Abstract
Background
Cystinuria, one of the first recognized inborn errors of metabolism, has been reported in many dog breeds.
Hypothesis/Objectives
To determine urinary cystine concentrations, inheritance and mutations in the SLC3A1 and SLC7A9 genes associated with cystinuria in 3 breeds.
Animals
Mixed and purebred Labrador Retrievers (n=6), Australian Cattle Dogs (6), Miniature Pinschers (4) and 1 mixed breed dog with cystine urolithiasis, relatives and control dogs.
Methods
Urinary cystinuria and aminoaciduria was assessed and exons of the SLC3A1 and SLC7A9 genes were sequenced from genomic DNA.
Results
In each breed, male and female dogs, independent of neuter status, were found to form calculi. A frameshift mutation in SLC3A1 (c.350delG) resulting in a premature stop codon was identified in autosomal-recessive (AR) cystinuria in Labrador Retrievers and mixed breed dogs. A 6 bp deletion (c.1095_1100del) removing 2 threonines in SLC3A1 was found in autosomal-dominant (AD) cystinuria with a more severe phenotype in homozygous than in heterozygous Australian Cattle Dogs. A missense mutation in SLC7A9 (c.964G>A) was discovered in AD cystinuria in Miniature Pinschers with only heterozygous affected dogs observed to date. Breed specific DNA tests were developed, but the prevalence of each mutation remains unknown.
Conclusions and clinical importance
These studies describe the first AD inheritance and the first putative SLC7A9 mutation to cause cystinuria in dogs and expand our understanding of this phenotypically and genetically heterogeneous disease, leading to a new classification system for canine cystinuria and better therapeutic management and genetic control in these breeds.
Keywords: Metabolic disease, urolithiasis, nephropathy, hereditary disease
Cystinuria (OMIA 000256-9615) is one of the first inborn errors of metabolism recognized by Sir Archibald Garrod 1,2 and is an inherited selective renal transport defect 3,4 involving cystine and the dibasic amino acids ornithine, lysine and arginine, collectively known as COLA.4 In contrast to the normal near complete reabsorption of COLA in the proximal renal tubules, these amino acids reach high concentrations in the urine in affected individuals, but only cystine causes a clinical problem.5 The low solubility of cystine in acidic and neutral urine may lead to the formation of cystine crystals and uroliths in the urinary tract,6 which result clinically in stranguria, hematuria, urinary obstruction and renal failure.7,8
In 1823, cystinuria was the first reported inborn error of metabolism in dogs,9 and now is known to affect >70 canine breeds according to reports of veterinary urolith analysis laboratories worldwide.10,11 Based upon the varied clinical presentations, metabolic derangements and genetic studies, we previously divided canine cystinuria into type I and non-type I cystinuria.12 Type I cystinuria has been characterized in Newfoundlands and Landseers,13 and causes massive aminoaciduria of COLA and juvenile to adult onset calculi formation. It is an autosomal recessive trait affecting both males and females independent of neutering status, although more often cystinuric males show clinical signs of urinary obstruction, presumably due to anatomical differences between males and females.13 In non-type I cystinuria, exemplified by Mastiffs and related breeds, Scottish Deerhounds, and Irish Terriers, only intact adult males show variable degrees of aminoaciduria and the average age of urolith formation is later than in Newfoundlands. The molecular basis and mode of inheritance of non-type I cystinuria remain unknown, but it is not an X-chromosomal disorder and appears to be testosterone dependent. a,14
Two genes, SLC3A1 and SLC7A9, encode the subunits of b0,+, the basic amino acid transporter system. 4,15,16 The SLC3A1 gene encodes the extracellular heavy chain referred to as rBAT, and the SLC7A9 gene the light chain called b0,+AT.3 The subunit b0,+AT has 12 transmembrane domains typical of cell membrane transporters and heterodimerizes with rBAT exclusively to form the COLA amino acid transporter.17 Among all the cystinuric dogs to date, only 1 mutation in SLC3A1, an early stop codon precluding the production of the rBAT protein, and leading to the loss of b0,+ function, was identified in cystinuric Newfoundlands in 2000.18 And although the disease was widely recognized in the breed, screening programs for the mutation have markedly decreased the incidence of cystinuria in Newfoundlands and Landseers worldwide.
We report here the clinical, metabolic and molecular genetic characterization of cystinuric Labrador Retrievers, Australian Cattle Dogs, Miniature Pinschers and mixed breed dogs. Because our data demonstrate multiple genetic etiologies and modes of inheritance for cystinuria, we propose a new expanded classification system for canine cystinuria.
Materials and Methods
Dogs of 3 different breeds and mixed breed dogs presented with cystine uroliths and relatives of these dogs were included in the study. Voided urine and EDTA-anticoagulated blood samples were sent chilled or frozen to the Metabolic Genetics Laboratory at the University of Pennsylvania.b Archival urine and EDTA-blood samples were used as controls. Medical records and pedigree information were reviewed, as provided. These studies were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Genetic nomenclature is in accordance with the Guidelines and Recommendations for Mutation Nomenclature by the Human Genome Variation Society.19 All laboratory procedures were performed by standard techniques that are described in the Supplementary Materials. Urine was evaluated for hexagonal crystalluria by microscopic sediment evaluation,20 crystallographic calculi analysis, urinary nitroprusside testing for cystine21 and urine amino acid quantitation. Molecular genetic studies included genomic DNA isolation, polymerase chain reaction (PCR) amplication and sequencing of the SLC3A1 and SLC7A9 genes and assays to detect specific DNA mutations identified in this study.
