Abstract
Aseptic implant loosening related to implant wear particle-induced inflammation is the most common cause of failure after joint replacement. Modulation of the inflammatory reaction to the wear products represents a rational approach for preventing aseptic implant failure. Long-term treatment using anti-inflammatory agents, however, can be associated with significant systemic side effects due to the drugs' lack of tissue specificity. To address this issue, N-(2-hydroxypropyl) methacrylamide (HPMA) copolymer-dexamethasone conjugate (P-Dex) was developed and evaluated for prevention of wear particle-induced osteolysis and the loss of fixation in a murine prosthesis failure model. Daily administration of free dexamethasone (Dex) was able to prevent wear particle-induced osteolysis, as assessed by micro-CT and histological analysis. Remarkably, monthly P-Dex administration (dose equivalent to free Dex treatment) was equally effective as free dexamethasone, but was not associated with systemic bone loss (a major adverse side effect of glucocorticoids). The reduced systemic toxicity of P-Dex is related to preferential targeting of the sites of wear particle-induced inflammation and its subcellular sequestration and retention by local inflammatory cell populations, resulting in sustained therapeutic action. These results demonstrate the feasibility of utilizing a macromolecular prodrug with reduced systemic toxicity to prevent wear particle-induced osteolysis.
Keywords: HPMA copolymer, prodrug, inflammation targeting, dexamethasone, implant loosening, ELVIS
1. Introduction
Total joint replacement has been performed in North America since the early 1970's and is considered to be the final treatment option to relieve pain and restore joint function in patients with advanced osteoarthritis or rheumatoid arthritis. There are over 600,000 total joint replacement surgeries performed annually in the United States, and the number is expected to increase to 4 million annually by 2030 [1, 2]. Despite the significant progress in the implant design/materials and surgery techniques, the overall 10-year success rate for total joint replacement is ~90 %, with close to 10% of patients ultimately requiring revision surgery [3]. Revision procedures are costly, surgically more challenging, and associated with a shorter duration of implant survival. Therefore, prevention of total joint replacement failure would be of great benefit to patients and the healthcare system.
Aseptic loosening of prosthetic implants due to peri-implant osteolysis is a common cause of failure after joint replacement [4]. Inflammation induced by wear particles generated from the prosthetic components is considered to be the major cause of osteolysis and aseptic implant loosening [5, 6]. Wear debris are phagocytosed primarily by macrophages, leading to activation of pro-inflammatory cascades, resulting in osteoclast-mediated osteolysis at the bone-implant interface and eventual loss of fixation [7, 8]. Thus, wear particle-induced inflammation plays a vital role in the development of wear particle-induced total joint replacement failure and consistent with this, modulation of inflammation has been proven to be an effective strategy to prevent wear particle-induced bone resorption [9–11].
Long-term modulation of inflammation is needed to prevent aseptic implant loosening. However, the lack of tissue specificity of anti-inflammatory drugs combined with their ubiquitous distribution may cause significant systemic adverse side effects after long-term use. For example, glucocorticoids (GC), a class of highly potent anti-inflammatory drugs, have been widely used in treating many inflammatory diseases, including rheumatoid arthritis, asthma, bronchospasm and inflammatory bowel diseases. The ubiquitous distribution of GC and their adverse systemic toxicities such as immunosuppression and secondary osteoporosis, however, have limited their therapeutic potential for many chronic inflammatory conditions.
In order to address the problem related to high off-target GC concentrations and associated systemic toxicity, we developed a N-(2-Hydroxypropyl) methacrylamide (HPMA) copolymer-dexamethasone conjugate (P-Dex) [12, 13]. In previous studies, we have found that HPMA copolymer conjugated with an imaging probe could preferentially target to the sites of particle-induced inflammation prior to detectable osteolysis in a murine calvaria osteolysis model [14]. Based on these preliminary results, we hypothesized that P-Dex would preferentially target the site of peri-implant inflammation to effectively prevent osteolysis and avert the typical side effects associated with GC systemic exposure. This study was designed to directly validate this hypothesis.
2. Material and methods
2.1 Synthesis of macromolecular prodrug
P-Dex was synthesized by reversible addition-fragmentation chain transfer (RAFT) copolymerization as described previously [12]. Briefly, N-(2-hydroxypropyl) methacrylamide (HPMA), N-methacryloylglycylglycylhydrazyl dexamethasone and trace amount of N-methacryloylaminopropyl fluorescein thiourea (for visualization in column purification) [15] were copolymerized at 40 °C under argon for 48 h with 2,2′-azobisisobutyronitrile as the initiator and S,S′-bis(α, α′-dimethyl-α″-acetic acid)-trithiocarbonate as the RAFT agent. Resulting polymers were purified by LH-20 column (GE HealthCare, Waukesha, WI) and lyophilized. N-(3-Aminopropyl)methacrylamide hydrochloride (APMA, Polysciences, Inc. Warrington, PA) was added to the aforementioned co-monomers to obtain the APMA-containing P-Dex. Alexa Fluor 488 labeled P-Dex (P-Dex-Alexa) and IRDye 800 CW labeled P-Dex (P-Dex-IRDye) were obtained via polymer analogous reaction between APMA-containing P-Dex and NHS esters of the dyes [14].
2.2 Establishment of a murine prosthesis failure model
Poly(methyl methacrylate) (PMMA) particles (1–10 μm, Bangs Laboratories, Fishers, IN) were soaked in 70 % ethanol overnight, then washed and suspended in sterile phosphate buffered saline (PBS). A limulus assay was performed using a Pyrosate® kit (Associates of Cape Cod, Inc., East Falmouth, MA) to confirm that the treated particles were endotoxin-free. All animal experiments were performed according to a protocol approved by University of Nebraska Medical Center Institutional Animal Care and Use Committee. Male Swiss Webster mice (10 weeks, Charles River Laboratories Inc., Wilmington, MA) were anesthetized with a mixture of xylazine and ketamine. The surgical site and wide margins (1 cm or more if possible) were clipped free of hair, scrubbed twice with betadine, and then wiped with 70% isopropyl alcohol. A distal to proximal incision along the medial aspect of the patella was made and the patella was reflected laterally to expose the femoral condyles. A 25-gauge needle was used to manually drill through the intercondylar notch to access the medullary cavity and the marrow cavity of the femur was reamed out to a depth of 5 mm from the condyles. The medullary canal was flushed with PBS. For the induction of osteolysis, PMMA particles (1 mg in 10 μL sterile PBS) were injected into the medullary canal before insertion of the pin. Then a 0.45mm × 5 mm stainless steel pin was inserted into the medullary chamber of the distal femur. The patella was reduced to its anatomical position. The wound was cleaned, rinsed with saline containing antibiotics and closed with sutures in layers. Antibiotics (cefazolin sodium, 25 mg/kg, oral) and analgesics (buprenorphine, 0.5 mg/kg, s.c.) were given twice daily for three days after surgery. The position of the implant was validated with plain X-ray. PMMA particles (1 mg in 10 uL PBS) were injected into the knee twice a month postoperatively to induce osteolysis. For the control group, PBS was injected at the time of surgery and twice a month postoperatively. A total of 75 male Swiss Webster mice were used in this experiment. For prevention study, 30 mice were implanted with stainless pin and injected with PMMA in both sides. As the control group, 6 mice were implanted with the pin and injected with PBS on both sides instead of PMMA particles. The rest of the animals (39 mice) were used for optical imaging (6 mice), flow cytometry (9 mice × 3 repetition) and immunohistochemical analysis (6 mice). These mice were implanted with the stainless steel pin in both femurs and injected with PMMA in one side and PBS in the other.
