Abstract
Non alcoholic fatty liver disease (NAFLD), hepatic insulin resistance and type 2 diabetes are all strongly associated and are all reaching epidemic proportions. Whether there is a causal link between NAFLD and hepatic insulin resistance is controversial. This review will discuss recent studies in both humans and animal models of NAFLD that have implicated increases in hepatic diacylglycerol content leading to activation of PKCε resulting in decreased insulin signaling in the pathogenesis of NAFLD associated hepatic insulin resistance and type 2 diabetes. The DAG-PKCε hypothesis can explain the occurrence of hepatic insulin resistance observed in most cases of NAFLD associated with obesity, lipodystrophy and type 2 diabetes.
Keywords: NAFLD, Hepatic, Insulin Resistance, Lipid, DAG, ceramides, endocanabinoid, PKCε
Introduction
NAFLD affects up to 30% of adults and up to 10% of children in developed countries (1, 2). The disease begins with the accumulation of triglyceride in the liver and is defined as the presence of cytoplasmic lipid droplets in more than 5% of hepatocytes or triglyceride levels exceeding the 95th percentile for lean, healthy individuals without significant alcohol consumption and negative viral and autoimmune liver disease (2–4). Ectopic accumulation of hepatic lipids has clearly been linked to the development of hepatic insulin resistance and type 2 diabetes (5, 6). The present review will focus on the physiological and cellular mechanisms that lead to NAFLD as well as the cellular and molecular mechanisms for lipid-induced hepatic insulin resistance.
Overview: Hepatic lipid metabolism
NAFLD develops when the rate of hepatic triglyceride synthesis, due to increased hepatic fatty acid uptake and esterification into triglyceride (TAG) as well as from de novo synthesis of TAG from carbohydrate and protein metabolism, exceeds the rate of hepatic TAG catabolism due to fatty acid oxidation and export of TAG as very low density lipoproteins (VLDL). The liver derives most of its energy for metabolism from fatty acid oxidation during both fasting and feeding and the contributions of fatty acid oxidation to hepatic energy metabolism approaches 100% with hepatic steatosis (7) Circulating fatty acids are taken up into the liver through specific membrane proteins, i.e. FATP2 and FATP5, FAT/CD36 and caveolins (3, 8). Part of the intracellular pathways of lipid storage, mobilization, synthesis, oxidation and export are portrayed in Figures 1 and 2.
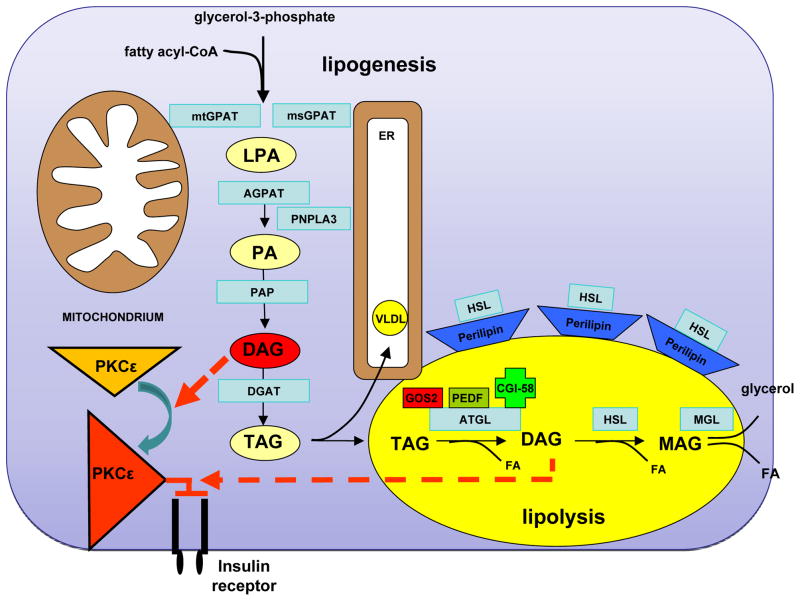
Figure 1. Molecular Regulation of Intrahepatic TAG and DAG Turnover.
The glycerol 3-phosphate (or phosphatidic acid) pathway represents the de novo lipogenesis route in the synthesis of triglycerides (TAG) and phospholipids. Acyl-CoA:glycerol-sn-3-phosphate acyltransferase (GPAT) catalyzes the acylation of sn-glycerol-3-phosphate with acyl-coenzyme A (acyl-CoA) to generate lysophosphatidic acid (LPA), which is thought to be the rate-controlling step in TAG synthesis. PNPLA3 seems to control this step through its transacetylation property. Fatty acyl-CoAs are then successively transferred to the glycerol backbone to from diacylglycerols (catalyzed by phosphatidic acid phosphatase (PAP)) and triacylglycerols (TAG) through diacylglycerol:acyl-CoA acyltransferases (DGAT). They can also esterify with sphingosine to form ceramides. LPA and PA require translocation through the cytosol for TAG synthesis at the endoplasmic reticulum if they are not synthesized in the endoplasmic reticulum. CGI-58 controls compartmentation of DAGs. DAGs in the plasma-membrane fraction activate PKCε, which in turn attenuates insulin receptor activation through its ligand. Hydrolysis from TAG to DAG is mediated by adipose triglyceride lipase (ATGL). DAG can be further hydrolyzed to monoacylglycerol (MAG) by hormone-sensitive lipase (HSL) and subsequently to glycerol by monoglyceride lipase (MGL). These reactions release fatty acids. Glycerol can be used as a substrate for gluconeogenesis. Plasma membrane DAG stimulate PKCε membrane translocation to inhibit the insulin receptor kinase.
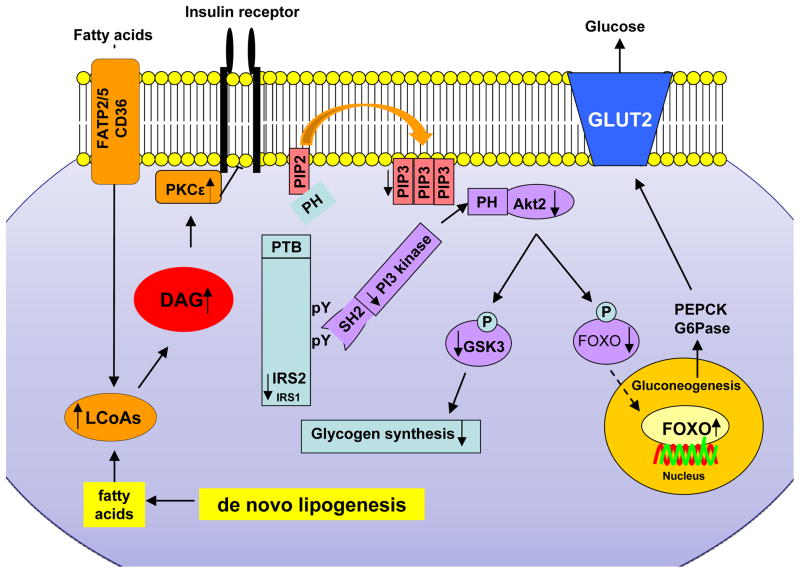
Figure 2. Mechanism of Diacylglycerol-PKCε Mediated Hepatic Insulin Resistance.
Membrane-near intracellular diacyglycerols lead to activation of PKC-ε which, in turn, inhibits the insulin receptor kinase. This then leads to decreased insulin-stimulated tyrosine phosphorylation (pY) of insulin receptor substrate-1 and -2 (IRS-1, IRS-2), PI3K activation and downstream insulin signaling. The net result is a decreased in hepatic glycogen synthesis, owing to decreased activation of glycogen synthase, and increased hepatic gluconeogenesis through reduced inactivation of FOXO1, which results in an exaggerated glucose release through glucose transporter 2 (GLUT2).
