Abstract
Background
For the premature infant, extrauterine life is a pathological condition which greatly amplifies the challenges to the brain in establishing functional oromotor behaviors. The extent to which suck can be entrained using a synthetically patterned orocutaneous input to promote its development in preterm infants who manifest chronic lung disease is unknown.
Objective
To evaluate the effects of a frequency-modulated orocutaneous pulse train delivered through a pneumatically-charged pacifier capable of enhancing non-nutritive suck (NNS) activity in tube-fed premature infants.
Methods
A randomized trial to evaluate the efficacy of pneumatic orocutaneous stimulation 3x/day on NNS development and length of stay (LOS) in the NICU among 160 newborn infants distributed among 3 subpopulations, including healthy preterm infants (HI), respiratory distress syndrome (RDS), and chronic lung disease (CLD). Study infants received a regimen of orocutaneous pulse trains through a PULSED pressurized silicone pacifier or a SHAM control (blind pacifier) during gavage feeds for up to 10 days.
Results
Mixed modeling, adjusted for the infant’s gender, gestational age, postmenstrual age, and birth weight, was used to handle interdependency among repeated measures within subjects. A significant main effect for stimulation mode (SHAM pacifier vs PULSED orosensory) was found among preterm infants for NNS Bursts/minute (p=.003), NNS events/minute (p=.033), and for Total Oral Compressions/minute [NNS+nonNNS] (p=.016). Pairwise comparison of adjusted means using Bonferroni adjustment indicated RDS and CLD infants showed the most significant gains on these NNS performance indices. CLD infants in the treatment group showed significantly shorter LOS by an average of 2.5 days.
Conclusion
Frequency-modulated PULSED orocutaneous pulse train stimuli delivered through a silicone pacifier are effective in facilitating NNS burst development in tube-fed RDS and CLD preterm infants, with an added benefit of reduced LOS for CLD infants.
Keywords: suck central pattern generator, mechanosensory experience, orofacial, NNS, critical period, prematurity
Introduction
Feeding competency is a frequent and serious challenge both to infants in the neonatal intensive care unit (NICU) and to their caregivers, healthcare staff and parents.1 One of the first steps employed by clinicians toward achieving ororhythmic patterning and assessment of feeding readiness involves the use of a finger or a silicone pacifier to induce non-nutritive suckling (NNS).1 This ororhythmic behavior appears between 28 and 33 weeks of gestational age (GA) and is remarkably stable by 34 weeks of post-menstrual age (PMA).2 Suck represents a complex sensorimotor behavior that can provide valuable insights into the integrity of the central nervous system in the human infant.3,4
Establishing the NNS provides the infant with numerous benefits, including improved pre-feed state control 5–7 and post-feed 8 growth, maturation, gastric motility, while decreasing stress 5,8,9 and enhancing oral feeds.10 NNS also has been shown to decrease the length of hospital stay in preterm infants, and in general there are no known short-term negative side effects.11 NNS may also allow critical aspects of oromotor and feeding development to progress through stimulation and to reduce the length of time spent on nasogastric (NG) tube feeding.12–15 Preterm infants born between 24 and 34 weeks of GA with higher NNS scores had a 3 day shorter transition to full oral feeds compared to infants with disorganized NNS.16 Gestational age at birth was inversely related with PMA at full oral feeds.
The purpose of the present investigation was to assess the effects of a new frequency-modulated (FM) orocutaneous intervention in tube-fed preterm infants on the development of NNS and length-of-stay (LOS) in 3 subpopulations, 2 of which are at significant risk for oromotor and feeding delays. It was hypothesized that repeated application of an FM orocutaneous stimulus that mimics the ‘burst-pause’ temporal dynamics of NNS would accelerate the development of this complex ororhythmic behavior, and potentially reduce the length of hospital stay.
Methods
Study Population
A randomized controlled trial design was used to evaluate the efficacy of pulsed orocutaneous stimulation on NNS development and length of stay among 162 newborn infants (73F/89M) distributed among 3 subpopulations, including an initial enrollment of 49 healthy preterm infants (HI), 39 with respiratory distress syndrome (RDS), and 74 whose respiratory condition evolved into chronic lung disease (CLD) (Power =.80, p<.05). A total of 2 infants dropped out of the study following enrollment due to the development of necrotizing enterocolitis, a criterion for exclusion from our study (group assignments: RDS treatment and HI treatment). For 2 infants in the study, gastrostomy tubes (g-tubes) were placed following hospital discharge or transfers to other NICUs in the Kansas area. Each of these infants had a diagnosis of CLD (one control, one treatment). Participant characteristics for the 160 preterm infants analyzed in this study are given in Table 1. The human subjects committee at each performance site, including the Overland Park Regional Medical Center (Overland Park, Kansas USA), and Stormont-Vail HealthCare (Topeka, Kansas USA) approved the research protocol for this study. Written informed consent was obtained from the parents at each NICU prior to the participants’ enrollment into the study following consultation with the attending physician and the research nurse or study coordinator dedicated full-time to this project. Medical staff involved in nursing care of study participants were blinded to treatment condition for the duration of the intervention protocol. Newborn infants among each of the 3 clinical populations were randomly assigned to either the PULSED orocutaneous stimulation or the SHAM ‘non-pulsatile’ pacifier using a random integer number function. The expected ethnic proportion for Kansas, based on the US Federal Census was African American 5.8%, Asian American 1.7%, Hispanic American 5.5%, Native American 0.8%, and White 86.2%.
Table 1.