2.3 In vivo treatment study
As shown in Table 1, 30 mice with pin implantation and PMMA particle infusion on both sides were randomly assigned into 5 groups for prevention study. One-month post surgery, the animals were given the following treatments: group 1, HPMA homopolymer without Dex (PHPMA, the amount of polymer used was equivalent to that in group 4, monthly i.v. injection, two injections in total); group 2, PBS (monthly i.v. injection, two injections in total); group 3, free Dex (total dose = 10 mg/kg, daily i.p. injections for two months); group 4, P-Dex (equivalent Dex dose = 10 mg/kg, monthly i.v. injection, two injections in total); group 5, P-Dex (equivalent Dex dose = 5 mg/kg, monthly i.v. injection, two injections in total). The sixth group was designated as the control with pin implantation and PBS injection on both sides instead of PMMA particle infusion. At three months post-surgery, these mice were euthanized. The femurs and L4 lumbar vertebrae bodies of the animals were isolated and fixed with 4% buffered paraformadehyde and subjected to micro-CT analysis.
Table 1.
Animal treatment group arrangement
| Group | Challenged with | Treated with | Dosage | Administration frequency |
|---|---|---|---|---|
| 1 | PMMA | PHPMA | Equivalent to polymer amount in group 4 | Monthly i.v. injection, two injections in total |
| 2 | PMMA | PBS | 0.1 mL | Monthly i.v. injection, two injections in total |
| 3 | PMMA | Free Dex | Total Dex dose = 10 mg/kg | Daily i.p. injections for two months |
| 4 | PMMA | P-Dex | Equivalent Dex dose = 10 mg/kg | Monthly i.v. injection, two injections in total |
| 5 | PMMA | P-Dex | Equivalent Dex dose = 5 mg/kg | Monthly i.v. injection, two injections in total |
| 6 | PBS | - | - | - |
2.4 Micro-CT evaluation
A high-resolution Skyscan 1172 micro-CT (Skyscan, Aartselaar, Belgium) was used for qualitative and quantitative analyses. For femurs, micro-CT focused on the distal part of the femur. The X-ray source was set at a voltage of 70 kV and a current of 141 μA with a fixed exposure time of 480 ms. Eight frames were averaged with a rotation step of 0.7° following an angle of 180°. The resolution was 5.5 μm using a medium camera pixel size (2000 × 1336) with Al 0.5 mm filter. The region of interest (ROI) was selected from 300 slices below the growth plate and extending distally for 100 slices, defined as a ring from the implant axis and with a radius of 0.6 mm. The pin was excluded from the ROI by setting the specific threshold. Three-dimensional reconstructions of the scanned region by the system reconstruction software (NRecon, Skyscan) and bone background segmentation were then performed. For quantitative analysis of the particle-induced osteolysis, the resident software (CTAn, Skyscan) was used to obtain specific morphometric parameters within the ROI, including bone volume (BV), tissue volume (TV), bone volume fraction (BV/TV), bone surface density (BS/TV), trabecular number (Tb.N) and structure model index (SMI) in 3D. In the two-dimensional (2-D) slice-by-slice analysis, average bone fragment area, average bone fragment numbers and mean polar moment of inertia (MMI) were calculated. To produce a visual representation of the results, 3D images were developed using the resident CT-Vol software. Methods to calculate the above mentioned bone parameters are described in detail on the Skyscan website.
For the analysis of L4 lumbar vertebrae bodies, the X-ray source was set at a voltage of 50 kV and a current of 100 μA with a fixed exposure time of 480 ms. Eight frames were averaged with a rotation step of 0.7° following an angle of 180°. The resolution was 6 μm using medium camera pixel size (2000 × 1336) with Al 0.5 mm filter. The volume of interest (VOI) was selected 150 slices up and 150 slices down (300 slices in total) the central slice, which was the middle of the upper and lower margins of the starting point of trabecular bone network. The trabecular bone region was outlined for each micro-CT slice, excluding both the cranial and caudal endplate. Within these regions, trabecular bone (ROI) was separated from cortical bone with boundaries defined by the endocortical bone surfaces. Three-dimensional reconstructions of the scanned region by the system reconstruction software and bone background segmentation were then performed. For quantitative analysis, bone volume fraction (BV/TV) and bone mineral density (BMD) of the ROI were obtained using the resident software.
2.5 Histological evaluation
After micro-CT analysis, the femurs were decalcified in 10 % EDTA. The pins were carefully removed after decalcification was completed. The specimens were then paraffin embedded and sectioned (6 μm thickness). The sections were stained with hematoxylin and eosin to examine the bone loss around the prosthetic pin and to evaluate debris-associated inflammation. For identification of osteoclasts, paraffin sections were stained for tartrate-resistant acid phosphatase (TRAP) using a commercial staining kit (387A, Sigma-Aldrich, St. Louis, MO) according to a protocol provided by the vendor. Purple red-stained multinuclear cells are recognized as TRAP positive osteoclasts. To examine the bone collagen loss, modified trichrome staining was performed according to prior studies [16, 17]. Bone collagen stained dark blue, with color density proportional to the content of collagen deposit. This was further quantified for integrated optical density (IOD) using Image Pro Plus software. All the stained sections were examined with an Olympus BX51 microscopy (Olympus, Japan).
2.6 Near-infrared imaging analysis
One-month post surgery, 6 mice were administrated P-Dex-IRDye (0.3 mg polymer/mouse) by tail vein injection. They were imagined 1, 7 and 14 days later using Pearl® Impulse small animal imaging system (LI-COR, Lincoln, NE) to evaluate the distribution and retention of the IRDye-labeled prodrug.
2.7 Flow cytometry analysis
Alexa Fluor 488-labeled P-Dex (P-Dex-Alexa, 5.0 mg polymer/mouse) was given to 18 mice by tail vein injection 1-month post surgery. Another 9 mice without P-Dex-Alexa administration were used as control. At necropsy (24 h post injection), the distal femur and the adjacent soft tissue were isolated and minced aseptically. The tissues were further digested with collagenase type I (1 mg/mL, Sigma-Aldrich, St. Louis, MO) at 37 °C for 2 h. After passing through a 70 μm cell strainer, a single cell suspension (1 × 106 cells/mL) was obtained. ACK lysing buffer (Quality Biological, Gaithersburg, MD) was then used to remove the red blood cells. For fluorescence-activated cell sorting (FACS) evaluation of F4/80, CD11c, Ly-6G (Gr-1) and Ly-6B (7/4) positive cells, the samples were incubated with antibodies {Allophycocyanin (APC)-labeled rat anti-mouse F4/80 (AbD Serotec, Raleigh, NC), APC-labeled hamster anti-mouse CD11c (BD Pharmingen, San Jose, CA), Phycoerythrin (PE)-labeled rat anti-mouse Ly-6B (7/4) (AbD Serotec) and PE-labeled rat anti-mouse Ly-6G (Gr-1) (Ebioscience, San Diego, CA)} for 30 min on ice. For FACS evaluation of P4HB positive cells, the samples were first incubated with rabbit anti-mouse prolyl-4-hydroxylase (P4HB, Abcam, Cambridge, MA) for 30 min on ice and then incubated with Alexa 647-labeled donkey anti-rabbit IgG (Invitrogen) for another 30 min on ice. Isotype-matched APC-labeled hamster IgG1 (BD Biosciences, San Jose, CA), APC-labeled rat IgG2a (Ebioscience), APC-labeled rat IgG2b (BD Pharmingen) and purified rabbit IgG (Ebioscience) were used as negative controls. After the final wash, the cells were analyzed with FACSCalibur flow cytometer (BD, Franklin Lakes, NJ).
