Abstract
Age-related macular degeneration (AMD) represents the leading cause of blindness in the elderly, yet no definitive therapy exists for early, dry disease. Several lines of evidence have implicated oxidative stress-induced damage to the retinal pigment epithelium (RPE) in the pathogenesis of AMD, suggesting that the aging RPE may exhibit increased susceptibility to cell damage induced by exogenous stressors. The transcription factor Nrf2 serves as the master regulator of a highly coordinated antioxidant response in virtually all cell types. We compared Nrf2 signaling in the RPE of young (2 months) and old (15 months) mice under unstressed and stressed (sodium iodate) conditions. The aging RPE expressed higher levels of the Nrf2 target genes NQO1, GCLM, and HO1 compared with the RPE of younger mice under unstressed conditions, suggesting an age-related increase in basal oxidative stress. Moreover, the RPE of older mice demonstrated impaired induction of the protective Nrf2 pathway following oxidative stress induced with sodium iodate. The RPE of old mice exposed to sodium iodate also exhibited higher levels of superoxide anion and malondialdehyde than young mice, suggesting inadequate protection against oxidative damage. Induction of Nrf2 signaling in response to sodium iodate was partially restored in the RPE of aging mice with genetic rescue, using conditional knockdown of the Nrf2 negative regulator Keap1 (Tam-Cre;Keap1loxP) compared to Keap1loxP mice. These data indicate that the aging RPE is vulnerable to oxidative damage due to impaired Nrf2 signaling, and that Nrf2 signaling is a promising target for novel pharmacologic or genetic therapeutic strategies.
Keywords: Age-related macular degeneration, aging, Nrf2, oxidative stress, retinal pigment epithelium, Abbreviations: all are standard in this field
Age-related macular degeneration (AMD) represents the leading cause of vision impairment in the elderly, accounting for 54% of blindness in Caucasian Americans(Congdon et al., 2004). Moreover, as the population continues to age, the prevalence of AMD is projected to double over a 20-year period, increasing to 2.95 million by 2020(Friedman et al., 2004). While the advent of anti-VEGF therapy has revolutionized the management of wet AMD, there is still no definitive treatment for the nonexudative form of the disease, and no intervention to slow the progression of the early stages. Understanding the molecular pathophysiology of dry AMD is critical for identifying potential novel therapeutic targets.
Several lines of epidemiologic, genetic, and clinical evidence have implicated cellular oxidative stress in the pathogenesis of AMD. In epidemiologic studies, cigarette smoking, a powerful chemical oxidant, is the most strongly linked modifiable risk factor, with a direct association between pack-years and prevalence of geographic atrophy or CNV(Khan et al., 2006; Smith et al., 2001; Tomany et al., 2004). Single-nucleotide polymorphisms in mitochondrial genes have been identified in isolated retinal tissue from patients with AMD, suggesting that perturbations in oxygen-dependent energy metabolism may contribute to the development of RPE and retinal pathology(Udar et al., 2009). Finally, the multi-center randomized controlled AREDS trial showed that high dose antioxidant supplementation with vitamins C, E, beta-carotene, and zinc reduces progression to advanced AMD in patients with intermediate or late dry AMD(Age-related Eye Disease Study Research Group, 2001).
The transcription factor Nrf2 serves as the master regulator of a highly conserved protective molecular response to oxidative stress in all cell types, driving expression of a coordinated suite of several antioxidant genes. Under basal conditions, Nrf2 physically interacts with the negative regulator Keap1, which targets the Nrf2 protein for ubiquitination and proteasomal degradation within the cytoplasm, thus limiting its activity. However, under conditions of oxidative stress, Keap1 undergoes a conformational modification and releases Nrf2 for translocation to the nucleus where it binds to antioxidant response elements (AREs), thus activating transcription of its target genes, including glutamate cysteine ligase subunit m (GCLM), heme oxygenase-1 (HO1), and NAD(P)H:quinone oxidoreductase (NQO1)(Zhang, 2006).
