Abstract
Inorganic arsenic is methylated in the body by arsenic (III) methyltransferase. Arsenic methylation is thought to play a role in arsenic-related epigenetic phenomena including aberrant DNA and histone methylation. However, it is unclear whether the promoter of the AS3MT gene, which codes for arsenic (III) methyltransferase, is differentially methylated as a function of arsenic exposure. In this study we evaluated AS3MT promoter methylation according to exposure, assessed by urinary arsenic excretion in a stratified random sample of 48 participants from the Strong Heart Study who had urine arsenic measured at baseline and DNA available from 1989–1991 and 1998–1999. For this study, all data are from the 1989–1991 visit. We measured AS3MT promoter methylation at its 48 CpG loci by bisulphite sequencing. We compared mean % methylation at each CpG locus by arsenic exposure group using linear regression adjusted for study centre, age and sex. A hypomethylated region in the AS3MT promoter was associated with higher arsenic exposure. In vitro, arsenic induced AS3MT promoter hypomethylation and it increased AS3MT expression in human peripheral blood mononuclear cells. These findings may suggest that arsenic exposure influences the epigenetic regulation of a major arsenic metabolism gene.
Keywords: arsenic, epigenetics, DNA methylation, AS3MT, arsenic(III) methyltransferase, arsenic metabolism
INTRODUCTION
Inorganic arsenic, a widespread environmental toxicant, is an established carcinogen (IARC, 2004) that may play a role in cardiovascular disease (Agusa et al., 2010; Chen et al., 1996; States et al., 2009) and diabetes (Del Razo et al., 2011; Gribble et al., 2012; Navas-Acien et al., 2008; Navas-Acien et al., 2006). The relationship between arsenic exposure and disease outcomes is thought to be related to arsenic metabolism (Chen et al., 2003; Huang et al., 2008; Huang et al., 2009; Lindberg et al., 2008; Vahter and Concha, 2001). Arsenic (III) methyltransferase (AS3MT), coded by the AS3MT gene, is a major enzyme in arsenic methylation reactions. The role of AS3MT in the metabolism of arsenic has been confirmed by functional genomics (Wood et al., 2006) and experimental assays (Drobna et al., 2010; Drobna et al., 2006). Genetic polymorphisms in the AS3MT gene are associated with differences in AS3MT gene expression (Engstrom et al., 2011) and with arsenic metabolism (Agusa et al., 2011; Engstrom et al., 2011). Due to the importance of the AS3MT gene for arsenic metabolism, a growing body of population genetics research has evaluated population differences in AS3MT polymorphism frequencies (Fujihara et al., 2009; Fujihara et al., 2008; Fujihara et al., 2010; Fujihara et al., 2011).
Epigenetic modifications, including DNA and histone methylation and histone acetylation, may alter gene expression and are increasingly recognized as potential mechanisms mediating arsenic health effects (Arita and Costa, 2009; Baccarelli and Bollati, 2009; Hou et al., 2012; Martinez-Zamudio and Ha, 2011). Arsenic exposure has been associated with gene specific promoter methylation of several genes related to tumourigenesis in human-derived cell lines or human epidemiologic samples including Tumour protein P53 (TP53) and Ras association domain-containing protein 1 (RASSF1A) (Jensen et al., 2009; Lu et al., 2001; Mass and Wang, 1997). However, little research in human populations has evaluated the possible relationship between arsenic exposure and AS3MT promoter methylation. Recently, methylation status of a few CpG sites located at the 5’AS3MT region was examined in samples from Argentina and Bangladesh using the Illumina Infinium HumanMethylation 450K BeadChip (Engstrom et al., 2013). No studies have measured methylation status of the AS3MT promoter region using bisulphite sequencing, a “gold standard” for the measurement of DNA methylation. We hypothesized that arsenic exposure would influence DNA methylation of the AS3MT promoter, with possible consequences for arsenic metabolism.
In this study we used bisulphite sequencing to evaluate the association of arsenic exposure with differences in AS3MT promoter methylation among a sample of adult men and women from Arizona, Oklahoma and North and South Dakota who participated in the Strong Heart Study in 1989–1991. In this population, arsenic exposure in drinking water ranged from low (<10 µg/L) to moderate (≥50 µg/L), resulting in urine arsenic concentrations that remained stable over the 10-year duration of the Strong Heart Study follow-up examinations (Navas-Acien et al., 2009). In addition, we conducted in vitro experiments exposing human peripheral blood mononuclear cells to arsenic to evaluate the role of arsenic in AS3MT promoter methylation and gene expression. We also evaluated the impact of AS3MT knock-down for the expression and methylation of genes potentially related to arsenic health effects.
MATERIALS AND METHODS
Study Population
The Strong Heart Study is a population-based prospective cohort study of cardiovascular disease and its risk factors in American Indian men and women from Arizona, Oklahoma, and North or South Dakota (Lee et al., 1990). Men and women 45 to 74 years of age listed on tribal rolls in these three areas were invited to participate. A total of 4,549 participants were recruited in 1989–1991, with an overall response rate of 62%. Participants gave informed consent and provided specimens from that visit for analysis of biomarkers of interest for cardiovascular disease risk, including spot urine samples and blood samples. The Strong Heart Study protocol and consent form were approved by local institutional review boards, participating tribes and the Indian Health Service.
