Abstract
Few preclinical models accurately depict normal human prostate tissue or primary prostate cancer (PCa). In vitro systems typically lack complex cellular interactions among structured prostatic epithelia and a stromal microenvironment, and genetic and molecular fidelity are concerns in both in vitro and in vivo models. “Tissue slice cultures” (TSC) provide realistic preclinical models of diverse tissues and organs, but have not been fully developed or widely utilized for prostate studies. Problems encountered include degeneration of differentiated secretory cells, basal cell hyperplasia, and poor survival of PCa. Here, we optimized, characterized, and applied a TSC model of primary human PCa and benign prostate tissue that overcomes many deficiencies of current in vitro models. Tissue cores from fresh prostatectomy specimens were precision-cut at 300-µm and incubated in a rotary culture apparatus. The ability of varied culture conditions to faithfully maintain benign and cancer cell and tissue structure and function over time was evaluated by immunohistological and biochemical assays. After optimization of the culture system, molecular and cellular responses to androgen ablation and to piperlongumine, purported to specifically reduce androgen signaling in PCa, were investigated. Optimized culture conditions successfully maintained the structural and functional fidelity of both benign and PCa TSCs for 5 days. TSCs exhibited androgen-dependence, appropriately undergoing ductal degeneration, reduced proliferation, and decreased prostate-specific antigen expression upon androgen ablation. Furthermore, TSCs revealed cancer-specific reduction of androgen receptor and increased apoptosis upon treatment with piperlongumine, validating data from cell lines. We demonstrate a TSC model that authentically recapitulates the structural, cellular, and genetic characteristics of the benign and malignant human prostate, androgen-dependence of the native tissue, and cancer-specific response to a potential new therapeutic for PCa. The work described herein provides a basis for advancing the experimental utility of the TSC model.
Keywords: ex vivo, model development, prostate, prostate cancer, tissue slice culture
Prostate cancer (PCa) cell lines and animal models have been extremely valuable in PCa research, but inherent drawbacks hinder their clinical relevance.1, 2 Cell lines, often artificially immortalized, acquire mutations in culture and lack the cellular interactions with a prostate microenvironment that are critical for prostate function and tumorigenesis.3 Animal models may not physiologically or genetically represent true human prostate pathology.1, 2, 4 There are currently few models of primary PCa, which historically has been difficult to maintain in culture or in vivo.5
To address these limitations, we and others have developed an ex vivo “tissue slice culture” (TSC) model of the benign and malignant human prostate. TSC purports to be an authentic model because it preserves native tissue architecture and functional differentiation, maintaining cellular heterogeneity and complex cell-cell interactions within the intact microenvironment. TSC has been a useful practice with other organs,6–8 and the advantages of TSC compared to monolayer cell culture are illustrated in many studies.9–11 Of note, the intact tumor microenvironment allows stromal-epithelial interactions that are critical for realistic studies of tumor metabolism.12, 13 With collaborators we found that TSCs exhibit steady-state glycolytic and phospholipid metabolism that mirrors that of human PCa in vivo but is not exhibited in PCa cell lines.14 Such deviations from human physiology often result in inaccurate preclinical assessment of drug responses in cell lines or animal models, leading to wasted efforts on clinical trials with drugs that are unlikely to work. TSCs, however, show promise in better-predicting drug responses in humans.13, 15