Nrf2 deficiency in vivo increases susceptibility to oxidative stress in all tissues, including the RPE. For example, cigarette smoke exposure induces more severe RPE damage in Nrf2−/− mice compared with wild type controls(Cano et al., 2010). Moreover, simply with aging, Nrf2−/− mice develop several histologic and fundoscopic hallmarks of AMD, including sub-RPE drusen deposition, lipofuscin accumulation, and ultrastructural features suggestive of choroidal neovascularization(Zhao et al., 2011). We hypothesize that dysregulation of the normal protective Nrf2 response occurs with age, resulting in increased susceptibility of RPE cells to oxidative stress, and that enhanced Nrf2 signaling via genetic reduction of its inhibitor Keap1 may rescue the aging RPE from oxidative stress-induced damage.
Young (2-month old) and old (15-month old) C57Bl6/J mice were housed and maintained according to institutional guidelines. Animals were kept in a 12 h light cycle with food and water at libitum. Mice expressing the Cre recombinase transgene under the control of a tamoxifen-inducible promoter (Tam-Cre) were mated with mice engineered to harbor loxP sequences flanking the Keap1 gene to generate Tam-Cre;Keap1loxP progeny. These mice were maintained on a C57Bl6/J background. Keap1 deletion was induced by daily intraperitoneal administration of 1 mg tamoxifen (reconstituted in DMSO and corn oil) for five consecutive days. One week later, oxidative stress was induced with NaIO3 as described below.
NaIO3 (5mg/kg; Sigma-Aldrich, St. Louis, MO) in a final volume of 100ul (or 100ul of PBS as vehicle alone) was injected intravenously into each mouse via tail vein. Seven days later, eyes were either harvested for histological studies (n=6 eyes each group) or RPE/choroid were dissected, homogenized, and stored in RLT buffer with b-mercaptoethanol at −80 ºC for RNA isolation and analysis of gene expression (n=6 eyes each group).
Mouse eyes were enucleated and lightly fixed in 2% paraformaldehyde (Sigma- Aldrich) for 1 hour, cryopreserved, and sectioned for immunohistochemistry. Assessment of reactive oxygen species (ROS) was carried out by incubating sections in dihydroethidium (DHE; Invitrogen-Molecular Probes, Eugene, OR) at a 1:1000 dilution for 30 minutes at 37 ºC. Images were captured using confocal microscopy (Zeiss ZEN LSM 710, Zeiss, Inc., Thornwood, NY).
Mouse cryosections (10 μm) were treated with a mouse on mouse blocking reagent (MOM; anti-mouse IgG blocking reagent, Vector Labs, Burlingame, CA) for 1 hour at room temperature. Sections were then incubated with MDA2, a murine IgG monoclonal antibody that binds to malondialdehyde (MDA)-lysine epitopes present on modified LDL or other MDA-modified proteins but not to native LDL or unmodified proteins (Courtesy, J Witztum, MD, UC San Diego(Rosenfeld et al., 1990)), overnight at 4 ºC, washed with PBS, and then MOM biotinylated anti-mouse IgG, followed by rhodamine avidin D (Vector labs). Appropriate mouse IgG (Santa Cruz Biotechnologies, Santa Cruz, CA) was used as an isotype control. After adjusting for autofluorescence, sections were imaged using a Zeiss ZEN LSM 710 confocal microscope.
RPE tissue was homogenized using Qiashredder columns (Qiagen, Inc., Valencia, CA). RNA was purified with RNeasy Mini spin columns (Qiagen, Inc.) following the standard manufacturer’s protocol with elution in 30ul of RNase-free water. The concentration of isolated RNA was quantified using the NanoDrop spectrophotometer (Thermo Scientific, Waltham, MA) and the RNA was reverse transcribed using the High-Capacity RNA-to-cDNA kit (Applied Biosystems, Foster City, CA). Quantitative real-time PCR analyses were performed on the ABI StepOne Plus PCR system (Applied Biosystems) in accordance with the manufacturer’s recommendations using Taqman-labeled probes for mouse NQO1, GCLM, HO1, and Keap1, as well as GAPDH as internal control. Data were analyzed according to the delta Ct method.