Preterm infant characteristics [mean (SD)]
| Healthy Infants | Respiratory Distress Syndrome | Chronic Lung Disease | ||||
|---|---|---|---|---|---|---|
| HI (N=48) | RDS (N=38) | CLD (N=74) | ||||
| SHAM | PULSED | SHAM | PULSED | SHAM | PULSED | |
| N=25 | N=23 | N=20 | N=18 | N=42 | N=32 | |
| Gender (♂ : ♀) | 12:13 | 14:9 | 13:7 | 12:6 | 21:21 | 16:16 |
| GAbirth (wks) | 32.1 (1.8) | 31.3 (1.5) | 30.8 (1.6) | 30.5 (2.4) | 26.6 (2.1) | 27.2 (1.7) |
| BW (gms) | 1736.7 (468.2) | 1645.2 (327.2) | 1491.3 (293.1) | 1438.9 (521.1) | 884.2 (353.5) | 860.8 (247.8) |
| PMAstart (wks) | 34.6 (1.4) | 34.2 (1.5) | 33.6 (1.4) | 34.5 (1.9) | 34.9 (1.9) | 35.7 (2.5) |
| %POstart | 26 (33) | 26 (30) | 7 (16) | 16 (28) | 12 (18) | 14 (17) |
| O2 Hx (days) | 4.3 (8.1) | 2.5 (3.9) | 16.4 (12.3) | 23.6 (14.9) | 69.8 (27.0) | 67.2 (26.6) |
Inclusion criteria for specific treatment groups
For HI, RDS and CLD infants: Born between 23 0/7 and 36 6/7 weeks of GA as determined by obstetric ultrasound and clinical examination. HI designates preterm infants (N=48; 23 treatment, 25 control) with no specific diagnosis who were otherwise medically stable, and who had minimal or no oxygen history (≤ 5 days of ventilator, CPAP, and nasal cannula). RDS infants (N=38; 18 treatment, 20 control) had a diagnosis of respiratory distress syndrome confirmed by X-ray earlier in their hospital stay and required respiratory support with oxygen (days on ventilator + CPAP + nasal cannula) that was no longer necessary by 28 days of age or 36 wks PMA. CLD includes sicker preterm infants (N=74; 32 treatment, 42 control) who still required respiratory support with oxygen at 36 weeks PMA.
General inclusion criteria: no functional suck and tube-fed at time of enrollment, head circumference within 10–90th percentile of mean for PMA, neurological examination showing no anomalies for PMA, and with stable vital signs (heart rate, blood pressure, age appropriate respiratory rate, and oxygen saturation >92 SpO2) to allow for NNS. General exclusion criteria: IVH grades III or IV, other intracranial hemorrhage, PVL, necrotizing enterocolitis, neonatal seizures and culture positive sepsis or meningitis at time of testing, chromosomal anomalies or craniofacial malformation, nervous system anomalies, cyanotic congenital heart disease, gastroschisis, omphalocele, diaphragmatic hernia and/or other major gastrointestinal anomalies, or not ready for oral feedings as determined by the health care team.
PULSED Pneumatic Orocutaneous and SHAM Stimulation Conditions
Infants assigned to the PULSED treatment group received three 3-minute epochs of pneumatically pulsed pacifier stimulation during gavage feeds using the NTrainer System® (Innara Health, Inc., Olathe, Kansas USA). This PULSED stimulus was programmed to mimic the temporal features of a NNS burst. Each 3-minute stimulation epoch consisted of 34 oral cutaneous pulse trains. The 3-minute PULSED stimulation periods were separated by 5.5 minute pause periods during which the stimulator was switched off and the pneumatically-charged pacifier was removed from the infant’s mouth (see Table 2).
Table 2.
Stimulation schedule.
| Periods | P1 | P2 | P3 | P4 | P5 |
|---|---|---|---|---|---|
| Treatment | PULSED- On | Off | PULSED- On | Off | PULSED- On |
| SHAM- On | Off | SHAM- On | Off | SHAM- On | |
| Duration (min) | 3 | 5.5 | 3 | 5.5 | 3 |
| NG Feed
|
|||||
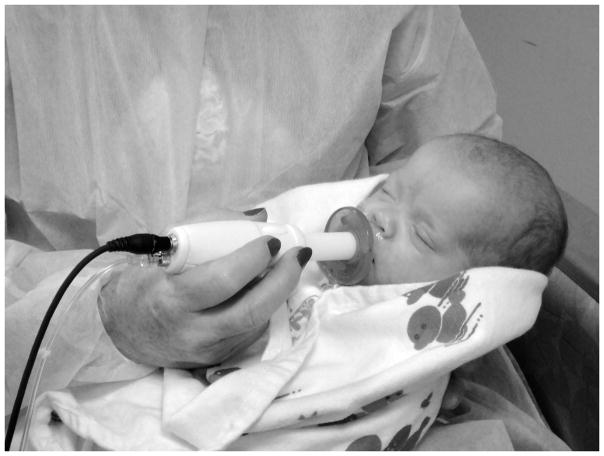
Infants assigned to the SHAM treatment were offered the same type of Soothie® pacifier for the same duration but without the pneumatically patterned stimulus. Figure 1 shows the NTrainer handpiece and silicone pacifier assembly with an infant as used for the SHAM or PULSED conditions.
Figure 1.
The NTrainer System handpiece and silicone pacifier assembly with an infant. Handpiece can be configured for the SHAM or PULSED conditions.
The treatment regimen for both SHAM and PULSED pacifier conditions was repeated 3 times per day for up to 10 days according to their 3-hour feed cycles, or until the infant attained 90% oral feeds at a volume of 140–160 ml/kg/d for two consecutive days. Infants were swaddled with limbs at midline, and in a quiet-awake to drowsy state during stimulation.17 Observers in the NICU could not differentiate which babies received the pulsed orocutaneous stimulation since the same cribside NTrainer System® workstation was used for NNS assessment and treatment conditions.
Advancement to Oral Feeding
All infants in the study, regardless of their assignment to either the NTrainer or the Control group, were fed with the Volu-feed® disposable nurser – Strong Babies System (Abbott Laboratories) in combination with periodic breast feeding attempts. The percentage of infants among the HI, RDS, and CLD groups who attempted breastfeeding was 54.2%, 54.3%, and 47.2%, respectively. Infants were monitored by their nurses and physicians for pulse and O2 saturation (pulse-oximetry), along with respiration patterns during feeds for safety. Infants were allowed to attempt oral feeds as long as their SpO2 levels were maintained at or above 92%, and heart rate was stable. Babies exhibiting O2 desaturations, breathing difficulties, spit-ups, postural distress, and bradycardia during oral feed attempts were then fed by NG tube. Nutritional content was specific to each infant as ordered by the physician to satisfy caloric demands.