Hepatic lipid metabolism, DAGs and hepatic insulin resistance
Numerous studies have demonstrated a strong relationship between intramyocellular lipids and muscle insulin resistance (3, 9, 10). Studies in normal weight, nondiabetic adults found that intramyocellular triglyceride content is a far stronger predictor of muscle insulin resistance than circulating fatty acids (11), suggesting that intramyocellular lipids may be playing a causal role in muscle insulin resistance. In fact, insulin sensitive and resistant obese subjects can be separated on the basis of muscle and liver lipid accumulation (12). In rodent models when plasma fatty acids were increased by infusing Liposyn along with heparin to activate lipoprotein lipase muscle insulin resistance developed at ~3 hours into the infusion when diacylglycerols (DAG) increased and PKCθ was activated (13). In contrast there were no changes in muscle triglyceride or ceramide content at this time thus disassociating these lipids as causal factors in the pathogenesis of lipid-induced muscle insulin resistance. DAGs are second messengers activating members of novel protein kinase C (nPKC) family. These findings of DAG-mediated muscle insulin resistance have subsequently been translated and confirmed in humans (14–16).
Hepatic steatosis and hepatic insulin resistance can be induced in mice and rats with 3 days of high-fat diet (HFD) before the development of obesity (17). Livers of these 3-day HFD fed rats showed increases in hepatic DAG species originating mainly from dietary sources. Similar to the muscle studies there were no alterations in liver ceramide content thus disassociating hepatic ceramide content from hepatic insulin resistance in these studies. The connection between hepatic DAG accumulation and hepatic insulin resistance could be attributed to activation of PKCε, which is highly expressed in liver (17). These changes were associated with reductions in insulin-stimulated insulin receptor substrate-2 (IRS-2) tyrosine phosphorylation by the insulin receptor kinase leading to reductions in insulin stimulation of hepatic glycogen synthesis and suppression of hepatic glucose production (Figure 2). The fact that hepatic insulin resistance occurred prior to any changes in systemic insulin resistance, inflammation or adipose tissue mass, argues strongly in support of a primary causal role of DAG-PKCε in mediating hepatic insulin resistance (18).
The specific role of PKCε in causing hepatic insulin resistance was also directly examined. Knock-down of hepatic expression of PKCε using antisense oligonucleotides in rats as well as PKCε gene knockout mice were both found to protected from lipid-induced hepatic insulin resistance when fed a HFD despite the development of hepatic steatosis (18, 19).
Complementary lines of evidence confirm a pivotal role of DAGs in the development of hepatic insulin resistance. First, mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase (mtGPAT) catalyzes the formation of lysophosphatidic acid (LPA) from fatty acyl CoA and glycerol 3-phosphate (Figure 1). When mtGPAT-deficient (mtGPAT1−/−) mice are placed on a high-fat diet, they accumulate hepatic fatty acyl-CoA, but not hepatic diacylglycerol and triglyceride (20). Furthermore they are protected from lipid-induced hepatic insulin resistance thus dissociating hepatic fatty acyl-CoA content from hepatic insulin resistance. Second, DGAT proteins catalyze the addition of fatty acids to DAGs, and it is highly expressed with increased fat content on lipid droplet membranes (21). Mice overexpressing hepatic DGAT2 develop hepatic steatosis associated with hepatic, but not peripheral, insulin resistance (22). Hepatic insulin resistance in these mice is associated with increased hepatic DAG content, PKCε activation and impaired insulin signaling at the level of the insulin receptor kinase. DGAT2 knock down in rat liver, using antisense oligonucelotides, leads to protection from high fat feeding-induced hepatic steatosis (23). Surprisingly, hepatic DAG content was found to be reduced along with decreased PKCε activation and protection from lipid-induced hepatic insulin resistance. This reduction in hepatic DAG content in the presence of decreased hepatic DGAT2 expression could be explained by down regulation of SREBP1c and the lipogenic pathway as an adaptive response to blocking the lipogenic pathway at DGAT 2 leading to increases in intrahepatic fatty acids (23).
An emerging role has been described for the endocannabinoid system in the development of hepatic lipid accumulation and hepatic insulin resistance. Endocannabinoid are taken up into hepatocytes by specific transporters, such as fatty acid binding proteins (FABP) FABP5, FABP7 (24, 25). The arachidonic acid derivatives act upon cannabinoid receptor 1 (CB1) and 2 (CB2). Both, the receptors as well as specific endocannabinoids, such as 2-AE, are increased in mouse models of dietary triggered obesity in the liver (26–28). Activation of CB1 has been proposed to induce a lipogenic program via the induction of ER stress (26) as well as of transcription factors SREBP1c and CREBH, activating the phosphatidic acid phosphatase Lipin-1 (29). This increases the formation of DAGs, which induce PKC-ε and inhibit insulin receptor signaling. Intriguingly DAG may be transformed into 2-AE again, potentially initiating a feed forward loop leading to and aggravating hepatic steatosis and insulin resistance (30).
Confirmation of this key interaction between DAG, activation of PKCε, and insulin resistance has been demonstrated in numerous other rodent models of NAFLD-associated hepatic insulin resistance (7, 18, 20, 23, 31–38).
Development of NAFLD and hepatic insulin resistance
Increased caloric intake
The most common cause of NAFLD in developed countries can most likely be attributed to increased caloric intake exceeding rates of caloric expenditure (3, 9, 39). This relationship between the nutritional state and NAFLD is reflected by the high prevalence of NAFLD and insulin resistance among obese individuals (40–43). The relationship raises the question why excess calories are not exclusively stored in adipose tissue, the primary storage site of triglycerides (44)?
Several lines of evidence suggest that regional mobilization of circulating triglycerides and fatty acid transport is altered in obese patients with NAFLD. Lipoprotein lipase (LpL) hydrolyzes circulating TAG, followed by tissue uptake through fatty acid transport proteins (FATPs) together with FAT/CD36 (45). LPL activity in adipose tissue in response to insulin seems to be blunted in obese patients (46), while NAFLD is associated with increased hepatic LPL and FATP expression (47). FATPs and FAT/CD36 have been shown to be increased in liver but decreased in adipose tissue of obese patients with NAFLD compared to obese subjects with normal intrahepatic lipid content (IHLC)(48, 49).
Hepatic overexpression of LpL (50, 51) or viral gene delivery of hepatic CD36 causes specific accumulation of lipids in the liver as well as hepatic insulin resistance (52), while deletion of hepatic fatty acid transport proteins, protects against the development of hepatic steatosis and insulin resistance. (53) Taken together, these studies suggest that in nutritionally-induced obesity, fatty acids are misrouted from adipose tissue to liver and skeletal muscle, where they are re-esterified into diacylglycerols, activating PKCε in liver and PKCθ in skeletal muscle, mediating hepatic insulin resistance in these organs.
Lipid storage defects: Lessons Learned from Lipodystrophies
Lipodystrophies are diseases characterized by a loss of adipose tissue, including visceral fat and serve as excellent models to study the effect of reduced lipid storage capacity on the distribution of lipids in other, ectopic sites (e.g. liver and muscle). Mice expressing the dominant-negative protein A-ZIP/F-1 lack white adipose tissue. They develop hypertriglyceridemia, hepatic steatosis and severe insulin resistance in liver and skeletal muscle (54). Transplantation of white adipose tissue in these mice decreases hepatic steatosis and improves hepatic and peripheral insulin sensitivity (54). Leptin is also able to correct many of the metabolic defects associated with lipodystrophy in mice and humans (55, 56). Leptin replacement in lipodystrophic patients markedly reduces both, hepatic and intramyocellular lipid content, which mostly could be attributed to reduction in caloric intake, with concomitant improvement in both hepatic and peripheral insulin sensitivity (56). Taken together these studies clearly dissociate the quantity of body fat as well as visceral fat from insulin resistance and suggest that it is the tissue specific distribution of fat in liver and skeletal muscle, not the whole body quantity of fat, that determines liver and muscle insulin resistance.