Ex vivo culture of the human prostate has been problematic, with benign tissues often exhibiting degradation of luminal epithelial cells and hyperproliferation of basal cells.16–18 Maintenance of PCa tissue in vitro has presented even more challenges than benign tissue.16, 19 Relatively recent technological innovations, particularly the practice of precision-cut slicing,6 have led to the current form of the prostate TSC model in which 250–500 µm thick slices of tissue, 5–8 mm in diameter, are cultured under defined conditions.18–23 Precision-cutting reduces sources of error due to variations in slice thickness and damage to cut surfaces, which both contribute to uneven gas and nutrient exchange throughout tissue slices. It enhances reproducibility when working with heterogeneous tissues such as prostate, allowing adjacent slices to be evaluated for histology and compared pair-wise under different experimental conditions. In addition, benign and PCa tissues may be compared from the same specimen. Variations of prostate TSC have been reported with mixed results.18, 19 With collaborators, we were the first to report the experimental implementation of a normal prostate TSC model, identifying altered DNA damage response mechanisms by which prostatic epithelia may be predisposed to malignant transformation.21, 22, 24
While these studies underscore the novel experimental potential of prostate TSCs, the model remains underutilized. This is in large part due to the need for further optimization and thorough characterization of the model as well as for additional feasibility studies to encourage its use. The ability to culture primary PCa is a unique feature of TSC that will confer greater authenticity to preclinical studies. One of the few reports involving PCa TSC was a pharmacodynamic profiling study reporting that TSCs were reproducible and accurate models for preclinical evaluation of drug responses.15 As evidenced in other TSC systems, extending viability in culture beyond 2–3 days allows modeling of chronic as well as acute toxicity studies, including the metabolic activity of the tissue.25–27
Our objectives were to systematically optimize and comprehensively characterize both benign and PCa TSCs in order to potentiate the utility of the model. Modifications of the culture system permitted maintenance of benign differentiated secretory epithelia in the absence of basal cell hyperplasia for up to 5 days. Primary PCa of Gleason grades 3, 4, and 5 also remained viable under optimized conditions. We showed that TSCs are androgen-responsive and maintained the microenvironment and heterogeneity of the native tissue, qualities that support their use as an authentic preclinical model for PCa. Finally, we used TSCs to test the cancer-specific effects of the natural compound piperlongumine, which was recently evaluated in PCa cell lines.28
MATERIALS AND METHODS
Culture of Prostate Tissue Slices
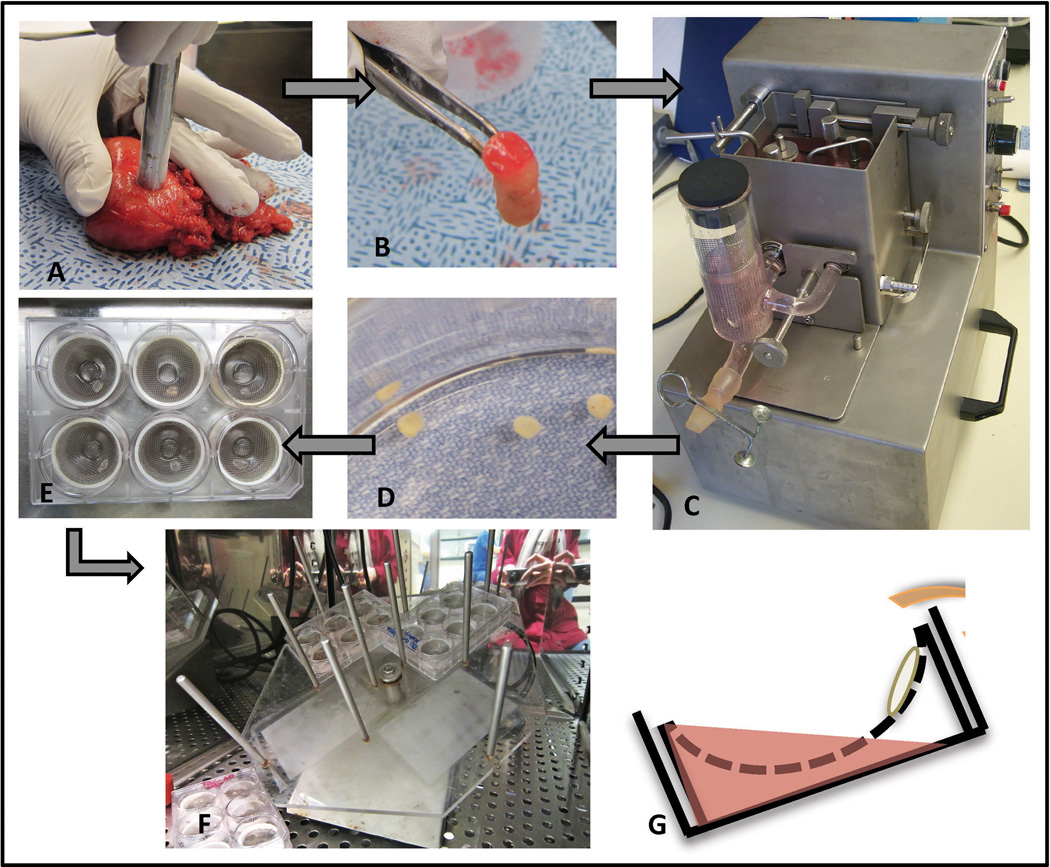
