Abstract
Inherent recipient factors, including pre-transplant diagnosis, obesity, and elevated pulmonary pressures are established PGD risks. We evaluated the relationship between pre-operative lung injury biomarkers and PGD to gain further mechanistic insight in recipients. We performed a prospective cohort study of recipients in the lung transplant outcomes group enrolled between 2002 and 2010. Our primary outcome was grade 3 PGD on day 2 or 3. We measured pre-operative plasma levels of 5 biomarkers (CC-16, sRAGE, ICAM-1, IL-8, and Protein C) that were previously associated with PGD when measured at the post-operative timepoint. We used multivariable logistic regression to adjust for potential confounders. Of 714 subjects, 130 (18%) developed PGD. Median CC-16 levels were elevated in subjects with PGD (10.1 vs. 6.0, p<0.001). CC-16 was associated with PGD in non-IPF subjects (OR for highest quartile of CC-16: 2.87, 95% CI: 1.37, 6.00, p=0.005) but not in subjects with IPF (OR 1.38, 95% CI: 0.43, 4.45, p=0.59). After adjustment, pre-operative CC-16 levels remained associated with PGD (OR: 3.03, 95% CI: 1.26, 7.30, p=0.013) in non-IPF subjects. Our study suggests the importance of pre-existing airway epithelial injury in PGD. Markers of airway epithelial injury may be helpful in pre-transplant risk stratification in specific recipients.
Keywords: Biomarkers, Acute Lung Injury, CC-16, Primary Graft Dysfunction, Lung Transplantation
Introduction
Primary graft dysfunction (PGD) is a form of acute lung injury occurring within 72 hours of lung transplantation (1, 2). It is the leading cause of early morbidity and mortality after transplant (3, 4), yet the mechanisms driving the development of PGD remain unclear. Prior work evaluating post-operative timepoints has identified plasma biomarkers associated with concurrent PGD, including soluble receptor for advanced glycation end products (sRAGE), club cell (Clara) secretory protein (CC-16), protein C, and intercellular adhesion molecule-1 (ICAM-1) (5–8). These markers have helped establish potential mechanisms occurring during clinical PGD, and have demonstrated discriminant validity as a quantitative measure of PGD (9). However, there is a lack of knowledge of mechanisms occurring prior to transplant in the recipient that may be important in the development of PGD, and better pre-operative recipient risk stratification may allow for changes in management or therapy prior to transplantation to reduce the risk of PGD.
Recently, we and others have established several recipient-related clinical risk factors for primary graft dysfunction, including obesity, presence of pulmonary hypertension, and predisposing diagnosis (4). Identification of the biologic processes underlying these clinical PGD associations is important because it will give insight into potentially modifiable factors prior to transplantation. For example, although predisposing diagnosis is not modifiable prior to transplantation, enhanced understanding of what is driving the increased risk of a particular diagnosis may provide targets for therapy to decrease PGD risk prior to transplantation. Additionally, studying biologic markers within known risk groups is important as there are likely several different mechanisms contributing to the risk of PGD and measurement of biomarkers may allow for individualized management decisions to decrease PGD risk.
In order to further study potential mechanisms underlying previously established clinical risk factors and identify potential biologic targets prior to transplantation to reduce the risk of development of PGD, we tested the association between five known PGD lung injury biomarkers measured pre-operatively in the recipient and the subsequent risk of development of PGD.
Methods
Study Population
The Lung Transplant Outcomes Group (LTOG) cohort is a multi-center, prospective study that has been previously described (5, 6). In prior studies, we have measured post-operative biomarkers in smaller subsets of this cohort study (6–8). In this study, we measured five pre-operative biomarkers in a large cohort of subjects that is expanded and distinct from previously studied cohorts. We included subjects transplanted between July 2002 and May 2010 with at least one biomarker measurement at the pre-operative time point. The majority of samples were collected immediately prior to transplantation during the transplant admission, however, a fraction were collected at the time of listing. Samples were processed within 60 minutes and then stored at −80° C for subsequent analysis, and clinical data were collected prospectively for all subjects as described previously (5, 7, 10). Mortality information was collected from each center and supplemented with data from UNOS (11). IRB approval was obtained from each participating center. Informed consent was obtained from each subject enrolled in the cohort.
Determination of PGD Grade
Our primary outcome was grade 3 PGD at 48 or 72 hours after transplantation. PGD grade was determined using the ISHLT consensus definition (2, 10, 12). Two blinded physicians examined chest radiographs to assess for the presence of PGD. Radiographs qualified for PGD if the transplanted lung(s) had diffuse infiltrates. Radiographs and arterial blood gases were assessed at the time of admission to the ICU after transplantation (T0), and 24, 48, and 72 hours after transplantation. The severity of PGD was graded according to the PaO2/FiO2 ratio, with a PaO2/FiO2 ratio less than 200 defining grade 3 PGD (13).