Results
Clinicopathological Findings
Dogs with cystine urolithiasis included 4 purebred (LABR 1-4) and 2 mixed breed (LABX 1-2) Labrador Retrievers, 6 Australian Cattle Dogs (AUCD 1-6), 1 mixed breed dog (MIXB 1), and 4 Miniature Pinschers (MINP 1-4). Control dogs with no clinical signs of cystinuria included Labrador Retrievers (LABR 5-7 related to LABR 4 and LABR 8-11), Australian Cattle Dogs (AUCD 7-10 related to AUCD 2) and Miniature Pinschers (MINP 5-13, all related to MINP 1-4) and historical urine amino acid analyses. Uroliths, surgically removed by cystotomy, consisted of 100% cystine, based on crystallographic analyses by various commercial urolith laboratories.
All 5 male mixed and purebred Labrador Retrievers, 4 of them castrated at least 3 months before testing, developed voiding problems due to cystine calculi at ages ranging from 6 to 14 months. The spayed female LABR 4 showed no evidence of cystine calculi in bladder and kidney until 7 years of age, requiring unilateral nephrectomy and cystotomy at that time (Table 1). Available dogs related to LABR 4 did not show any urinary tract signs and were 7, 12 and 13 years old at the time of testing. Dogs in an additional Labrador family (LABR 8-11), related to a cystine stone former that was not available for the study, also showed no urinary tract signs.
Table 1.
Comparison of clinicalpathologic, metabolic and genetic findings in cystinuric Labrador Retrievers, Labrador mixed breed dogs, Australian Cattle Dogs and Miniature Pinschers (M=male, MC=male castrated, F=female, FS=female spayed, 1/1=normal genotype, 1/2=heterozygous for the mutation, 2/2=homozygous for the mutation);
| Dog # |
Sex
(n) |
Initial Signs
(years) |
Amino Acid Quantitation in the Urine (μmol/g Creatinine) * | Genotype | ||||
|---|---|---|---|---|---|---|---|---|
| Cystine | Ornithine | Lysine | Arginine | COLA | ||||
| Labrador Retriever |
SLC3A1
c.350delG |
|||||||
| LABR 1-3 | M/MC (1/2) |
0.7 | 355 ** | 289 ** | 5,708 ** | 7,213 ** | 13,565 ** | 2 / 2 |
| LABR 4 | F (1) |
7 | 5 | 1,099 | 2,143 | 81 | 3,328 | 2 / 2 |
| LABX 1-2 | MC (2) |
1.1 | 834 | 557 | 4,660 | 4,200 | 10,250 | 2 / 2 |
| Australian Cattle Dog |
SLC3A1
c.1095_1100del |
|||||||
| AUCD 1, 3-4 | M/MC (1/2) |
0.7 | 716 ± 474 | 723 ± 179 | 5,852 ± 3,151 | 5,376 ± 1,163 |
12,667 ± 4,068 |
2 / 2 |
| AUCD 6 | FS (1) |
4 | 822 | 510 | 4,052 | 4,852 | 10,236 | 2 / 2 |
| AUCD 2,5 | M/MC (1/1) |
5 | 1,253** | 214** | 2,339** | 228** | 4,034 ** | 2 / 1 |
| MIXB 1 | MC (1) |
2 | 98 | 2,756 | 3,912 | 4,211 | 10,977 | 2 / 2 |
| Miniature Pinscher |
SLC7A9
c.964G>A |
|||||||
| MINP 1-4 | M/MC (3/1) |
1.7 | 386 ± 234 | 149 ± 14 | 255 ± 118 | 96 ± 66 | 737 ± 272 | 2 / 1 |
| MINP 5-8 | F (4) |
4 | 485 ± 305 | 67 ± 5 | 355 ± 385 | 61 ± 38 | 918 ± 719 | 2 / 1 |
|
Normal
Upper Limit |
≤ 178 | ≤ 100 | ≤ 200 | ≤ 100 | ≤ 500 | 1 / 1 | ||
Mean value (± SD for ≥ 3 dogs);
COLA values only available for LABR 1 / AUCD 2
Among the Australian Cattle Dogs studied, all from different parts of the United States, 2 neutered males and 1 intact male developed urinary tract signs due to cystine uroliths within their first year of life, whereas the 2 other affected males and the female did not show signs of urinary obstruction until 4-5 years of age (Table 1). The available dogs related to AUCD 2 did not show any clinical signs of cystinuria.
In addition, a male castrated mixed-breed dog (MIXB 1), obtained from a shelter, experienced urinary obstruction due to cystine calculi twice by 2 years of age. This dog has been managed with urinary alkalization, diet, and tioproninc and has since not become obstructed again as of the time of this study, at age 5 years.
The family of 13 Miniature Pinschers (MINP 1-13) was comprised of 7 male dogs, 1 of them neutered, and 6 intact females. Two males, 1 intact and 1 neutered, as well as 2 females had been diagnosed with problems urinating due to cystine uroliths and crystalluria. MINP 1 first developed signs of cystinuria at 11 months of age, whereas the other male and the females were first recognized as affected at the age of 2 years and older (Table 1). All other related dogs showed no clinical signs of cystinuria.
Metabolic Urine Analyses
Urine samples from Labrador Retrievers and Australian Cattle Dogs with a history of cystine calculi formation all were strongly positive by the cyanide-nitroprusside test, whereas this test was negative for the related dogs of either breed and also all unrelated dogs, if tested. The stone formers included male and females dogs and the cystine test results were positive even after neutering. For the Miniature Pinschers urine samples were not available for the cyanide-nitroprusside test.
