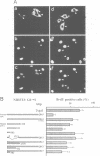
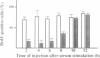
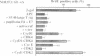Abstract
The APC gene is mutated in familial adenomatous polyposis (FAP) as well as in sporadic colorectal tumours. The product of the APC gene is a 300 kDa cytoplasmic protein associated with the adherence junction protein catenin. Here we show that overexpression of APC blocks serum-induced cell cycle progression from G0/G1 to the S phase. Mutant APCs identified in FAP and/or colorectal tumours were less inhibitory and partially obstructed the activity of the normal APC. The cell-cycle blocking activity of APC was alleviated by the overexpression of cyclin E/CDK2 or cyclin D1/CDK4. Consistent with this result, kinase activity of CDK2 was significantly down-regulated in cells overexpressing APC although its synthesis remained unchanged, while CDK4 activity was barely affected. These results suggest that APC may play a role in the regulation of the cell cycle by negatively modulating the activity of cyclin-CDK complexes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Ohuchi T., Sumida S., Matsumoto K., Toyoshima K. Phosphorylation of the retinoblastoma protein by cdk2. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):7900–7904. doi: 10.1073/pnas.89.17.7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer W. F., Bailey C. J., Bodmer J., Bussey H. J., Ellis A., Gorman P., Lucibello F. C., Murday V. A., Rider S. H., Scambler P. Localization of the gene for familial adenomatous polyposis on chromosome 5. Nature. 1987 Aug 13;328(6131):614–616. doi: 10.1038/328614a0. [DOI] [PubMed] [Google Scholar]
- Burt R. W., Samowitz W. S. The adenomatous polyp and the hereditary polyposis syndromes. Gastroenterol Clin North Am. 1988 Dec;17(4):657–678. [PubMed] [Google Scholar]
- Cross S. M., Sanchez C. A., Morgan C. A., Schimke M. K., Ramel S., Idzerda R. L., Raskind W. H., Reid B. J. A p53-dependent mouse spindle checkpoint. Science. 1995 Mar 3;267(5202):1353–1356. doi: 10.1126/science.7871434. [DOI] [PubMed] [Google Scholar]
- Dowdy S. F., Hinds P. W., Louie K., Reed S. I., Arnold A., Weinberg R. A. Physical interaction of the retinoblastoma protein with human D cyclins. Cell. 1993 May 7;73(3):499–511. doi: 10.1016/0092-8674(93)90137-f. [DOI] [PubMed] [Google Scholar]
- Ewen M. E., Sluss H. K., Sherr C. J., Matsushime H., Kato J., Livingston D. M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993 May 7;73(3):487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- Ewen M. E., Sluss H. K., Whitehouse L. L., Livingston D. M. TGF beta inhibition of Cdk4 synthesis is linked to cell cycle arrest. Cell. 1993 Sep 24;74(6):1009–1020. doi: 10.1016/0092-8674(93)90723-4. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Fodde R., Edelmann W., Yang K., van Leeuwen C., Carlson C., Renault B., Breukel C., Alt E., Lipkin M., Khan P. M. A targeted chain-termination mutation in the mouse Apc gene results in multiple intestinal tumors. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8969–8973. doi: 10.1073/pnas.91.19.8969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDNER E. J., RICHARDS R. C. Multiple cutaneous and subcutaneous lesions occurring simultaneously with hereditary polyposis and osteomatosis. Am J Hum Genet. 1953 Jun;5(2):139–147. [PMC free article] [PubMed] [Google Scholar]
- Geng Y., Weinberg R. A. Transforming growth factor beta effects on expression of G1 cyclins and cyclin-dependent protein kinases. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10315–10319. doi: 10.1073/pnas.90.21.10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M., Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groden J., Gelbert L., Thliveris A., Nelson L., Robertson M., Joslyn G., Samowitz W., Spirio L., Carlson M., Burt R. Mutational analysis of patients with adenomatous polyposis: identical inactivating mutations in unrelated individuals. Am J Hum Genet. 1993 Feb;52(2):263–272. [PMC free article] [PubMed] [Google Scholar]
- Groden J., Thliveris A., Samowitz W., Carlson M., Gelbert L., Albertsen H., Joslyn G., Stevens J., Spirio L., Robertson M. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991 Aug 9;66(3):589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- Gu Y., Turck C. W., Morgan D. O. Inhibition of CDK2 activity in vivo by an associated 20K regulatory subunit. Nature. 1993 Dec 16;366(6456):707–710. doi: 10.1038/366707a0. [DOI] [PubMed] [Google Scholar]