NNS progression
The evolution of the NNS compression pressure waveform was documented through daily 3-minute digital recordings completed at the beginning of a gavage feed. During these NNS recording sessions, infants did not receive the pulsed orocutaneous stimulation. Infants remained connected to their bedside monitors at all times for observation of respiration, heartbeat and oxygen saturation. The same Soothie® silicone pacifier type used during the stimulation sessions was coupled to the NTrainer handpiece which was instrumented to record nipple pressure and permitted the study nurse to start and stop computer program operation.
Following a brief examination of physiologic state, each infant, regardless of group, was held in a developmentally supportive semi-inclined posture. Background/overhead lighting was dimmed in the immediate area to promote eye contact with the nurse/study coordinator. Sampling of NNS behavior was not initiated until the infant achieved an optimal behavioral state, i.e., drowsy to active alert (state 3, 4 or 5 as described by the Naturalistic Observation of Newborn Behavior, Newborn Individualized Developmental Care and Assessment Program; NIDCAP).17
Automated NNS digital signal processing and feature extraction
A 2-minute subsample reflecting the most active period of NNS oromotor behavior was automatically extracted from each 3-minute suck assessment data file using a custom software algorithm on the NTrainer System®. An NNS burst was defined as two or more NNS events occurring within 1200 milliseconds. If this amount of time is exceeded, then a new NNS burst is classified. This algorithm permits objective identification of NNS burst activity as distinct from non-organized mouthing compressions. A detailed description of the NNS burst software algorithm is included in the online supplement. Five measures were extracted from the indexed records of suck, including: (1) Total Oral Compressions defined as the sum of all pressure events per minute, (2) NNS Cycles defined as suck compression cycles with cycle periods less than 1200 milliseconds and occurring within the NNS burst structure per minute, (3) the number of NNS Bursts which consisted of two or more nipple compression cycles. The two remaining NNS performance measures included the (4) mean number NNS Cycles/Burst, and (5) NNS pressure amplitude (cmH2O).
Statistical analysis
The primary endpoints included a longitudinal comparison of non-nutritive suck performance and length-of-stay (LOS) between the two treatments (SHAM, PULSED), each consisting of three preterm infant groups (HI, RDS, CLD). Mixed modeling was used to handle interdependency among repeated measures within subjects. Adjusting for the infants’ gender, gestational age, postmenstrual age, and birth weight, mixed models estimated (linear) growth over time as well as stimulus group, infant group, and their interaction effects on each outcome, using restricted maximum likelihood estimator which produces unbiased estimates under the conditions of unbalanced sample and/or incomplete data. Adjusted means were pairwise compared using Bonferroni adjustment for inflated Type I error. A compound symmetric error covariance structure provided better model fit than other error covariance structures according to Akaike Information Criterion and Bayesian Information Criterion and thus was chosen for the mixed models. All analyses were conducted using SAS 9.3.18
RESULTS
The PULSED orocutaneous stimuli delivered through a silicone pacifier was effective in facilitating NNS burst development in tube-fed preterm infants. Within each group, no substantial deviation from normality was found for any of the dependent variables. The assumptions of normality, linearity, homogeneity of variance-covariance matrices, and sphericity were satisfactory for the multivariate analysis.
Distribution of GAbirth and Treatment Start among Preterm Groups
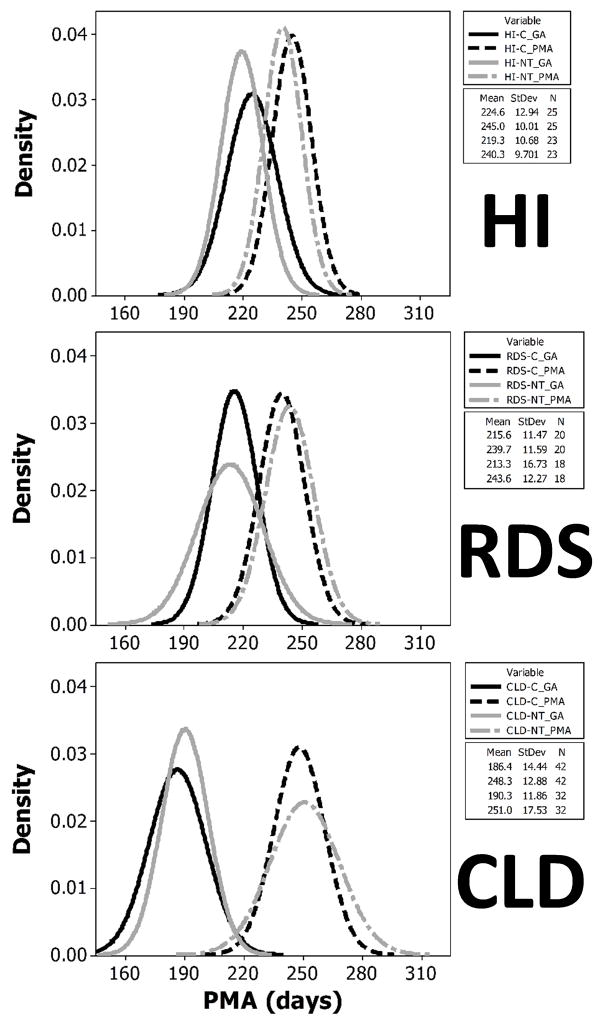
The resulting distribution of preterm infants for the 3 preterm populations exposed to either of the treatment conditions is shown in Figure 2. HI infants began the treatment at 20.6 days of age (mean GAbirth=222.1 days), whereas RDS infants, who were born approximately one week earlier (mean GAbirth=214.5 days), began treatment at 27.0 days of age. CLD infants were born the earliest (mean GAbirth=188.1 days) and began the orosensory treatments at 61.4 days of age. Within preterm groups, there were no significant differences in GAbirth or the PMA at the start of treatment conditions, however the starting PMA for the treatment conditions between preterm infant groups was significantly different [F(1,157), p<.0001] and as shown follows a logical progression from HI>RDS>CLD.