Measurement of sRAGE, ICAM-1, Protein C, IL-8, and CC-16
Biomarkers were chosen because of previously reported associations with acute lung injury or PGD (5, 7, 14, 15). Protein C was measured using the Actichrome protein C assay (American Diagnostica, Greenwich, CT). The intra-assay coefficient of variation was 5.5%. sRAGE, ICAM-1, and IL-8 were measured by ELISA (R&D, Minneapolis, MN). The intra-assay coefficients of variation were 7%, 5%, and 3%, respectively. CC-16 levels were measured using a commercially available ELISA (Biovender, Candler, NC). The intra-assay coefficient of variation was 4%. All analytes were measured in duplicate.
Statistical Analysis
Biomarkers were analyzed either continuously or using quartiles, based on fractional polynomial fit plots evaluating the relationship between each biomarker and predicted probabilities of PGD (16); as well as categorizing each biomarker into quartiles as a dummy variable in logistic regression models with PGD as the outcome. We evaluated CC-16 stratified by diagnosis (IPF vs. non-IPF) based on our previous finding that diagnosis is an effect modifier of the relationship between CC-16 and PGD (8, 17). We also analyzed each biomarker stratified by presence of pulmonary hypertension (defined by mean PA pressure (mPAP) >40) to obtain further information about mechanisms within this high-risk sub-group. We performed a sensitivity analysis of the association of biomarkers by time of collection, repeating the analyses in those subjects who had plasma collected at the time of listing, defined as greater than 24 hours prior to transplantation.
We used multivariable logistic regression to evaluate the relationship between each biomarker and PGD while evaluating for confounding using variables previously demonstrated to be risk factors for PGD, including BMI, mPAP, transplant type, ischemic time, FiO2 at reperfusion, female sex and parity, pre-transplant diagnosis, donor smoking (defined as any history of smoking) and use of cardiopulmonary bypass (18). Using a prediction model previously developed (19) for PGD using bootstrap resampling methods, which incorporated pre-transplant diagnosis, obesity, and pulmonary artery pressure, we evaluated significant biomarkers for incremental predictive utility by comparing area under the curve (AUC) for the model with an individual biomarker to the model without. A likelihood ratio test was used to evaluate for significant differences in AUC. Multiple imputation was used to account for missing data in the covariates (20). Imputation was not used in either the exposure (biomarker) or outcome (grade of PGD) variables; the very few individuals with missing biomarker values were excluded from analyses. P values of less than 0.05 were considered significant. Analyses were performed using STATA version 12.0 (STATA Corp., College Station, TX).
Results
There were 714 subjects in the study, of which 130 (18%, 95% CI: 15%, 21%) developed PGD. The majority of plasma samples were obtained at the time of transplantation, however, there were 126 subjects (19%) who had samples collected more than 24 hours prior to transplantation. In those subjects, plasma samples were collected at the time of listing. The average time between collection and transplantation in those subjects was 80 ± 96 days. Missing biomarker values (n=2) were due to assay failure.
Subjects with PGD more frequently received a lung from a smoking donor, were more obese, and had IPF and PAH more often as a pre-transplant diagnosis (10). Additionally, subjects with PGD had higher mPAP, more frequent RBC transfusions, and more frequent use of cardiopulmonary bypass (Table 1). The percentage of missing data for each covariate is listed in Table 1. There were no significant differences in plasma levels of sRAGE, ICAM-1, IL-8, and Protein C between those with PGD and those without (Table 2).
Table 1.
Univariate analysis of donor, recipient in peri-operative variables stratified by Primary Graft Dysfunction (PGD) status. PGD is defined as grade 3 PGD on day 2 or 3 after lung transplantation. Continuous variables are listed as mean ± standard deviation.