This notion is further supported by the role peroxisome proliferators-activated receptor γ (PPARγ) in hepatic steatosis. PPARγ is highly expressed in adipose tissue and plays a key role in promoting fatty acid uptake into adipocytes and adipocyte differentiation (57). Patients with dominant negative mutations in PPARγ develop NAFLD and the metabolic syndrome suggesting increased triglyceride delivery to the liver (58). PPARγ is present in the liver to a much lesser degree than in adipose tissue. Liver-specific PPARγ deficient mice are protected against the development of hepatic steatosis suggesting a role for hepatic PPARγ in the regulation of hepatic triglyceride accumulation (59, 60). Thiazolidinediones (TZDs), therapeutic ligands for PPARγ, decrease hepatic TAG and intramyocellular fat content, promote a redistribution of fat to subcutaneous fat tissue and increase hepatic and muscle insulin sensitivity (61–63). Together, these data show that the ability of adipose tissue to adapt to overfeeding by means of hypertrophy and triglyceride storage plays a protective role in the development and maintenance of NAFLD. Activation of related target genes in adipocytes, such as PPARγ, can indirectly alter hepatic fat content.
Muscle insulin resistance
Primary insulin resistance in skeletal muscle can also lead to a redistribution of substrates towards the liver, resulting in hepatic steatosis and subsequently hepatic insulin resistance through the accumulation of hepatic DAGs with activation of PKCε. Evidence in support of this hypothesis comes from mice with muscle-specific inactivation of the insulin receptor gene (MIRKO-mice) (64). In these mice insulin-stimulated muscle glucose transport and glycogen synthesis were suppressed by more than 80%, and insulin-stimulated glucose transport in adipocytes was increased threefold, demonstrating that selective insulin resistance in skeletal muscle can lead to compensatory hyperinsulinemia leading to the rerouting of substrates toward ectopic sites, such as the liver. These observations were confirmed in mice lacking muscle glucose transporter 4 (GLUT4). These mice have a near-complete loss of insulin-stimulated muscle glucose uptake associated with hepatic steatosis and insulin resistance (65). These findings have been translated to humans. In individuals with selective insulin resistance in skeletal muscle, as observed in healthy, young, lean, individuals in the bottom quartile of whole-body insulin sensitivity, ingested carbohydrates are diverted away from muscle glycogen synthesis and toward hepatic de novo lipogenesis, predisposing these individuals to NALFD and hepatic insulin resistance (66). Furthermore, a recent study showed that a single bout of exercise reversed defects in insulin-stimulated muscle glucose transport and glycogen synthesis (67). This improvement resulted in decreased hepatic de novo lipogenesis and reduced net hepatic triglyceride synthesis following a high carbohydrate meal, demonstrating that skeletal muscle insulin resistance is an early therapeutic target for the prevention and treatment of NAFLD and subsequently, hepatic insulin resistance (67). These findings are supported by a study that showed that the effect of improved physical fitness on insulin sensitivity in overweight to obese subjects is mediated through a reduction in hepatic fat content (68). Chronic exercise without reduction in body weight or whole body fat also leads to a reduction in hepatic fat content, without changing VLDL secretion rates (69). Together, these studies in mice and humans support the concept that selective muscle insulin resistance, which is one of the earliest metabolic abnormalities detected in young lean offspring of parents with type 2 diabetes, can be an important and early factor in the pathogenesis of NAFLD and hepatic insulin resistance.
Genetics and NAFLD
ApoC3, hepatic steatosis and hepatic insulin resistance
Apolipoprotein C3 (ApoC3) is a protein component of VLDL, inhibiting lipoprotein lipase to regulate lipid distribution to different organs and tissues. Two common gene variants (e.g., C-482T/T-455C) in the insulin response element of the apoCIII gene promoter lead to a loss of the suppression effect of insulin on ApoC3, resulting in increased hepatic gene expression of apo CIII leading to increased plasma concentrations of ApoC3 and hypertriglyceridemia (70). Recently, Petersen et al. showed that healthy lean male Asian Indians with these common gene variants (e.g., C-482T/T-455C) were at higher risk of developing NAFLD and insulin resistance (71). The carriers of these polymorphisms have approximately 30% higher plasma concentrations of ApoC3, as well as postprandial hypertriglyceridemia, compared to individuals who are wild-type homozygotes for ApoC3 (C-482/T-455). Through the exaggerated inhibition of lipoprotein lipase activity, carriers of the polymorphism have decreased triglyceride clearance following an intravenous infusion of lipids and increased postprandial hypertriglyceridemia and chylomicrons remnants, leading to NAFLD and hepatic insulin resistance (71). Furthermore, modest weight reduction in these subjects reversed hepatic steatosis and insulin resistance (71). It is important to note that these ApoC3 gene variants, which result in a 30% increase in plasma ApoC3 concentrations, do not directly cause hepatic steatosis but represent a predisposing condition for individuals who carry them. Therefore, when exposed to a toxic environment (i.e., increased dietary fat and calorie dense foods), lean, Asian Indian males with ApoC3 gene variants (C-482T/T-455C) are more susceptible to develop NAFLD and hepatic insulin resistance. It is important to note that this ApoC3 gene variant is a predisposing gene-environment interaction and will typically be observed only in lean individuals, who normally have a low prevalence of NAFLD and not in obese individuals who invariably already have NAFLD (72, 73). Furthermore this gene variant only appears to confer a predisposition to NAFLD in male individuals and not in young, lean, premenopausal women suggesting that estrogen may have a protective effect that negates the effects of ApoC3 to inhibit LPL (74). The effect of the ApoC3 gene variant (C-482T/T-455C) to increase the susceptibility to NAFLD in healthy, lean, individuals of European ancestry has recently been independently confirmed by Peter et al. (75).
Transgenic mice that have hepatic overexpression of human ApoC3 mice (ApoC3 transgenic mice) offer strong genetic evidence demonstrating that increased plasma levels of Apo C3 levels represent a gene environment-interaction promoting hepatic steatosis and hepatic insulin resistance. Since ApoC3 can inhibit LpL activity and limit peripheral fat uptake, ApoC3 transgenic mice have marked hypertriglyceridemia, but normal hepatic lipid content when fed a regular chow-fed diet compared to wild-type littermate mice fed the same diet (34). However, when placed on a high-fat diet, ApoC3 TAG mice develop severe hepatic steatosis and hepatic insulin resistance compared to the wildtype littermate mice (34). Hepatic steatosis could be attributed to a mismatch between hepatic lipid uptake and lipid export in the high fat-fed mice. Taken together these studies demonstrate that alterations in plasma ApoC3 concentrations can alter the postprandial distribution of triglyceride and under conditions of increased fat ingestion and serve as a predisposing condition for the development of NAFLD and hepatic insulin resistance.
Perilipin mutations
Perilipin-1 is the most abundant protein of the lipid droplet mantle in adipose tissue and it functions as a shell regulating specialized TAG- and DAG- hydrolases (76). Recently, two different C-terminal PLIN1 frame shift mutations (Leu-404fs and Val-398fs) were identified in patients partial lipodystrophy, leading to reduced fat mass, hepatic steatosis and elevated plasma triglyceride levels as well as type 2 diabetes (77). Subsequent work has shown that perilipin-1 regulated the molecular interaction of comparative gene identification-58 (CGI-58/Abhd5) with its target, adipose tissue triglyceride lipase (ATGL), and thus, inhibits lipolysis (76). Together, these data show the important role of adipose tissue lipolysis in fatty acid partitioning throughout the body (78). Impairments in the ability to properly control these processes misroutes fatty acids away from adipose tissue and towards ectopic sites, resulting in hepatic steatosis.