Information about donors and tissues is presented in Supplementary Table 1. Figure 1 illustrates the acquisition, generation, and culture of prostate TSCs. Radical prostatectomy specimens were obtained immediately post-surgery and 8-mm diameter cores of putative benign and PCa regions were taken from the peripheral zones according to needle biopsy maps and gross analysis (Figure 1A, B). The cores were aseptically precision-cut to 300-µm thickness in the Krumdieck Tissue Slicer (Alabama Research and Development, Mundford, AL, USA),8, 29 from which they emerged in a sequential manner (Figure 1C, D). Alternating slices were formalin-fixed for paraffin-embedding or frozen and sectioned for histological verification by hematoxylin and eosin (H&E) staining (Supplementary Figure 1). Adjacent slices were transferred with sterile forceps on to titanium mesh inserts in 6-well plates with 2.5 mL of culture medium and one to five tissue slices per well (Figure 1E). The plates were incubated at 37°C with 95% air/5% CO2 on a rotating platform set at a 30° angle (Alabama Research and Development, Figure 1F). Intermittent submersion in the medium caused by the angled rotation facilitates nutrient and gas diffusion throughout the 300-µm slices (Figure 1G), which is critical for maintaining cell viability over time. M199, Keratinocyte Serum-Free Medium (KSFM), and antibiotic/antimycotic were from Gibco Life Technologies (Grand Island, NY, USA) and were prepared as in Blauer et al.19 Complete PFMR-4A was prepared as previously reported.30 Dihydrotestosterone (DHT, Steraloids Inc., Newport, RI, USA) and R1881 (Perkin Elmer, Waltham, MA, USA) stocks were prepared in ethanol (Sigma-Aldrich, St. Louis, MO, USA). Piperlongumine (Indofine Chemical Company Inc., Hillsborough, NJ, USA) was prepared in dimethyl sulfoxide (DMSO, Fisher Scientific Inc., Hampton, NH, USA).
Figure 1.
Schematic for tissue slice acquisition and culture. Cores 8-mm in diameter were taken from radical prostatectomy specimens (A, B) and placed in the Krumdieck tissue slicer (C) from which slices emerged in a sequential manner (D). Slices were placed on titanium mesh inserts in 6-well plates with 2.5 mL culture medium (E). Plates were mounted on an angled rotating platform in a tissue culture incubator (F). The angled rotation caused intermittent submersion of the tissue slices, facilitating diffusion of gas and nutrients from all sides of the slices (G).
Tissue Histology and Immunohistochemistry
Tissue slices were fixed in 10% buffered formalin overnight at room temperature. The slices were then either embedded in OCT compound and frozen at −80°C or dehydrated and embedded in paraffin according to standard practices. Serial 5-µm slices were cut for histological and immunohistochemical procedures. Formalin-fixed, paraffin-embedded (FFPE) sections were deparaffinized, rehydrated, and prepared for immunohistochemistry (IHC) as described previously.31 The 5-µm sections were incubated with primary and secondary antibodies listed in Table 1 and labeled with alkaline phosphatase (Biocare Medical, Concord, CA, USA), Warp Red Chromogen (Biocare Medical), and/or 3,3-diaminobenzidine (Dako, Carpinteria, CA, USA) according to specifications and counterstained with hematoxylin. Immunohistochemical quantification was performed by blindly counting total cells and IHC-positive cells from three to six random 40× fields from each of three tissue slices per experimental condition. The percent IHC-positive cells per field was calculated and presented as the mean ± standard deviation. Statistical significance was determined by paired Student’s t-tests.
Table 1.