2.8 Immunohistochemical analysis
P-Dex-Alexa (5.0 mg polymer/mouse) was given to 6 mice by tail vein injection 1-month post surgery. The animals were sacrificed and the distal femurs were collected 24 h post injection. The femurs were fixed and decalcified. The pin was carefully removed when decalcification was completed. The specimens were then paraffin embedded and sectioned (6 μm thickness). The slides obtained were first incubated with 10% goat serum (Sigma-Aldrich) for 30 min at room temperature. After addition of the primary antibodies {rat anti-mouse F4/80 (Invitrogen, Camarillo, CA), rat anti-mouse Ly-6G (Gr-1) (EBioscience), rat anti-mouse Ly-6B (7/4) (AbD Serotec), Armenian hamster anti-mouse CD11c (AbD Serotec), and rabbit anti-mouse prolyl-4-hydroxylase (P4HB) (Abcam)}, the sections were incubated at room temperature for 1 h in a humidified chamber. After washing with PBS, diluted secondary antibody {Alexa 647 labeled goat anti-rat IgG (Invitrogen), Alexa 647 labeled goat anti-Armenian hamster IgG (Jackson ImmunoResearch, West Grove, PA) or Alexa 647 labeled donkey anti-rabbit IgG (Invitrogen)} was added and incubated for another 30 min at room temperature in the dark. In control experiments, primary antibodies were replaced by corresponding isotype controls {purified rabbit IgG (Sigma-Aldrich), purified rat IgG (Sigma-Aldrich) and purified hamster IgG (Jackson ImmunoResearch)} and the samples were processed similarly as described above. The tissue sections were then evaluated with a Nikon Swept Field confocal microscope (Nikon Instruments Inc. Melville, NY).
2.9 Statistical analysis
Comparisons were made by one-way ANOVA followed by a post hoc test (Tukey) for multiple comparisons using SPSS software. Data were expressed as means ± standard deviation. A value of P < 0.05 was considered to be statistically significant.
3. Results
3.1 Characterization of HPMA copolymer conjugates
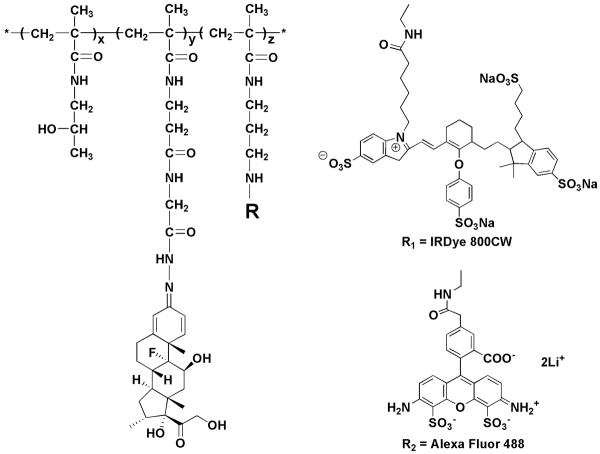
The general structures of P-Dex, P-Dex-Alexa and P-Dex-IRDye are shown in Figure 1. The weight average molecular weight (Mw = 3.68 × 104) and number average molecular weight (Mn = 2.69 × 104) of copolymers were determined based on HPMA homopolymer calibration using an ÄKTA FPLC system (GE HealthCare, Piscataway, NJ) equipped with UV and RI detectors. To obtain Dex content (140 mg/g of P-Dex), P-Dex was hydrolyzed in 0.1 N HCl (1 mg/mL) overnight, neutralized and then analyzed with HPLC [12]. The Alexa Fluor 488 content in P-Dex-Alexa was determined to be 2.0×10−5 mol/g and the IRDye-800 content in P-Dex-IRDye was 1.1×10−5 mol/g using a Lambda 10 UV/vis spectrometer. For in vitro release kinetics, Dex was found to gradually release from P-Dex (in acetate buffer, 0.01 M with 0.15 M NaCl, pH 5.0, 37°C) at a rate of approximately 1% of the conjugated Dex per day during the 14 days release study [12].
Figure 1.
The structures of P-Dex (z = 0), P-Dex-IRDye (R1) and P-Dex-Alexa (R2)
3.2 Prevention of osteolysis by P-Dex – micro-CT analysis
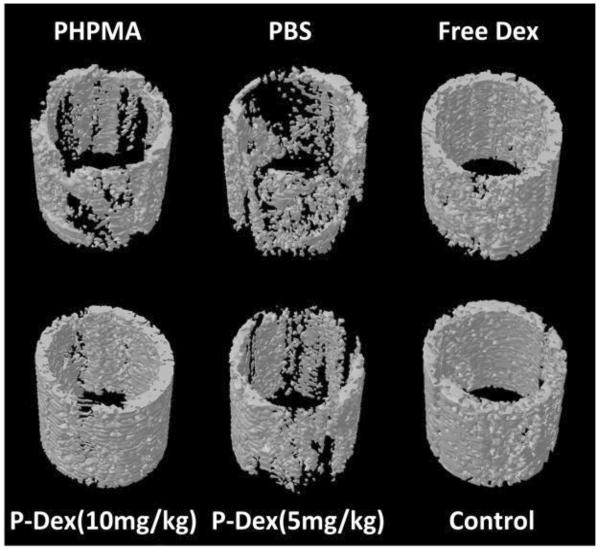
Three months after initial surgery and particle implantation, the femurs were recovered from all groups and the periprosthetic bone analyzed using high-resolution micro-CT. Representative 3D images from all 6 treatment groups are shown in Figure 2. As expected, animals that were not given PMMA particles showed no signs of bone loss (group 6, control). However, animals with PMMA but without any treatment (i.e. no free Dex or prodrug, group 2) demonstrated significant bone loss, confirming that these particles are sufficient to induce periprosthetic osteolysis in this model. As expected, plain drug-free polymer (PHPMA, group 1) was unable to prevent the PMMA-induced osteolysis. Both daily treatment with unconjugated dexamethasone (free Dex, group 3) and monthly treatment with P-Dex (10 mg/kg, group 4, dose equivalent to free Dex group) resulted in preservation of peri-implant bone integrity. Low dose P-Dex (5 mg/kg, group 5) treatment resulted in partial prevention of bone loss.