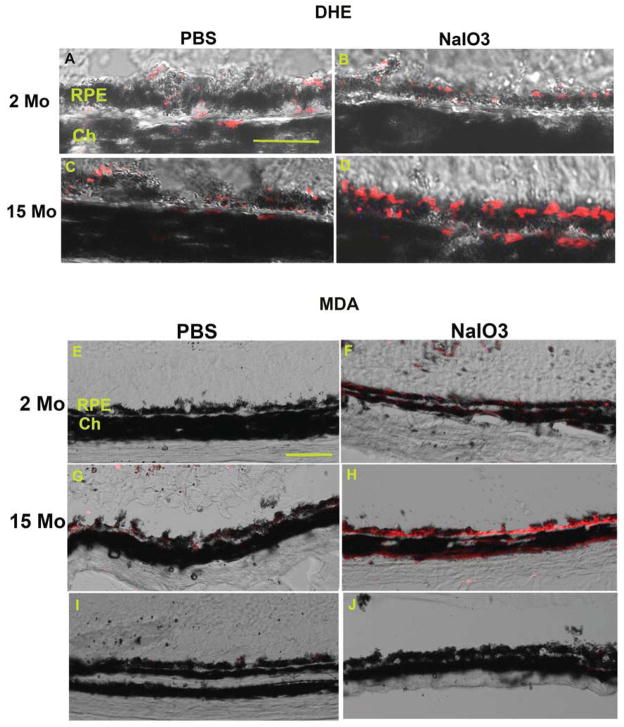
To address our hypothesis, we first employed DHE histochemical labeling to identify superoxide anion production as a measure of oxidative stress, and fluorescence immunohistochemistry for malondialdehyde (MDA) as a measure of oxidative damage. To induce oxidative stress in vivo, the pharmacologic agent sodium iodate (NaIO3) was administered intravenously to mice, and the RPE was evaluated after 7 days. In unstressed mice, DHE labeling was similar in the RPE of 2-month-old (young) and 15-month-old (old) mice (Figure 1A, C). In the RPE of young mice, NaIO3 treatment did not increase DHE staining, compared with vehicle-treated 2-month old controls (Figure 1A, B). In contrast, the RPE of 15-month-old mice given NaIO3 (Figure 1D) demonstrated the highest levels of DHE staining when compared with old mice given vehicle controls or young mice given either vehicle control or NaIO3. To establish whether these levels of superoxide anion were associated with oxidative damage to the RPE, we performed immunolabeling for malondialdehyde (MDA), a known lipid peroxidation product that has been identified in AMD(Weismann et al., 2011). In unstressed mice, MDA labeling in the RPE of 2-month old mice were absent (Figure 1E), and minimal in the RPE of 15-month (Figure 1G) old mice while the RPE had MDA immunolabeling in the RPE of 2-month old mice treated with NaIO3 (Figure 1F). More consistent and stronger labeling was seen in 15-month old mice treated with NaIO3 (Figure 1H).
Figure 1. Pharmacologic induction of oxidative stress in the RPE of young and old mice.
Oxidative stress was pharmacologically induced with NaIO3. The RPE of 2-month old mice treated with PBS (A) has similar DHE fluorescence (red), a marker of superoxide anion, as the RPE of 2-month-old mice given NaIO3 (B). The RPE of 15-month old mice treated with PBS (C) display similar DHE staining as their young counterparts treated also with PBS (A), indicating adequate neutralization of ROS in the RPE of aging mice at baseline, i.e. under unstressed conditions. The RPE of 15-month old mice treated with NaIO3 (D) have increased DHE labeling compared with their age-matched vehicle-treated controls (C) or with 2-month old mice treated with NaIO3 (B), which suggests inadequate neutralization of ROS. Bar=25um. RPE, retinal pigment epithelium; Ch, choroid.