Antibody information
| Company | Name | Specifications | Dilution | Incubation |
|---|---|---|---|---|
| Santa Cruz Biotechnology, Santa Cruz, CA, USA | CK18 | Mouse monoclonal | 1:500 | 4°C overnight |
| AR | Rabbit polyclonal | 1:500 | 4°C overnight | |
| PSA | Mouse monoclonal | 1:100 | 4°C overnight | |
| SYP | Mouse monoclonal | 1:100 | 30 min. room temp. | |
| ERG | Rabbit polyclonal | 1:100 | 1 hour room temp. | |
| Biocare Medical, Concord, CA, USA | Ki67 | Mouse monoclonal | Pre-diluted | 1 hour room temp. |
| CC3 | Rabbit polyclonal | 1 hour room temp. | ||
| CD31 | Mouse monoclonal | 1 hour room temp. | ||
| AMACR/p63/CK5 | Mouse mono/rabbit poly | 1 hour room temp. | ||
| 4plus HRP Universal 2° | Anti-mouse/anti-rabbit | 30 min. room temp. | ||
| Abcam, Cambridge, MA, USA | p63 | Mouse monoclonal | 1:200 | 4°C overnight |
| SMA | Mouse monoclonal | 1:1000 | ||
| CD68 | Mouse monoclonal | 1:500 | ||
| PSMA | Mouse monoclonal | 1:200 | ||
| Cell Signaling Technology, Danvers, MA, USA | PTEN | Rabbit monoclonal | 1:100 | 4°C overnight |
Lactate Dehydrogenase and MTS Assays
Conditioned media were collected, spun down and stored at −80°C. The Cytotoxicity Detection Kit Plus (LDH) (Roche, Indianapolis, IN, USA) was used to measure lactate dehydrogenase (LDH) activity in conditioned media as directed. The CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega, Madison, WI, USA) was used to measure tissue slice viability. Slices were incubated in culture medium containing the MTS reagent for 2.5 hours at 37°C and absorbances were measured as directed. LDH and MTS activity readouts were normalized to the weights in mg of the corresponding tissue slices and presented as means ± standard deviations (n = 3). Statistical significance was determined by paired Student’s t-tests.
RESULTS
Optimization of TSC Conditions
The lab previously developed a serum-free medium, “Complete PFMR-4A,” for primary culture of human prostatic cells.30 This medium, with the addition of 10 nM of the synthetic androgen R1881, kept benign prostate TSCs viable for ~2 days before luminal degeneration occurred. We used these conditions as a starting point to further optimize prostate TSC to extend viability and maintain authentic prostate structure and function in vitro for up to 5 days.
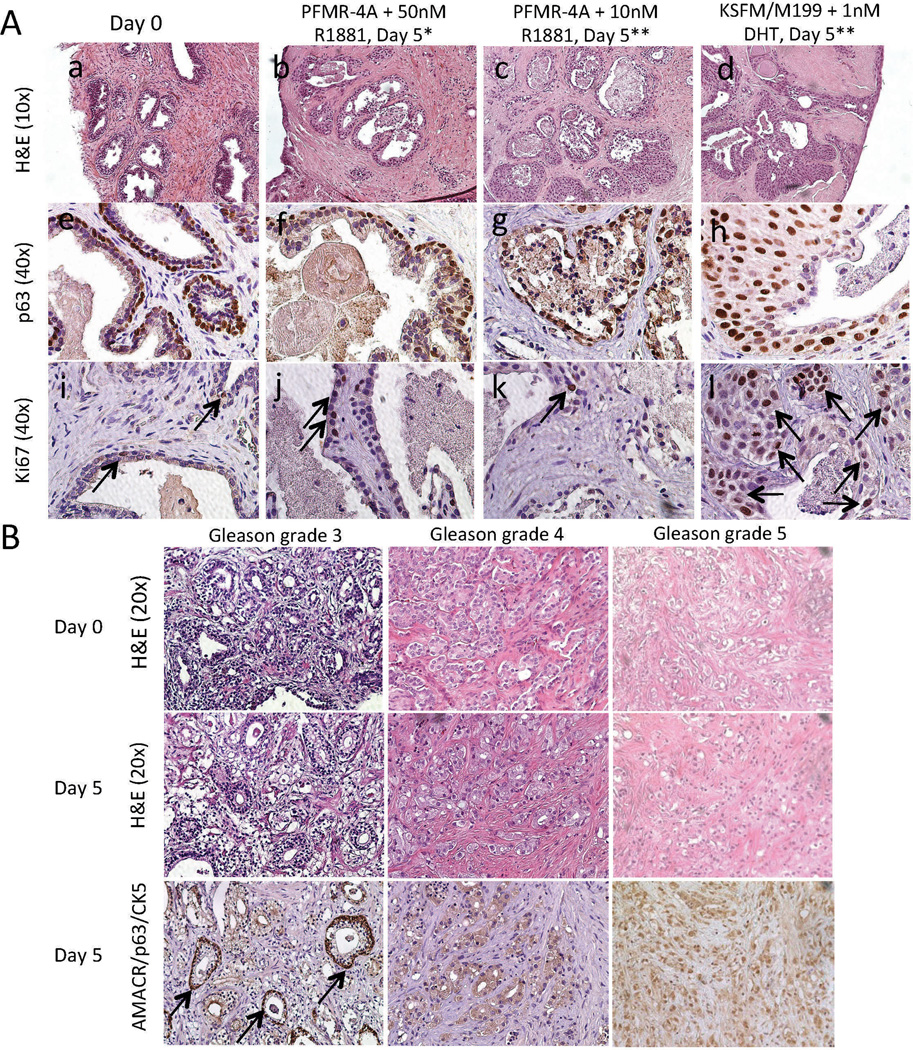
Complete PFMR-4A medium with 10 nM R1881 was compared to a medium composed of KSFM/M199 with 1 nM DHT; this medium was previously reported to maintain normal prostate TSCs up to 7 days.19 The media were changed every other day and TSCs from multiple prostate specimens were evaluated (Supplementary Tables 1 and 2). In our hands, the KSFM/M199-based medium caused basal cell hyperplasia and occasional luminal degeneration after 5 days of culture, while TSCs in Complete PFMR-4A with 10 nM R1881 exhibited luminal degeneration as