Similarly, quantitative amino acid determination identified increased urinary cystine, ornithine, lysine and arginine concentrations and thus high COLA values (Table 1), but normal urinary concentrations for other amino acids (not shown). In 1 stone-forming dog (LABR 4), cystine was found by the nitroprusside test, but had a normal quantitative cystine result, perhaps due to precipitation of excess cystine in the urine during storage or laboratory error. Interestingly, among the early Australian Cattle Dog stone formers the COLA values were at least twice as high as those from the male with later stone formation. Urinary nitroprusside test results were negative (COLA values were not determined) for all related Labrador Retrievers and Australian Cattle Dogs that showed no clinical signs of cystinuria. In the Miniature Pinschers, all 4 cystine stone formers and 1 other male were strongly cystinuric, and 1 male and 2 females had moderately increased COLA concentrations. The degree of increase of urinary COLA concentrations did not correlate with the observation of stone formation or age. Of note, some cystinuric Miniature Pinschers showed higher urine cystine concentrations than the combined concentrations for ornithine, lysine and arginine.
DNA Sequencing and Mutation Analyses
All exonic DNA sequence differences in SLC3A1 and SLC7A9 genes between the CanFam 3.1 canine reference genome assembly and cystine stone-forming dogs LABR 2, LABX 1, AUCD 1 and 5, MIXB 1 as well as MINP 8 are shown in Table 2. Neither the mutation found to cause cystinuria in the Newfoundlands,18 nor the formerly described amino acid polymorphisms in exon 6 (I192V) and 10 (S698G) of SLC3A1 and exon 5 (A217T) of SLC7A9 in English Bulldogs were observed in any of the dogs studied here.22 The sequence differences resulting in cystinuria are detailed below for each breed studied.
Table 2.
Differences in the coding and flanking intronic sequences of SLC3A1 and SLC7A9 of 5 cystine stone formers (LABR 2, LABX 1, AUCD 1, AUCD 5, MIXB 1 and MINP 8) to the canine genome reference sequence.
| Gene | Exon/ Intron |
SNP | Protein | Heterozygous | Homozygous |
|---|---|---|---|---|---|
| SLC3A1 | Exon 1 | c.350delG | p.Gly117Alafs*41 |
LABR 2
LABX 1 |
|
| Exon 1 | c.378G>A | Silent | LABR 2 LABX 1 |
||
| Exon 6 | c.1095_1100del | p.Thr366_Thr367del | AUCD 5 |
AUCD 1
MIXB 1 |
|
| Exon 7 | c.1308G>C | Silent | AUCD 5 | AUCD 1 MIXB 1 MINP 8 |
|
| SLC7A9 | Intron2 | c.96+8T>C | LABR 2 LABX 1 |
||
| Exon 4 | c.267C>T | Silent | AUCD 5 | AUCD 1 LABX 1 |
|
| Intron 4 |
c.561+11A>G
a. +13G>A |
MIXB 1 MINP 8 |
|||
| Intron 7 | c.759-8G>A | LABX 1 | |||
| Exon 9 | c.928C>T | Silent | AUCD 1 | ||
| Exon 9 | c.964G>A | p.Gly322Arg | MINP 8 | ||
| Intron 12 | c.1409-25G>T | LABR 2 | LABX 1 AUCD 1 |
Mixed and Purebred Labrador Retrievers
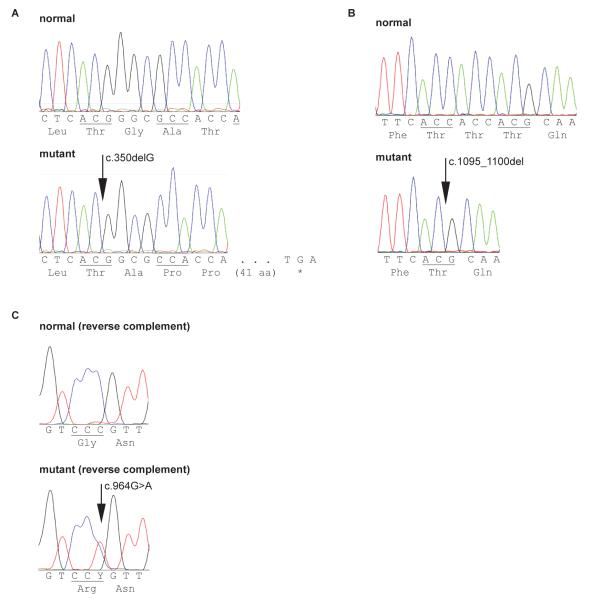
A homozygous 1 bp deletion (c.350delG) in exon 1 of the SLC3A1 gene was found in the DNA from the 1 cystinuric Labrador Retriever and 1 Labrador mixed breed dog (LABR 2 and LABX 1). The deleted guanine in codon GGC (coding for glycine) causes a shift of the open reading frame (p.Gly117Alafs*41) leading to a premature stop codon 41 codons later (Fig. 1A).
Fig. 1.
Sequencing chromatograms displaying the mutations found and their effect on the protein sequence. A) A 1 bp deletion in SLC3A1 exon 1 in purebred and mixed breed Labrador Retriever and causing a frameshift and a premature stop codon to truncate the translated protein. B) A 6 bp in frame deletion in SLC3A1 exon 6 in Australian Cattle Dogs and a mixed breed dog deleting 2 threonines in a repeat of 3. C) A single nucleotide exchange in SLC7A9 exon 9 found heterozygous in cystinuric Miniature Pinschers, changing the amino acid from glycin to arginine.