NaIO3 induces oxidative damage, as indicated by MDA immunolabeling. The RPE of 2-month old mice treated with PBS had no MDA immunolabeling (E) while the RPE of 2- month old mice treated with NaIO3 have mild MDA staining (F). This labeling pattern suggests that the RPE’s antioxidant response prevents oxidative damage after an oxidative stress stimulus in young mice. The RPE of 15-month old mice treated with PBS also have very little MDA labeling (G) while the RPE of 15-month old mice given NaIO3 (H) show the strongest MDA labeling among the experimental groups, suggesting inadequate neutralization of ROS that promotes oxidative damage. IgG control of 2-month (I) and 15- month (J) old mouse treated with NaIO3. Bar=50um. RPE, retinal pigment epithelium; Ch, choroid.
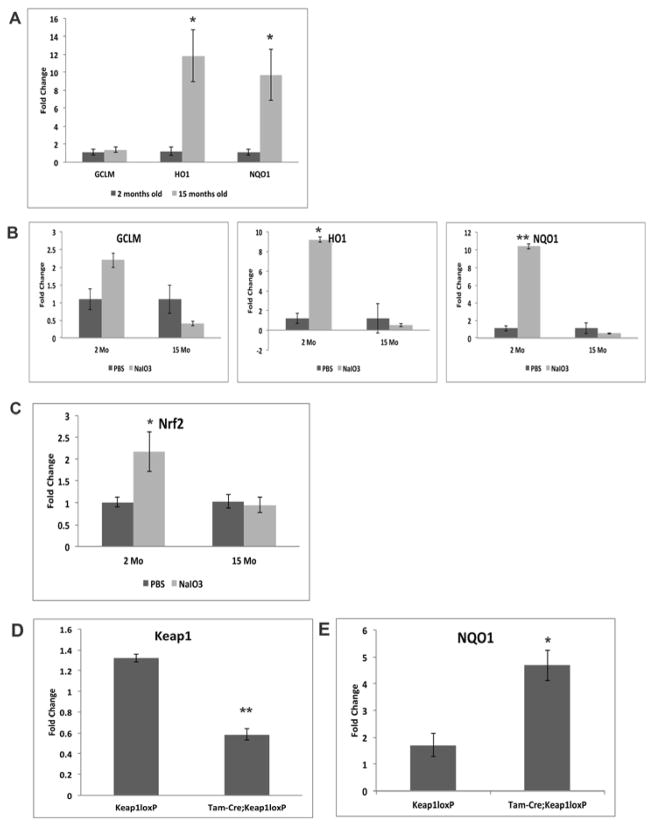
We next measured the expression of three well-known Nrf2 responsive antioxidant genes in young and old mice using quantitative real-time RT-qPCR. RPE tissue isolated from older mice had an approximately 10-fold increase in HO1 (p<0.05) and NQO1 (p<0.05) transcript compared with their younger counterparts, indicating increased basal antioxidant transcriptional activity with aging even without administration of an oxidant (Figure 2A). To determine whether aging alters the induction of the protective Nrf2 response in the RPE under stressed conditions, we compared GCLM, HO1, and NQO1 expression in both young and old mice after stimulation with NaIO3. In young mice, NaIO3 induced the expression of GCLM, HO1, and NQO1 by 2-fold, 9-fold (p<0.05), and 10-fold (p<0.005), respectively, indicating a robust stimulation of Nrf2 activity in response to pharmacological oxidative stress (Figure 2B). In contrast, NaIO3 failed to increase transcript levels of these Nrf2 downstream target genes in older mice and, in fact, there was a trend towards an approximately 2-fold reduction in GCLM, HO1, and NQO1 expression in NaIO3-treated older mice compared with their age-matched vehicle-treated controls (Figure 2B). NaIO3 administration also increased transcript levels of Nrf2 itself in young mice relative to age-matched vehicle treated controls (p<0.05), but this stress-related induction in Nrf2 expression was not seen in 15-month old mice (Figure 2C). These data suggest an age-related impaired induction of the Nrf2 antioxidant response in the RPE after oxidative stress.
Figure 2. Increased basal Nrf2 activity in the RPE of aging mice.