PNPLA 3 variants
Patients with a Hispanic background are at risk of developing NAFLD and hepatic insulin resistance (79). In this population, the Met148Ile allele of the lipid droplet protein patatin-like phospholipase domain containing protein 3 (PNPLA3, adiponutrin) markedly correlates with the development of NAFLD (80, 81). PNPLA3 possesses both lipase and transacylation activity (82) and the rs738409 polymorphism has been suggested to cause a loss of lipolytic activity. However, overexpression of PNPLA3 did not alter liver lipid content (83, 84). In contrast, knockdown of hepatic and adipose tissue PNPLA3 expression in rats with antisense oligonucleotides actually reduced hepatic TAG and DAG content and prevented lipid-induced hepatic insulin resistance when adult rats were fed a HFD diet for 5 weeks (15). The effect could be attributed to reduced esterification of lysophosphatidic acid (LPA) to phosphatidic acid through LPA acyltransferase activity. In contrast, in a whole body PNPLA3-knockout mouse model this effect was not observed (85). The difference between these models might be explained either by the variances in genetic targeting (whole body leading to complete knockdown of PNPLA3 vs. ASO treatment leading to partial knockdown of PNPLA3 in liver and adipose tissue) or the time course of high fat feeding, which was extremely long in the PNPLA3-knockout mouse model (85). Taken together these data suggest that PNPLA3 might be involved in hepatic lipogenesis at the level of LPA esterification to phosphatidic acid.
In contrast, adipose triglyceride lipase (ATGL, PNPLA2) is a potent lipase that catalyzes the hydrolysis of triglycerides into diacylglycerols (86, 87). Mice lacking ATGL have marked alterations in lipid metabolism with ectopic lipid accumulation in most tissues (88). Liver-specific deletion of ATGL renders mice prone to hepatic steatosis and decreased lipid oxidation (89). In comparison, adenoviral overexpression of hepatic ATGL reduces liver lipids (including DAGs and ceramides), possibly due to decreased lipogenesis and increased lipid oxidation (90).
Critical Role of DAG Intracellular Compartmentation in Causing Hepatic Insulin Resistance
Animal models have clearly demonstrated that compartmentation of DAGs within the hepatocyte is a major factor in determining whether PKCε is activated by DAGs, leading to hepatic insulin resistance (22). Recently Cantley et al. (91) demonstrated the importance of subcellular localization of DAGs on their ability to interact as signaling molecules with PKCε. In this study Cantley et al. examined the role of intracellular lipid compartmentation in causing hepatic insulin resistance in a rat model where hepatic and adipose tissue expression of the lipid droplet associated protein comparative gene identification-58 (CGI-58) protein was knocked down with antisense oligonucleotides (ASO). CGI-58 stimulates the hydrolysis of stored lipids by activating ATGL. The knockdown of CGI-58 in HFD fed rats led to severe lipid accumulation in the liver, increasing levels of TAG, DAG and ceramides. However, CGI-58-ASO rats remained insulin sensitive. This seemingly paradoxical effect could be explained by a specific compartmentation pattern of DAGs. DAGs were found to be increased mainly in lipid droplets/lipid-droplet associated endoplasmic reticula of CGI-58-ASO rats but not in the plasma-membrane fractions. In this setting, the plasma membrane associated protein kinase PKCε was not activated. In the HFD fed control rats, DAGs accumulated mainly in the hepatic plasma-membrane fractions. In this setting, PKCε was activated and rats developed hepatic insulin resistance. Together, these data strongly suggest that intracellular compartmentation of DAGs is essential in order for them to interact with PKCε and to induce hepatic insulin resistance. Differences in compartmental DAG accumulation and the ability to induce hepatic insulin resistance between patients and animal models might be related to differences in the time course of the development of NAFLD or due to species differences. It is likely that similar differences in DAG compartmentalization may explain the dissociation between hepatic steatosis and hepatic insulin resistance in other transgenic mouse models of NAFLD as well in some patients with NAFLD, who appear to manifest normal hepatic insulin responsiveness. Further studies are needed to address this key question.
The Role of Ceramides in NAFLD associated Hepatic Insulin Resistance
In addition to DAGs, other lipid species have been suggested to contribute to insulin resistance. Ceramides are intermediates of sphingomyelin metabolism and can derive from intracellular saturated fatty acids and inflammatory stimuli. Ceramides accumulate in peripheral tissues of obese subjects (92). Inhibition of sphingomyelin synthesis with reduced levels of ceramides prevents and improves insulin resistance in animal models of dietary induced obesity. The effect of ceramides on insulin signaling has been proposed to be mediated via a direct interaction with AKT (92). However, recent evidence suggests that ceramides are not a primary event in the development of lipid induced hepatic insulin resistance. A three day high fat diet with both, saturated and unsaturated fatty acids in rats induced hepatic insulin resistance with DAG, but not ceramide, accumulation. In the same study, ablation of the TLR-4 receptor did not protect from a short term high fat diet induced hepatic insulin resistance (93). In mammals, ceramides are generated by at least 6 different ceramide synthases (CerS) 1–6, which are encoded by longevity assurance (lass) genes 1–6. CerS 1–6 generate distinct sets of ceramides with specific chain lengths. It will be important to characterize the effect of each of the isoforms in order to better understand the significance of ceramides with distinct chain lengths on insulin signaling as well as to examine if ceramide content is increased in liver of patients with NAFLD and related to hepatic insulin resistance. In this regard, two studies have found that in contrast to hepatic diacylglycerol content, which was strongly associated with hepatic insulin resistance, there was no relationship between hepatic ceramide content and hepatic insulin resistance in humans suggesting that ceramides do not play a major role in mediating hepatic insulin resistance in humans (15, 16).
Endoplasmic reticulum stress and the Innate Immune Response
Endoplasmic reticulum stress has also been implied in the pathogenesis of hepatic steatosis and hepatic insulin resistance. The ER folds unfolded polypeptide chains into proteins with distinct functions. Accumulation of unfolded proteins in the ER activates at least three transmembrane signal transducers, namely inositol requiring protein-1 (IRE-1) splicing and activating its effector X-box binding protein 1 (XBP1), activating transcription factor-6 (ATF-6), and protein kinase RNA – like ER kinase (PERK)(94–96). Disequilibrium between the unfolded protein load entering the ER and the actual ER capacity to properly fold these proteins causes ER stress, which ultimately leads to a cell suicide signal mediated by the pro-apoptoic factor CHOP. Prior to this event, a negative feedback loop consisting of eIF2α and ATF4, inducing CHOP is initiated. CHOP increases transcription of the phosphatase GADD34, which dephosphorylates and inactivates eIF2α, tuning in the strength of the UPR upon chronic ER stress (95).
The UPR regulates lipogenesis, allowing for expansion of the ER membrane and increasing the capacity of the ER to handle proteins. Wild-type mice, treated with chemical activators of the UPR develop a transient hepatic steatosis. Conversely, the induction of chaperone molecules, guiding unfolded proteins through the ER (such as GRP78/BiP) suppressed activation of the UPR and reduced liver lipid content (97). This is attributed to a decreased expression in SREBP1c and ChREBP, two key transcriptional regulators of lipogenesis. Liver specific inhibition of the PERK pathway has been achieved by transgenic expression of the C-terminal fragment of GADD34/ PPP1R15a under an albumin promoter (Alb:GC). This gene encodes a phosphatase that specifically dephosphorylates eIF2a, effectively terminating the signal from PERK. Alb:GC mice have reduced body weight and reduced liver triglyceride content when challenged with 4 months of high-fat feeding (98). In order to study the role of this pathway in the early steps in the development of hepatic steatosis we first studied eIF2α signaling in mice fed a high fat diet for 3 days (99). This intervention specifically increases hepatic lipid content, and the eIF2α pathway was markedly induced by the 3-day high fat diet, pointing to an important role of this ER stress pathway in the pathogenesis of hepatic steatosis. Constitutive dephosphorylation of eIF2α reduced the accumulation of the 3 day high fat diet through reduced expression of key transcription factors C/EBPα, C/EBPβ, suggesting that one of the early events in the induction of a high fat diet induced hepatic steatosis is the activation of eIF2α (99).
In liver samples from patients with NAFLD, components of the UPR only partially correlated with measures of insulin resistance. While eIF2α activation and its downstream factor CHOP correlated with HOMA-IR, components of the other arms, namely XBPs and ATF6, did not show this association. Also, JNK activity did not correlate with hepatic insulin resistance (15). Taken together, these data suggest that the UPR contributes to the regulation of hepatic lipogenesis, and by these means might contribute to hepatic insulin resistance in specific settings.