Figure 2.
Probability distribution of GA and treatment starting PMA’s among preterm groups. [C = control SHAM, and NT = PULSED treatment conditions].
NNS Bursts/minute
A significant main effect for treatment condition (SHAM pacifier versus PULSED pacifier) was found among preterm infants independent of clinical group (p<.001, d=.58). For all preterm groups combined, this translates to a 17.2% increase from 7.67 to 8.99 NNS Bursts/minute. As shown in Figure 3, the adjusted means from the mixed model showed an increase of 12.5% from 7.20 (SHAM) to 8.10 NNS bursts/minute in the presence of the PULSED pacifier entrainment condition. RDS infants exposed to the PULSED treatment condition showed an increase of 20.7%, 7.62 to 9.20 NNS bursts/minute (p<.05, d=.36). Infants with CLD also showed moderate gains from the treatment condition with an increase of 17.9% from 8.19 NNS bursts/minute (SHAM) to 9.66 NNS bursts/minute during the PULSED entrainment condition (p<.01, d=.46).
Figure 3.
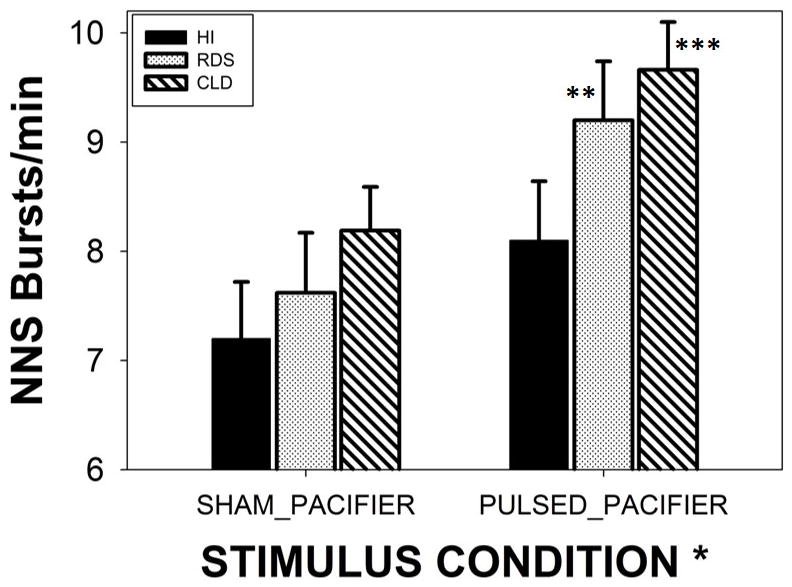
Minute-rate for NNS burst production among three preterm groups (HI-healthy infants, RDS-respiratory distress syndrome, and CLD-chronic lung disease). Groups were randomized to either SHAM or PULSED pacifier treatments. Significant main effect for stimulus condition (* p<.05, d=.41), and Bonferroni pairwise comparisons (** p<.05, d=.36; *** p<.01, d=.46). Error bars depict standard deviation.
NNS Events/minute
A significant main effect for treatment condition was found among preterm subjects independent of clinical group (p<.05, d=.36). For all preterm groups combined, this translates to a 17.2% increase from 65.6 to 73.0 NNS Events/minute. As shown in Figure 4, CLD infants showed the most significant gains from the treatment condition with an increase of 15.0% from 68.75 NNS events/minute (SHAM) to 79.04 NNS events/minute for PULSED stimulation (p<.05, d=.42). Moreover, CLD babies outpaced healthy preterm infants by 20.7% in the PULSED treatment condition (p<.05).
Figure 4.
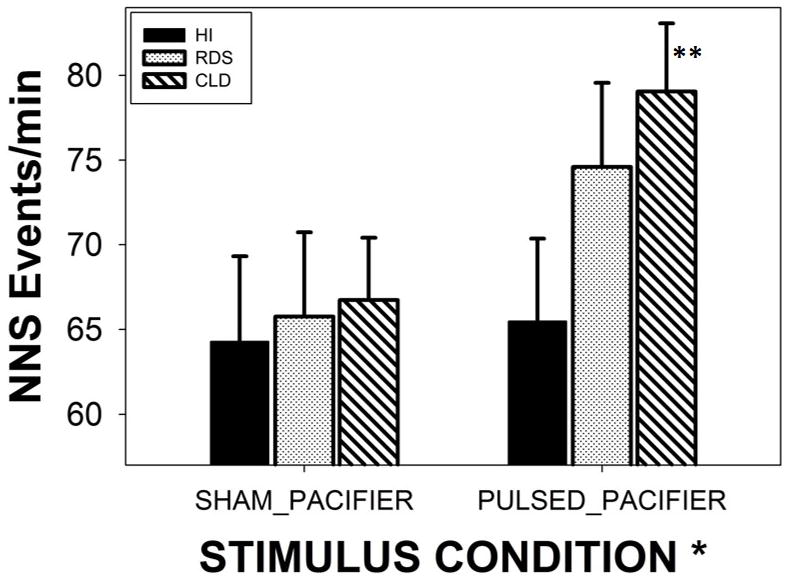
Minute-rate for NNS Events among three preterm groups (HI-healthy infants, RDS-respiratory distress syndrome, and CLD-chronic lung disease). Groups were randomized to either SHAM or PULSED pacifier treatments. Significant main effect for stimulus condition (* p<.05, d=.36), and Bonferroni pairwise comparison for CLD infants (** p<.05, d=.42). Error bars depict standard deviation.