Two-month-old and 15-month-old C57Bl6J mice were treated with either NaIO3 or vehicle (PBS), and RNA was isolated from the RPE for quantitative RT-qPCR analyses of expression of Nrf2 target genes GCLM, HO1, and NQO1. (A) HO1 and NQO1 expression was approximately 11-fold and 10- fold higher, respectively, in aging RPE compared with their young counterparts. *p<0.05, n = 4 mice per group. (B) NaIO3 induced a 2-fold, 9-fold, and 10-fold increase in transcript levels of GCLM, HO1, and NQO1 respectively, in the RPE of young mice. NaIO3 treatment failed to stimulate an Nrf2 response in the RPE of older mice (15mo). *p<0.05; **p<0.005, n= 4 mice per group. (C) NaIO3 induced a 2.2-fold increase in Nrf2 mRNA in the RPE of young mice, but did not stimulate induction of Nrf2 mRNA in the RPE of older mice (15mo). *p<0.05; n= 4 mice per group. (D) After injection of tamoxifen, 2-month-old and 15-monthold Tam-Cre;Keap1loxP and Keap1loxP mice were treated with either PBS or NaIO3. Tamoxifen decreased Keap1 expression by 50%, as assayed by quantitative RT-qPCR in Tam-Cre;Keap1loxP mice compared with Keap1loxP mice which lack the tamoxifen-inducible Cre recombinase transgene. (E) NaIO3 treatment induced 2.5-fold higher levels of the Nrf2 target gene NQO1 in old Tam-Cre;Keap1loxP mice compared with age-matched Keap1loxP mice expressing wild type levels of Keap1. *p<0.05, **o<0.005. n= 6 mice per group.
To determine whether enhanced Nrf2 signaling could rescue the impaired response to oxidative stress in the RPE of aging mice, we employed a Cre/loxP strategy to knock down expression of the Nrf2 inhibitor Keap1. Since Global Keap1 deficient mice are embryologically lethal(Wakabayashi et al., 2003), we used tamoxifen inducible Cre recombinase (Tam-Cre) mice that were crossed with floxed keap1 (Keap1loxP) mice to generate inducible Keap1 knockout mice (Tam-Cre;Keap1loxP). To “rescue” Nrf2 signaling, we knocked down Keap1 with tamoxifen in 15-month old mice. Quantitative RT-qPCR analyses of Keap1 transcript levels confirmed a 50% reduction in Keap1 expression in tamoxifen-treated Tam-Cre;Keap1loxP mice compared with control Keap1loxP mice lacking the Cre recombinase transgene (Figure 2D). In old mice with wild-type Keap1 levels (Keap1loxP), NaIO3 treatment failed to induce expression of NQO1, consistent with data shown above. In the RPE of aging Tam-Cre;Keap1loxP mice, with genetic knockdown of Keap1, NQO1 transcript was increased 2.5 fold in NaIO3-treated aging mice (p<0.05), suggesting partial restoration of the impaired Nrf2 response to oxidative stress in the RPE (Figure 2E). GCLM and HO1 transcripts were not altered by knockdown of Keap1 in 15- month old mice (data not shown).
An aging cell is known to exhibit high levels of oxidative stress, as reviewed in(Orr et al., 2013). Here, we show that the RPE of aging mice has elevated constitutive antioxidant gene expression compared with young mice under basal conditions, which is associated with superoxide anion levels and malondialdehyde labeling similar to young mice. These findings suggest that under basal conditions, the antioxidant response in the aging RPE undergoes an adapative upregulation that provides adequate antioxidant protection. Despite the high baseline antioxidant gene expression in the RPE of aging mice, importantly, our findings also demonstrate a lack of transcriptional induction of these Nrf2 responsive genes in the RPE of aging mice exposed to an exogenous oxidative stressor, with an associated increase in DHE labeling, an indicator of increased ROS levels, and increased MDA, a marker of oxidative damage, compared with young mice subject to the same oxidative stress.