The notion that inflammatory responses impact on insulin signaling and lipid metabolism derived from studies in obese rodent models of NAFLD which demonstrated an increase in TNFα in adipose tissue obtained from these animals (100). Adipose TNFα secretion is thought to be mainly released from adipose tissue macrophages that accumulate in obese adipose tissue and are recruited via chemokine signaling (101). Subsequently, TNFα was shown to activate jun-N-terminal kinase 1 (JNK1), which leads to serine-307 residue phosphorylation of IRS1 in adipose tissue, explaining the attenuation of insulin signaling at the post-receptor level. Adipose tissue overexpression of chemokine ligand CCL2 (also known as monocyte chemo attractant protein 1 [MCP1]) increases adipose tissue macrophages, hepatic steatosis, and hepatic insulin resistance (102). Consistent with this adipose tissue specific model, deletion of CCL2 protects from high-fat diet-induced hepatic lipid accumulation (102). These effects may at least in part be related to the induction of adipose tissue lipolysis by adipokines. TNFα, as well as IL6 have been shown to induce lipolysis, even though the mechanism seems not be fully understood. As pointed out earlier, the derivation of fatty acids away from adipose tissue towards the liver can result in re-esterification of these fatty acids in the liver and thus, lipid accumulation leading to hepatic insulin resistance.
Cytokine associated signaling within the liver does not necessarily appear to reflect the situation in adipose tissue. While mice with tissue specific knockout of JNK1 (FKO) are protected from HFD induced hepatic steatosis (103) and insulin resistance, presumably through reduced release of IL-6 and reduced SOCS3 activation (103, 104), liver specific deletion of JNK1 also leads to hepatic steatosis, hepatic insulin resistance and increased insulin clearance (105). Continuing along these lines, indirect activation of JNK1 in the liver increases lipid oxidation and decreases lipid synthesis (106). Also, increase JNK1 levels and activity, through ablation of IRE1 in the liver, is paradoxically associated with protection from fructose induced hepatic steatosis and hepatic insulin resistance thus dissociating increased hepatic JNK1 activity from hepatic insulin resistance (96). It is also unclear if IRS1 serine-307 phosphorylation is actually inducing insulin resistance, or rather beneficial for insulin sensitivity (107). Thus, it seems reasonable to conclude that the innate immune system is able to interfere with insulin responsiveness, but the underlying mechanism is not fully understood. One paradigm that covers most models of modulated inflammatory immune responses is that inflammatory response can lead to alterations in insulin action indirectly by altering energetics (energy intake, energy expenditure) and/or lipid delivery to liver and muscle thus leading to alterations in intracellular lipid (DAG) metabolism and insulin action. However, it remains to be conclusively shown, whether or not ER stress as well innate immune responses cause or occur in parallel with the development of hepatic insulin resistance. Further studies are needed to examine this hypothesis.
Summary
NAFLD develops when the rate of fatty acid input (fatty acid uptake and de novo lipogenesis) exceeds the rate of fatty acid output (fatty acid oxidation and secretion of VLDL). Genetic and physiological mechanisms regulating these processes have developed during evolution to cope with starvation but become deregulated in the face of nutritional oversupply. In this setting, they converge to promote the accumulation of ectopic lipids leading to the development of NAFLD and hepatic insulin resistance. Numerous studies in both humans and animal models of NAFLD have demonstrated that hepatic accumulation of the lipid moiety, diacylglycerols, leads to activation of PKCε, resulting in hepatic insulin resistance. Furthermore recent studies have found that intracellular compartmentation of DAG is a critical factor in determining whether increased hepatic DAG content results in hepatic insulin resistance and will likely explain why some patients with NAFLD and some genetic mouse models of NAFLD are not associated with hepatic insulin resistance. Given these findings suggesting that the DAG-PKCε mechanism may represent the root cause of NAFLD associated hepatic insulin resistance, novel therapies targeted to prevent hepatic DAG accumulation and PKCε activation would be predicted to be most efficacious for treating the root cause of NAFLD associated hepatic insulin resistance and type 2 diabetes.
Acknowledgments
Financial Support:
This work was supported by grants from the United States Public Health Service (R24 DK-085638, R01 DK-40936, DK-49230, U24 DK-059635, P30 DK-45735, P30 DK-034989) and grants from the German Research Foundation (DFG, Bl1292/4-1, BI1292/5-1) and the Fritz-Thyssen-Foundation (Az 10.12.2.140).
List of Abbreviations
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- PKCε
novel protein kinase Cε
- FATP
fatty acid transfer protein
- AGPAT
acyl-CoA: 1-acylglycerol-sn-3-phosphate acyltransferase
- MAG
monoacylglcerol
- DAG
diacylglycerol
- TAG
triacylglycerol
- PAP
phosphatidic acid phosphatase
- DGAT
diacylglycerol:acyl-CoA acyltransferase
- ACC
acetyl-CoA carboxylase
- CPT
carnitine-palmityl-transferases
- VLDL
very low density lipoproteins
- LpL
lipoprotein lipase
- PPARγ
role peroxisome proliferators-activated receptor γ
- TZD
thiazolidinediones
- PLIN1
perilipin 1
- ATGL
adipose triglyceride lipase
- CGI-58
comparative gene identification-58
- PNPLA3
patatin-like phospholipase domain containing protein 3
- AMPK
AMP-activated protein kinase
- SREBP-1c
sterol-regulatory element binding protein 1c
- IRS
insulin-receptor-substrate
- ChREBP
carbohydrate responsive element binding protein
- ApoC3
apolipoprotein C3
- INDY
I’m not dead yet
- FOXO
forkhead box protein O
- G6Pase
glucose-6- phosphatase
- GSK3
glycogen synthase kinase-3
- LCoAs
long-chain fatty acids
- PDK
pyruvate dehydrogenase kinase
- PEPCK
phosphoenolpyruvate carboxykinase
- PIP2
phosphatidylinositol bisphosphate
- PIP3
phosphatidylinositol trisphosphate
- PH
pleckstrin homology domain
- PTB
phosphotyrosine binding domain
- SH2
src homology domain
Footnotes
The authors have no conflict of interest to disclose
Contributor Information
Andreas L. Birkenfeld, Email: andreas.birkenfeld@charite.de.
Gerald I. Shulman, Email: gerald.shulman@yale.edu.