| Covariate | Number Imputed n (%) | PGD (n=130) | Non-PGD (n=584) | p-value |
|---|---|---|---|---|
|
| ||||
| Donor Variables | ||||
| Male Gender, n, (%) | 2 (0.3) | 71 (55) | 341 (58) | 0.43 |
| Age | 7 (1) | 35.4 ± 14.8 | 34.8 ± 14.1 | 0.70 |
| Mode of Death, n (%) | 1 (<1) | 0.87 | ||
| Trauma | 49 (38) | 238 (41) | ||
| Stroke | 56 (43) | 235 (40) | ||
| Anoxia | 9 (7) | 46 (8) | ||
| Other | 16 (12) | 65 (11) | ||
| Race, n (%) | 9 (1) | 0.66 | ||
| Caucasian | 87 (67) | 365 (63) | ||
| African American | 26 (20) | 117 (20) | ||
| Other | 17 (13) | 102 (17) | ||
| Any smoking, yes | 27 (4) | 70 (54) | 246 (43) | 0.001 |
| Recipient Variables | ||||
| Male Gender, n (%) | 3 (<1) | 73 (56) | 311 (53) | 0.55 |
| Age | 6 (1) | 52.1 ± 12.7 | 53.0 ± 12.8 | 0.47 |
| BMI | 14 (2) | 26.0 ± 4.7 | 24.6 ± 4.5 | 0.002 |
| BMI category, n (%) | 0.008 | |||
| <18.5 | 11 (8) | 59 (10) | ||
| 18.5–25 | 40 (31) | 258 (44) | ||
| 25–30 | 52 (40) | 196 (34) | ||
| >30 | 27 (21) | 71 (12) | ||
| Pulmonary Diagnosis, n (%) | 3 (<1) | <0.001 | ||
| Chronic obstructive pulmonary disease | 33 (26) | 256 (44) | ||
| Idiopathic pulmonary fibrosis | 56 (43) | 180 (31) | ||
| Cystic Fibrosis | 13 (10) | 96 (16) | ||
| Sarcoidosis (3) | 3 (2) | 15 (3) | ||
| Pulmonary arterial hypertension (4) | 13 (10) | 15 (3) | ||
| Other | 12 (9) | 22 (4) | ||
| mPAP | 136 (19) | 35.0 ± 17.6 | 27.9 ± 10.2 | <0.001 |
| mPAP severity category, n (%) | <0.001 | |||
| <25 mmHg (normal) | 41 (32) | 253 (43) | ||
| 25–40 mmHg (mild) | 45 (35) | 271 (46) | ||
| 41–55 mmHg (moderate) | 33 (25) | 51 (9) | ||
| >55 mmHg (severe) | 11 (8) | 9 (2) | ||
| Race, n (%) | 3 (<1) | 0.018 | ||
| Caucasian | 103 (79) | 506 (87) | ||
| African American | 21 (16) | 44 (8) | ||
| Other | 6 (5) | 34 (6) | ||
| Operative Variables | ||||
| Ischemic time, min | 22 (3) | 329 ± 92.2 | 305 ± 92.3 | 0.008 |
| Transplant type, single, n (%) | 4 (1) | 39 (30) | 190 (33) | 0.58 |
| PRBC >1L, n (%) | 0 (0) | 46 (35) | 124 (21) | 0.002 |
| Cardiopulmonary bypass use, n (%) | 4 (1) | 78 (60) | 196 (34) | <0.001 |
BMI: Body mass index
mPAP: mean pulmonary artery pressure
PRBC: Packed red blood cells
Percentages may not exactly equal 100% because of rounding.
Table 2.