Figure 2.
3D images of the region of interest of peri-implant bone in the mouse femurs from different treatment groups. Free Dex and P-Dex (10 mg/kg) treatments successfully prevented the peri-implant osteolysis, in which the bone qualities were the same as that in control group without PMMA particle challenge. In contrast, there was significant bone loss in PHPMA and PBS treated groups.
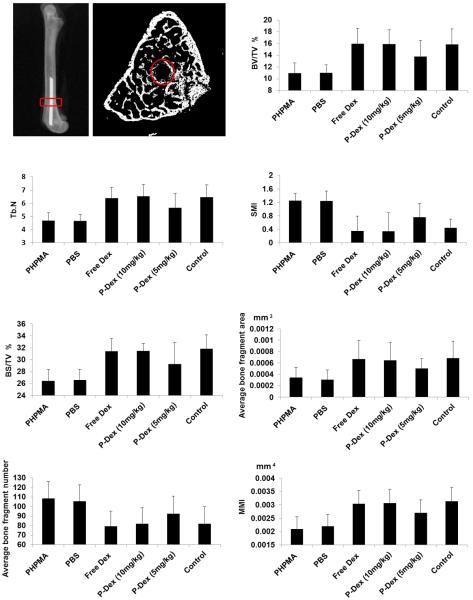
Quantitative analyses (2D and 3D) for bone quality of the region of interest (ROI) are shown in Figure 3. These data are consistent with the images shown in Figure 2. Specifically, compared to the control group (group 6), animals implanted with PMMA particles and treated with PBS (group 2) or PHPMA (group 1) showed clear bone loss, as indicated by significantly lower bone volume fraction (BV/TV), bone surface density (BS/TV) trabecular number (Tb. N), and significant reduction of the normal plate-like trabecular structure, as revealed by higher structure model index (SMI). In the slice-by-slice 2D analysis, PMMA challenge (groups 1 and 2) resulted in loss of peri-implant bone quality, as suggested by significant decreases in average bone fragment area, increases in average bone fragment number, and reduced torsional rigidity, as evident by their mean polar moment of inertia (MMI) values, when compared to the control animals without PMMA particle challenge (group 6). In contrast, animals implanted with PMMA followed by free Dex (group 3) or P-Dex (10 mg/kg, group 4) treatment demonstrated preservation of bone quantity, quality and rigidity, with no significant differences compared to the sham operated animals in any of these parameters, whereas lower doses of P-Dex (5 mg/kg, group 5) resulted in partial preservation of bone.
Figure 3.
Microstructure parameters of the harvested femurs evaluated by micro-CT. The region of interest (ROI) for micro-CT analysis is shown by the red circle. There was no significant difference among free Dex, P-Dex (10 mg/kg) and control groups for all the parameters, suggesting that free Dex and P-Dex (10 mg/kg) can prevent osteolysis. PHPMA and PBS did not show any prevention effect, as evidenced by the significant differences among PHPMA, PBS and control group without particle challenge.
Collectively, these data demonstrate that the PMMA particles-induced periprosthetic osteolysis, as assessed by multiple micro-CT parameters, can be efficiently prevented by treatment with dose equivalent daily free Dex or monthly P-Dex.
3.3 Prevention of osteolysis by P-Dex – histological evaluation
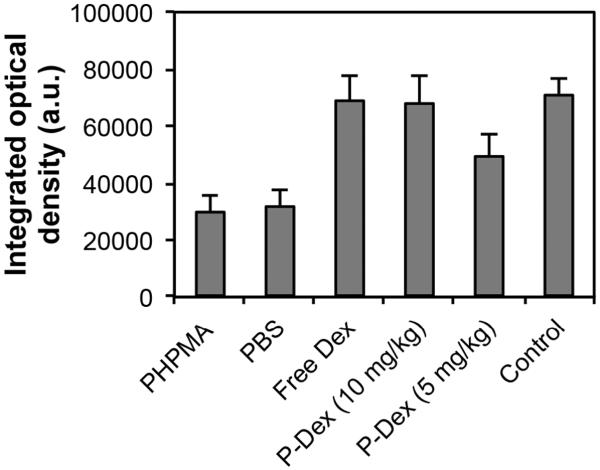
To further define the periprosthetic tissue reactions within the various treatment groups, sections were prepared for histological analysis (Figure 4–7). H&E staining showed that PMMA particle challenge followed by either PHPMA (group 1) or PBS (group 2) led to osteolysis around the implanted pin compared to the sham operated animals (group 6) (Figure 4 A, B, F). In contrast, there was no significant bone loss in PMMA challenged animals treated with free Dex (group 3) or high dose P-Dex (10 mg/kg, group 4) groups (Figure 4 C & D). Tartrate-resistant acid phosphatase (TRAP) is a glycosylated monomeric metalloenzyme highly expressed in osteoclasts [18–20]. It is a biomarker for the presence of osteoclasts, which are responsible for bone resorption [21, 22]. As shown in Figure 5, multiple TRAP-positive cells were present on the bone surfaces in PHPMA and PBS treated groups (Figure 5A–1, B-1, arrows), consistent with active osteoclast-mediated bone resorption. In contrast, very few TRAP-positive cells were observed in free Dex and P-Dex treated and control groups (Figure 5 C–F). Modified trichrome staining (Figure 6) indicated significant bone collagen loss in animals challenged with PMMA followed by either PHPMA (group 1, Figure 6A) or PBS (group 2, Figure 6B) compared to control (group 6, Figure 6F), which could be prevented by treatment with free Dex (group 3, Figure 6C) or high dose P-Dex (10 mg/kg, group 4, Figure 6D). Low dose P-Dex (5 mg/kg, group 5) had some prevention effect against loss of bone collagen (Figure 6E) but the effect was less than that observed in the high dose group. The changes of the blue color in these trichrome stained sections were further quantified for integrated optical density (IOD) using Image-Pro Plus software according to literature [16, 23]. As shown in Figure 7, the IODs for the plain polymer (PHPMA) and PBS treatment groups were significantly lower (P < 0.05) than the control group. No statistic significant difference can be found among the free Dex, P-Dex (10 mg/kg, dose equivalent to free Dex group) and the control. These results further validate the observation made in Figure 6.
Figure 4.
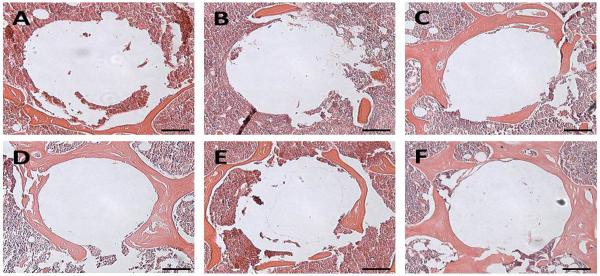
Representative images of H&E staining of the peri-implant bone. A: PHPMA treated group; B: PBS treated group; C: Free Dex treated group; D: P-Dex (10 mg/kg) treated group; E: P-Dex (5 mg/kg) treated group; F: Control without PMMA particle challenge. Bars = 100 μm.
Figure 7.