To assess arsenic exposure in the population, urine arsenic species including inorganic arsenic, methylarsonate (MMA), dimethylarsinate (DMA), arsenobetaine and other arsenic cations were measured in 3,974 Strong Heart Study study participants with available urine samples using anion-exchange high performance liquid chromatography-inductively coupled plasma mass spectrometry (HPLC-ICPMS), as described previously (Scheer, 2012). Strong Heart Study participants were eligible for this study if they had arsenic levels measured in 1998–1991, had available DNA collected in 1989–1991 and 1998–1999, and participated in an ancillary study called the Strong Heart Family Study (North et al., 2003), resulting in a final number of 512 eligible participants. For this study, all data are from the 1989–1991 visit and were collected on the same day for each participant.
To maximize efficiency of this relatively small epigenetic study and reduce exposure misclassification (Stuart and Hanna, 2013; Zubizarreta et al., 2013), we used a stratified random sample to select eight participants with moderate arsenic exposure and eight participants with low arsenic exposure from each region (16 from Arizona, 16 from Oklahoma and 16 from North or South Dakota), resulting in a total of 48 participants. Based on the Strong Heart Study distributions, relatively low and moderate arsenic exposures were defined as urine concentrations < 7.2 µg/L (tertile 1) and ≥ 14.0 µg/L (tertile 3), respectively, for the sum of inorganic and methylated arsenic species in 1989 – 1991. For each participant, detailed socio-demographic and health status information were obtained from detailed in-person interviews, physical examinations and biological specimens.
Laboratory Methods
AS3MT promoter methylation analysis was performed at the Department of Environmental Health Sciences, Johns Hopkins Bloomberg School of Public Health. DNA from whole blood collected in 1989–1991 was solvent-extracted, treated with proteinase K, and stored at the Penn Medical Laboratory of Medstar Health Research, Washington, DC. Genomic DNA (200 ng) was bisulphite-modified using the EZ DNA Methylation kit (Zymo Research, Irvine, CA) prior to PCR. In silico analyses and detailed database searches were used to predict the 5’-CGI(s) of AS3MT (NC_000010.10: 104626210-104630210) (www.urogene.org/methprimer/). Primers were designed to amplify a 695 bp (−240 to +454) fragment encompassing the predicted 5’-CGI(s) of AS3MT (hBS-AS3MT-Forward, 5’ TGATTTTATTTTTAAAAAGGGGGAG 3’ and hBS-AS3MT-Reverse, 5’ CAAACCAAACTTCCATTAACCTAAA 3’) from bisulphite-modified DNA. Amplicons were generated from individual samples using Platinum Taq DNA polymerase (Life Technologies, Grand Island, New York) and inserted into the pCR2.1 vector (Life Technologies, Grand Island, New York). Six isolates were picked and sequenced for each sample (Macrogen, Rockville, Maryland) to obtain direct measures of DNA methylation at each CpG locus in the AS3MT promoter region. An example of the resulting data is shown in Appendix 1. The DNA methylation data from sequencing were analyzed with the BiQ analyzer. Genomic DNA with 0%, 50% and 100% methylation were used as a quality control to ensure bisulphite conversion quality on sample. Sequences with low conversion rate (<90%) were excluded from further analysis. In one participant, one isolate failed quality control, and so only 5 isolates were used to calculate mean % methylation for that participant.
Statistical Analyses
We analyzed each of the 48 CpG loci in the AS3MT promoter region separately using the estimated mean % DNA methylation from 5–6 isolates as the methylation metric of interest per individual at each CpG locus. Histograms were examined to evaluate the distribution of mean % DNA methylation at each CpG locus (Appendix 2) and scatter plots to describe mean % DNA methylation across loci according to study centre, sex, and arsenic methylation profile (i.e. above or below median % MMA, and above or below median %DMA). The scatter plots of % DNA methylation for %DMA are similar to those for %MMA and are not shown. We analyzed the relationship between % DNA methylation at each CpG locus by arsenic exposure group (moderate vs. low) using linear regression adjusting for sex, age and study centre (Arizona, Oklahoma and North or South Dakota). In a sensitivity analysis, we further adjusted for body mass index, smoking status and drinking status. Given the limited number of observations and the departure of residuals from normality, we used a bootstrap to estimate bias-corrected and accelerated 95% confidence intervals, resampling observations from within study centres (DiCiccio and Efron, 1996).
In vitro experiments
To evaluate the potential impact of arsenic on AS3MT promoter methylation and gene expression, we exposed human peripheral blood mononuclear cells (Lonza, Walkersville, Maryland) to 200nM non-targeting small interfering RNA (siRNA) control (siCTL) or on-target plus siRNA against AS3MT (Dharmacon-ThermoScientific, Pittsburgh, PA) for 3 days prior exposure to 5µM aqueous sodium arsenite for another 48h. RNA and DNA were isolated from all treatment groups. In addition to AS3MT, we evaluated gene expression and methylation status of other genes related to arsenic exposure in previous studies, including tumour suppressor genes (TP53 and RASSF1A) and peroxisome proliferator-activated receptor gamma (PPARG). The mRNA levels of AS3MT, RASSF1A, TP53 and PPARG were quantified by TaqMan-based real-time PCR. The delta-delta cycle threshold (Ct) method was used to calculate the relative expression level of transcripts normalized to 18S (Wong and Medrano, 2005). Gene specific methylation of AS3MT, RASSF1A, TP53, and PPARG were assayed by MethyLight assay as previously described (Weisenberger et al., 2006). We measured global DNA methylation in genomic DNA (200ng) isolated from each treatment group by 5’-mC enzyme-linked immunosorbent assay (Zymo Research, Irvine, California). Four independent experiments with siRNA treatment were employed. Results of gene expression and methylation analysis were statistically evaluated using GraphPad (GraphPad Software Inc., San Diego, CA). All data groups were analyzed by one-way ANOVA, followed by Tukey’s multiple comparison test, and differences between groups were accepted at α = 0.05.