Based on SLC3A1 exon 1 sequencing, the additional 3 cystinuric Labrador Retrievers as well as the cystinuric Labrador-mixed breed dog also were homozygous for the single bp. Among the related non-cystinuric Labrador Retrievers tested, the parents of the affected LABR 4 were heterozygous for the deletion, whereas the 1 available littermate had 2 normal alleles. In the unrelated Labrador family (LABR 5-8), 1 parent and 1 female offspring were heterozygous for the mutation. Exon 1 of SLC3A1 contains 2 polymorphic hexamer repeat sequences, GAGCCC and GTCGGG, which have been shown to be polymorphic within and among dog breeds.18,22 All sequenced mixed and purebred Labrador Retrievers were homozygous and had 8 copies of the GAGCCC sequence and 6 copies of the GTCGGG sequence (referred to as haplotype 8/6).
Australian Cattle Dogs
A heterozygous deletion of 6 bases (c.1095_1100del) was found in exon 6 of the SLC3A1 gene from AUCD 2. This deletion removes 2 of 3 consecutive threonines (p.Thr366_Thr367del) from the rBAT polypeptide (Fig. 1B). Sequencing of this exon in other cystinuric Australian Cattle Dogs showed that 4 were homozygous (AUCD 1, 3-4 and 6) and 2 were heterozygous (AUCD 2 and AUCD 5) for this deletion. Two dogs related to AUCD 2 were homozygous for the normal allele, whereas 1 young male also was heterozygous for the deletion (lost to follow-up and no urine available for study). Only the 7/6 haplotype for the hexamer repeats in exon 1 was observed in the sequenced Australian Cattle Dogs.
Mixed Breed Dog
Interestingly, the cystinuric dog MIXB 1 had the same homozygous 6 bp deletion in the SLC3A1 gene as found in the Australian Cattle Dogs. This was an unexpected finding, given the physical appearance of MIXB 1. Because of the simple sequence repeat nature of the 6 bp deletion, potentially predisposing this region for a high mutation rate, we examined the possibility that the same deletion event occurred independently in Australian Cattle Dogs and in the ancestors of MIXB 1. Examination of all exons and some flanking intronic regions in the SLC3A1 gene indicated that this dog was entirely homozygous for the same silent and intronic polymorphisms (Table 2) observed in the homozygous affected Australian Cattle Dogs, compared to the canine reference genome sequence. The hexameric repeat haplotype was 7/6 as seen in the Australian Cattle Dogs.
Miniature Pinschers
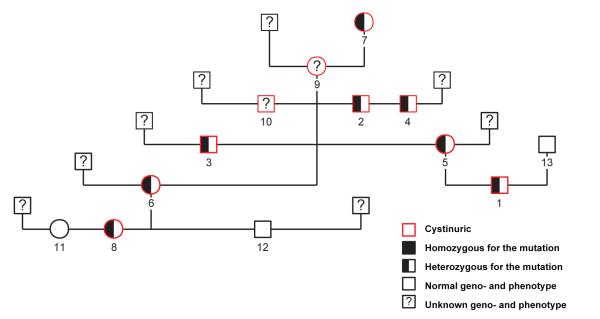
In exon 9 of the SLC7A9 gene, a single base change (c.964G>A) was detected, causing the substitution of a large positively charged, hydrophilic arginine for the very small, hydrophobic glycine residue (p.Gly322Arg); (Fig. 1C). All additional cystinuric Miniature Pinschers (as defined by stone formation or increased urine COLA concentrations) studied were found to be heterozygous for this missense mutation, whereas all dogs homozygous for the normal allele had normal urinary COLA concentrations and no history of calculi formation (Fig. 2). The exonic sequence of the SLC3A1 gene did not indicate any mutations, and the observed number of hexameric repeats in exon 1 was 7/5 in all Miniature Pinschers tested.
Fig. 2.
Pedigree of the Miniature Pinscher family displaying the genotype and the phenotype of the dogs studied, showing their correlation and the inheritance in an autosomal dominant trait
DNA Mutation Tests
The presence of the mutations in Labrador Retrievers and Miniature Pinschers can be easily detected by the established TaqMan SNP genotyping assaysd , and retesting all studied DNA samples by this method confirmed the genotype results from sequencing.
A simple DNA amplification of a small region surrounding the 6 bp deletion found in Australian Cattle Dogs readily differentiates between the normal and mutant allele due to the 6 bp fragment size difference and can be used as a screening test to discriminate heterozygous or homozygous affected or normal genotypes (Fig. 3).
Fig. 3.
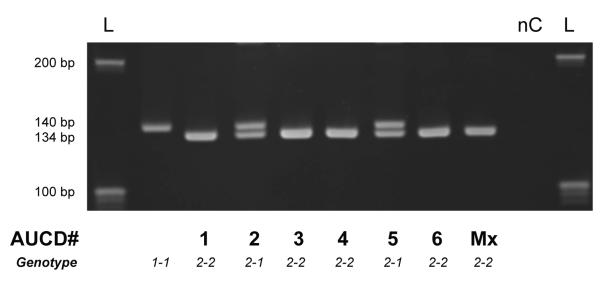
Fragment length analysis on a 5% polyacrylamid gel, discriminating the normal DNA amplicon of 140 bp from the smaller fragment of 134 bp in presence of the 6 bp deletion in SLC3A1 exon 6 found in cystinuric Australian Cattle Dogs (see AUCD #) and a mixed breed dog (here Mx)
Breed and Mixed Breed Determination
Because these molecular genetic studies included purebred and mixed breed dogs, the breed composition of some dogs was examined using the Wisdom Panel Teste. The cystinuric Labrador mixed breed dogs were reported to have Labrador contributions. The 2 heterozygous cystinuric Australian Cattle Dogs were found to be purebred Australian Cattle Dogs, as stated by their owners.
The mixed breed dog (MIXB 1), weighing 18.5 lbs and measuring 16.5 inches at the shoulder with a white soft long hair coat, had a breed constitution of 1/4 each from Miniature Poodle, Chihuahua, and Shi Tzu, with several other breeds constituting the last quarter, but no evidence of Australian Cattle Dog. Despite the many breeds represented, this dog had a high degree of homozygosity (69%) for the informative 312 single nucleotide polymorphism (SNP) markers, which typically is seen with inbred and purebred dogs according to an extended report of the breed testing results.