previously observed as well as occasional basal cell hyperplasia (Figure 2A and Supplementary Tables 1 and 2). IHC for markers of basal cells (p63) and actively proliferating cells (Ki67) confirmed basal cell hyperplasia in the tissues cultured in the KSFM/M199-based medium (Figure 2A). Representative negative controls for IHC are included in Supplementary Figure 2. Antioxidants N-acetyl cysteine and L-arginine have each been reported to aid survival in organotypic and cell cultures.32–34 Each was added to the Complete PFMR-4A medium and tissue slices were evaluated histologically over time, but neither compound prevented luminal epithelial degeneration (data not shown).
Figure 2.
Histology of benign and PCa TSCs. (A) H&E (a – d) and IHC for p63 (e – h) and Ki67 (i – l, arrows indicate positive nuclei) showed benign TSCs undergoing luminal cell degeneration and/or basal cell hyperproliferation compared to the native tissue (“Day 0”) when cultured in PFMR-4A with 10 nM R1881 (c, g, k) or KSFM/M199 with 1 nM DHT (d, h, l), but not in PFMR-4A with 50 nM R1881 (b, f, j) for 5 days. * = Medium changed every 24 hours. ** = Medium changed every 48 hours. (B) Gleason grades 3, 4, and 5 PCa TSCs cultured in PFMR-4A medium with 50 nM R1881 for 5 days exhibited histologic fidelity to the native “Day 0” tissue as evidenced by H&E staining. AMACR/p63/CK5 staining revealed regions of cancer and some interspersed benign glands (arrows). Representative images were from patient specimens #12 (A), 18, 27, and 30 (B, Table S1).
Increasing the concentration of R1881 in Complete PFMR-4A revealed that 50 nM prevented luminal epithelial degeneration up to 5 days in culture (Figure 2A, Supplementary Figure 3, Supplementary Tables 1 and 2). A single layer of p63-positive basal cells topped by secretory luminal cells, with one-to-two Ki67-positive cells per gland, accurately reflected native (“Day 0”) tissue histology and phenotype (Figure 2A). While we have not yet extensively characterized TSCs beyond 5 days, preliminary studies indicate that basal cell hyperproliferation and overall degeneration of both benign and PCa tissues become apparent after one week.
It has long been understood that tissue viability is enhanced when the culture medium is periodically refreshed to maintain sufficient levels of supplements and to remove metabolic waste products that can be damaging when present in the cellular environment.35, 36 This is particularly important for metabolic studies, and perfusion culture systems in bioreactors have been shown to enhance survival and realistically maintain cellular metabolism.14, 36, 37 For our purposes, changing the culture medium every day appeared to enhance prostate TSC survival over changing the culture medium every other day. There was no perceived benefit to changing the medium more than once per day (data not shown).