Median (interquartile range) pre-operative biomarker levels by PGD status. P values were calculated using the Wilcoxon-rank sum test. The number of subjects with a valid biomarker measurement is listed next to the biomarker.
| Biomarker | n | PGD (n=130) | Non-PGD (n=584) | p-value |
|---|---|---|---|---|
|
| ||||
| CC-16 (ng/mL) | 712 | 10.1 (5.2, 19.0) | 6.0 (3.4, 12.8) | <0.001 |
| sRAGE (pg/mL) | 712 | 743.3 (438.9, 2030.8) | 725.6 (391.7, 1462.6) | 0.27 |
| ICAM-1 (ng/mL) | 714 | 223 (135, 333) | 214 (137, 340) | 0.97 |
| IL-8 (pg/mL) | 714 | 5.5 (3.3, 12.9) | 5.3 (3, 10) | 0.36 |
| Protein C (% Control) | 713 | 110 (77, 140) | 105 (79, 134) | 0.44 |
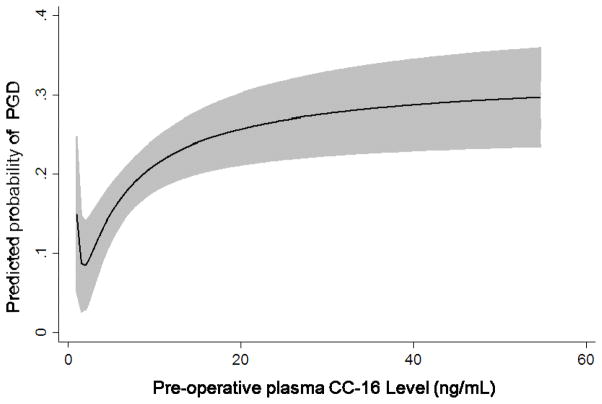
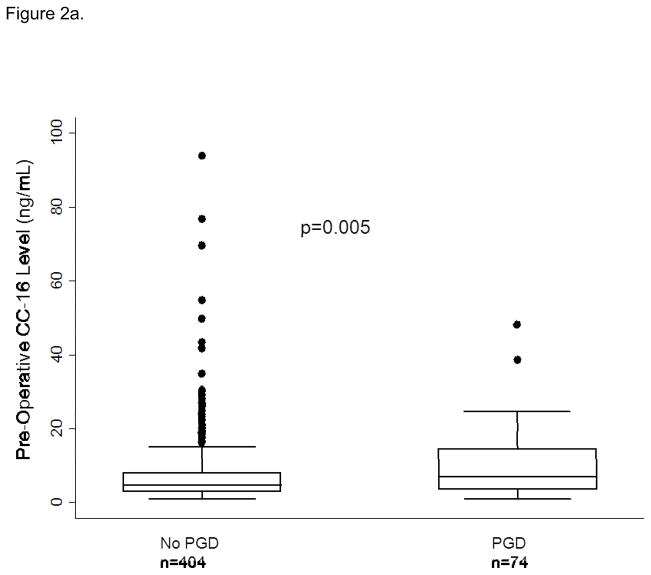
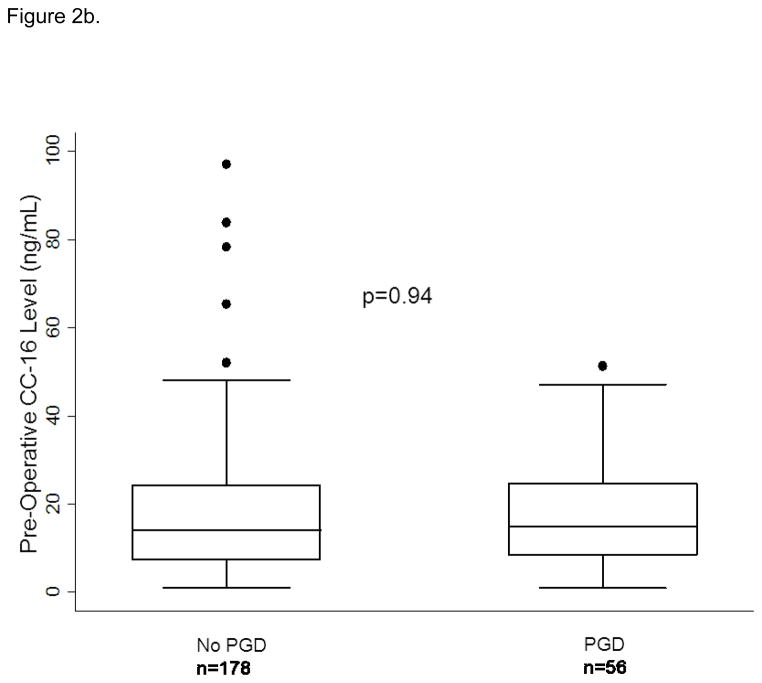
Median plasma CC-16 levels were higher in subjects with PGD compared to those without (10.1 (IQR: 5.2, 19) vs. 6.0 (IQR: 3.4, 12.8), p<0.001). We analyzed CC-16 categorically, in quartiles, based on the relationship of CC-16 with predicted probability of PGD generated from the fractional polynomial fit plot (Figure 1). There was an increased odds of PGD in subjects in the third quartile of CC-16 (OR: 1.89, 95% CI: 1.08, 3.32, p=0.03) and the highest quartile of CC-16 (OR: 2.35, 95% CI: 1.35, 4.08, p=0.002) compared to the lowest quartile of CC-16. When we evaluated CC-16 and pre-transplant diagnosis (stratified as IPF vs. non-IPF) we found that the highest quartile of CC-16 had more subjects with IPF compared to other diagnoses (118 vs. 60, p<0.001). There was no detectable association between CC-16 and PGD in subjects with IPF (OR for highest quartile of CC-16 1.38, 95% CI: 0.43, 4.45, p=0.59). Plasma CC-16 levels were higher in subjects with PGD than without in the sub-group of non-IPF subjects (Figure 2a and b). The association between CC-16 and PGD was unchanged in subjects without IPF (OR for third quartile: 1.90 95% CI: 0.97, 3.72, p=0.06 and highest quartile OR 2.87, 95% CI: 1.37, 6.01, p=0.005). In a multivariable model with previously identified risk factors for PGD, the association between CC-16 and PGD in non-IPF subjects remained (OR for third quartile: 2.16, 95% CI: 1.00, 4.63, p= 0.049 and for fourth quartile: 3.03, 95% CI: 1.26, 7.30, p=0.013) (Table 3).
Figure 1.