Nrf2 has been implicated as the master regulator of the protective response to cellular oxidative stress, coordinating expression of a large program of antioxidant genes and promoting cell survival under these conditions. Our results suggest that Nrf2 transcriptional activation may contribute to the Nrf2-mediated response to NaIO3 in young mice, and might provide a partial explanation for the lack of response in old mice. However, oxidative stress predominantly activates Nrf2 post-translationally, with the release of the Nrf2 protein from cytosolic Keap1 to allow for nuclear translocation and the initiation of the cytoprotective response via binding to AREs, with minimal variable direct effects on Nrf2 transcription(Braun et al., 2002; Itoh et al., 2003; Vargas et al., 2005). As such, alterations in post-translational Nrf2 regulation are likely to explain the diminished induction of Nrf2 activity in elderly RPE. Nrf2 is one of several antioxidant regulatory elements. The high baseline antioxidant gene expression in aging RPE could result from high constitutive Nrf2 activity. Alternatively, other oxidant-sensitive transcription factors such as AP1, activating transcription factor (ATF) family, forkhead box O (FOXO) family, or Nf-kB could play a role in the higher constitutive antioxidant gene expression(Biswas and Rahman, 2008). Further work characterizing the complete transcriptional and posttranscriptional Nrf2 response in the context of other antioxidant transcription factors in the RPE during aging is warranted.
Nrf2 deficiency has been associated with various disease processes caused by increased susceptibility to oxidative stress. Several lines of evidence suggest that oxidative damage plays a role in the pathogenesis of early, dry AMD, a disease for which no treatment currently exists. Our results indicate that the aging RPE has a limited capacity to respond to perturbations in oxidative stress levels, and suggest that an impaired antioxidant response by aging RPE after an oxidative stimulus could induce RPE dysfunction that may contribute to development of AMD. Pharmacologic or genetic attempts to restore a normal Nrf2 response may protect the aging RPE from damage, degeneration, and death. Indeed, we found that genetic enhancement of Nrf2 activity via knockdown of its negative regulator Keap1 partially restores Nrf2 signaling in the RPE of old mice under oxidative stress conditions. This partial response also raises that possibility that other antioxidant regulators work in concert with Nrf2. Regardless, our findings suggest that the Nrf2 signaling response can be rejuvenated in an adverse environment which provides a rationale for targeted pharmacologic therapy that utilizes Nrf2 activators such as the naturally-occurring sulforaphane or triterpenoids, or their more potent chemically-modified derivatives, for dry AMD. Based on our results, further study into the role of Nrf2 signaling with aging and strategies to invigorate Nrf2 signaling is warranted.
Highlights.
Nrf2 mediated transcription by the RPE of young and old mice was compared.
Aging RPE had elevated superoxide and malondialdehyde after sodium iodide compared to young RPE.
Aging RPE expressed higher basal levels of NQO1, GCLM, and HO1 than young RPE.
Aging RPE exhibited impaired induction of NQO1, GCLM, and HO1 after sodium iodide.
Genetic rescue by Keap1 knock down in old mice restored NQO1 expression in the RPE.
Acknowledgments
NEI EY019904 (JTH), EY14005 (JTH), Thome Foundation Award in AMD (JTH), RPB Senior Scientist Award (JTH), Wilmer Core Grant, EY001765, Unrestricted RPB grant to Wilmer Eye Institute, generous donations by the Merlau Family. JTH is the Robert Bond Welch Professor. We thank Hong Wei, Sonny Dike, and Brad Barnett for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Age-related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas SK, Rahman I. Environmental toxicity, redox signaling and lung inflammation: The role of glutathione. Molecular aspects of medicine. 2008 doi: 10.1016/j.mam.