References
- 1.WHO. Obesity and Overweight Fact sheet N°311. 2012. [Google Scholar]
- 2.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stefan N, Haring HU. The metabolically benign and malignant fatty liver. Diabetes. 2011;60:2011–2017. doi: 10.2337/db11-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jornayvaz FR, Shulman GI. Diacylglycerol activation of protein kinase Cepsilon and hepatic insulin resistance. Cell Metab. 2012;15:574–584. doi: 10.1016/j.cmet.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142:711–725. e716. doi: 10.1053/j.gastro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Alves TC, Befroy DE, Kibbey RG, Kahn M, Codella R, Carvalho RA, Falk Petersen K, et al. Regulation of hepatic fat and glucose oxidation in rats with lipid-induced hepatic insulin resistance. Hepatology. 2011;53:1175–1181. doi: 10.1002/hep.24170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375:2267–2277. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cusi K. Nonalcoholic fatty liver disease in type 2 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2009;16:141–149. doi: 10.1097/MED.0b013e3283293015. [DOI] [PubMed] [Google Scholar]
- 11.Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42:113–116. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 12.Stefan N, Kantartzis K, Haring HU. Causes and metabolic consequences of Fatty liver. Endocr Rev. 2008;29:939–960. doi: 10.1210/er.2008-0009. [DOI] [PubMed] [Google Scholar]
- 13.Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, Slezak LA, et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103:253–259. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51:2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 15.Kumashiro N, Erion DM, Zhang D, Kahn M, Beddow SA, Chu X, Still CD, et al. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc Natl Acad Sci U S A. 2011;108:16381–16385. doi: 10.1073/pnas.1113359108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magkos F, Su X, Bradley D, Fabbrini E, Conte C, Eagon JC, Varela JE, et al. Intrahepatic diacylglycerol content is associated with hepatic insulin resistance in obese subjects. Gastroenterology. 2012;142:1444–1446. e1442. doi: 10.1053/j.gastro.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 18.Samuel VT, Liu ZX, Wang A, Beddow SA, Geisler JG, Kahn M, Zhang XM, et al. Inhibition of protein kinase Cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest. 2007;117:739–745. doi: 10.1172/JCI30400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frangioudakis G, Burchfield JG, Narasimhan S, Cooney GJ, Leitges M, Biden TJ, Schmitz-Peiffer C. Diverse roles for protein kinase C delta and protein kinase C epsilon in the generation of high-fat-diet-induced glucose intolerance in mice: regulation of lipogenesis by protein kinase C delta. Diabetologia. 2009;52:2616–2620. doi: 10.1007/s00125-009-1543-0. [DOI] [PubMed] [Google Scholar]
- 20.Neschen S, Morino K, Hammond LE, Zhang D, Liu ZX, Romanelli AJ, Cline GW, et al. Prevention of hepatic steatosis and hepatic insulin resistance in mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase 1 knockout mice. Cell Metab. 2005;2:55–65. doi: 10.1016/j.cmet.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Stone SJ, Levin MC, Zhou P, Han J, Walther TC, Farese RV., Jr The endoplasmic reticulum enzyme DGAT2 is found in mitochondria-associated membranes and has a mitochondrial targeting signal that promotes its association with mitochondria. J Biol Chem. 2009;284:5352–5361. doi: 10.1074/jbc.M805768200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jornayvaz FR, Birkenfeld AL, Jurczak MJ, Kanda S, Guigni BA, Jiang DC, Zhang D, et al. Hepatic insulin resistance in mice with hepatic overexpression of diacylglycerol acyltransferase 2. Proc Natl Acad Sci U S A. 2011;108:5748–5752. doi: 10.1073/pnas.1103451108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi CS, Savage DB, Kulkarni A, Yu XX, Liu ZX, Morino K, Kim S, et al. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J Biol Chem. 2007;282:22678–22688. doi: 10.1074/jbc.M704213200. [DOI] [PubMed] [Google Scholar]
- 24.Kaczocha M, Vivieca S, Sun J, Glaser ST, Deutsch DG. Fatty acid-binding proteins transport N-acylethanolamines to nuclear receptors and are targets of endocannabinoid transport inhibitors. J Biol Chem. 2012;287:3415–3424. doi: 10.1074/jbc.M111.304907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newberry EP, Kennedy SM, Xie Y, Luo J, Crooke RM, Graham MJ, Fu J, et al. Decreased body weight and hepatic steatosis with altered fatty acid ethanolamide metabolism in aged L-Fabp −/− mice. J Lipid Res. 2012;53:744–754. doi: 10.1194/jlr.M020966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, Zhou L, Xiong K, Godlewski G, Mukhopadhyay B, Tam J, Yin S, et al. Hepatic cannabinoid receptor-1 mediates diet-induced insulin resistance via inhibition of insulin signaling and clearance in mice. Gastroenterology. 2012;142:1218–1228. e1211. doi: 10.1053/j.gastro.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tam J, Cinar R, Liu J, Godlewski G, Wesley D, Jourdan T, Szanda G, et al. Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab. 2012;16:167–179. doi: 10.1016/j.cmet.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silvestri C, Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 2013;17:475–490. doi: 10.1016/j.cmet.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Chanda D, Kim YH, Kim DK, Lee MW, Lee SY, Park TS, Koo SH, et al. Activation of cannabinoid receptor type 1 (Cb1r) disrupts hepatic insulin receptor signaling via cyclic AMP-response element-binding protein H (Crebh)-mediated induction of Lipin1 gene. J Biol Chem. 2012;287:38041–38049. doi: 10.1074/jbc.M112.377978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeong WI, Osei-Hyiaman D, Park O, Liu J, Batkai S, Mukhopadhyay P, Horiguchi N, et al. Paracrine activation of hepatic CB1 receptors by stellate cell-derived endocannabinoids mediates alcoholic fatty liver. Cell Metab. 2008;7:227–235. doi: 10.1016/j.cmet.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Birkenfeld AL, Lee HY, Guebre-Egziabher F, Alves TC, Jurczak MJ, Jornayvaz FR, Zhang D, et al. Deletion of the mammalian INDY homolog mimics aspects of dietary restriction and protects against adiposity and insulin resistance in mice. Cell Metab. 2011;14:184–195. doi: 10.1016/j.cmet.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Erion DM, Ignatova ID, Yonemitsu S, Nagai Y, Chatterjee P, Weismann D, Hsiao JJ, et al. Prevention of hepatic steatosis and hepatic insulin resistance by knockdown of cAMP response element-binding protein. Cell Metab. 2009;10:499–506. doi: 10.1016/j.cmet.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jornayvaz FR, Jurczak MJ, Lee HY, Birkenfeld AL, Frederick DW, Zhang D, Zhang XM, et al. A high-fat, ketogenic diet causes hepatic insulin resistance in mice, despite increasing energy expenditure and preventing weight gain. Am J Physiol Endocrinol Metab. 2010;299:E808–815. doi: 10.1152/ajpendo.00361.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee HY, Birkenfeld AL, Jornayvaz FR, Jurczak MJ, Kanda S, Popov V, Frederick DW, et al. Apolipoprotein CIII overexpressing mice are predisposed to diet-induced hepatic steatosis and hepatic insulin resistance. Hepatology. 2011;54:1650–1660. doi: 10.1002/hep.24571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jurczak MJ, Lee HY, Birkenfeld AL, Jornayvaz FR, Frederick DW, Pongratz RL, Zhao X, et al. SGLT2 deletion improves glucose homeostasis and preserves pancreatic beta-cell function. Diabetes. 2011;60:890–898. doi: 10.2337/db10-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumashiro N, Yoshimura T, Cantley JL, Majumdar SK, Guebre-Egziabher F, Kursawe R, Vatner DF, et al. Role of patatin-like phospholipase domain-containing 3 on lipid-induced hepatic steatosis and insulin resistance in rats. Hepatology. 2012 doi: 10.1002/hep.26170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown WH, Gillum MP, Lee HY, Camporez JP, Zhang XM, Jeong JK, Alves TC, et al. Fatty acid amide hydrolase ablation promotes ectopic lipid storage and insulin resistance due to centrally mediated hypothyroidism. Proc Natl Acad Sci U S A. 2012;109:14966–14971. doi: 10.1073/pnas.1212887109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumashiro N, Beddow SA, Vatner DF, Majumdar SK, Cantley JL, Guebre-Egziabher F, Fat I, et al. Targeting pyruvate carboxylase reduces gluconeogenesis and adiposity and improves insulin resistance. Diabetes. 2013 doi: 10.2337/db12-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 40.Hilden M, Christoffersen P, Juhl E, Dalgaard JB. Liver histology in a ‘normal’ population--examinations of 503 consecutive fatal traffic casualties. Scand J Gastroenterol. 1977;12:593–597. doi: 10.3109/00365527709181339. [DOI] [PubMed] [Google Scholar]