The Miniature Pinschers were not subjected to breed testing because these dogs were registered with official pedigrees showing common ancestry. Interestingly, these dogs were from Europe (mostly from Germany) and belonged to a single 4-generation pedigree (Fig. 2).
Discussion
Although cystinuria is one of the classic inborn errors of metabolism and has been described in several species,12 the disease is phenotypically heterogeneous in humans and animals, and its molecular basis is not yet completely understood.12,23,24
The genetic studies in 3 breeds presented here document some of the heterogeneity in canine cystinuria. We identified a new mutation in the SLC3A1 gene in an autosomal recessive form of the disease, phenotypically and genetically similar to that previously described in Newfoundland dogs.13,18 We also characterized the first examples of both SLC3A1 and SLC7A9 gene mutations that are associated with autosomal dominant patterns of inheritance of cystinuria in dogs. These genes together encode the heteromeric b0,+ amino acid transporter.16,17 Unfortunately, we did not have access to kidneys of any cystinuric dogs for protein and mRNA studies, but computer modeling of the observed mutations and current knowledge of the protein structure and functional domains strongly suggest defective reabsorption by the transporter system b0,+. Based upon the genetic findings, we propose a novel classification nomenclature for cystinuria in dogs (Table 3), which also has important implications for management and genetic control.
Table 3.
New expanded classification system for canine cystinuria according to the novel metabolic and genetic findings (nd = not determined).
| Phenotype | Type I - A | Type II - A | Type II - B | Type III - | |
|---|---|---|---|---|---|
| Inheritance | Autosomal recessive | Autosomal dominant | Autosomal dominant | Sex limited | |
| Gene | SLC3A1 | SLC3A1 | SLC7A9 | Undetermined | |
| Sex | Males and Females | Males and Females | Males and Females | Intact Adult Males | |
| Androgen dependence | No | No | No | Yes | |
|
COLA
μmol/g creatinine (normal ≤ 500) |
homozygous | ≥ 8,000 | ≥ 8,000 | nd | ≤ 4,000 |
| heterozygous | ≤ 500 | ≥ 3,000 | ≥ 700 | ||
| Breeds |
Newfoundland
Landseer Labrador |
Australian Cattle Dog | Miniature Pinscher |
Mastiff &
Related Breeds Scottish Deerhound Irish Terrier |
|
Similar to cystinuria in the Newfoundland and Landseer breeds, cystinuria in Labrador Retrievers is caused by an early frameshift mutation (c.350delG) in the SLC3A1 gene, leading to a premature stop codon that truncates the rBAT protein to 157 amino acids instead of 784 (Fig. 1A). The early termination likely causes accelerated RNA decay and decreased or no protein synthesis. Regardless of the amount of protein, the truncated protein would only contain 13 amino acids of the extracellular domain, and would likely be unstable and unable to dimerize precluding normal function. Several human type I cystinuric patients with premature stop mutations in the amino terminal region of the protein show partial or complete loss of the rBAT protein.25 Only Labrador Retrievers homozygous for the deletion were cystinuric and both males and females, regardless of neuter status, were cystinuric and developed cystine calculi early in life, albeit more frequently and earlier in males (by 1.1 years in this study) than females, presumably due to anatomic differences (Table 1). Interestingly, the spayed female Labrador Retriever dog developed nephrolithiasis, which also has been reported in cystinuric Newfoundland dogs,13 but generally is rarely reported in dogs with any type of uroliths.
Labrador Retrievers that were heterozygous for this nonsense mutation neither showed clinical urinary tract signs nor positive nitroprusside urine test results, consistent with an autosomal recessive mode of inheritance as previously reported for the Newfoundland breed.13,18 Based upon information from urolith laboratories and particularly when considering the popularity of the Labrador Retriever breed, the incidence of cystinuria in this breed appears low.8,26 Although the information presented here can be used to devise genetic tests differentiating among affected, carrier (asymptomatic) and normal dogs, general screening of the population of pedigreed Labrador Retrievers currently is not recommended. Those Labrador Retrievers showing clinical signs of cystinuria should be screened by urine testing, followed by DNA testing of related dogs. And because the mutation seems rare, it is reasonable to consider elimination of carrier and cystinuric dogs from the large breeding stock of Labrador Retrievers without depleting the gene pool of the breed.