RESULTS
Mean (standard deviation) for the sum of inorganic and methylated arsenic species was 5.2 (1.7) µg/L for participants with low arsenic exposure and 29.2 (23.4) µg/L for participant with moderate arsenic exposure (Table 1). Within the same study centre, participants of low and high arsenic levels were similar in age, body mass index, and education, although in Oklahoma participants with low arsenic were more likely to be smokers than those with high arsenic and in North and South Dakota participants with low arsenic had higher mean body mass index. Within the same study centre, participants of low and high arsenic levels were not balanced on sex.
Table 1. Participant characteristics by study centre and arsenic exposure levels.
Summary statistics are for the observed sample from which bootstrap samples redrawn.
| Overall | Arizona | Oklahoma | North or South Dakota |
|||||
|---|---|---|---|---|---|---|---|---|
| Moderate As |
Low As | Moderate As |
Low As |
Moderate As |
Low As |
Moderate As |
Low As |
|
| N | 24 | 24 | 8 | 8 | 8 | 8 | 8 | 8 |
| Mean Age (SD) | 55.9 (6.8) | 54.0 (7.5) | 53.9 (5.1) | 53.8 (5.5) | 55.2 (6.1) | 53.0 (8.5) | 58.7 (8.7) | 55.0 (8.8) |
| % Male | 29.2 | 33.3 | 25 | 37.5 | 50 | 25 | 12.5 | 37.5 |
| Mean BMI (SD) | 30.6 (6.0) | 31.5 (5.8) | 33.1 (8.3) | 29.2 (4.7) | 29.4 (4.8) | 30.6 (5.1) | 29.2 (3.6) | 35.1 (6.7) |
| Mean Education (SD) | 11.7 (3.3) | 11.4 (2.9) | 10.9 (3.7) | 9.6 (2.3) | 12.3 (2.8) | 13.4 (1.4) | 11.9 (3.6) | 11.3 (3.4) |
| % Current Smoker | 20.8 | 37.5 | 12.5 | 12.5 | 12.5 | 50 | 37.5 | 50 |
| Mean %MMA (SD) | 14.9 (5.5) | 14.5 (4.4) | 13.2 (3.2) | 13.9 (6.0) | 17.0 (7.9) | 14.2 (3.0) | 14.6 (4.1) | 15.6 (3.9) |
| Mean Urine Arsenic* (SD) | 29.2 (23.4) | 5.2 (1.7) | 32.5 (31.9) | 5.6 (1.5) | 17.9 (3.4) | 5.5 (1.1) | 37.3 (23.0) | 4.5 (2.2) |
Sum of inorganic and methylated arsenic species.
Moderate arsenic refers to urine arsenic concentrations in the highest tertile (≥14.0 µg/L) and low arsenic to concentrations in the lowest tertile (< 7.2 µg/L).
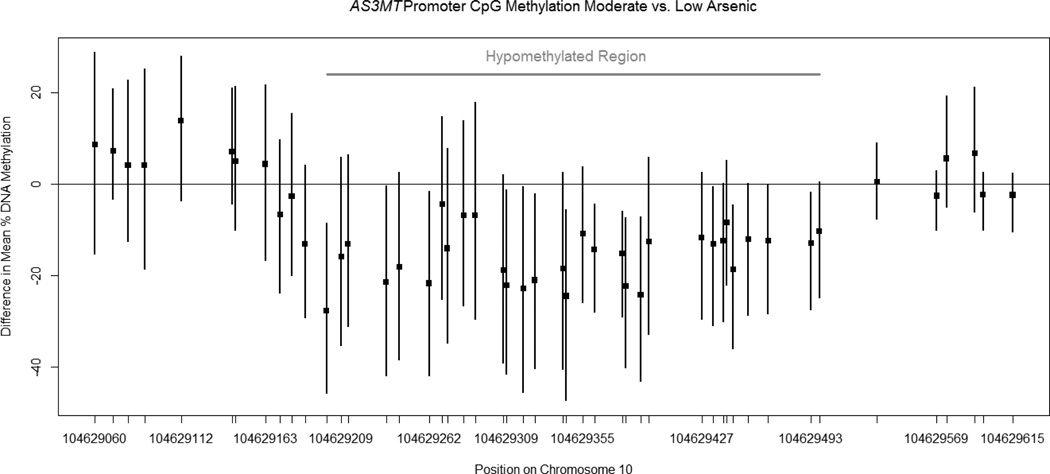
Across an approximately 30-CpG-loci region of the AS3MT promoter, mean levels of % DNA methylation were lower comparing participants with moderate versus low arsenic exposure and in participants from Arizona and North and South Dakota compared to Oklahoma (Appendix 3). In regression analyses adjusted for sex, age and study centre, hypomethylation of an approximately 30-CpG-loci region in the promoter was observed in participants exposed to moderate versus low arsenic levels (Figure 1). We evaluated mean regional methylation differences over CpG 12 to 42 using the same linear regression bootstrap methods. Participants with moderate arsenic exposure had 16 (95% CI −28, −5) percent lower mean regional methylation after adjustment for study centre and sex. Further adjustment for body mass index, drinking status and smoking status resulted in 17 (95% CI −30, −2) percent lower mean regional DNA methylation in the highest versus lowest arsenic tertile.
Figure 1. Difference in mean % DNA methylation at each AS3MT promoter CpG locus after adjustment for study centre, age and sex.
Bias-corrected and accelerated 95% confidence intervals were calculated from 1000 bootstrap replicates, except for CpG loci 30, 47 and 48 where only 999 replicates yielded estimable parameters. Observations were resampled from within each study centre. The gray line represents the hypomethylated region. The difference in mean regional methylation comparing moderate vs. low arsenic was −0.16 (95% CI −0.28, −0.05) percent.