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun S, Hanselmann C, Gassmann MG, auf dem Keller U, Born-Berclaz C, Chan K, Kan YW, Werner S. Nrf2 transcription factor, a novel target of keratinocyte growth factor action which regulates gene expression and inflammation in the healing skin wound. Molecular and cellular biology. 2002;22:5492–5505. doi: 10.1128/MCB.22.15.5492-5505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano M, Thimmalappula R, Fujihara M, Nagai N, Sporn M, Wang AL, Neufeld AH, Biswal S, Handa JT. Cigarette smoking, oxidative stress, the anti-oxidant response through Nrf2 signaling, and Age-related Macular Degeneration. Vision Res. 2010;50:652–664. doi: 10.1016/j.visres.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon N, O’Colmain B, Klaver CC, Klein R, Munoz B, Friedman DS, Kempen J, Taylor HR, Mitchell P. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- Friedman DS, O’Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- Itoh K, Wakabayashi N, Katoh Y, Ishii T, O’Connor T, Yamamoto M. Keap1 regulates both cytoplasmic-nuclear shuttling and degradation of Nrf2 in response to electrophiles. Genes to cells : devoted to molecular & cellular mechanisms. 2003;8:379–391. doi: 10.1046/j.1365-2443.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- Khan JC, Thurlby DA, Shahid H, Clayton DG, Yates JR, Bradley M, Moore AT, Bird AC. Smoking and age related macular degeneration: the number of pack years of cigarette smoking is a major determinant of risk for both geographic atrophy and choroidal neovascularisation. Br J Ophthalmol. 2006;90:75–80. doi: 10.1136/bjo.2005.073643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr WC, Radyuk SN, Sohal RS. Involvement of Redox State in the Aging of Drosophila melanogaster. Antioxid Redox Signal. 2013 doi: 10.1089/ars.2012.5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld ME, Palinski W, Yla-Herttuala S, Butler S, Witztum JL. Distribution of oxidation specific lipid-protein adducts and apolipoprotein B in atherosclerotic lesions of varying severity from WHHL rabbits. Arteriosclerosis. 1990;10:336–349. doi: 10.1161/01.atv.10.3.336. [DOI] [PubMed] [Google Scholar]
- Smith W, Assink J, Klein R, Mitchell P, Klaver CC, Klein BE, Hofman A, Jensen S, Wang JJ, de Jong PT. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology. 2001;108:697–704. doi: 10.1016/s0161-6420(00)00580-7. [DOI] [PubMed] [Google Scholar]
- Tomany SC, Wang JJ, Van Leeuwen R, Klein R, Mitchell P, Vingerling JR, Klein BE, Smith W, De Jong PT. Risk factors for incident age-related macular degeneration: pooled findings from 3 continents. Ophthalmology. 2004;111:1280–1287. doi: 10.1016/j.ophtha.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Udar N, Atilano SR, Memarzadeh M, Boyer DS, Chwa M, Lu S, Maguen B, Langberg J, Coskun P, Wallace DC, Nesburn AB, Khatibi N, Hertzog D, Le K, Hwang D, Kenney MC. Mitochondrial DNA haplogroups associated with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50:2966–2974. doi: 10.1167/iovs.08-2646. [DOI] [PubMed] [Google Scholar]
- Vargas MR, Pehar M, Cassina P, Martinez-Palma L, Thompson JA, Beckman JS, Barbeito L. Fibroblast growth factor-1 induces heme oxygenase-1 via nuclear factor erythroid 2-related factor 2 (Nrf2) in spinal cord astrocytes: consequences for motor neuron survival. J Biol Chem. 2005;280:25571–25579. doi: 10.1074/jbc.M501920200. [DOI] [PubMed] [Google Scholar]
- Wakabayashi N, Itoh K, Wakabayashi J, Motohashi H, Noda S, Takahashi S, Imakado S, Kotsuji T, Otsuka F, Roop DR, Harada T, Engel JD, Yamamoto M. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet. 2003;35:238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- Weismann D, Hartvigsen K, Lauer N, Bennett KL, Scholl HP, Charbel Issa P, Cano M, Brandstatter H, Tsimikas S, Skerka C, Superti-Furga G, Handa JT, Zipfel PF, Witztum JL, Binder CJ. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature. 2011;478:76–81. doi: 10.1038/nature10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug metabolism reviews. 2006;38:769–789. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Chen Y, Wang J, Sternberg P, Freeman ML, Grossniklaus HE, Cai J. Age-related retinopathy in NRF2-deficient mice. PLoS One. 2011;6:e19456. doi: 10.1371/journal.pone.0019456. [DOI] [PMC free article] [PubMed] [Google Scholar]