Quantitative analysis of the peri-implant bone collagen after different treatments. A modified trichrome staining procedure was used to stain the sections. The presence of bone collagen (indicated by the blue color) was quantified for integrated optical density (IOD) using Image Pro Plus software. No significant difference was found when comparing free Dex and P-Dex (10 mg/kg) with the control group, suggesting that free Dex and P-Dex (10 mg/kg) can prevent bone collagen loss. PHPMA and PBS did not show any prevention effect, as evidenced by the significant (P < 0.05) differences between PHPMA vs. control and PBS vs. control.
Figure 5.
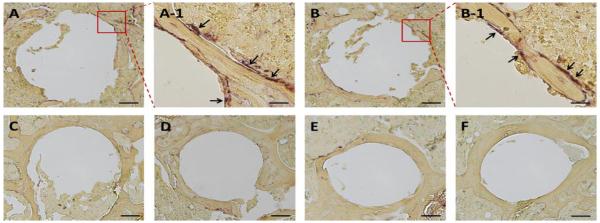
Representative TRAP staining images of the peri-implant bone. A: PHPMA treated group; B: PBS treated group; C: Free Dex treated group; D: P-Dex (10 mg/kg) treated group; E: P-Dex (5 mg/kg) treated group; F: Control without PMMA particle challenge; Bars = 100 μm; A-1 and B-1 represented the enlarged area in A and B; Bars = 20 μm. There were multiple TRAP-positive cells present on the bone surfaces in A-1 and B-1 as indicated by arrows.
Figure 6.
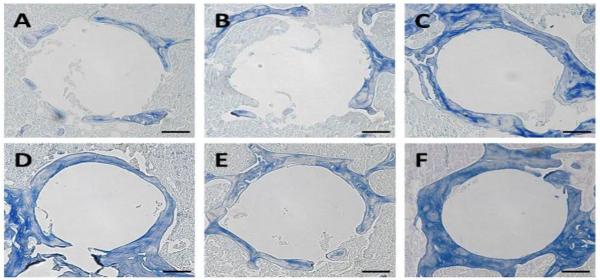
Representative images of modified trichrome staining of the peri-implant bone. A: PHPMA treated group; B: PBS treated group; C: Free Dex treated group; D: P-Dex (10 mg/kg) treated group; E: P-Dex (5 mg/kg) treated group; F: Control without PMMA particle challenge. Bars = 100 μm.
3.4 Targeting and retention of P-Dex at the peri-implant inflammation site
A strong near-infrared (NIR) signal from P-Dex-IRDye was observed at the PMMA particle deposition site one-month post surgery compared with the contralateral side injected with PBS (Figure 8). After a single injection, the P-Dex-IRDye signal persisted for at least 14 days, consistent with sustained retention of the prodrug at the site of PMMA-induced inflammation.
Figure 8.
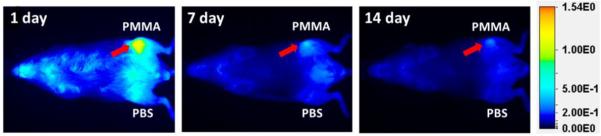
Representative optical images of mice challenged with PMMA particles on one femur and PBS on the contralateral side. Images were obtained 1, 7 and 14 days after one intravenous injection of P-Dex-IRDye. There was obvious retention of the P-Dex-IRDye signal at the PMMA particle-induced inflammation site as the red arrows indicated.
3.5 Delineation of P-Dex' retention mechanism
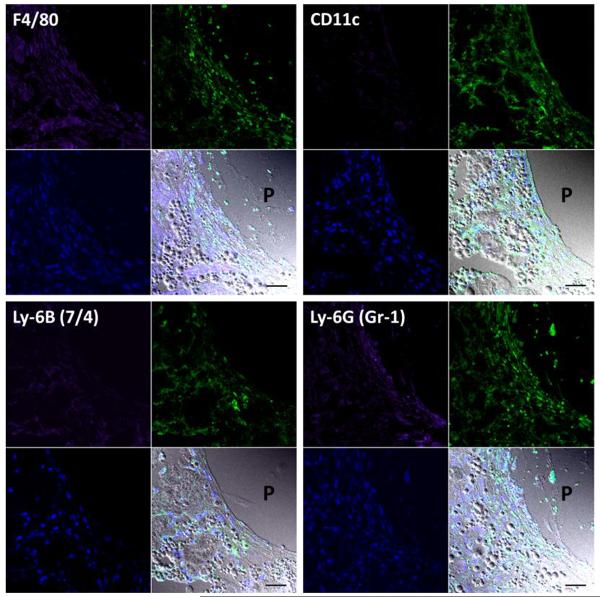
To identify the pattern of cellular sequestration within peri-implant tissues responsible for the retention of P-Dex, immunohistochemical staining with a series of markers of specific cell types was performed. As shown in Figure 9, there were numerous P-Dex-Alexa positive cells (green fluorescence) in the peri-implant area of PMMA-infused femur. Co-localization with Alexa-647 labeled cell type markers indicated that among those cells that had sequestered P-Dex-Alexa, the majority were F4/80 (macrophage marker), Ly-6B (7/4) (neutrophil marker) and Ly-6G (Gr-1) (neutrophil and monocyte marker) positive. Weak co-localization was observed for CD11c (dendritic cell marker) positive cells. P4HB (fibroblast marker) positive cells did not show evidence of P-Dex-Alexa internalization (data not shown).
Figure 9.
Representative confocal images of anti-F4/80, anti-CD11c, anti-Ly-6B (7/4) and anti-Ly-6G (Gr-1) antibodies stained sections of decalcified distal femur from PMMA particle injected group (treated with P-Dex-Alexa). Each panel was composed of four subpanels: Alexa-647 labeled antibody stained purple, P-Dex-Alexa exhibited green fluorescence, DAPI stained blue and the overlap of the three with DIC. The co-localization of the purple and green color confirmed the internalization of the P-Dex-Alexa by F4/80, CD11c, Ly-6B (7/4) or Ly-6G (Gr-1) positive cells at the sites of inflammation. `P' indicated the location of the pin. The spherical structures in DIC images were PMMA particles. Bar = 30 μm.
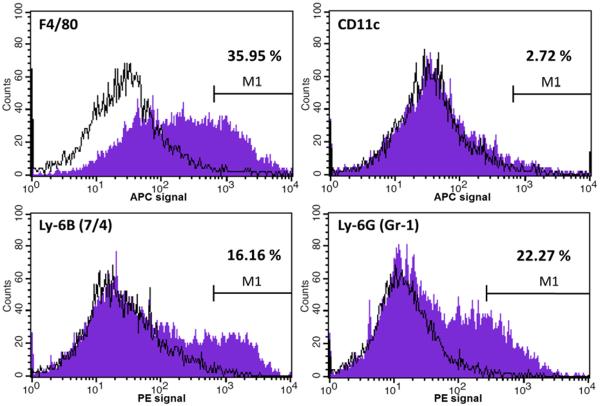
Flow cytometry analysis revealed that approximately 4% of the cells isolated from the implantation sites were P-Dex-Alexa positive. When gated for further analysis (Figure 10), it was found that among the P-Dex-Alexa positive cells, 35.95% were F4/80 positive; 2.72% were CD11c positive; 16.16% were Ly-6B (7/4) and 22.27% were Ly-6G (Gr-1) positive. Less than 1% of the P-Dex-Alexa positive cells were P4HB positive, and the antibodies stained histogram plots were almost exactly the same as the isotype control (data not shown). All data presented were isotype-control corrected.