In in vitro experiments, sodium arsenite exposure induced AS3MT and RASSF1A gene expression along with a decrease in promoter methylation (Table 2). In contrast, sodium arsenite exposure decreased TP53 gene expression with an increase in promoter methylation. Arsenite-treated cells showed downregulation of PPARG gene expression but no changes in promoter methylation. Knockdown of AS3MT by small interfering RNA reversed the arsenic-induced RASSF1A promoter hypomethylation and TP53 hypermethylation (Table 2). Knockdown of AS3MT also inhibited arsenic-induced global DNA hypomethylation (Table 3).
Table 2.
Gene expression and promoter methylation for AS3MT, RASSF1A, PPARG and TP53 in human peripheral blood mononuclear cells under controlled conditions, exposed to arsenite (5 µM) and with AS3MT knockdown*
| Treatment group | Gene expression Relative expression ratio mean (min, max) |
p-value** | Promoter methylation % methylation reference mean (min, max) |
p-value** |
|---|---|---|---|---|
| AS3MT | ||||
| siCTL | 0.90 (0.70, 1.00) | 25.44 (22.57, 30.94) | ||
| As+siCTL | 1.93 (1.41, 2.27) | 0.02 | 5.58 (5.33, 5.83) | 0.02 |
| As+siAS3MT | 0.57 (0.43, 0.70) | 0.02 | 49.08 (48.90, 49.15) | 0.01 |
| RASSF1A | ||||
| siCTL | 0.94 (0.81, 1.00) | 59.88 (50.08, 69.69) | ||
| As+siCTL | 3.56 (2.78, 4,56) | 0.05 | 25.97 (20.80, 35.70) | 0.05 |
| As+siAS3MT | 0.91 (0.90, 0.92) | 0.04 | 54.20 (51.27, 56.46) | 0.05 |
| PPARG | ||||
| siCTL | 1.12 (1.00, 1.35) | 5.33 (2.92, 6.98) | ||
| As+siCTL | 0.22 (0.19, 0.27) | 0.02 | 6.07 (4.44, 5.45) | 0.4 |
| As+siAS3MT | 0.25 (0.16, 0.34) | 0.07 | 9.52 (5.05, 14.00) | 0.43 |
| TP53 | ||||
| siCTL | 1.07 (1.00, 1.22) | 2.60 (2.47, 2.68) | ||
| As+siCTL | 0.51 (0.38, 0.49) | 0.04 | 7.26 (6.36, 8.34) | 0.02 |
| As+siAS3MT | 0.82 (0.78, 0.89) | 0.07 | 3.48 (2.21, 4.91) | 0.05 |
Human peripheral blood mononuclear cells were exposed to 200nM of non-targeting small interfering RNA control (siCTL) or on-target plus siRNA against AS3MT (siAS3MT) for 3 days prior exposure to 5µM aqueous sodium arsenite for another 48h. 4 independent sets of experiments were performed.
For each gene, the first p-value is for As+siCTL compared to siCTL, and the second one is for As+siAS3MT vs. As+siCTL, based on one-way ANOVA followed by Tukey’s multiple comparison test.
AS3MT: Arsenic (III) methyltransferase gene
RASSF1A: Ras association domain-containing protein 1 gene
PPARG: Peroxisome proliferator-activated receptor gamma gene
TP53: Tumour protein P53 gene
Table 3.
Knockdown effect of AS3MT on global DNA methylation in response to sodium arsenite
| global 5-methylcytosine ng, Mean (Min, Max) |
p-value | |
|---|---|---|
| siCTL | 12.54 (12.22, 12.74) | |
| As+siCTL | 6.38 (5.48, 7.13) | 0.01 vs. siCTL |
| As+siAS3MT | 10.08 (9.70, 10.37) | 0.01 vs. As+siCTL |
DISCUSSION
Arsenic exposure, as measured in urine, was associated with AS3MT promoter hypomethylation in a sample of Strong Heart Study participants exposed to low-moderate arsenic exposure. These associations were observed for many CpG loci after adjustment for sex, study centre, age, body mass index, smoking status and drinking status. In vitro, we confirmed that arsenic-induced hypomethylation of AS3MT increased its gene expression. Knockdown of AS3MT altered arsenic-induced gene-specific and global DNA methylation, supporting that AS3MT might be an important gene for epigenetic modifications induced by arsenic. While these study findings need to be interpreted with caution given the small sample size and the multiple comparisons, the findings are consistent with our initial hypothesis of arsenic-induced methylation changes in the AS3MT promoter. Overall, the study results underscore the need for future work evaluating the relationships and health implications of arsenic exposure, epigenetics, and arsenic metabolism.