TSC of Primary PCa of Different Gleason Grades
After extending the maintenance of benign prostate TSCs with the optimized PFMR-4A-based medium, we tested the efficacy of these conditions with PCa TSC of different Gleason grades. Using medium supplemented with 50 nM R1881 and changing media daily successfully maintained the native histology of Gleason grade 3 (low-grade), 4, and 5 (high-grade) PCa tissue slices up to 5 days in culture (Figure 2B, Supplementary Table 1). We used a triple IHC stain to verify the cancerous regions: the presence of cytoplasmic α methylacyl coenzyme A racemase (AMACR) staining and coincident absence of both nuclear p63 and cytoplasmic cytokeratin 5 (CK5) staining indicate malignancy, while the presence of nuclear p63 and cytoplasmic CK5 staining in basal cells in the absence of cytoplasmic AMACR indicate normal tissue.38 Benign glands can be interspersed in regions of cancer, as indicated by the arrows in Figure 2B.
Optimized Culture Conditions Maintain Viability of Benign and PCa TSCs
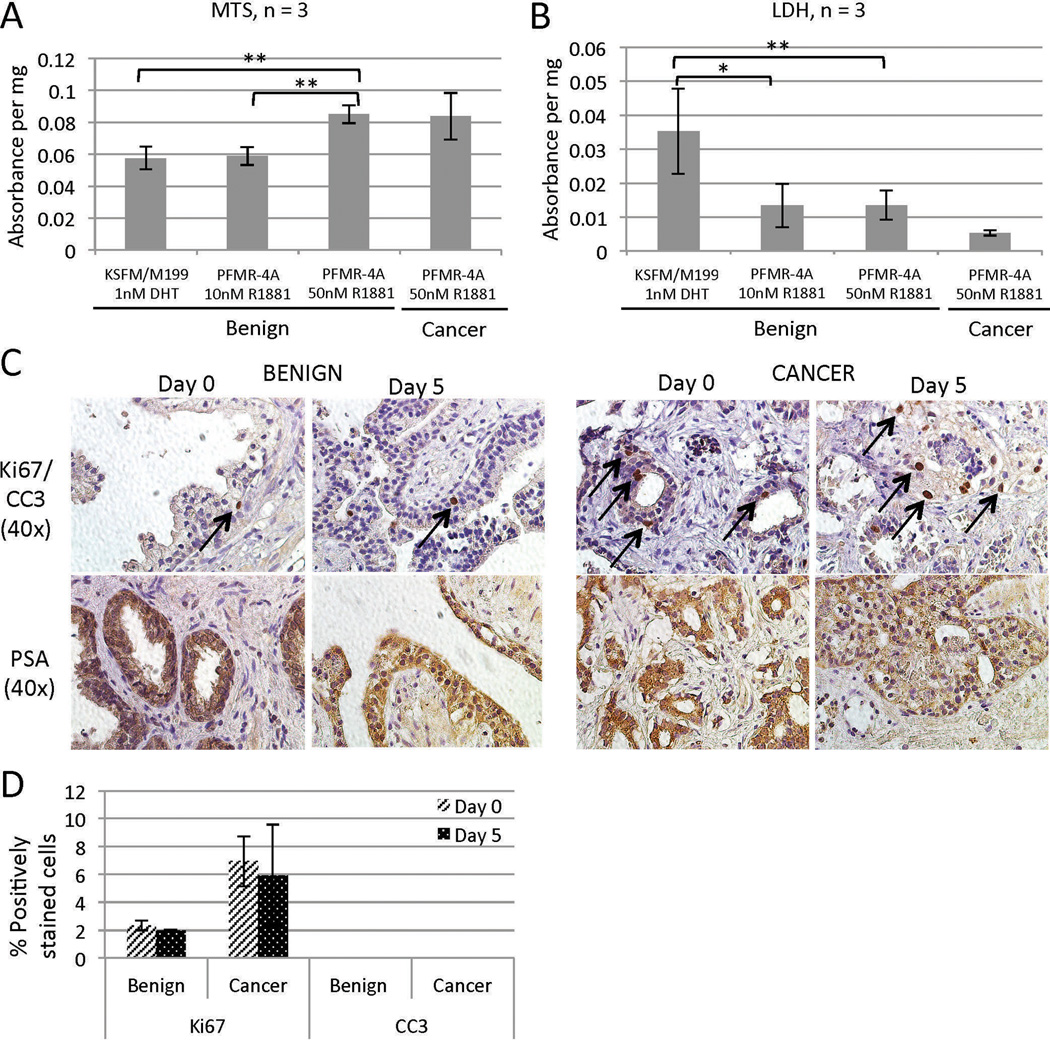
Quantitative assays were performed to evaluate overall cell viability and cytotoxicity in TSCs in different culture conditions. MTS assays, which are biochemical measures of viability, revealed a significant improvement in survival of benign TSCs under the optimized culture condition at day 5 compared to the other conditions tested (Figure 3A). LDH assays, which measure cytotoxicity, showed decreased cytotoxicity under optimized conditions (Figure 3B). PCa TSC viability and cytotoxicity levels were on par with those of the benign prostate TSCs in the optimized medium (Figure 3A and B). MTS and LDH assay results corresponded to the qualitative histological assessments represented in Figure 2. Furthermore, cleaved caspase 3 (CC3), a marker of apoptosis, was not detected in TSCs cultured for 5 days in the optimized culture system (Figure 3C, D). Ki67, a marker for proliferating cells, was present in ~2% of benign epithelial cells and ~7% of cancer cells in both the native tissue and day 5 TSCs (Figure 3D). The percentages of CC3- and Ki67-positive nuclei were quantified from three random 40× fields from each of three tissue slices per condition (average of 97 nuclei per field), and there were no statistically significant differences between days 0 and 5 (Figure 3D). Importantly, prostate-specific antigen (PSA) continued to be expressed in the benign luminal epithelial cells and cancer cells in TSCs at 5 days, suggesting sustained prostatic function in vitro (Figure 3C). Together, these histological, biochemical, and immunohistochemical assays confirm the functional viability of both benign and PCa TSCs up to 5 days in culture.
Figure 3.