Relationship of CC-16 level to predicted probability of grade 3 PGD on day 2 or 3 using fractional polynomial plot. The gray area describes the 95% confidence interval.
Figure 2.
Figure 2a: CC-16 levels in those with PGD and those without PGD in subjects without Idiopathic Pulmonary Fibrosis (IPF) as a pre-transplant diagnosis. Figure 2b. CC-16 levels in those with PGD and those without PGD in subjects with Idiopathic Pulmonary Fibrosis (IPF) as a pre-transplant diagnosis. The horizontal line in the middle of each box indicates the median, the top and bottom borders mark the 75th and 25th percentiles, respectively; and the whiskers mark the 90th and 10th percentiles.
Table 3.
Univariate and multivariate results of CC-16 association with PGD in subjects without IPF as a pre-transplant diagnosis
| Variable | Odds Ratio for third quartile of CC-16 | p-value | Odds ratio for fourth quartile of CC-16 | p-value |
|---|---|---|---|---|
| CC-16 | 1.90 (0.97, 3.72) | 0.06 | 2.87 (1.37, 6.01) | 0.005 |
| Adjusted for | ||||
| Body Mass Index | 1.87 (0.95, 3.66) | 0.07 | 2.83 (1.35, 5.94) | 0.006 |
| Mean PA pressure | 1.64 (0.82, 3.30) | 0.16 | 2.58 (1.20, 5.55) | 0.015 |
| Transplant type | 2.05 (1.04, 4.05) | 0.04 | 3.16 (1.49, 6.71) | 0.003 |
| Ischemic time | 1.88 (0.96, 3.69) | 0.07 | 2.85 (1.36, 5.97) | 0.005 |
| FiO2 at reperfusion | 1.95 (0.99, 3.84) | 0.05 | 2.82 (1.34, 5.95) | 0.006 |
| Female sex and parity | 1.99 (1.01, 3.92) | 0.05 | 3.00 (1.42, 6.35) | 0.004 |
| Packed red blood cell | 2.05 (1.04, 4.08) | 0.04 | 3.34 (1.56, 7.08) | 0.002 |
| Donor Smoking | 1.92 (0.98, 3.77) | 0.06 | 2.90 (1.38, 6.07) | 0.005 |
| Cardiopulmonary bypass | 1.68 (0.84, 3.33) | 0.14 | 2.31 (1.08, 4.94) | 0.031 |
| Multivariable model* | 2.16 (1.00, 4.63) | 0.049 | 3.03 (1.26, 7.30) | 0.013 |
Multivariable model includes BMI, mean PA pressure, transplant type, ischemic time, FiO2 at reperfusion, female sex and parity, PRBC, donor smoking, and CBP
PA=pulmonary artery
FiO2=fraction of inspired oxygen
Given the association between pre-operative plasma CC-16 and PGD, we evaluated CC-16 as a possible predictor for PGD in non-IPF subjects. First, we evaluated the predictive utility of CC-16 alone, which had an AUC of 0.60. Then, based on a previous study, we analyzed the predictive utility of pre-transplant diagnosis, BMI category and mean PA pressure as a base model. The negative predictive value of this model was 93% and the positive predictive value was 20%. With the addition of CC-16 to the model, there were no significant improvements in the negative or positive predictive values (90% and 15%, respectively), despite statistically significant improvement in the AUC (0.72 for model with CC-16 vs. 0.70 for base clinical model, p=0.04). Therefore, although elevated pre-operative plasma CC-16 is an independent risk factor and possible biomarker of PGD, it may not be clinically useful in prediction of PGD when added to known clinical predictor variables.
In sensitivity analyses, the relationship between the biomarkers and PGD did not change significantly by time of sample collection. In subjects who had samples collected at the time of transplantation, the relationship between CC-16 and PGD was unchanged (OR for highest quartile of CC-16: 2.07, 95% CI: 1.00, 4.31, p=0.05). In subjects who had samples collected greater than 24 hours from the time of transplantation (n=174), there was no change in the relationship between CC-16 and PGD (OR for the highest quartile of CC-16 was 3.8, 95% CI: 1.19, 12.14, p=0.02). There were not enough subjects to perform a stratified analysis by individual diagnosis category.
Discussion
In this study, we have demonstrated an association between plasma CC-16 levels measured pre-operatively and PGD. This association was strongest in subjects without IPF as a pre-transplant diagnosis, and in subjects in the highest quartile of plasma CC-16. The association was independent of adjustment from multiple known confounding variables, indicating that the level of epithelial injury, as represented by circulating CC-16 levels, may predispose to PGD prior to the transplant procedure. Although CC-16 was not a good predictor of PGD, we have demonstrated the utility of CC-16 as a pre-operative marker of PGD. This study builds on our prior work evaluating biomarkers in PGD (5, 6, 8) by exclusively evaluating the pre-operative timepoint in a large cohort of prospectively studied transplant recipients, with adequate power to evaluate the role of biomarkers in pre-specified sub-groups.