AS3MT is an important gene for arsenic metabolism, bioavailability of toxic arsenic species, and toxicological response to arsenic exposure (Drobna et al., 2009; Engström et al., 2010; Thomas, 2009; Thomas et al., 2007). AS3MT promoter methylation could be important for arsenic toxicity if arsenic metabolism is a mediator or a modifier of arsenic toxicity. It has been proposed that arsenic metabolism mediates the associations of arsenic exposure with aberrant DNA or histone methylation, perhaps through depletion of the methyl donor S-adenosyl methionine (Reichard et al., 2007) or dysregulation of DNA methyltransferases (Reichard et al., 2007). Over-expression of AS3MT via promoter hypomethylation may further deplete S-adenosyl methionine and inhibit DNA methyltransferase and histone methyltransferase activity. This arsenic-mediated epigenetic disruption (Reichard and Puga, 2010) may contribute to global DNA hypomethylation (Benbrahim-Tallaa et al., 2005; Chen et al., 2004) and promoter methylation at specific genes involved in tumourigenesis (Cui et al., 2006; Marsit et al., 2006; Okoji et al., 2002) and diabetes (Bailey et al., 2013). In addition to being a mediator, arsenic metabolism may also be an effect modifier. By changing chemical forms of arsenic, and thus bioavailable doses, arsenic metabolism could modify risk of toxicities specific to arsenic species (Chen et al., 2011). For example, arsenic is a powerful oxidant (Jomova et al., 2011), with differing redox catalytic potentials across different arsenic compounds. Therefore, population heterogeneity in arsenic metabolism could result in varying relationships between inorganic arsenic exposures and oxidative damage. Additional studies are needed to determine the impacts of gene regulation of AS3MT on overall arsenic toxicity.
To evaluate potential transcription factors binding sites, we performed in silico analysis (TRANSFAC®, Biobase Biological Databases, Beverly, Massachusetts) of the 5’ AS3MT promoter region. The putative transcription factor binding sites include hepatocyte nuclear factor (HNF), CCAAT-enhancer binding protein (C/EBP), GATA-1, -2–3, Sp1, transcription factor family E2F and homeobox protein NKX2. Interestingly, in previous studies, inorganic arsenic altered expression of C/EBP (Lin et al., 2005), SP1 (Jutooru et al., 2010), GATA-3 (Yao et al., 2008) and E2F (Yamamoto et al., 2008). Nevertheless, how site-specific methylation of AS3MT contributes to the binding with these transcription factors needs further investigation.
In this study, while arsenic exposure was associated with DNA hypomethylation of the AS3MT promoter, the pattern of arsenic metabolites in urine, as measured by %MMA or %DMA, was not associated with differences in DNA methylation. If arsenic exposure affects AS3MT promoter methylation and this promoter’s methylation status is functionally important, we would expect an association between the pattern of arsenic metabolites (i.e. %MMA) and DNA methylation of the AS3MT promoter. It is possible that either DNA hypomethylation in this promoter is not functionally important for AS3MT expression, or that increased AS3MT expression is not relevant for the pattern of arsenic metabolites in urine. Alternatively, the association of arsenic with AS3MT promoter hypomethylation in our relatively small study could be a spurious finding. A recent array-based study evaluating arsenic exposure levels and methylation status of AS3MT in populations from Bangladesh (N=127) and Argentina (N=103) found no association with a few CpG sites at 5’ AS3MT (Engstrom et al., 2013). Our study, however, focused precisely on the AS3MT promoter (30 CpG sites on a 695 base pair (−240 to +454) fragment that encompasses the predicted 5’CpG islands of AS3MT) and used bisulphite sequencing to access methylation status of each CpG site. However, the lack of apparent association between AS3MT promoter methylation and arsenic metabolism in our study may also be due to limitations of our measurements. First, arsenic metabolite patterns measured in urine are an imperfect proxy for internal metabolite patterns, and a subtle impact on internal metabolite patterns may not be apparent from the urine measures. Although less is known about blood arsenic species than urine arsenic species, there is some evidence that the two compartments may differ in the relative proportion of arsenic species (Hall et al., 2007). The differences between blood and urine could be related to differences in renal elimination of different arsenic species (Buchet et al., 1981; Kala et al., 2004; Vahter and Concha, 2001). Second, DNA samples came from blood rather than the cells primarily involved in arsenic metabolism processes (Styblo et al., 2002). Third, measurement error in arsenic speciation could result in some misclassification of %MMA, making the contrast between groups less distinct. Moreover, dichotomized %MMA might be an inadequate summary of population-level differences in arsenic metabolism. Another possible explanation is that one or more of the yet-to-be-identified arsenic methyltransferases (Drobna et al., 2006) is affected in the opposite way from AS3MT, leading to no apparent change in arsenic metabolism. Additional data including arsenic species in blood and the methylation status of other arsenic metabolism genes, as well as an increased sample size, are needed to clarify the relationships between gene-specific promoter methylation and arsenic metabolism in human populations. More detailed experimental studies will also be important to further evaluate the relevance of AS3MT promoter methylation and gene expression for arsenic metabolism.
This study had a number of strengths but also several limitations. Strengths include the high quality laboratory methods for arsenic speciation and for the DNA methylation. The study was conducted in humans instead of animal models which metabolize arsenic differently (Wang et al., 2002). We were able to detect an association between arsenic exposure and AS3MT promoter methylation over multiple CpG loci in a biologically plausible pattern (i.e. adjacent CpG often showed similar signals despite being analyzed separately). Our study compared extremes of arsenic exposure in the Strong Heart Study population, reducing potential biases from exposure misclassification (Stuart and Hanna, 2013; Zubizarreta et al., 2013). The major limitation was the sample size, which limited the study power and limited our ability to control for confounders. Also, the study was cross-sectional, and we cannot discard the possibility that AS3MT promoter methylation affects arsenic concentrations in urine. Another limitation is the measurement of DNA methylation in whole blood. Because arsenic affects multiple tissues in the body, whole blood DNA is a likely relevant tissue. Moreover, despite cell heterogeneity blood DNA is increasingly used to evaluate determinants of epigenetic modifications and also the association of epigenetic modifications with chronic diseases (Baccarelli et al., 2010; Seow et al., 2012; Toperoff et al., 2012). Finally, another limitation was the lack of gene expression data on participants at the time of the study because only DNA samples from the 1989–1991 baseline visit were available for this analysis. However, we evaluated gene expression of AS3MT in vitro.