Figure 10.
Representative fluorescence-activated cell sorting (FACS) analysis data of cells isolated from femurs with PMMA particles injection 24 h after systemic administration of P-Dex-Alexa. The histogram plots showed the intensity of staining with the specific antibodies designated on the x-axis (purple) with isotype control antibodies (black lines) on the same plots. The percentages represented the percent of antibody positive cells among P-Dex-Alexa positive cells.
Collectively, the data from Figures 8–10 demonstrate that P-Dex was efficiently targeted to sites of PMMA-induced inflammation, where it is sequestered in local cell populations, predominantly of the myeloid lineage, resulting in sustained retention over several weeks. of the purple and green color confirmed the internalization of the P-Dex-Alexa by F4/80, CD11c, Ly-6B (7/4) or Ly-6G (Gr-1) positive cells at the sites of inflammation. `P' indicated the location of the pin. The spherical structures in DIC images were PMMA particles. Bar = 30 μm.
3.6 P-Dex treatment is not associated with the development of osteoporosis
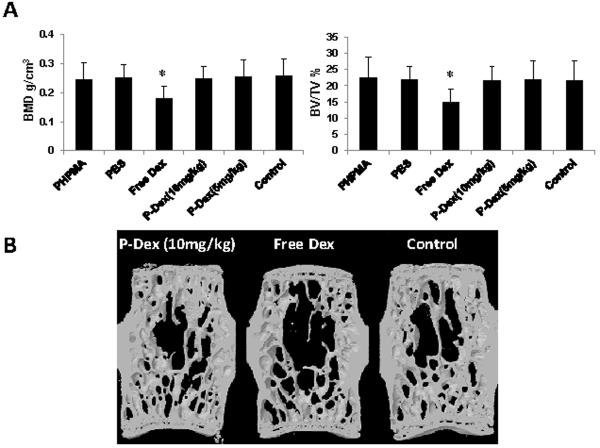
The above results demonstrate P-Dex localization and sustained retention at sites of PMMA-induced inflammation and osteolysis, such that monthly administration is sufficient to provide effective prevention of peri-implant bone loss, comparable to daily administration of free Dex. To establish whether this translates into reduced side effects of P-Dex treatment on the general skeleton, the bone quality of the L4 lumbar vertebral body was evaluated by micro-CT. Microstructural analysis of the vertebral trabecular bone is shown in Figure 10. There was significant reduction of vertebral trabecular bone volume fraction (BV/TV) and trabecular BMD in daily free Dex-treated group when compared with other groups, including free PHPMA, PBS, P-Dex treatment groups and control (P < 0.05). However, there was no significant difference in BMD and BV/TV when comparing the P-Dex-treated groups (both doses) with the PBS and PHPMA treated groups or the control (with pin implantation but no PMMA challenge or any form of treatment) groups (Figure 11A). As shown in Figure 11B, the reconstructed 3D images from micro-CT clearly showed there was less trabecular bone and trabecular connectivity in free Dex treated group. Thus, in contrast to free Dex, P-Dex did not lead to systemic osteopenia.
Figure 11.
Skeleton side effect evaluation analyzed by micro-CT. A: In the lumbar vertebral body, there were significant lower BMD and BV/TV in free Dex treated group when compared with all other groups (P < 0.05). B: Representative 3D images of one lumbar vertebral body from P-Dex (10 mg/kg), free Dex and control groups. It is obvious that there is less trabecular bone and trabecular connectivity in the free Dex treated group.
4. Discussion
Wear particle induced inflammation plays a critical role in the development of aseptic implant loosening. Phagocytosis of wear particles activates macrophages, resulting in activation of multiple intracellular inflammatory signaling cascades and the secretion of pro-inflammatory cytokines. In turn, this inflammatory milieu leads to the differentiation, maturation and activation of osteoclasts [24, 25], inhibiting osteoprogenitor cells as well as inducing apoptosis of osteoblasts [26, 27], resulting in osteoclast-mediated peri-implant osteolysis at the bone-implant interface and the loss of fixation [7]. Therefore, dampening the peri-implant inflammation represents a logical strategy for therapeutic intervention and has been proven to be an effective way of preventing osteolysis in animal models [9–11]. The efficacy of an anti-inflammatory drug depends on its specificity for the molecular target and the concentration at the inflammation site. The ubiquitous biodistribution of anti-inflammatory drugs such as GC after systemic administration is associated with significant off-target toxicities and this issue has limited their use in chronic inflammatory conditions. How to regulate the in vivo drug distribution in such a way as to preferentially deliver these therapeutic agents to the inflammatory lesion and avoid or reduced systemic exposure still remains a major clinical challenge. In an attempt to address this problem, we have developed macromolecular prodrugs of anti-inflammatory agents, which passively target to inflammation. This approach is based on a mechanism involving the prodrug's extravasation through leaky vasculature and inflammatory cell-mediated sequestration (ELVIS) and has been validated in several inflammatory disease models [13, 14, 28].
In a previous study we employed a murine calvaria particle implant model and demonstrated the utility of the HPMA copolymer system to detect early particle-induced inflammation [13]. Though the murine calvaria osteolysis model reflects many biological features of the wear debris-associated osteolysis [10, 29], the lack of a prosthetic implant and a prosthesis/medullar canal interaction does not recapitulate the actual clinical setting associated with peri-implant osteolysis after total joint replacement. In addition, the short-term duration (7 days in total) of the cavarial animal model precludes its application for the long-term evaluation of the drug's therapeutic efficacy and potential toxicities. In the present study, we employed a long-term murine knee prosthesis failure model that more accurately recapitulates the prosthetic peri-implant osteolysis associated with total joint replacement based on literatures [30–34]. A stainless steel pin was surgically inserted into the distal end of the femur. Inflammation was induced by periodic injection of PMMA particles, which led to bone loss around the pin three months after implantation. Using a combination of micro-CT and histological approaches, we have evaluated the tissue response to PMMA in this model and demonstrated that this accurately recapitulated the cellular pathogenesis seen in the human disease, characterized by enhanced osteoclast-mediated bone resorption at the implant-bone interface, leading to the gradual loss of local bone quantity and quality.
In this paper, we have exploited this new, more relevant, mouse model of osteolysis to investigate the potential advantages of macromolecular pro-drugs of anti-inflammatory agents for the treatment of this disorder. For these studies, we have focused upon HPMA conjugated dexamethasone (P-Dex), a prodrug that we have characterized before. Dex was conjugated to HPMA copolymer via an acid sensitive linker. In vitro release studies showed Dex was gradually released from the conjugate at pH 5.0 at a rate of about 1% per day [12]. P-Dex could passively target inflammation sites and provide sustained therapeutic benefit via ELVIS based mechanisms in rheumatoid arthritis animal model [35, 36]. In our study, using optical imaging, we confirmed that P-Dex could preferentially accumulate at sites of PMMA-induced inflammation and be retained for more than 14 days after a single i.v. injection. Immunohistochemical staining and FACS validated that Alexa Fluor 488 labeled P-Dex (P-Dex-Alexa) was internalized by cells at the inflammatory site, with the majority being myeloid lineage cells. This finding indicates that the retention of the prodrug was related to the cellular uptake of the macromolecular prodrug by activated inflammatory cells after extravasation at the inflammatory site, thus providing a cellular depot of anti-inflammatory prodrug within the very cells that are driving the inflammatory response.