Total Oral Compressions/minute [NNS+nonNNS Events]
A significant main effect for treatment condition (SHAM vs PULSED) was found among preterm subjects independent of clinical group (p<.05, d=.41). For all preterm groups combined, this translates to a 17.2% increase from 71.7 to 81.3 Oral Compressions/minute. CLD infants showed the most significant gains from the treatment with an increase of 22.2% from 74.07 in the SHAM condition to 90.53 Oral Compressions/minute in the PULSED condition (p<.01, d=.50) (Figure 5). Moreover, CLD babies outperformed healthy preterm infants by 27% in the PULSED treatment condition by the margin 90.53 versus 71.30 Oral Compressions/minute, respectively (p<.05).
Figure 5.
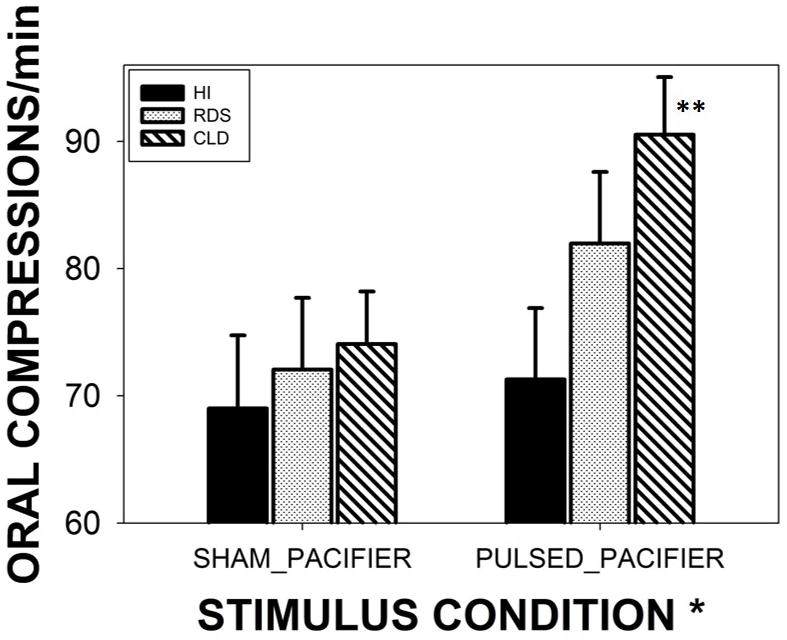
Minute-rate for NNS and nonNNS Oral Compressions among three preterm groups (HI-healthy infants, RDS-respiratory distress syndrome, and CLD-chronic lung disease). Groups were randomized to either SHAM or PULSED pacifier treatments. Significant main effect for stimulus condition (* p<.05, d=.41), and Bonferroni pairwise comparison for CLD infants (** p<.01, d=.50). Error bars depict standard deviation.
Mean NNS Pressure
No significant group effects, and no significant treatment effects emerged from the mixed model analysis.
Length of Stay (LOS)
A significant group by treatment interaction effect was present (p<.001). As shown in Figure 6, CLD infants given the PULSED pacifier treatment stayed in the NICU for a significantly shorter period of time compared to CLD infants on the SHAM pacifier, by an average of 2.5 days less (p<.05, d=.39). On the other hand, RDS infants given the PULSED pacifier treatment remained in the NICU 4.3 days longer than RDS infants on the SHAM pacifier (p<.01, d=.47). Overall, both CLD and RDS infants remained in the NICU longer than their HI counterparts, (p<.0001, d=.69; and p<.01, d=.55, respectively).
Figure 6.
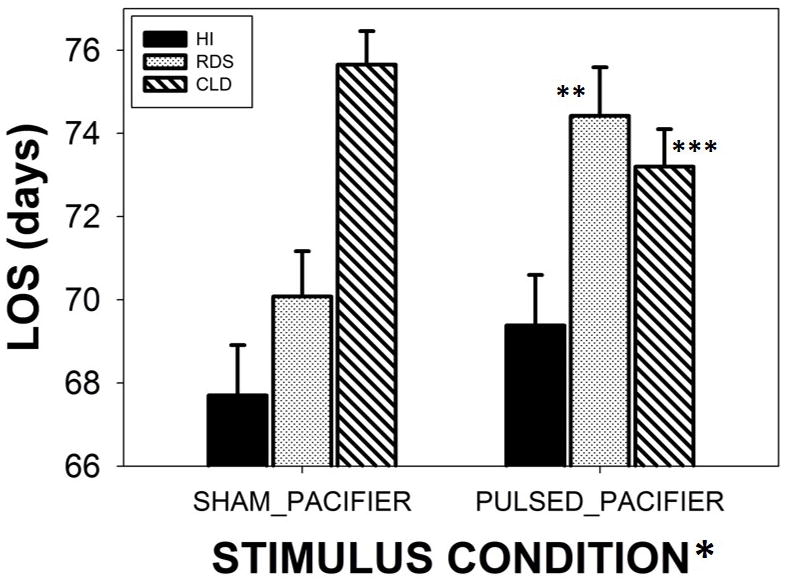
Length-of-stay (LOS) among three preterm groups (HI-healthy infants, RDS-respiratory distress syndrome, and CLD-chronic lung disease). Groups were randomized to either SHAM or PULSED pacifier treatments. A significant group by treatment interaction effect was present (p<.001), and Bonferroni pairwise comparisons for RDS (** p<.01, d=.47) and CLD infants (*** p<.05, d=.39). Error bars depict standard deviation.