CC-16 is secreted by epithelial cells in the distal respiratory tract and acts to protect the integrity of the epithelial lining against inflammation and oxidant stress (21). In sarcoidosis, CC-16 is a biomarker of parenchymal disease severity, with increased levels being reflective of increasing parenchymal disease (15, 22). CC-16 has also been evaluated as a biomarker of acute lung injury, and plasma levels measured at the time of injury are decreased compared to other causes of pulmonary edema (23, 24). In our study, increased plasma levels of CC-16 in the recipient prior to transplantation are associated with subsequent PGD. The difference in directionality from ALI may be because our measurement was taken prior to the development of lung injury, not during, indicating that pre-existing epithelial injury is associated with subsequent graft dysfunction. It is possible that systemic up-regulation of lung epithelial injury pathways prior to transplantation lead to an increased susceptibility of PGD. Future investigation on the systemic immune effectors of these pathways in the post-transplant period is important.
We found that the association was strongest in subjects without IPF as a pre-transplant diagnosis. Overall, subjects with IPF had a significantly higher CC-16 level compared to other pre-transplant diagnoses. The lack of association between CC-16 and PGD in IPF subjects may be that subjects with IPF already had such a strong signal of epithelial injury prior to transplantation that any subsequent injury related to PGD is difficult to detect as levels in IPF patients are so high (25). Alternatively, a recent study demonstrated that COPD patients with high levels of circulating inflammatory markers in a symptom-free period had a greater number of exacerbations (26). It may be that patients with COPD and other non-IPF diagnoses with high CC-16 levels are a sub-group of “exacerbators” that are at increased risk for epithelial injury after transplant, and that relationship is washed out in IPF where there are consistently high CC-16 levels in all patients.
When added to a predictive model using clinical covariates, CC-16 only had a slight increase in utility for predicting PGD. Our findings indicate that pre-operative CC-16 levels are independently associated with PGD, and worthy of further study into the mechanism of development of PGD in those subjects without IPF, although it has not been proven a useful predictor of those who will go on to develop PGD when measured pre-operatively.
We were unable to demonstrate an association between Protein C, lL-8, and sRAGE at the pre-operative timepoint and PGD. Protein C and sRAGE have established associations with PGD at post-operative timepoint (5, 7) and IL-8 is a marker of ALI (27); IL-8 was recently demonstrated to have good predictive utility for ALI when measured in the ED. Our inability to detect an association between these biomarkers and the subsequent development of PGD may be because these biomarkers reflect mechanisms that are activated by the process of IRI, and not mechanisms that are active in the recipient prior to transplantation. Additionally, a small proportion of our pre-operative biomarkers was measured at months prior to transplantation, and may have diluted our ability to detect an association using these biomarkers.
Our study has several limitations. First, not all of the plasma measurements were taken at the same timepoint, so there may have been other confounding factors associated with the earlier measurements. However, in sensitivity analyses, the association between CC-16 and PGD was unchanged in subjects who had plasma measurements at the time of listing or at the time of transplantation. This increases applicability of our findings, as the association was still present with biomarkers measured early, it supports the hypothesis that there may be time for potential interventions prior to transplantation. Second, we do not have available data on concomitant immunosuppressant medications at the time of pre-operative blood draws. Although prior studies have successfully measured these markers in the setting of concomitant steroid use, little is known about the effect of immunosuppressants on human plasma levels, thus residual confounding may account for some of our negative results (23, 28–30). Third, we used multiple imputation to account for missing data in the covariates; however, missing data on clinical covariates were rare and we had no missing PGD grade and minimal missing biomarker measurements within the cohort.
In conclusion, we have demonstrated an association between pre-operative levels of CC-16 and PGD in subjects without IPF as a pre-transplant diagnosis. This finding sheds light on pre-transplant, potentially modifiable factors that may lead to PGD. Further research is warranted focusing on the mechanisms of how recipient epithelial injury “primes” the lung for subsequent PGD.
Acknowledgments
This work was supported by the National Institutes of Health Grants R01-HL087115, HL103836, HL088263, T32 HL007568.