- Hamaguchi M., Matsuyoshi N., Ohnishi Y., Gotoh B., Takeichi M., Nagai Y. p60v-src causes tyrosine phosphorylation and inactivation of the N-cadherin-catenin cell adhesion system. EMBO J. 1993 Jan;12(1):307–314. doi: 10.1002/j.1460-2075.1993.tb05658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon G. J., Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994 Sep 15;371(6494):257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Hinds P. W., Mittnacht S., Dulic V., Arnold A., Reed S. I., Weinberg R. A. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992 Sep 18;70(6):993–1006. doi: 10.1016/0092-8674(92)90249-c. [DOI] [PubMed] [Google Scholar]
- Hunter T. Braking the cycle. Cell. 1993 Dec 3;75(5):839–841. doi: 10.1016/0092-8674(93)90528-x. [DOI] [PubMed] [Google Scholar]
- Hunter T., Pines J. Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell. 1994 Nov 18;79(4):573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- Inoue N., Kinoshita T., Orii T., Takeda J. Cloning of a human gene, PIG-F, a component of glycosylphosphatidylinositol anchor biosynthesis, by a novel expression cloning strategy. J Biol Chem. 1993 Apr 5;268(10):6882–6885. [PubMed] [Google Scholar]
- Joslyn G., Carlson M., Thliveris A., Albertsen H., Gelbert L., Samowitz W., Groden J., Stevens J., Spirio L., Robertson M. Identification of deletion mutations and three new genes at the familial polyposis locus. Cell. 1991 Aug 9;66(3):601–613. doi: 10.1016/0092-8674(81)90022-2. [DOI] [PubMed] [Google Scholar]
- Joslyn G., Richardson D. S., White R., Alber T. Dimer formation by an N-terminal coiled coil in the APC protein. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11109–11113. doi: 10.1073/pnas.90.23.11109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karantza V., Maroo A., Fay D., Sedivy J. M. Overproduction of Rb protein after the G1/S boundary causes G2 arrest. Mol Cell Biol. 1993 Nov;13(11):6640–6652. doi: 10.1128/mcb.13.11.6640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J., Matsushime H., Hiebert S. W., Ewen M. E., Sherr C. J. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993 Mar;7(3):331–342. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- Kinzler K. W., Nilbert M. C., Su L. K., Vogelstein B., Bryan T. M., Levy D. B., Smith K. J., Preisinger A. C., Hedge P., McKechnie D. Identification of FAP locus genes from chromosome 5q21. Science. 1991 Aug 9;253(5020):661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- Kudoh T., Ishidate T., Moriyama M., Toyoshima K., Akiyama T. G1 phase arrest induced by Wilms tumor protein WT1 is abrogated by cyclin/CDK complexes. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4517–4521. doi: 10.1073/pnas.92.10.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert M., Dobbs M., Scambler P., O'Connell P., Nakamura Y., Stauffer D., Woodward S., Burt R., Hughes J., Gardner E. The gene for familial polyposis coli maps to the long arm of chromosome 5. Science. 1987 Dec 4;238(4832):1411–1413. doi: 10.1126/science.3479843. [DOI] [PubMed] [Google Scholar]
- Marshall C. J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995 Jan 27;80(2):179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Matsushime H., Quelle D. E., Shurtleff S. A., Shibuya M., Sherr C. J., Kato J. Y. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994 Mar;14(3):2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsunobu F., Fukui M., Oda T., Yamamoto T., Toyoshima K. A mechanism of c-raf-1 activation: fusion of the lipocortin II amino-terminal sequence with the c-raf-1 kinase domain. Oncogene. 1989 Apr;4(4):437–442. [PubMed] [Google Scholar]
- Miyoshi Y., Ando H., Nagase H., Nishisho I., Horii A., Miki Y., Mori T., Utsunomiya J., Baba S., Petersen G. Germ-line mutations of the APC gene in 53 familial adenomatous polyposis patients. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4452–4456. doi: 10.1073/pnas.89.10.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi Y., Nagase H., Ando H., Horii A., Ichii S., Nakatsuru S., Aoki T., Miki Y., Mori T., Nakamura Y. Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum Mol Genet. 1992 Jul;1(4):229–233. doi: 10.1093/hmg/1.4.229. [DOI] [PubMed] [Google Scholar]
- Munemitsu S., Souza B., Müller O., Albert I., Rubinfeld B., Polakis P. The APC gene product associates with microtubules in vivo and promotes their assembly in vitro. Cancer Res. 1994 Jul 15;54(14):3676–3681. [PubMed] [Google Scholar]