DISCUSSION
The infant brain is a developing organ of enormous complexity, whose initial form is specified through genetic instruction, with pathway formation and network tuning subsequently modified and continuously refined by experience- and activity-dependent mechanisms.19 Unfortunately, extrauterine life is a pathological condition for the premature infant, which greatly amplifies the challenges to the brain in establishing functional motor behaviors.20 The brainstem suck central pattern generator (sCPG) in infants is responsive to peripheral input during this critical period of development 21 and adapts to changes in the local oral environment.22,23 The sCPG consists of networks of interneurons in the pontine and medullary reticular formation. These networks are premotor and influence lower motoneuron activity among trigeminal, facial, hypoglossal, glossopharyngeal, and vagal nuclei.24–26 In neurotypical infants, the NNS pattern is organized into alternating epochs of frequency-modulated bursts and pause periods. An NNS burst consists of 2 to 12 suck cycles that initiate at approximately 2.9 Hz and exponentially decay to approximately 1.6 Hz followed by pause periods of variable length to accommodate respiration.27
The results from the present study expand on our previous work in RDS preterm infants and demonstrate the beneficial effects of a pneumatically-pulsed pacifier nipple on the development of NNS in a larger and more diverse cohort of preterm infants with oromotor delays, including those with CLD. The pulsed orocutaneous experience has been enhanced to incorporate the frequency-modulated characteristics of a neurotypical NNS burst.27 This form of stimulation serves to entrain trigeminal mechanosensitive nerves in the lips, tongue, and jaw of the neonate which in turn influence the sCPG and oromotor activity. This approach is consistent with the role of sensory-driven neural activity in circuit formation and the notion that appropriate oral experiences may be critical in the final weeks of gestation in the formation of functional central neural circuits.28 Use of an orocutaneous entrainment stimulus also has the distinct advantage of being safe and pleasurable for the neonate, and easily administered cribside by the care team in the NICU.
In general, somatosensory stimulation strategies have proven beneficial in developing oral feeding skills in premature infants.15,29 In a recent study, relatively brief tactile-motion stimulation (15 minutes, twice/day) applied to the face-mouth and body before tube feedings among 75 clinically stable preterm infants beginning at 32.3 weeks of PMA (26–32 weeks GA) resulted in the attainment of independent oral feeding approximately 9–10 days earlier than those in the control group. 30 This form of somatosensory stimulation also serves to improve the dynamics of swallow–respiration coordination.31
Factors contributing to the development of CLD include immaturity of lung structure itself, prolonged oxygen and/or higher levels of respiratory support that cause free radicals exposure and damage to the developing lung, inflammatory cytokine cascades that may originate with maternal infection during pregnancy, and potentially genetic factors. As pulmonary function improves, these infants often manifest oromotor dyscoordination, absent or weak non-nutritive suck, poor airway protection, dysphagia, and poor state control.32,33 The invasiveness of lengthy intubation and oxygen supplementation procedures associated with prematurity and lung disease cost the baby precious sensory and motor experiences during a critical period of brain development for oromotor pattern generation.34–37 Even the presence of a nasogastric (NG) feeding tube has negative effects on sucking and breathing.36 This translates to longer NICU stays until the infants achieve these life-essential skills. Therefore, it appears that preterm infants with significant lung disease may benefit from oral somatosensory therapy to improve their sucking and feeding skills. Preterm infants with significant lung disease have many reasons to explain their relative delays in sucking and feeding skills, and are therefore excellent candidates for oral somatosensory therapy to improve these skills.
The richness of the frequency-modulated orocutaneous experience offered by a PULSED entrainment pacifier nipple presents a new and exciting neurotherapeutic application to promote suck and feeding skills. Exposure to this orosensory experience over the course of 3 gavage feeds per day for up to 10 days in the NICU provides the preterm infant with a neural entrainment experience that facilitates the development and strengthening of central pathways which regulate suck. Recent evidence has shown the same PULSED orocutaneous stimulation, as used in the present study, is highly effective in modulating brain activity as reflected in amplitude-integrated EEG and range-EEG measurements sampled in preterm infants at 32 weeks PMA.38,39 One unexpected finding in the present study was the increased LOS for RDS infants who received the PULSED orocutaneous treatment and manifest significant NNS performance increases. One likely reason is the relatively late introduction of the PULSED orosensory treatment (~ 34.5 weeks PMA). On average, RDS infants began extrauterine life a week earlier than their HI counterparts, and undoubtedly gained additional oromotor experience moving them ever closer to discharge from the NICU before our PULSED orocutaneous treatment was initiated. Future work with this population will introduce the PULSED orosensory treatment 10 to 14 days earlier which also is consistent with somatosensory stimulation strategies described in two recent papers.30,31
Another key question to be addressed in future studies concerns the dose of orocutaneous stimulation per gavage session and whether this should be adjusted in a progressive manner based on the infant’s PMA and suck performance levels. For example, in our sicker preterm infants (i.e., CLD), the administration of the third 3-minute block of pulsed orocutaneous stimulation towards the end of a 20-minute gavage feed was sometimes judged by the study nurse/coordinator to exceed the energy capacity of the infant to support NNS activity. This is an important observation since overstimulation of trigeminal afferents may negatively reinforce ororhythmic pattern formation. Our previous experience with RDS and preterm infants 40 utilized a single 3-minute orosensory period which was tolerated well by the majority of infants. Thus, it seems reasonable to consider a graduated oral somatosensory therapy schedule for preterm infants, based in part, on their medical diagnosis, motor capacity and experience. In the present study, CLD infants had an average GAbirth of approximately 270/7 weeks, and began orosensory entrainment therapy at approximately 355/7 weeks PMA, whereas RDS infants were in the womb 3 weeks longer with an average GAbirth of 304/7 weeks, and began their entrainment therapy a week earlier at approximately 345/7 weeks PMA. In a graduated schedule, one could initiate the CLD infant with a single 3-minute orosensory entrainment condition during 3 gavage sessions on days 1 and 2, and then increase to two 3-minute orosensory entrainment conditions on successive days (e.g., days 3–10). Preterm infants with somewhat more mature gestational ages at birth (e.g., 30 wks compared with 27 wks) can be initiated at higher levels of orosensory entrainment therapy to promote NNS and ororythmic pattern stabilization.
The study results show a significant improvement in NNS in preterm infants with various degrees of illness through the use of the pulsed pacifier controlled by the NTrainer device. RDS and CLD babies increased several NNS performance measures as a result of the PULSED treatment condition. These findings may relate to the gestational age at birth, with the CLD babies being born at a younger average GAbirth. This may also be a function of the very different NICU cares and therapies required in these babies. Brain development and responses to oral stimulation have different characteristics in these babies. Understanding these characteristics can lead to tailored clinical strategies, using the N Trainer device, according to the baby’s clinical condition and history. Algorithms could be developed that could be specific for different disease states. The NTrainer device used in this study is easily used and is very portable. This study shows that the NTrainer device is promising as a potential therapeutic strategy for babies in NICU with various clinical disorders who need to develop oral feeding skills in order to be safely discharged home with adequate oral nutrition.