To prevent progression of osteolysis in response to PMMA, treatment was started one month after surgery when the inflammation was observed. Consistent with its known anti-inflammatory properties, daily administration of free Dex was able to prevent PMMA particle-induced osteolysis, as assessed by micro-CT and histological analysis. Remarkably, at a dose of 10 mg/kg (Dex equivalent), P-Dex was equally effective as free Dex, but with an administration frequency of once per month as opposed to daily administration of free Dex. This reflects the enhanced targeting and sustained retention of the prodrug at the sites of inflammation compared to the ubiquitous distribution and rapid clearance of the free drug, and accentuates the advantages of prodrug delivery systems for chronic conditions such as peri-implant osteolysis.
In addition to the potential for enhanced efficacy, a second advantage of P-Dex over free Dex for the treatment of chronic inflammatory disorders such as peri-implant osteolysis, lies in the possibility of reducing off-target toxicities. Since it is known that long-term use of free Dex can lead to systemic osteoporosis, we have addressed this by comparing the effects of Dex and P-Dex on the properties of lumbar vertebral bodies. Importantly, these studies demonstrate that the decrease in BMD and BV/TV seen with free Dex can be avoided by the use of dose equivalent P-Dex. Thus P-Dex can effectively target sites of inflammation and osteolysis while sparing other skeletal sites.
There are limitations to our study. First, the murine knee prosthesis failure model was established by periodic injection of PMMA particles. In patients with peri-implant osteolysis, wear debris is produced slowly and gradually over an extended period of time. To more closely approximate the clinical situation, it would be advantageous to use a murine intramedullary model with continuous delivery of wear particles to the implant site. Second, as GC are known to be associated with many sides effects in addition to skeletal complications, further studies are needed to more comprehensively establish the safety profile of P-Dex. Third, dexamethasone was used as a prototype drug candidate for the HPMA copolymer-based drug delivery system. Other anti-inflammatory agents, such as COX-2 inhibitors, kinase inhibitors, and therapeutic agents that inhibit osteoclast-mediated bone resorption may also be explored as additional candidates for prodrug development.
5. Conclusions
In this study, a macromolecular prodrug for prevention of wear particle-induced osteolysis was evaluated. P-Dex (10 mg/kg) and equivalent dosage of free Dex could effectively prevent PMMA particle-induced peri-implant osteolysis in a murine knee prosthesis failure model. Compared to the free Dex treatment, however, P-Dex significantly reduced adverse skeleton side effects. Additional data confirm that the optimal combination of efficacy and safety of the P-Dex is due to its passive targeting and sustained retention at the particle-induced peri-implant inflammation, which is in agreement with the ELVIS mechanism we reported previously. Our results demonstrate the feasibility of delivering an anti-inflammatory drug to the site of peri-implant inflammation to prevent bone resorption with reduced side effects, providing a potential practical approach for non-surgical intervention in patients at risk for wear particle-induced osteolysis and implant failure.
Acknowledgements
This study was supported in part by National Institutes of Health/National Institute of Arthritis, Musculoskeletal and Skin Diseases with Grant Number R01 AR053325 and R01 AR062680 to D.W. and ACRREF: Within Our Reach Grant to S.R.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Teeny SM, York SC, Mesko JW, Rea RE. Long-term follow-up care recommendations after total hip and knee arthroplasty: results of the American Association of Hip and Knee Surgeons' member survey. The Journal of arthroplasty. 2003;18:954–62. doi: 10.1016/j.arth.2003.09.001. [DOI] [PubMed] [Google Scholar]
- [2].Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. The Journal of bone and joint surgery American volume. 2007;89:780–5. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- [3].Labek G, Thaler M, Janda W, Agreiter M, Stockl B. Revision rates after total joint replacement: cumulative results from worldwide joint register datasets. The Journal of bone and joint surgery British volume. 2011;93:293–7. doi: 10.1302/0301-620X.93B3.25467. [DOI] [PubMed] [Google Scholar]
- [4].Hallab NJ, Jacobs JJ. Biologic effects of implant debris. Bulletin of the NYU hospital for joint diseases. 2009;67:182–8. [PubMed] [Google Scholar]
- [5].Harris WH. Wear and periprosthetic osteolysis: the problem. Clinical orthopaedics and related research. 2001:66–70. doi: 10.1097/00003086-200112000-00007. [DOI] [PubMed] [Google Scholar]
- [6].Jiang Y, Jia T, Wooley P, Yang S-Y. Current research in the pathogenesis of aseptic implant loosening associated with particulate wear debris. Acta orthopaedica Belgica. 2013;79:1–9. [PubMed] [Google Scholar]
- [7].Howling GI, Barnett PI, Tipper JL, Stone MH, Fisher J, Ingham E. Quantitative characterization of polyethylene debris isolated from periprosthetic tissue in early failure knee implants and early and late failure Charnley hip implants. Journal of biomedical materials research. 2001;58:415–20. doi: 10.1002/jbm.1036. [DOI] [PubMed] [Google Scholar]
- [8].Noordin S, Masri B. Periprosthetic osteolysis: genetics, mechanisms and potential therapeutic interventions. Canadian journal of surgery Journal canadien de chirurgie. 2012;55:408–17. doi: 10.1503/cjs.003711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schwarz EM, Benz EB, Lu AP, Goater JJ, Mollano AV, Rosier RN, et al. Quantitative small-animal surrogate to evaluate drug efficacy in preventing wear debris-induced osteolysis. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2000;18:849–55. doi: 10.1002/jor.1100180602. [DOI] [PubMed] [Google Scholar]
- [10].Zhang X, Morham SG, Langenbach R, Young DA, Xing L, Boyce BF, et al. Evidence for a direct role of cyclo-oxygenase 2 in implant wear debris-induced osteolysis. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2001;16:660–70. doi: 10.1359/jbmr.2001.16.4.660. [DOI] [PubMed] [Google Scholar]
- [11].Im GI, Kwon BC, Lee KB. The effect of COX-2 inhibitors on periprosthetic osteolysis. Biomaterials. 2004;25:269–75. doi: 10.1016/s0142-9612(03)00523-4. [DOI] [PubMed] [Google Scholar]