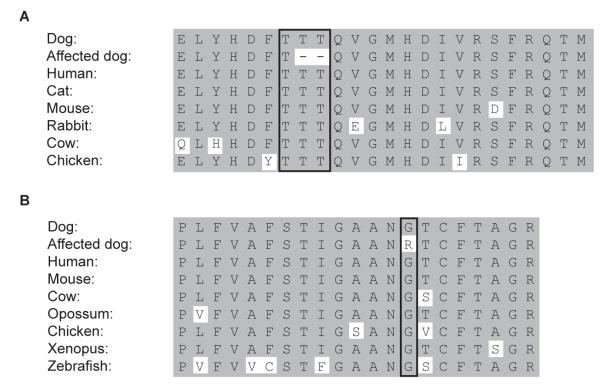
The cystinuric Australian Cattle Dogs had an in-frame 6 bp deletion removing 2 of the 3 adjacent threonine residues in exon 6 of the SLC3A1 gene (Fig. 1B). This mutation may have occurred during DNA replication by mispairing or polymerase slippage within the repeat of a CAC sequence. This portion of the rBAT protein is found in a highly conserved portion of the extracellular domain (Fig. 4A). This domain is responsible for substrate trafficking,27 suggesting the deletion may interfere with normal transport function. All dogs, male and female, homozygous or heterozygous for this deletion were cystinuric, but all homozygous males and the 1 homozygous female had higher urinary cystine and COLA concentrations than the heterozygous dogs (Table 1). Moreover, the homozygous males showed obstruction earlier in life than heterozygous male Australian Cattle Dogs. Again, the later age of onset of clinical signs in the homozygous female Australian Cattle Dog may be explained by the anatomic differences between the sexes. The heterozygous offspring of AUCD 2 was 3 months old when it tested negative by nitroprusside test and this dog unfortunately was not available for follow-up cystine and COLA testing. As the tested dam showed a normal genotype and cyanide nitroprusside test results, the sire of AUCD 2 likely was heterozygous for the deletion and therefore cystinuric, but this dog also was not available for study. Failure to detect any other mutations in the SLC3A1 or SLC7A9 genes excludes the possibility of compound heterozygotes with 2 different mutations in the same gene (common in people) or 1 in the SLC3A1 and 1 in the SLC7A9 gene.28 Therefore, cystinuria in the Australian Cattle Dog is inherited in an autosomal dominant trait; a similar mutation has not been found in humans. Although mutations in the SLC3A1 gene typically are inherited recessively in human patients, rare dominant traits have been documented.24
Fig. 4.
Alignment of the amino acid sequences from different species, surrounding A) the deletion of two threonines, found in SLC3A1 exon 6 in cystinuric Australian Cattle Dogs and a mixed breed dog, and B) the exchange of a glycine to an arginine, found in cystinuric Miniature Pinschers, both showing a highly conserved sequence in the protein.
More Australian Cattle Dogs need to be studied to determine the mutant allele prevalence in this breed. However, the cystinuric Australian Cattle Dogs studied here were from very different parts of the United States, ranging from the Southeast to the Northwest, suggesting that the defect may be somewhat widespread in the United States. Therefore, it might be appropriate to screen any Australian Cattle Dog intended for breeding or those showing clinical signs involving the urinary tract. Australian Cattle Dogs can be simply screened for cystinuria by examining urine for cystine crystals and performing the nitroprusside test, followed by using the established DNA test (Fig. 3) to differentiate between cystinuric heterozygotes and homozygotes.
Surprisingly, the mixed breed dog (MIXB 1) that formed cystine calculi early in life was also found to be homozygous for the same 6 bp deletion in SLC3A1 (c.1095_1100del). However, phenotypically, with small stature and somewhat curly white fur, this dog did not resemble an Australian Cattle Dog. Breed testing identified more than 3 breed contributions (but no Australian Cattle Dog) and also high homozygosity for the analyzed SNPs, suggesting close inbreeding. Moreover, this MIXB 1 dog also was homozygous for other variations within the SLC3A1 gene and identical to the homozygous cystinuric Australian Cattle Dogs (Table 2). These findings suggest that the SLC3A1 haplotypes in MIXB 1 and Australian Cattle Dogs are identical and they share a common ancestry rather than being caused by 2 separate events leading to the same mutation.
Finally we describe here the first putative mutation in the SLC7A9 gene associated with autosomal dominant cystinuria, in Miniature Pinschers from Europe. It is a single base missense mutation (c.964G>A) changing the small hydrophobic glycine residue to the larger, charged basic amino acid arginine (G322R) in transmembrane domain 9 of the light subunit bo,+AT (Fig. 1C), which is highly conserved in vertebrates as evolutionarily distant as zebrafish (Fig 4B). According to SIFTf analysis, which predicts the consequences of gene mutations on protein function based on known evolutionary conservation and 3-dimensional structure, this substitution is not compatible with normal function.
In the small family of Miniature Pinschers studied, only cystinuric heterozygous dogs were observed. The mutation segregated with increased urinary cystine concentrations throughout the entire pedigree, tracing back to a common heterozygous dam. Therefore, this missense mutation appears to cause an autosomal dominant form of cystinuria (Fig. 2). Although for dominantly inherited traits, homozygosity for the disease-causing allele can be lethal, this seems unlikely in cystinuria because homozygous individuals with SLC7A9 mutations have been observed in humans.24,25
Although we have no direct evidence explaining why this mutation causes a dominant type of cystinuria in dogs, we hypothesize that because this missense mutation does not affect the binding site to rBAT, it likely results in a malfunctional transporter channel, rather than inhibiting heterodimerization of the 2 subunits. Having a malfunctioning and normal functioning b0,+AT protein anchored in the membrane, may result in defective transport. Furthermore, it has been suggested that the COLA transporter is a heterotetramer formed from 2 heterodimers, potentially explaining abnormal or diminished transport when mutant and normal dimers interact.29 As indicated, all Miniature Pinschers heterozygous for the missense mutation in the SLC7A9 gene were cystinuric, and some repeatedly developed cystine calculi independent of sex and neuter status. The extent of COLA excretion was less severe than with the SLC3A1 mutations described above, but surprisingly seemed to cause relatively higher urinary concentrations of cystine compared to ornithine, lysine and arginine (Table 1). This is in contrast to observations in cystinuric Newfoundlands and dogs of other breeds studied here, where the sum of the dibasic amino acids always exceeded that of cystine alone. Additional studies are needed to define the molecular mechanism of this mutation altering the b0,+AT protein.