Abbreviations
- PGD
primary graft dysfunction
- CC-16
club (clara) cell secretory protein
- sRAGE
soluble receptor for advanced glycation end product
- ICAM-1
intracellular adhesion molecule-1
- UNOS
united network for organ sharing
- IPF
idiopathic pulmonary fibrosis
- mPAP
mean pulmonary arterial pressure
- COPD
chronic obstructive pulmonary disease
Footnotes
Disclosures
Disclosures: Drs. R. Shah, Palmer, Cantu, Flesch, Diamond, Kawut, Localio, Bellamy, Lama, Bhorade, Crespo, Sonnett, Wille, A. Shah, Weinacker, Arcasoy, P. Shah, Christie, and Ware have no conflicts of interest to disclose as described by the American Journal of Transplantation. Dr. Lederer consults for ImmuneWorks on lung transplantation and primary graft dysfunction. Dr. Wilkes is the co-founder and Chief Scientific Officer of ImmuneWorks.
References
- 1.Christie JD, Bavaria JE, Palevsky HI, Litzky L, Blumenthal NP, Kaiser LR, et al. Primary graft failure following lung transplantation. Chest. 1998 Jul;114(1):51–60. doi: 10.1378/chest.114.1.51. [DOI] [PubMed] [Google Scholar]
- 2.Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2005 Oct;24(10):1454–9. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 3.Christie JD, Kotloff RM, Ahya VN, Tino G, Pochettino A, Gaughan C, et al. The effect of primary graft dysfunction on survival after lung transplantation. American journal of respiratory and critical care medicine. 2005 Jun 1;171(11):1312–6. doi: 10.1164/rccm.200409-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christie JD, Kotloff RM, Pochettino A, Arcasoy SM, Rosengard BR, Landis JR, et al. Clinical risk factors for primary graft failure following lung transplantation. Chest. 2003 Oct;124(4):1232–41. doi: 10.1378/chest.124.4.1232. [DOI] [PubMed] [Google Scholar]
- 5.Covarrubias M, Ware LB, Kawut SM, De Andrade J, Milstone A, Weinacker A, et al. Plasma intercellular adhesion molecule-1 and von Willebrand factor in primary graft dysfunction after lung transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007 Nov;7(11):2573–8. doi: 10.1111/j.1600-6143.2007.01981.x. [DOI] [PubMed] [Google Scholar]
- 6.Christie JD, Robinson N, Ware LB, Plotnick M, De Andrade J, Lama V, et al. Association of protein C and type 1 plasminogen activator inhibitor with primary graft dysfunction. American journal of respiratory and critical care medicine. 2007 Jan 1;175(1):69–74. doi: 10.1164/rccm.200606-827OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christie JD, Shah CV, Kawut SM, Mangalmurti N, Lederer DJ, Sonett JR, et al. Plasma levels of receptor for advanced glycation end products, blood transfusion, and risk of primary graft dysfunction. American journal of respiratory and critical care medicine. 2009 Nov 15;180(10):1010–5. doi: 10.1164/rccm.200901-0118OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diamond JM, Kawut SM, Lederer DJ, Ahya VN, Kohl B, Sonett J, et al. Elevated plasma clara cell secretory protein concentration is associated with high-grade primary graft dysfunction. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011 Mar;11(3):561–7. doi: 10.1111/j.1600-6143.2010.03431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah RJ, Bellamy SL, Localio AR, Wickersham N, Diamond JM, Weinacker A, et al. A panel of lung injury biomarkers enhances the definition of primary graft dysfunction (PGD) after lung transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2012 Sep;31(9):942–9. doi: 10.1016/j.healun.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diamond JM, Lee JC, Kawut SM, Shah RJ, Localio AR, Bellamy S, et al. Clinical Risk Factors for Primary Graft Dysfunction after Lung Transplantation. American journal of respiratory and critical care medicine. 2013 Jan 10; doi: 10.1164/rccm.201210-1865OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Department of Health and Hunman Services HRaSA, Healthcare Systems Bureau, Division of Transplantation, Rockville, MD. Transplant Data 1994–2012. United Network for Organ Sharing; Richmond, VA: 2012. 2012 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients. [Google Scholar]
- 12.Cypel M, Yeung JC, Liu M, Anraku M, Chen F, Karolak W, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. The New England journal of medicine. 2011 Apr 14;364(15):1431–40. doi: 10.1056/NEJMoa1014597. [DOI] [PubMed] [Google Scholar]