Supplementary Material
Acknowledgments
This study was supported by grants NIH R01 DC003311 (SM Barlow), NIH P30 HD02528, and the Sutherland Foundation. The authors express gratitude to Joy Carlson, NNP and Mimi Burch, MS, for clinical support in patient recruitment and data collection, Drs. Meredith P. Harold and Emily Zimmerman for experimental support, Kenny Aron for software technical support, and the scores of families who participated in this project. This paper is dedicated to Anna M. Dusick, MD (1955–2012), an outstanding clinical scientist and neurodevelopmentalist, who encouraged the first author to pursue studies on oral sensorimotor development in the preterm infant.
Footnotes
FINANCIAL DISCLOSURE
None of the authors have a direct financial relation with the manufacturers of the Soothie® pacifier, Honeywell Sensors, nor with the SAS statistical software. Dr. Barlow is the inventor of the NTrainer System® which is registered and licensed by the University of Kansas to Innara Health, Incorporated (Shawnee, Kansas USA).
References
- 1.Lau C. Oral feeding in the preterm infant. Neoreviews. 2006;7:19–27. [Google Scholar]
- 2.Hack M, Estabrook MM, Robertson SS. Development of sucking rhythm in preterm infants. Early Hum Dev. 1985;11:133–140. doi: 10.1016/0378-3782(85)90100-8. [DOI] [PubMed] [Google Scholar]
- 3.Mizuno K, Ueda A. Neonatal feeding performance as a predictor of neurodevelopmental outcome at 18 months. Dev Med Child Neurol. 2005;47:299–304. doi: 10.1017/s0012162205000587. [DOI] [PubMed] [Google Scholar]
- 4.Medoff-Cooper B, Shults J, Kaplan J. Sucking behavior of preterm neonates as a predictor of developmental outcomes. J Dev Behav Pediatrics. 2009;30:16–22. doi: 10.1097/DBP.0b013e318196b0a8. [DOI] [PubMed] [Google Scholar]
- 5.DiPietro JA, Cusson RM, Caughy MO, Fox NA. Behavioral and physiologic effects of nonnutritive sucking during gavage feeding in preterm infants. Pediatr Res. 1994;36 :207–214. doi: 10.1203/00006450-199408000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Gill NE, Behnke M, Conlon M, Anderson GC. Nonnutritive sucking modulates behavioral state for preterm infants before feeding. Scand J Caring Sci. 1992;6:3–7. doi: 10.1111/j.1471-6712.1992.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 7.Pickler RH, Frankel HB, Walsh KM, Thompson NM. Effects of nonnutritive sucking on behavioral organization and feeding performance in preterm infants. Nurs Res. 1996;45:135. doi: 10.1097/00006199-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Pickler RH, Higgins KE, Crummette BD. The effect of nonnutritive sucking on bottlefeeding stress in preterm infants. J Obstet Gynecol Neonatal Nurs. 1993;22:230–234. doi: 10.1111/j.1552-6909.1993.tb01804.x. [DOI] [PubMed] [Google Scholar]
- 9.Field T. Sucking for stress reduction, growth and development during infancy. Pediatric Basics. 1993;64:13–16. [Google Scholar]
- 10.McCain GC. Promotion of preterm infant nipple feeding with nonnutritive sucking. J Pediatr Nurs. 1995;10:3–8. doi: 10.1016/S0882-5963(05)80093-4. [DOI] [PubMed] [Google Scholar]
- 11.Pinelli J, Symington AJ. The Cochrane Collaboration. Non-nutritive sucking for promoting physiologic stability and nutrition in preterm infants. Vol. 6. John Wiley & Sons, Ltd The Cochrane Library; 2010. pp. 1–34. [Google Scholar]
- 12.Boiron M, Da Nobrega L, Roux S, Nenrot A, Saliba E. Effects of oral stimulation and oral support on non-nutritive sucking and feeding performance in preterm infants. Dev Med Child Neurol. 2007;49:439–444. doi: 10.1111/j.1469-8749.2007.00439.x. [DOI] [PubMed] [Google Scholar]
- 13.Harding C. An evaluation of the benefits of non-nutritive sucking for premature infants as described in the literature. Arch Dis Child. 2009;94:636–640. doi: 10.1136/adc.2008.144204. [DOI] [PubMed] [Google Scholar]
- 14.Pickler RH, Reyna B. Effects of non-nutritive sucking on nutritive sucking, breathing, and behavior during bottle feedings of preterm infants. Advances in Neonatal Care. 2004;4:226–234. doi: 10.1016/j.adnc.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Rocha AD, Moreira MEL, Pimenta HP, Ramos JRM, Lucena SL. A randomized study of the efficacy of sensory-motor-oral stimulation and non-nutritive sucking in very low birth weight infant. Early Hum Dev. 2007;83:385–388. doi: 10.1016/j.earlhumdev.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Bingham P, Ashikaga T, Abbasi S. Prospective study of non-nutritive sucking and feeding skills in premature infants. Arch Dis Child Fetal Neonatal Ed. 2010;95:F194–F200. doi: 10.1136/adc.2009.164186. [DOI] [PubMed] [Google Scholar]
- 17.Als H. A manual for naturalistic observation of the newborn (preterm and full term infants) In: Goldson E, editor. Nurturing the Premature Infant, Developmental Interventions in the Neonatal Intensive Care Nursery. Oxford University Press; New York, NY: 1995. pp. 77–85. [Google Scholar]
- 18.SAS Institute. SAS/STAT 9.2 user’s guide. Cary, NC: SAS Institute Inc; 2002–2012. [Google Scholar]
- 19.Penn AA, Shatz CJ. Brain waves and brain wiring: the role of endogenous and sensory-driven neural activity in development. Pediatr Res. 1999;45:447–458. doi: 10.1203/00006450-199904010-00001. [DOI] [PubMed] [Google Scholar]