- [12].Liu XM, Quan LD, Tian J, Alnouti Y, Fu K, Thiele GM, et al. Synthesis and evaluation of a well-defined HPMA copolymer-dexamethasone conjugate for effective treatment of rheumatoid arthritis. Pharmaceutical research. 2008;25:2910–9. doi: 10.1007/s11095-008-9683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yuan F, Nelson RK, Tabor DE, Zhang Y, Akhter MP, Gould KA, et al. Dexamethasone prodrug treatment prevents nephritis in lupus-prone (NZB × NZW)F1 mice without causing systemic side effects. Arthritis and rheumatism. 2012;64:4029–39. doi: 10.1002/art.34667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ren K, Purdue PE, Burton L, Quan LD, Fehringer EV, Thiele GM, et al. Early detection and treatment of wear particle-induced inflammation and bone loss in a mouse calvarial osteolysis model using HPMA copolymer conjugates. Molecular pharmaceutics. 2011;8:1043–51. doi: 10.1021/mp2000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Omelyanenko V, Kopeckova P, Gentry C, Kopecek J. Targetable HPMA copolymer-adriamycin conjugates. Recognition, internalization, and subcellular fate. Journal of controlled release : official journal of the Controlled Release Society. 1998;53:25–37. doi: 10.1016/s0168-3659(97)00235-6. [DOI] [PubMed] [Google Scholar]
- [16].Yang SY, Mayton L, Wu B, Goater JJ, Schwarz EM, Wooley PH. Adeno-associated virus-mediated osteoprotegerin gene transfer protects against particulate polyethylene-induced osteolysis in a murine model. Arthritis and rheumatism. 2002;46:2514–23. doi: 10.1002/art.10527. [DOI] [PubMed] [Google Scholar]
- [17].Zhang T, Yu H, Gong W, Zhang L, Jia T, Wooley PH, et al. The effect of osteoprotegerin gene modification on wear debris-induced osteolysis in a murine model of knee prosthesis failure. Biomaterials. 2009;30:6102–8. doi: 10.1016/j.biomaterials.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lamp EC, Drexler HG. Biology of tartrate-resistant acid phosphatase. Leukemia & lymphoma. 2000;39:477–84. doi: 10.3109/10428190009113378. [DOI] [PubMed] [Google Scholar]
- [19].Bonucci E, Nanci A. Alkaline phosphatase and tartrate-resistant acid phosphatase in osteoblasts of normal and pathologic bone. Italian journal of anatomy and embryology = Archivio italiano di anatomia ed embriologia. 2001;106:129–33. [PubMed] [Google Scholar]
- [20].Igarashi Y, Lee MY, Matsuzaki S. Acid phosphatases as markers of bone metabolism. Journal of chromatography B, Analytical technologies in the biomedical and life sciences. 2002;781:345–58. doi: 10.1016/s1570-0232(02)00431-2. [DOI] [PubMed] [Google Scholar]
- [21].Reynolds JJ. Degradation processes in bone and cartilage. Calcified tissue research. 1970;(Suppl):52–6. doi: 10.1007/BF02152350. [DOI] [PubMed] [Google Scholar]
- [22].Belanger LF. Osteocytic osteolysis. Calcified tissue research. 1969;4:1–12. doi: 10.1007/BF02279101. [DOI] [PubMed] [Google Scholar]
- [23].Zhang L, Jia TH, Chong AC, Bai L, Yu H, Gong W, et al. Cell-based osteoprotegerin therapy for debris-induced aseptic prosthetic loosening on a murine model. Gene therapy. 2010;17:1262–9. doi: 10.1038/gt.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Goodman SB, Huie P, Song Y, Schurman D, Maloney W, Woolson S, et al. Cellular profile and cytokine production at prosthetic interfaces. Study of tissues retrieved from revised hip and knee replacements. The Journal of bone and joint surgery British volume. 1998;80:531–9. doi: 10.1302/0301-620x.80b3.8158. [DOI] [PubMed] [Google Scholar]
- [25].Fang HW, Yang CB, Chang CH, Huang CH, Liu HL, Fang SB. The potential role of phagocytic capacity in the osteolytic process induced by polyethylene wear particles. The Journal of international medical research. 2006;34:655–64. doi: 10.1177/147323000603400611. [DOI] [PubMed] [Google Scholar]
- [26].Vermes C, Glant TT, Hallab NJ, Fritz EA, Roebuck KA, Jacobs JJ. The potential role of the osteoblast in the development of periprosthetic osteolysis: review of in vitro osteoblast responses to wear debris, corrosion products, and cytokines and growth factors. The Journal of arthroplasty. 2001;16:95–100. doi: 10.1054/arth.2001.28719. [DOI] [PubMed] [Google Scholar]
- [27].Pioletti DP, Leoni L, Genini D, Takei H, Du P, Corbeil J. Gene expression analysis of osteoblastic cells contacted by orthopedic implant particles. Journal of biomedical materials research. 2002;61:408–20. doi: 10.1002/jbm.10218. [DOI] [PubMed] [Google Scholar]
- [28].Wang D, Miller SC, Liu XM, Anderson B, Wang XS, Goldring SR. Novel dexamethasone-HPMA copolymer conjugate and its potential application in treatment of rheumatoid arthritis. Arthritis research & therapy. 2007;9:R2. doi: 10.1186/ar2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rao AJ, Nich C, Dhulipala LS, Gibon E, Valladares R, Zwingenberger S, et al. Local effect of IL-4 delivery on polyethylene particle induced osteolysis in the murine calvarium. Journal of biomedical materials research Part A. 2012 doi: 10.1002/jbm.a.34486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jiang Y, Jia T, Gong W, Wooley P, Yang S-Y. Titanium particle-challenged osteoblasts promote osteoclastogenesis and osteolysis in a murine model of periprosthestic osteolysis. Acta biomaterialia. 2013;9:7564–72. doi: 10.1016/j.actbio.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhang T, Yu H, Gong W, Zhang L, Jia T, Wooley P, et al. The effect of osteoprotegerin gene modification on wear debris-induced osteolysis in a murine model of knee prosthesis failure. Biomaterials. 2009;30:6102–8. doi: 10.1016/j.biomaterials.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yang S-Y, Yu H, Gong W, Wu B, Mayton L, Costello R, et al. Murine model of prosthesis failure for the long-term study of aseptic loosening. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2007;25:603–11. doi: 10.1002/jor.20342. [DOI] [PubMed] [Google Scholar]
- [33].Howie D, Vernon-Roberts B, Oakeshott R, Manthey B. A rat model of resorption of bone at the cement-bone interface in the presence of polyethylene wear particles. The Journal of bone and joint surgery American volume. 1988;70:257–63. [PubMed] [Google Scholar]
- [34].Warme B, Epstein N, Trindade M, Miyanishi K, Ma T, Saket R, et al. Proinflammatory mediator expression in a novel murine model of titanium-particle-induced intramedullary inflammation. Journal of biomedical materials research Part B, Applied biomaterials. 2004;71:360–6. doi: 10.1002/jbm.b.30120. [DOI] [PubMed] [Google Scholar]
- [35].Yuan F, Quan LD, Cui L, Goldring SR, Wang D. Development of macromolecular prodrug for rheumatoid arthritis. Advanced drug delivery reviews. 2012;64:1205–19. doi: 10.1016/j.addr.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Quan LD, Purdue PE, Liu XM, Boska MD, Lele SM, Thiele GM, et al. Development of a macromolecular prodrug for the treatment of inflammatory arthritis: mechanisms involved in arthrotropism and sustained therapeutic efficacy. Arthritis research & therapy. 2010;12:R170. doi: 10.1186/ar3130. [DOI] [PMC free article] [PubMed] [Google Scholar]