- 13.Lee JC, Christie JD. Primary graft dysfunction. Proceedings of the American Thoracic Society. 2009 Jan 15;6(1):39–46. doi: 10.1513/pats.200808-082GO. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman SA, Wang L, Shah CV, Ahya VN, Pochettino A, Olthoff K, et al. Plasma cytokines and chemokines in primary graft dysfunction post-lung transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009 Feb;9(2):389–96. doi: 10.1111/j.1600-6143.2008.02497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hermans C, Petrek M, Kolek V, Weynand B, Pieters T, Lambert M, et al. Serum Clara cell protein (CC16), a marker of the integrity of the air-blood barrier in sarcoidosis. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2001 Sep;18(3):507–14. doi: 10.1183/09031936.01.99102601. [DOI] [PubMed] [Google Scholar]
- 16.Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. International journal of epidemiology. 1999 Oct;28(5):964–74. doi: 10.1093/ije/28.5.964. [DOI] [PubMed] [Google Scholar]
- 17.Lederer DJ, Kawut SM, Wickersham N, Winterbottom C, Bhorade S, Palmer SM, et al. Obesity and primary graft dysfunction after lung transplantation: the Lung Transplant Outcomes Group Obesity Study. American journal of respiratory and critical care medicine. 2011 Nov 1;184(9):1055–61. doi: 10.1164/rccm.201104-0728OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. American journal of epidemiology. 1993 Dec 1;138(11):923–36. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 19.Shah RJDJ, Cantu E, Localio AR, Bellamy S, Flesch J, Kawut SM, Lederer DJ, Lama VN, Bhorade SM, Crespo M, Sonett J, Wille KM, Orens J, Shah A, Weinacker A, Wilkes DS, Palmer SM, Ware LB, Christie JD. Use Of A Simple Prediction Model Improves Pre-Transplant Risk Stratification For Primary Graft Dysfunction After Lung Transplantation. American journal of respiratory and critical care medicine. 2013;187(A3773) [Google Scholar]
- 20.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. Bmj. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broeckaert F, Clippe A, Knoops B, Hermans C, Bernard A. Clara cell secretory protein (CC16): features as a peripheral lung biomarker. Annals of the New York Academy of Sciences. 2000;923:68–77. doi: 10.1111/j.1749-6632.2000.tb05520.x. [DOI] [PubMed] [Google Scholar]
- 22.Janssen R, Sato H, Grutters JC, Bernard A, van Velzen-Blad H, du Bois RM, et al. Study of Clara cell 16, KL-6, and surfactant protein-D in serum as disease markers in pulmonary sarcoidosis. Chest. 2003 Dec;124(6):2119–25. doi: 10.1378/chest.124.6.2119. [DOI] [PubMed] [Google Scholar]
- 23.Lesur O, Langevin S, Berthiaume Y, Legare M, Skrobik Y, Bellemare JF, et al. Outcome value of Clara cell protein in serum of patients with acute respiratory distress syndrome. Intensive care medicine. 2006 Aug;32(8):1167–74. doi: 10.1007/s00134-006-0235-1. [DOI] [PubMed] [Google Scholar]
- 24.Kropski JA, Fremont RD, Calfee CS, Ware LB. Clara cell protein (CC16), a marker of lung epithelial injury, is decreased in plasma and pulmonary edema fluid from patients with acute lung injury. Chest. 2009 Jun;135(6):1440–7. doi: 10.1378/chest.08-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sims MW, Beers MF, Ahya VN, Kawut SM, Sims KD, Lederer DJ, et al. Effect of single vs bilateral lung transplantation on plasma surfactant protein D levels in idiopathic pulmonary fibrosis. Chest. 2011 Aug;140(2):489–96. doi: 10.1378/chest.10-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomsen MITMJ, et al. INflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA : the journal of the American Medical Association. 2013;309(22):2353–61. doi: 10.1001/jama.2013.5732. [DOI] [PubMed] [Google Scholar]
- 27.Pires-Neto RC, Morales MMB, Lancas T, Inforsato N, Duarte MIS, Amato MBP, et al. Expression of acute-phase cytokines, surfactant proteins, and epithelial apoptosis in small airways of human acute respiratory distress syndrome. J Crit Care. 2013 Feb;28(1) doi: 10.1016/j.jcrc.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Seam N, Meduri GU, Wang H, Nylen ES, Sun J, Schultz MJ, et al. Effects of methylprednisolone infusion on markers of inflammation, coagulation, and angiogenesis in early acute respiratory distress syndrome. Critical care medicine. 2012 Feb;40(2):495–501. doi: 10.1097/CCM.0b013e318232da5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jorens PG, Sibille Y, Goulding NJ, van Overveld FJ, Herman AG, Bossaert L, et al. Potential role of Clara cell protein, an endogenous phospholipase A2 inhibitor, in acute lung injury. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 1995 Oct;8(10):1647–53. doi: 10.1183/09031936.95.08101647. [DOI] [PubMed] [Google Scholar]
- 30.Panwar R, Venkatesh B, Kruger P, Bird R, Gill D, Nunnink L, et al. Plasma protein C levels in immunocompromised septic patients are significantly lower than immunocompetent septic patients: a prospective cohort study. Journal of hematology & oncology. 2009;2:43. doi: 10.1186/1756-8722-2-43. [DOI] [PMC free article] [PubMed] [Google Scholar]