- 20.Limperopoulos C, Gauvreau KK, O’Leary H, Moore M, Bassan H, Eichenwald EC, Soul JS, Ringer SA, Di Salvo DN, du Plessis AJ. Cerebral hemodynamic changes during intensive care of preterm infants. Pediatrics. 2008;122:1009–1013. doi: 10.1542/peds.2008-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finan DS, Barlow SM. Mechanosensory modulation of non-nutritive sucking in human infants. Early Hum Dev. 1998;52:181–197. doi: 10.1016/s0378-3782(98)00029-2. [DOI] [PubMed] [Google Scholar]
- 22.Zimmerman E, Barlow SM. Pacifier stiffness alters the dynamics of the suck central pattern generator. J Neonatal Nurs. 2008;14:79–86. doi: 10.1016/j.jnn.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oder A, Stalling D, Barlow SM. Effects of pacifier texture on NNS in neurotypical infants. Int J Pediatrics. 2013 doi: 10.1155/2013/168459. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ihara Y, Nakayama K, Nakamura S, Mochizuki A, Takahashi K, Inoue T. Coordination of NMDA-induced rhythmic activity in the trigeminal and hypoglossal nerves of neonatal mice in vitro. Neuroscience Research. 2013;75:138–149. doi: 10.1016/j.neures.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Ishihama K, Kogo M, Koizumi H, Nomura K, Tanaka S, Yamanishi T, Enomoto A. Oral-motor patterns of rhythmic trigeminal activity generated in fetal rat brainstem in vitro. Dev Brain Res. 2003;145:163–166. doi: 10.1016/s0165-3806(03)00220-7. [DOI] [PubMed] [Google Scholar]
- 26.Barlow SM, Lund JP, Estep M, Kolta A. Central pattern generators for speech and orofacial activity. In: Brudzynski SM, editor. Handbook of Mammalian Vocalization. Elsevier; Oxford: 2010. pp. 351–370. [Google Scholar]
- 27.Barlow SM, Urish M, Venkatesan L, Harold M, Zimmerman E. Frequency modulation (FM) and spatiotemporal stability of the sCPG in preterm infants with RDS. Int Journal Pediatrics. 2012 doi: 10.1155/2012/581538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bosma JF. Summarizing and perspective comments: Part V. Form and function in the infant’s mouth and pharynx. In: Bosma JF, editor. Second Symposium on Oral Sensation and Perception. Charles C. Thomas; Springfield IL: 1970. pp. 550–555. [Google Scholar]
- 29.Fucile S, Gisel E, Lau C. Effect of an oral stimulation program on sucking skill maturation of preterm infants. Dev Med Child Neurol. 2005;47:158–162. doi: 10.1017/s0012162205000290. [DOI] [PubMed] [Google Scholar]
- 30.Fucile S, Gisel EG, McFarland DH, Lau C. Oral and non-oral sensorimotor interventions enhance oral feeding performance in preterm infants. Dev Med Child Neurology. 2011;53:829–835. doi: 10.1111/j.1469-8749.2011.04023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fucile S, McFarland DH, Gisel EG, Lau C. Oral and nonoral sensorimotor interventions facilitate suck-swallow-respiration functions and their coordination in preterm infants. Early Hum Dev. 2012;88:345–350. doi: 10.1016/j.earlhumdev.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gewolb IH, Vice FL. Abnormalities in the coordination of respiration and swallow in preterm infants with bronchopulmonary dysplasia. Dev Med Child Neurology. 2006;48 :595–599. doi: 10.1017/S0012162206001241. [DOI] [PubMed] [Google Scholar]
- 33.White-Traut RC, Berbaum ML, Lessen B, McFarlin B, Cardenas L. Feeding readiness in preterm infants: the relationship between preterm behavioral state and feeding readiness behaviors and efficiency during transition from gavage to oral feeding. Am J Matern Child Nurs. 2005;30:52–59. [PubMed] [Google Scholar]
- 34.Estep M, Barlow SM, Vantipalli R, Finan D, Lee J. Non-nutritive suck parameters in preterm infants with RDS. J Neonatal Nurs. 2008;14:28–34. doi: 10.1016/j.jnn.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bosma JF. Prologue to the symposium. In: Bosma JF, editor. Fourth Symposium on Oral Sensation and Perception. Charles C. Thomas; Bethesda MD: 1973. p. 7. [PubMed] [Google Scholar]
- 36.Shiao S-YPK, Youngblut JM, Anderson GC, DiFiore JM, Martin RJ. Nasogastric tube placement: effects on breathing and sucking in very-low-birth-weight infants. Nurs Res. 1995;44:82–88. [PubMed] [Google Scholar]
- 37.Stumm S, Barlow SM, Estep M, Lee J, Cannon S, Gagnon K, Carlson J, Finan D. The relation between respiratory distress syndrome and the fine structure of the non-nutritive suck in preterm infants. J Neonatal Nursing. 2008;14(1):9–16. doi: 10.1016/j.jnn.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song D, Jegatheesan P, Weiss S, Govindaswami B, Wang J, Lee J, Oder A, Barlow SM. Spectral edge frequency of EEG during patterned pneumatic oral stimulation in preterm infants. Pediatric Research. 2013 doi: 10.1038/pr.2013.179. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barlow SM, Jegatheesan P, Weiss S, Govindaswami B, Wang J, Lee J, Oder A, Song D. Amplitude-integrated EEG and range-EEG modulation associated with pneumatic orocutaneous stimulation in preterm infants. J Perinatology. 2013 doi: 10.1038/jp.2013.150. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barlow SM, Finan DS, Chu S, Lee J. Patterns for the premature brain: Synthetic orocutaneous stimulation entrains preterm infants with feeding difficulties to suck. J Perinatology. 2008;28:541–548. doi: 10.1038/jp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.