10.1073/pnas.1100992108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Olivecrona G, Beisiegel U. Lipid binding of apolipoprotein CII is required for stimulation of lipoprotein lipase activity against apolipoprotein CII-deficient chylomicrons. Arteriosclerosis, thrombosis, and vascular biology. 1997;17:1545–1549. doi: 10.1161/01.atv.17.8.1545. [DOI] [PubMed] [Google Scholar]
- 94.Sukonina V, et al. Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17450–17455. doi: 10.1073/pnas.0604026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schoonjans K, et al. PPARalpha and PPARgamma activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. The EMBO journal. 1996;15:5336–5348. [PMC free article] [PubMed] [Google Scholar]
- 96.Davies BS, et al. The expression of GPIHBP1, an endothelial cell binding site for lipoprotein lipase and chylomicrons, is induced by peroxisome proliferator-activated receptor-gamma. Molecular endocrinology. 2008;22:2496–2504. doi: 10.1210/me.2008-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jun JC, et al. Acute hypoxia induces hypertriglyceridemia by decreasing plasma triglyceride clearance in mice. American journal of physiology. Endocrinology and metabolism. 2012;303:E377–388. doi: 10.1152/ajpendo.00641.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mead JR, et al. Lipoprotein lipase: structure, function, regulation, and role in disease. Journal of molecular medicine. 2002;80:753–769. doi: 10.1007/s00109-002-0384-9. [DOI] [PubMed] [Google Scholar]