In conclusion, this study found statistically significant DNA hypomethylation of the AS3MT promoter with moderate arsenic exposure, after adjusting for socio-demographic factors, body mass index, smoking status and drinking status, suggesting that arsenic exposure may affect the epigenetic regulation of a major arsenic metabolism gene. It is possible that dysregulation of arsenic methyltransferases in response to arsenic may contribute to epigenetic changes throughout the genome. Future research in larger studies is needed to clarify the importance of this association for arsenic speciation in blood and at target organs, and how arsenic exposure, arsenic metabolism, and DNA methylation may relate to disease risks.
Supplementary Material
Acknowledgments
FUNDING
Supported by grants from the National Heart Lung and Blood Institute (R01HL090863 and by Strong Heart Study grants HL41642, HL41652, HL41654 and HL65520) and the National Institute of Environmental Health Sciences (R01ES021367, R00ES016817 and P30ES03819). M. Gribble was supported by T32 training grants from the National Heart, Lung, and Blood Institute (5T32HL007024) and the National Institute of Diabetes and Digestive and Kidney Diseases (5T32DK062707-10).
Footnotes
SUPPLEMENTARY DATA DISCUSSION
The supplemental materials illustrate the raw data used to generate individual % methylation variables at each CpG locus (Supplement 1), the distribution of % methylation values across individuals at each CpG locus (Supplement 2) and mean level of %DNA methylation in a 30-CpG-loci region of the AS3MT promoter (Supplement 3).
REFERENCES
- Agusa T, et al. Individual Variations in Inorganic Arsenic Metabolism Associated with AS3MT Genetic Polymorphisms. Int J Mol Sci. 2011;12:2351–2382. doi: 10.3390/ijms12042351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agusa T, et al. Exposure, metabolism, and health effects of arsenic in residents from arsenic-contaminated groundwater areas of Vietnam and Cambodia: a review. Rev Environ Health. 2010;25:193–220. doi: 10.1515/reveh.2010.25.3.193. [DOI] [PubMed] [Google Scholar]
- Arita A, Costa M. Epigenetics in metal carcinogenesis: nickel, arsenic, chromium and cadmium. Metallomics. 2009;1:222–228. doi: 10.1039/b903049b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarelli A, Bollati V. Epigenetics and environmental chemicals. Curr Opin Pediatr. 2009;21:243–251. doi: 10.1097/mop.0b013e32832925cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarelli A, et al. Ischemic heart disease and stroke in relation to blood DNA methylation. Epidemiology. 2010;21:819–828. doi: 10.1097/EDE.0b013e3181f20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey KA, et al. Arsenic and the epigenome: interindividual differences in arsenic metabolism related to distinct patterns of DNA methylation. J Biochem Mol Toxicol. 2013;27:106–115. doi: 10.1002/jbt.21462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L, et al. Molecular events associated with arsenic-induced malignant transformation of human prostatic epithelial cells: aberrant genomic DNA methylation and K-ras oncogene activation. Toxicol Appl Pharmacol. 2005;206:288–298. doi: 10.1016/j.taap.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Buchet JP, et al. Comparison of the urinary excretion of arsenic metabolites after a single oral dose of sodium arsenite, monomethylarsonate, or dimethylarsinate in man. Int Arch Occup Environ Health. 1981;48:71–79. doi: 10.1007/BF00405933. [DOI] [PubMed] [Google Scholar]
- Chen B, et al. Mouse arsenic (+3 oxidation state) methyltransferase genotype affects metabolism and tissue dosimetry of arsenicals after arsenite administration in drinking water. Toxicol Sci. 2011;124:320–326. doi: 10.1093/toxsci/kfr246. [DOI] [PubMed] [Google Scholar]
- Chen CJ, et al. Dose-response relationship between ischemic heart disease mortality and long-term arsenic exposure. Arterioscler Thromb Vasc Biol. 1996;16:504–510. doi: 10.1161/01.atv.16.4.504. [DOI] [PubMed] [Google Scholar]
- Chen H, et al. Chronic inorganic arsenic exposure induces hepatic global and individual gene hypomethylation: implications for arsenic hepatocarcinogenesis. Carcinogenesis. 2004;25:1779–1786. doi: 10.1093/carcin/bgh161. [DOI] [PubMed] [Google Scholar]
- Chen YC, et al. Arsenic methylation and skin cancer risk in southwestern Taiwan. J Occup Environ Med. 2003;45:241–248. doi: 10.1097/01.jom.0000058336.05741.e8. [DOI] [PubMed] [Google Scholar]
- Cui X, et al. Arsenic trioxide inhibits DNA methyltransferase and restores methylation-silenced genes in human liver cancer cells. Hum Pathol. 2006;37:298–311. doi: 10.1016/j.humpath.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Del Razo LM, et al. Exposure to arsenic in drinking water is associated with increased prevalence of diabetes: a cross-sectional study in the Zimapan and Lagunera regions in Mexico. Environ Health. 2011;10:73. doi: 10.1186/1476-069X-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCiccio TJ, Efron B. Bootstrap Confidence Intervals. Statistical Science. 1996;11:189–212. [Google Scholar]