The prevalence of cystinuria in the Miniature Pinscher breed currently is unknown, but it is not a breed that has been reported to have a higher incidence of cystine urolithiasis by any urolith laboratory.11,26 All cystinuric Miniature Pinschers studied belonged to a single family. The single common ancestor was 11 years old when tested and its parents or other informative relatives were not available. Hence we do not know if the mutation originated in this dog, any of its ancestors or even in the line of the common sire with an unknown genotype (Fig. 2). Based upon our current knowledge, all Miniature Pinschers related to this family in Europe should be screened by the mutation-specific DNA test, particularly when related to any cystinuric dogs. Because it is a dominant trait, all dogs homozygous or heterozygous for the mutation should be excluded from breeding.
We had previously divided cystinuria in dogs into 2 types, with type I referring to an SLC3A1 mutation showing autosomal recessive inheritance and non-type I to cystinuria exhibiting a milder degree of cystinuria observed in mature, non-neutered male dogs of various breeds (unpublished data).a,14 Although the metabolic and genetic studies reported here in 3 additional canine breeds clearly expand our understanding of the heterogeneity of cystinuria in dogs, the molecular basis of cystinuria in many breeds has not been elucidated. For example, the fact that the average age of urolith-formation in the breeds with known associated mutations is much lower than the reported mean age of dogs when cystine calculi are removed (4.8 years to 5.6 years),7,30,31 and the fact that several breeds have been shown to be free from protein-coding mutations in the SLC3A1 and SCL7A9 genes,12,22 indicates that the basis for additional heterogeneity in canine cystinuria remains to be elucidated. And although cystinuria also is complex in humans and several classification systems have been proposed, no sex-linked or sex-limited forms of the disease have been identified.3,24 We therefore propose a classification system that encompasses both discriminating aspects of the phenotype (e.g., sex related, androgen dependence, mode of inheritance) and the gene associated with the disease (Table 3). We designate type I cystinuria when the disease shows autosomal recessive inheritance, type II when it shows autosomal dominant inheritance, and type III for sex-limited inheritance (unpublished data). Specific mutations within each type should lead to phenotypes that are sufficiently similar that the same medical management and breeding advice applies to all cases within that type. Involvement of the SLC3A1 gene is indicated by adding A, and similarly B indicates mutations in SLC7A9. If no letter is appended, the genetic basis is unknown. Alternatively, stating simply the mode of inheritance and mutant gene could be used. These recent discoveries will have major clinical impact including selection of the best clinical management and genetic control of cystinuria in future generations.
Supplementary Material
Acknowledgments
Supported in part by grants from the AKC Canine Health Foundation and NIH OD 010939. The authors thank the various referring clinicians and owners for their assistance with sample collection and LaboKlin, Germany for handling some of the samples.
This study was performed at the School of Veterinary Medicine, University of Pennsylvania, in part as fulfillment of the doctoral thesis work of the first author Ann-Kathrin Brons at the University of Zürich, Switzerland.
Abbreviations
- AD
autosomal dominant
- AR
autosomal recessive
- bp
base pair(s)
- DNA
deoxyribonucleic acid
- COLA
cystine, ornithine, lysine and arginine
- PCR
polymerase chain reaction
- SNP
single nucleotide polymorphism
Footnotes
Urs Giger, Jessica W. Lee, Cait Fitzgerald et al, Characterization Of Non-Type I Cystinuria In Irish Terriers, J Vet Int Med, 2011 Am Col Vet Int Med Forum Abstracts, 2011:25:718
Captimer®, Biokanol Pharma, Rastatt, Germany
Applied Biosystems, Foster City, CA
PennGen Laboratories, Philadelphia, PA
Mars Synbiotics, Gaithersburg, PA
Conflict of Interest Declaration: Authors disclose no conflict of interest.
References
- 1. [Last accessed: March 23, 2013];OMIA - Online Mendelian Inheritance in Animals. http://omia.angis.org.au/
- 2.Garrod A. The Croonian Lectures ON INBORN ERRORS OF METABOLISM. Lancet. 1908;172:1–7. [Google Scholar]
- 3.Chillaron J, Font-Llitjos M, Fort J, et al. Pathophysiology and treatment of cystinuria. Nat Rev Nephrol. 2010;6:424–434. doi: 10.1038/nrneph.2010.69. [DOI] [PubMed] [Google Scholar]
- 4.Palacin M, Goodyer P, Nunes V, et al. Cystinuria. In: Scriver CR, editor. The metabolic and molecular bases of inherited disease. 8th ed McGraw-Hill; New York: 2001. pp. 4909–4932. [Google Scholar]
- 5.Calonge MJ, Gasparini P, Chillaron J, et al. Cystinuria caused by mutations in rBAT, a gene involved in the transport of cystine. Nat Genet. 1994;6:420–425. doi: 10.1038/ng0494-420. [DOI] [PubMed] [Google Scholar]
- 6.Treacher RJ. Urolithiasis in the dog. II. Biochemical aspects. J Small Anim Pract. 1966;7:537–547. doi: 10.1111/j.1748-5827.1966.tb04483.x. [DOI] [PubMed] [Google Scholar]
- 7.Hoppe A, Denneberg T. Cystinuria in the dog: clinical studies during 14 years of medical treatment. J Vet Intern Med. 2001;15:361–367. [PubMed] [Google Scholar]