- 41.Gholam PM, Kotler DP, Flancbaum LJ. Liver pathology in morbidly obese patients undergoing Roux-en-Y gastric bypass surgery. Obes Surg. 2002;12:49–51. doi: 10.1381/096089202321144577. [DOI] [PubMed] [Google Scholar]
- 42.Haufe S, Engeli S, Kast P, Bohnke J, Utz W, Haas V, Hermsdorf M, et al. Randomized comparison of reduced fat and reduced carbohydrate hypocaloric diets on intrahepatic fat in overweight and obese human subjects. Hepatology. 2011;53:1504–1514. doi: 10.1002/hep.24242. [DOI] [PubMed] [Google Scholar]
- 43.Haufe S, Haas V, Utz W, Kast P, Bohnke J, Mähler A, Mehling H, et al. Long Lasting Improvements in Liver Fat and Function Despite Body Weight Regain Following a Six Months Hypocaloric Diet. Diabtetes Care. 2013 doi: 10.2337/dc13-0102. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sunny NE, Parks EJ, Browning JD, Burgess SC. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab. 2011;14:804–810. doi: 10.1016/j.cmet.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldberg IJ, Eckel RH, Abumrad NA. Regulation of fatty acid uptake into tissues: lipoprotein lipase- and CD36-mediated pathways. J Lipid Res. 2009;50 (Suppl):S86–90. doi: 10.1194/jlr.R800085-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sadur CN, Yost TJ, Eckel RH. Insulin responsiveness of adipose tissue lipoprotein lipase is delayed but preserved in obesity. J Clin Endocrinol Metab. 1984;59:1176–1182. doi: 10.1210/jcem-59-6-1176. [DOI] [PubMed] [Google Scholar]
- 47.Westerbacka J, Kolak M, Kiviluoto T, Arkkila P, Siren J, Hamsten A, Fisher RM, et al. Genes involved in fatty acid partitioning and binding, lipolysis, monocyte/macrophage recruitment, and inflammation are overexpressed in the human fatty liver of insulin-resistant subjects. Diabetes. 2007;56:2759–2765. doi: 10.2337/db07-0156. [DOI] [PubMed] [Google Scholar]
- 48.Greco D, Kotronen A, Westerbacka J, Puig O, Arkkila P, Kiviluoto T, Laitinen S, et al. Gene expression in human NAFLD. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1281–1287. doi: 10.1152/ajpgi.00074.2008. [DOI] [PubMed] [Google Scholar]
- 49.Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, Okunade A, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A. 2009;106:15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim JK, Fillmore JJ, Chen Y, Yu C, Moore IK, Pypaert M, Lutz EP, et al. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc Natl Acad Sci U S A. 2001;98:7522–7527. doi: 10.1073/pnas.121164498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Merkel M, Weinstock PH, Chajek-Shaul T, Radner H, Yin B, Breslow JL, Goldberg IJ. Lipoprotein lipase expression exclusively in liver. A mouse model for metabolism in the neonatal period and during cachexia. J Clin Invest. 1998;102:893–901. doi: 10.1172/JCI2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koonen DP, Jacobs RL, Febbraio M, Young ME, Soltys CL, Ong H, Vance DE, et al. Increased hepatic CD36 expression contributes to dyslipidemia associated with diet-induced obesity. Diabetes. 2007;56:2863–2871. doi: 10.2337/db07-0907. [DOI] [PubMed] [Google Scholar]
- 53.Falcon A, Doege H, Fluitt A, Tsang B, Watson N, Kay MA, Stahl A. FATP2 is a hepatic fatty acid transporter and peroxisomal very long-chain acyl-CoA synthetase. Am J Physiol Endocrinol Metab. 2010;299:E384–393. doi: 10.1152/ajpendo.00226.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim JK, Gavrilova O, Chen Y, Reitman ML, Shulman GI. Mechanism of insulin resistance in A-ZIP/F-1 fatless mice. J Biol Chem. 2000;275:8456–8460. doi: 10.1074/jbc.275.12.8456. [DOI] [PubMed] [Google Scholar]
- 55.Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401:73–76. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- 56.Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109:1345–1350. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schupp M, Lazar MA. Endogenous ligands for nuclear receptors: digging deeper. J Biol Chem. 2010;285:40409–40415. doi: 10.1074/jbc.R110.182451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Savage DB, Tan GD, Acerini CL, Jebb SA, Agostini M, Gurnell M, Williams RL, et al. Human metabolic syndrome resulting from dominant-negative mutations in the nuclear receptor peroxisome proliferator-activated receptor-gamma. Diabetes. 2003;52:910–917. doi: 10.2337/diabetes.52.4.910. [DOI] [PubMed] [Google Scholar]
- 59.Matsusue K, Haluzik M, Lambert G, Yim SH, Gavrilova O, Ward JM, Brewer B, Jr, et al. Liver-specific disruption of PPARgamma in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J Clin Invest. 2003;111:737–747. doi: 10.1172/JCI17223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gavrilova O, Haluzik M, Matsusue K, Cutson JJ, Johnson L, Dietz KR, Nicol CJ, et al. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem. 2003;278:34268–34276. doi: 10.1074/jbc.M300043200. [DOI] [PubMed] [Google Scholar]
- 61.Mayerson AB, Hundal RS, Dufour S, Lebon V, Befroy D, Cline GW, Enocksson S, et al. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes. 2002;51:797–802. doi: 10.2337/diabetes.51.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bajaj M, Baig R, Suraamornkul S, Hardies LJ, Coletta DK, Cline GW, Monroy A, et al. Effects of pioglitazone on intramyocellular fat metabolism in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2010;95:1916–1923. doi: 10.1210/jc.2009-0911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luedtke A, Boschmann M, Colpe C, Engeli S, Adams F, Birkenfeld AL, Haufe S, et al. Thiazolidinedione Response in Familial Lipodystrophy Patients with LMNA Mutations: A Case Series. Horm Metab Res. 2012 doi: 10.1055/s-0031-1301284. [DOI] [PubMed] [Google Scholar]
- 64.Kim JK, Michael MD, Previs SF, Peroni OD, Mauvais-Jarvis F, Neschen S, Kahn BB, et al. Redistribution of substrates to adipose tissue promotes obesity in mice with selective insulin resistance in muscle. J Clin Invest. 2000;105:1791–1797. doi: 10.1172/JCI8305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kotani K, Peroni OD, Minokoshi Y, Boss O, Kahn BB. GLUT4 glucose transporter deficiency increases hepatic lipid production and peripheral lipid utilization. J Clin Invest. 2004;114:1666–1675. doi: 10.1172/JCI21341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S, Cline GW, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104:12587–12594. doi: 10.1073/pnas.0705408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rabol R, Petersen KF, Dufour S, Flannery C, Shulman GI. Reversal of muscle insulin resistance with exercise reduces postprandial hepatic de novo lipogenesis in insulin resistant individuals. Proc Natl Acad Sci U S A. 2011;108:13705–13709. doi: 10.1073/pnas.1110105108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haufe S, Engeli S, Budziarek P, Utz W, Schulz-Menger J, Hermsdorf M, Wiesner S, et al. Cardiorespiratory fitness and insulin sensitivity in overweight or obese subjects may be linked through intrahepatic lipid content. Diabetes. 2010;59:1640–1647. doi: 10.2337/db09-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sullivan S, Kirk EP, Mittendorfer B, Patterson BW, Klein S. Randomized trial of exercise effect on intrahepatic triglyceride content and lipid kinetics in nonalcoholic fatty liver disease. Hepatology. 2012;55:1738–1745. doi: 10.1002/hep.25548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li WW, Dammerman MM, Smith JD, Metzger S, Breslow JL, Leff T. Common genetic variation in the promoter of the human apo CIII gene abolishes regulation by insulin and may contribute to hypertriglyceridemia. J Clin Invest. 1995;96:2601–2605. doi: 10.1172/JCI118324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Petersen KF, Dufour S, Hariri A, Nelson-Williams C, Foo JN, Zhang XM, Dziura J, et al. Apolipoprotein C3 gene variants in nonalcoholic fatty liver disease. N Engl J Med. 2010;362:1082–1089. doi: 10.1056/NEJMoa0907295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Valenti L, Nobili V, Al-Serri A, Rametta R, Leathart JB, Zappa MA, Dongiovanni P, et al. The APOC3 T-455C and C-482T promoter region polymorphisms are not associated with the severity of liver damage independently of PNPLA3 I148M genotype in patients with nonalcoholic fatty liver. J Hepatol. 2011;55:1409–1414. doi: 10.1016/j.jhep.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 73.Hyysalo J, Stojkovic I, Kotronen A, Hakkarainen A, Sevastianova K, Makkonen J, Lundbom N, et al. Genetic variation in PNPLA3 but not APOC3 influences liver fat in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2012;27:951–956. doi: 10.1111/j.1440-1746.2011.07045.x. [DOI] [PubMed] [Google Scholar]