- Drobna Z, et al. Disruption of the arsenic (+3 oxidation state) methyltransferase gene in the mouse alters the phenotype for methylation of arsenic and affects distribution and retention of orally administered arsenate. Chem Res Toxicol. 2009;22:1713–1720. doi: 10.1021/tx900179r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobna Z, et al. Interspecies differences in metabolism of arsenic by cultured primary hepatocytes. Toxicol Appl Pharmacol. 2010;245:47–56. doi: 10.1016/j.taap.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobna Z, et al. shRNA silencing of AS3MT expression minimizes arsenic methylation capacity of HepG2 cells. Chem Res Toxicol. 2006;19:894–898. doi: 10.1021/tx060076u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrom K, et al. Polymorphisms in arsenic(+III oxidation state) methyltransferase (AS3MT) predict gene expression of AS3MT as well as arsenic metabolism. Environ Health Perspect. 2011;119:182–188. doi: 10.1289/ehp.1002471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström K, et al. Polymorphisms in Arsenic(+III Oxidation State) Methyltransferase (<italic>AS3MT</italic>) Predict Gene Expression of <italic>AS3MT</italic> as Well as Arsenic Metabolism. Environ Health Perspect. 2010:119. doi: 10.1289/ehp.1002471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrom KS, et al. Efficient Arsenic Metabolism - The AS3MT Haplotype Is Associated with DNA Methylation and Expression of Multiple Genes Around AS3MT. PLoS One. 2013;8:e53732. doi: 10.1371/journal.pone.0053732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihara J, et al. Ethnic differences in five intronic polymorphisms associated with arsenic metabolism within human arsenic (+3 oxidation state) methyltransferase (AS3MT) gene. Toxicol Appl Pharmacol. 2009;234:41–46. doi: 10.1016/j.taap.2008.09.026. [DOI] [PubMed] [Google Scholar]
- Fujihara J, et al. Asian specific low mutation frequencies of the M287T polymorphism in the human arsenic (+3 oxidation state) methyltransferase (AS3MT) gene. Mutat Res. 2008;654:158–161. doi: 10.1016/j.mrgentox.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Fujihara J, et al. Global analysis of genetic variation in human arsenic (+3 oxidation state) methyltransferase (AS3MT) Toxicol Appl Pharmacol. 2010;243:292–299. doi: 10.1016/j.taap.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Fujihara J, et al. Genetic variants associated with arsenic metabolism within human arsenic (+3 oxidation state) methyltransferase show wide variation across multiple populations. Arch Toxicol. 2011;85:119–125. doi: 10.1007/s00204-010-0568-y. [DOI] [PubMed] [Google Scholar]
- Gribble MO, et al. Arsenic Exposure, Diabetes Prevalence, and Diabetes Control in the Strong Heart Study. American Journal of Epidemiology. 2012;176:865–874. doi: 10.1093/aje/kws153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M, et al. Determinants of arsenic metabolism: blood arsenic metabolites, plasma folate, cobalamin, and homocysteine concentrations in maternal-newborn pairs. Environ Health Perspect. 2007;115:1503–1509. doi: 10.1289/ehp.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, et al. Environmental chemical exposures and human epigenetics. International Journal of Epidemiology. 2012;41:79–105. doi: 10.1093/ije/dyr154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YK, et al. Arsenic exposure, urinary arsenic speciation, and the incidence of urothelial carcinoma: a twelve-year follow-up study. Cancer Causes Control. 2008;19:829–839. doi: 10.1007/s10552-008-9146-5. [DOI] [PubMed] [Google Scholar]
- Huang YL, et al. Urinary arsenic methylation capability and carotid atherosclerosis risk in subjects living in arsenicosis-hyperendemic areas in southwestern Taiwan. Sci Total Environ. 2009;407:2608–2614. doi: 10.1016/j.scitotenv.2008.12.061. [DOI] [PubMed] [Google Scholar]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 84. Lyon, France: 2004. Some Drinking-Water Disinfectants and Contaminants, including Arsenic. [PMC free article] [PubMed] [Google Scholar]
- Jensen TJ, et al. Arsenicals produce stable progressive changes in DNA methylation patterns that are linked to malignant transformation of immortalized urothelial cells. Toxicol Appl Pharmacol. 2009;241:221–229. doi: 10.1016/j.taap.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jomova K, et al. Arsenic: toxicity, oxidative stress and human disease. Journal of Applied Toxicology. 2011;31:95–107. doi: 10.1002/jat.1649. [DOI] [PubMed] [Google Scholar]
- Jutooru I, et al. Arsenic trioxide downregulates specificity protein (Sp) transcription factors and inhibits bladder cancer cell and tumor growth. Exp Cell Res. 2010;316:2174–2188. doi: 10.1016/j.yexcr.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kala SV, et al. Formation and urinary excretion of arsenic triglutathione and methylarsenic diglutathione. Chem Res Toxicol. 2004;17:243–249. doi: 10.1021/tx0342060. [DOI] [PubMed] [Google Scholar]
- Lee ET, et al. The Strong Heart Study. A study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol. 1990;132:1141–1155. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- Lin L, et al. Arsenite induces a cell stress-response gene, RTP801, through reactive oxygen species and transcription factors Elk-1 and CCAAT/enhancer-binding protein. Biochem J. 2005;392:93–102. doi: 10.1042/BJ20050553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg AL, et al. The risk of arsenic induced skin lesions in Bangladeshi men and women is affected by arsenic metabolism and the age at first exposure. Toxicol Appl Pharmacol. 2008;230:9–16. doi: 10.1016/j.taap.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Lu G, et al. [Effects of inorganic arsenicals on the methylation of p16 gene CpG islands and the expression of p16 gene in BEP2D cells] Zhonghua Yi Xue Za Zhi. 2001;81:1238–1241. [PubMed] [Google Scholar]