- 8.Osborne CA, Sanderson SL, Lulich JP, et al. Canine cystine urolithiasis. Cause, detection, treatment, and prevention. Vet Clin North Am Small Anim Pract. 1999;29:193–211. xiii. doi: 10.1016/s0195-5616(99)50011-9. [DOI] [PubMed] [Google Scholar]
- 9.Lassaigne J. Observation sur l’existence de l’oxide cystique dans un calcul vesical du chien, et essai analytique sur la composition élémentaire de cette substance particulière. Ann Chim Phys. 1823;23:328–334. [Google Scholar]
- 10.Ling GV, Franti CE, Ruby AL, et al. Urolithiasis in dogs. II: Breed prevalence, and interrelations of breed, sex, age, and mineral composition. Am J Vet Res. 1998;59:630–642. [PubMed] [Google Scholar]
- 11.Osborne CA, Lulich JP, Kruger JM, et al. Analysis of 451,891 canine uroliths, feline uroliths, and feline urethral plugs from 1981 to 2007: perspectives from the Minnesota Urolith Center. Vet Clin North Am Small Anim Pract. 2009;39:183–197. doi: 10.1016/j.cvsm.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Henthorn PS, Giger U. Cystinuria. In: Ostrander EA, Giger U, Lindblad-Toh K, editors. Cold Spring Harbor monograph series: The dog and its genome. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N.Y.: 2006. pp. 349–364. [Google Scholar]
- 13.Casal ML, Giger U, Bovee KC, et al. Inheritance of cystinuria and renal defect in Newfoundlands. J Am Vet Med Assoc. 1995;207:1585–1589. [PubMed] [Google Scholar]
- 14.Giger U, Sewell AC, Lui J, et al. Update on Fanconi Syndrome and Cystinuria in Dogs: Amino Acidurias. Am Coll Vet Intern Med Forum; Denver, CO: 2011. [Google Scholar]
- 15.Palacin M, Estevez R, Bertran J, et al. Molecular biology of mammalian plasma membrane amino acid transporters. Physiol Rev. 1998;78:969–1054. doi: 10.1152/physrev.1998.78.4.969. [DOI] [PubMed] [Google Scholar]
- 16.Palacin M, Nunes V, Font-Llitjos M, et al. The genetics of heteromeric amino acid transporters. Physiology (Bethesda) 2005;20:112–124. doi: 10.1152/physiol.00051.2004. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez E, Carrascal M, Rousaud F, et al. rBAT-b(0,+)AT heterodimer is the main apical reabsorption system for cystine in the kidney. Am J Physiol Renal Physiol. 2002;283:F540–548. doi: 10.1152/ajprenal.00071.2002. [DOI] [PubMed] [Google Scholar]
- 18.Henthorn PS, Liu J, Gidalevich T, et al. Canine cystinuria: polymorphism in the canine SLC3A1 gene and identification of a nonsense mutation in cystinuric Newfoundland dogs. Hum Genet. 2000;107:295–303. doi: 10.1007/s004390000392. [DOI] [PubMed] [Google Scholar]
- 19. [Last accessed: March 23, 2013];Guidelines and recommendations for mutation nomenclature. http://www.hgvs.org/mutnomen/
- 20.Osborne CA, O’Brien TD, Ghobrial HK, et al. Crystalluria. Observations, interpretations, and misinterpretations. Vet Clin North Am Small Anim Pract. 1986;16:45–65. doi: 10.1016/s0195-5616(86)50004-8. [DOI] [PubMed] [Google Scholar]
- 21.Brand E, Harris MM, Biloon S. Cystinuria. The excretion of a cystine complex which decomposes in the urine with the liberation of free cystine. J Biol Chem. 1930;86:315–331. [Google Scholar]
- 22.Harnevik L, Hoppe A, Soderkvist P. SLC7A9 cDNA cloning and mutational analysis of SLC3A1 and SLC7A9 in canine cystinuria. Mamm Genome. 2006;17:769–776. doi: 10.1007/s00335-005-0146-4. [DOI] [PubMed] [Google Scholar]
- 23.Bannasch D, Henthorn PS. Changing paradigms in diagnosis of inherited defects associated with urolithiasis. Vet Clin North Am Small Anim Pract. 2009;39:111–125. doi: 10.1016/j.cvsm.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eggermann T, Venghaus A, Zerres K. Cystinuria: an inborn cause of urolithiasis. Orphanet J Rare Dis. 2012;7:19. doi: 10.1186/1750-1172-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bisceglia L, Fischetti L, Bonis PD, et al. Large rearrangements detected by MLPA, point mutations, and survey of the frequency of mutations within the SLC3A1 and SLC7A9 genes in a cohort of 172 cystinuric Italian patients. Mol Genet Metab. 2010;99:42–52. doi: 10.1016/j.ymgme.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Houston DM, Moore AE. Canine and feline urolithiasis: examination of over 50 000 urolith submissions to the Canadian veterinary urolith centre from 1998 to 2008. Can Vet J. 2009;50:1263–1268. [PMC free article] [PubMed] [Google Scholar]
- 27.Broer S, Wagner CA. Structure-function relationships of heterodimeric amino acid transporters. Cell Biochem Biophys. 2002;36:155–168. doi: 10.1385/CBB:36:2-3:155. [DOI] [PubMed] [Google Scholar]
- 28.Dello Strologo L, Pras E, Pontesilli C, et al. Comparison between SLC3A1 and SLC7A9 cystinuria patients and carriers: a need for a new classification. J Am Soc Nephrol. 2002;13:2547–2553. doi: 10.1097/01.asn.0000029586.17680.e5. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez E, Jimenez-Vidal M, Calvo M, et al. The structural and functional units of heteromeric amino acid transporters. The heavy subunit rBAT dictates oligomerization of the heteromeric amino acid transporters. J Biol Chem. 2006;281:26552–26561. doi: 10.1074/jbc.M604049200. [DOI] [PubMed] [Google Scholar]
- 30.Brown NO, Parks JL, Greene RW. Canine urolithiasis: retrospective analysis of 438 cases. J Am Vet Med Assoc. 1977;170:414–418. [PubMed] [Google Scholar]
- 31.Ling GV, Franti CE, Ruby AL, et al. Urolithiasis in dogs. I: Mineral prevalence and interrelations of mineral composition, age, and sex. Am J Vet Res. 1998;59:624–629. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.