- Nagase H., Miyoshi Y., Horii A., Aoki T., Ogawa M., Utsunomiya J., Baba S., Sasazuki T., Nakamura Y. Correlation between the location of germ-line mutations in the APC gene and the number of colorectal polyps in familial adenomatous polyposis patients. Cancer Res. 1992 Jul 15;52(14):4055–4057. [PubMed] [Google Scholar]
- Nishida E., Gotoh Y. The MAP kinase cascade is essential for diverse signal transduction pathways. Trends Biochem Sci. 1993 Apr;18(4):128–131. doi: 10.1016/0968-0004(93)90019-j. [DOI] [PubMed] [Google Scholar]
- Nishisho I., Nakamura Y., Miyoshi Y., Miki Y., Ando H., Horii A., Koyama K., Utsunomiya J., Baba S., Hedge P. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991 Aug 9;253(5020):665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- Noda A., Ning Y., Venable S. F., Pereira-Smith O. M., Smith J. R. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res. 1994 Mar;211(1):90–98. doi: 10.1006/excr.1994.1063. [DOI] [PubMed] [Google Scholar]
- Olschwang S., Laurent-Puig P., Groden J., White R., Thomas G. Germ-line mutations in the first 14 exons of the adenomatous polyposis coli (APC) gene. Am J Hum Genet. 1993 Feb;52(2):273–279. [PMC free article] [PubMed] [Google Scholar]
- Polyak K., Kato J. Y., Solomon M. J., Sherr C. J., Massague J., Roberts J. M., Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994 Jan;8(1):9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- Polyak K., Lee M. H., Erdjument-Bromage H., Koff A., Roberts J. M., Tempst P., Massagué J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994 Jul 15;78(1):59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- Powell S. M., Zilz N., Beazer-Barclay Y., Bryan T. M., Hamilton S. R., Thibodeau S. N., Vogelstein B., Kinzler K. W. APC mutations occur early during colorectal tumorigenesis. Nature. 1992 Sep 17;359(6392):235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B., Souza B., Albert I., Müller O., Chamberlain S. H., Masiarz F. R., Munemitsu S., Polakis P. Association of the APC gene product with beta-catenin. Science. 1993 Dec 10;262(5140):1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- Sherr C. J. G1 phase progression: cycling on cue. Cell. 1994 Nov 18;79(4):551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- Shibata T., Gotoh M., Ochiai A., Hirohashi S. Association of plakoglobin with APC, a tumor suppressor gene product, and its regulation by tyrosine phosphorylation. Biochem Biophys Res Commun. 1994 Aug 30;203(1):519–522. doi: 10.1006/bbrc.1994.2213. [DOI] [PubMed] [Google Scholar]
- Smith K. J., Johnson K. A., Bryan T. M., Hill D. E., Markowitz S., Willson J. K., Paraskeva C., Petersen G. M., Hamilton S. R., Vogelstein B. The APC gene product in normal and tumor cells. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2846–2850. doi: 10.1073/pnas.90.7.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. J., Levy D. B., Maupin P., Pollard T. D., Vogelstein B., Kinzler K. W. Wild-type but not mutant APC associates with the microtubule cytoskeleton. Cancer Res. 1994 Jul 15;54(14):3672–3675. [PubMed] [Google Scholar]
- Su L. K., Johnson K. A., Smith K. J., Hill D. E., Vogelstein B., Kinzler K. W. Association between wild type and mutant APC gene products. Cancer Res. 1993 Jun 15;53(12):2728–2731. [PubMed] [Google Scholar]
- Su L. K., Kinzler K. W., Vogelstein B., Preisinger A. C., Moser A. R., Luongo C., Gould K. A., Dove W. F. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992 May 1;256(5057):668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- Su L. K., Vogelstein B., Kinzler K. W. Association of the APC tumor suppressor protein with catenins. Science. 1993 Dec 10;262(5140):1734–1737. doi: 10.1126/science.8259519. [DOI] [PubMed] [Google Scholar]
- Toyoshima H., Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994 Jul 15;78(1):67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- Varesco L., Gismondi V., James R., Robertson M., Grammatico P., Groden J., Casarino L., De Benedetti L., Bafico A., Bertario L. Identification of APC gene mutations in Italian adenomatous polyposis coli patients by PCR-SSCP analysis. Am J Hum Genet. 1993 Feb;52(2):280–285. [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K. W. The multistep nature of cancer. Trends Genet. 1993 Apr;9(4):138–141. doi: 10.1016/0168-9525(93)90209-z. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Hannon G. J., Zhang H., Casso D., Kobayashi R., Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993 Dec 16;366(6456):701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]