- Marsit CJ, et al. Carcinogen exposure and gene promoter hypermethylation in bladder cancer. Carcinogenesis. 2006;27:112–116. doi: 10.1093/carcin/bgi172. [DOI] [PubMed] [Google Scholar]
- Martinez-Zamudio R, Ha HC. Environmental epigenetics in metal exposure. Epigenetics. 2011;6:820–827. doi: 10.4161/epi.6.7.16250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mass MJ, Wang L. Arsenic alters cytosine methylation patterns of the promoter of the tumor suppressor gene p53 in human lung cells: a model for a mechanism of carcinogenesis. Mutat Res. 1997;386:263–277. doi: 10.1016/s1383-5742(97)00008-2. [DOI] [PubMed] [Google Scholar]
- Navas-Acien A, et al. Arsenic exposure and prevalence of type 2 diabetes in US adults. JAMA. 2008;300:814–822. doi: 10.1001/jama.300.7.814. [DOI] [PubMed] [Google Scholar]
- Navas-Acien A, et al. Arsenic exposure and type 2 diabetes: a systematic review of the experimental and epidemiological evidence. Environ Health Perspect. 2006;114:641–648. doi: 10.1289/ehp.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, et al. Urine arsenic concentrations and species excretion patterns in American Indian communities over a 10-year period: the Strong Heart Study. Environ Health Perspect. 2009;117:1428–1433. doi: 10.1289/ehp.0800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North KE, et al. Genetic and environmental contributions to cardiovascular disease risk in American Indians: the strong heart family study. Am J Epidemiol. 2003;157:303–314. doi: 10.1093/aje/kwf208. [DOI] [PubMed] [Google Scholar]
- Okoji RS, et al. Sodium arsenite administration via drinking water increases genomewide and Ha-ras DNA hypomethylation in methyl-deficient C57BL/6J mice. Carcinogenesis. 2002;23:777–785. doi: 10.1093/carcin/23.5.777. [DOI] [PubMed] [Google Scholar]
- Reichard JF, Puga A. Effects of arsenic exposure on DNA methylation and epigenetic gene regulation. Epigenomics. 2010;2:87–104. doi: 10.2217/epi.09.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard JF, et al. Long term low-dose arsenic exposure induces loss of DNA methylation. Biochem Biophys Res Commun. 2007;352:188–192. doi: 10.1016/j.bbrc.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer JFS, Goessler W, Francesconi KA, Howard B, Umans JH, Pollak J, Tellez-Plaza M, Silbergeld EK, Guallar E, Navas-Acien A. Arsenic species and selected metals in human urine: validation of HPLC/ICPMS and ICPMS procedures for a long-term population-based epidemiological study. Analytical Methods. 2012;4:406–413. doi: 10.1039/C2AY05638K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seow WJ, et al. Urinary benzene biomarkers and DNA methylation in Bulgarian petrochemical workers: study findings and comparison of linear and beta regression models. PLoS One. 2012;7:e50471. doi: 10.1371/journal.pone.0050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- States JC, et al. Arsenic and cardiovascular disease. Toxicol Sci. 2009;107:312–323. doi: 10.1093/toxsci/kfn236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart EA, Hanna DB. Should epidemiologists be more sensitive to design sensitivity? Epidemiology. 2013;24:88–89. doi: 10.1097/EDE.0b013e3182782468. [DOI] [PubMed] [Google Scholar]
- Styblo M, et al. The role of biomethylation in toxicity and carcinogenicity of arsenic: a research update. Environ Health Perspect. 2002;110(Suppl 5):767–771. doi: 10.1289/ehp.110-1241242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DJ. Unraveling arsenic-glutathione connections. Toxicol Sci. 2009;107:309–311. doi: 10.1093/toxsci/kfn257. [DOI] [PubMed] [Google Scholar]
- Thomas DJ, et al. Arsenic (+3 oxidation state) methyltransferase and the methylation of arsenicals. Exp Biol Med (Maywood) 2007;232:3–13. [PMC free article] [PubMed] [Google Scholar]
- Toperoff G, et al. Genome-wide survey reveals predisposing diabetes type 2-related DNA methylation variations in human peripheral blood. Hum Mol Genet. 2012;21:371–383. doi: 10.1093/hmg/ddr472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahter M, Concha G. Role of metabolism in arsenic toxicity. Pharmacol Toxicol. 2001;89:1–5. doi: 10.1034/j.1600-0773.2001.d01-128.x. [DOI] [PubMed] [Google Scholar]
- Wang JP, et al. A review of animal models for the study of arsenic carcinogenesis. Toxicology Letters. 2002;133:17–31. doi: 10.1016/s0378-4274(02)00086-3. [DOI] [PubMed] [Google Scholar]
- Weisenberger DJ, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787–793. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
- Wong ML, Medrano JF. Real-time PCR for mRNA quantitation. Biotechniques. 2005;39:75–85. doi: 10.2144/05391RV01. [DOI] [PubMed] [Google Scholar]
- Wood TC, et al. Human arsenic methyltransferase (AS3MT) pharmacogenetics: gene resequencing and functional genomics studies. J Biol Chem. 2006;281:7364–7373. doi: 10.1074/jbc.M512227200. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, et al. Selective activation of NF-kappaB and E2F by low concentration of arsenite in U937 human monocytic leukemia cells. J Biochem Mol Toxicol. 2008;22:136–146. doi: 10.1002/jbt.20222. [DOI] [PubMed] [Google Scholar]
- Yao X, et al. Inhibition of interleukin-13 gene expression in T cells through GATA-3 pathway by arsenic trioxide. Chin Med J (Engl) 2008;121:2346–2349. [PubMed] [Google Scholar]
- Zubizarreta JR, et al. Effect of the 2010 Chilean earthquake on posttraumatic stress: reducing sensitivity to unmeasured bias through study design. Epidemiology. 2013;24:79–87. doi: 10.1097/EDE.0b013e318277367e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.