- 74.Coban N, Onat A, Guclu-Geyik F, Komurcu-Bayrak E, Sansoy V, Hergenc G, Can G, et al. Gender- and obesity-specific effect of apolipoprotein C3 gene (APOC3) -482C>T polymorphism on triglyceride concentration in Turkish adults. Clin Chem Lab Med. 2012;50:285–292. doi: 10.1515/CCLM.2011.747. [DOI] [PubMed] [Google Scholar]
- 75.Peter A, Kantartzis K, Machicao F, Machann J, Wagner S, Templin S, Konigsrainer I, et al. Visceral obesity modulates the impact of apolipoprotein C3 gene variants on liver fat content. Int J Obes (Lond) 2012;36:774–782. doi: 10.1038/ijo.2011.154. [DOI] [PubMed] [Google Scholar]
- 76.Gandotra S, Lim K, Girousse A, Saudek V, O’Rahilly S, Savage DB. Human frame shift mutations affecting the carboxyl terminus of perilipin increase lipolysis by failing to sequester the adipose triglyceride lipase (ATGL) coactivator AB-hydrolase-containing 5 (ABHD5) J Biol Chem. 2011;286:34998–35006. doi: 10.1074/jbc.M111.278853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gandotra S, Le Dour C, Bottomley W, Cervera P, Giral P, Reznik Y, Charpentier G, et al. Perilipin deficiency and autosomal dominant partial lipodystrophy. N Engl J Med. 2011;364:740–748. doi: 10.1056/NEJMoa1007487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rohm M, Sommerfeld A, Strzoda D, Jones A, Sijmonsma TP, Rudofsky G, Wolfrum C, et al. Transcriptional cofactor TBLR1 controls lipid mobilization in white adipose tissue. Cell Metab. 2013;17:575–585. doi: 10.1016/j.cmet.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 79.Liska D, Dufour S, Zern TL, Taksali S, Cali AM, Dziura J, Shulman GI, et al. Interethnic differences in muscle, liver and abdominal fat partitioning in obese adolescents. PLoS One. 2007;2:e569. doi: 10.1371/journal.pone.0000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kantartzis K, Peter A, Machicao F, Machann J, Wagner S, Konigsrainer I, Konigsrainer A, et al. Dissociation between fatty liver and insulin resistance in humans carrying a variant of the patatin-like phospholipase 3 gene. Diabetes. 2009;58:2616–2623. doi: 10.2337/db09-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jenkins CM, Mancuso DJ, Yan W, Sims HF, Gibson B, Gross RW. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J Biol Chem. 2004;279:48968–48975. doi: 10.1074/jbc.M407841200. [DOI] [PubMed] [Google Scholar]
- 83.He S, McPhaul C, Li JZ, Garuti R, Kinch L, Grishin NV, Cohen JC, et al. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J Biol Chem. 2010;285:6706–6715. doi: 10.1074/jbc.M109.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qiao A, Liang J, Ke Y, Li C, Cui Y, Shen L, Zhang H, et al. Mouse patatin-like phospholipase domain-containing 3 influences systemic lipid and glucose homeostasis. Hepatology. 2011;54:509–521. doi: 10.1002/hep.24402. [DOI] [PubMed] [Google Scholar]
- 85.Basantani MK, Sitnick MT, Cai L, Brenner DS, Gardner NP, Li JZ, Schoiswohl G, et al. Pnpla3/Adiponutrin deficiency in mice does not contribute to fatty liver disease or metabolic syndrome. J Lipid Res. 2011;52:318–329. doi: 10.1194/jlr.M011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 87.Villena JA, Roy S, Sarkadi-Nagy E, Kim KH, Sul HS. Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J Biol Chem. 2004;279:47066–47075. doi: 10.1074/jbc.M403855200. [DOI] [PubMed] [Google Scholar]
- 88.Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 89.Wu JW, Wang SP, Alvarez F, Casavant S, Gauthier N, Abed L, Soni KG, et al. Deficiency of liver adipose triglyceride lipase in mice causes progressive hepatic steatosis. Hepatology. 2011;54:122–132. doi: 10.1002/hep.24338. [DOI] [PubMed] [Google Scholar]
- 90.Turpin SM, Hoy AJ, Brown RD, Rudaz CG, Honeyman J, Matzaris M, Watt MJ. Adipose triacylglycerol lipase is a major regulator of hepatic lipid metabolism but not insulin sensitivity in mice. Diabetologia. 2011;54:146–156. doi: 10.1007/s00125-010-1895-5. [DOI] [PubMed] [Google Scholar]
- 91.Cantley JL, Yoshimura T, Camporez JP, Zhang D, Jornayvaz FR, Kumashiro N, Guebre-Egziabher F, et al. CGI-58 knockdown sequesters diacylglycerols in lipid droplets/ER-preventing diacylglycerol-mediated hepatic insulin resistance. Proc Natl Acad Sci U S A. 2013;110:1869–1874. doi: 10.1073/pnas.1219456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab. 2012;15:585–594. doi: 10.1016/j.cmet.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 93.Galbo T, Perry RJ, Jurczak MJ, Camporez JP, Alves TC, Kahn M, Guigni BA, et al. Saturated and unsaturated fat induce hepatic insulin resistance independently of TLR-4 signaling and ceramide synthesis in vivo. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1311176110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fu S, Watkins SM, Hotamisligil GS. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 2012;15:623–634. doi: 10.1016/j.cmet.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 95.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 96.Jurczak MJ, Lee AH, Jornayvaz FR, Lee HY, Birkenfeld AL, Guigni BA, Kahn M, et al. Dissociation of inositol-requiring enzyme (IRE1alpha)-mediated c-Jun N-terminal kinase activation from hepatic insulin resistance in conditional X-box-binding protein-1 (XBP1) knock-out mice. J Biol Chem. 2012;287:2558–2567. doi: 10.1074/jbc.M111.316760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferre P, et al. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119:1201–1215. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oyadomari S, Harding HP, Zhang Y, Oyadomari M, Ron D. Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008;7:520–532. doi: 10.1016/j.cmet.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Birkenfeld AL, Lee HY, Majumdar S, Jurczak MJ, Camporez JP, Jornayvaz FR, Frederick DW, et al. Influence of the hepatic eukaryotic initiation factor 2alpha (eIF2alpha) endoplasmic reticulum (ER) stress response pathway on insulin-mediated ER stress and hepatic and peripheral glucose metabolism. J Biol Chem. 2011;286:36163–36170. doi: 10.1074/jbc.M111.228817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 101.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, Barrett T, et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Henkel J, Neuschafer-Rube F, Pathe-Neuschafer-Rube A, Puschel GP. Aggravation by prostaglandin E2 of interleukin-6-dependent insulin resistance in hepatocytes. Hepatology. 2009;50:781–790. doi: 10.1002/hep.23064. [DOI] [PubMed] [Google Scholar]
- 105.Sabio G, Cavanagh-Kyros J, Ko HJ, Jung DY, Gray S, Jun JY, Barrett T, et al. Prevention of steatosis by hepatic JNK1. Cell Metab. 2009;10:491–498. doi: 10.1016/j.cmet.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Flach RJ, Qin H, Zhang L, Bennett AM. Loss of mitogen-activated protein kinase phosphatase-1 protects from hepatic steatosis by repression of cell death-inducing DNA fragmentation factor A (DFFA)-like effector C (CIDEC)/fat-specific protein 27. J Biol Chem. 2011;286:22195–22202. doi: 10.1074/jbc.M110.210237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Copps KD, Hancer NJ, Opare-Ado L, Qiu W, Walsh C, White MF. Irs1 serine 307 promotes insulin sensitivity in mice. Cell Metab. 2010;11:84–92. doi: 10.1016/j.cmet.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]