TSC viability and cellular activity. Benign TSCs cultured in PFMR-4A with 50 nM R1881 for 5 days exhibited increased viability by MTS assay (A) and reduced cytotoxicity by LDH assay (B) compared to those cultured under different conditions. PCa TSCs exhibited levels of viability (A) and cytotoxicity (B) similar to those of benign TSCs. ** = p < 0.001, * = p < 0.01. (C) Benign and PCa TSCs cultured for 5 days in PFMR-4A with 50 nM R1881 maintained the same proliferative and apoptotic profiles as the native tissue (“Day 0”) as evidenced by the presence of Ki67-positive cells (arrows) and the absence of CC3 staining, respectively (top row, quantified in D). They also continued to express PSA (bottom row). (D) The percentages of CC3- and Ki67-positive nuclei were quantified from three random 40× fields from each of three tissue slices per condition (average of 97 nuclei per field). Experiments were performed on patient specimens #19, 20, 21 (A, B), 14, and 18 (C).
Benign and PCa TSCs Maintain the Cellular Complexity of the Native Tissue
One of the major advantages of the TSC model over current in vitro models is its potential to preserve the structural and cellular diversity of the human prostate. Cellular and molecular heterogeneity and dependence on endocrine and paracrine signaling are important elements of human PCa that are lacking in current in vitro models. Crosstalk between the epithelia and the stromal microenvironment, which includes fibroblasts, vasculature, nerves, and cells of the immune system, is critical to functions of the normal prostate as well as to cancer progression.3, 39, 40 Benign and cancer tissues were obtained from the same specimen and IHC was performed for lineage-specific markers of the various cell types within the human prostate at Day 0 (immediately post-surgery) and in adjacent TSCs after 5 days (Figure 4). The basal epithelial cell marker (p63) was present in a single layer in benign glands but was absent from malignant glands as expected, while cytokeratin 18 (CK18) was present in both benign luminal epithelia and cancer, as appropriate. Smooth muscle α-actin (SMA) was present throughout the stroma as expected in both benign and cancer tissues. CD31 staining of endothelial cells showed evidence of vasculature in the stroma, more so in regions of cancer as has been previously observed.41 CD68 is a cell surface glycoprotein expressed by macrophages and monocytes, and its increased presence in cancer compared to benign tissue reflects an inflammatory phenotype common to PCa.40 Finally, a marker for neuroendocrine cells, synaptophysin, was expressed in distinct cells within the basal cell layer in the benign tissue and in sporadic cancer cells as expected (Figure 4).42 Overall, these results demonstrate that benign and cancer tissue slices cultured for 5 days in our system retain much of the cellular complexity of native tissues, validating the fidelity of the TSC model of the human prostate.
Figure 4.
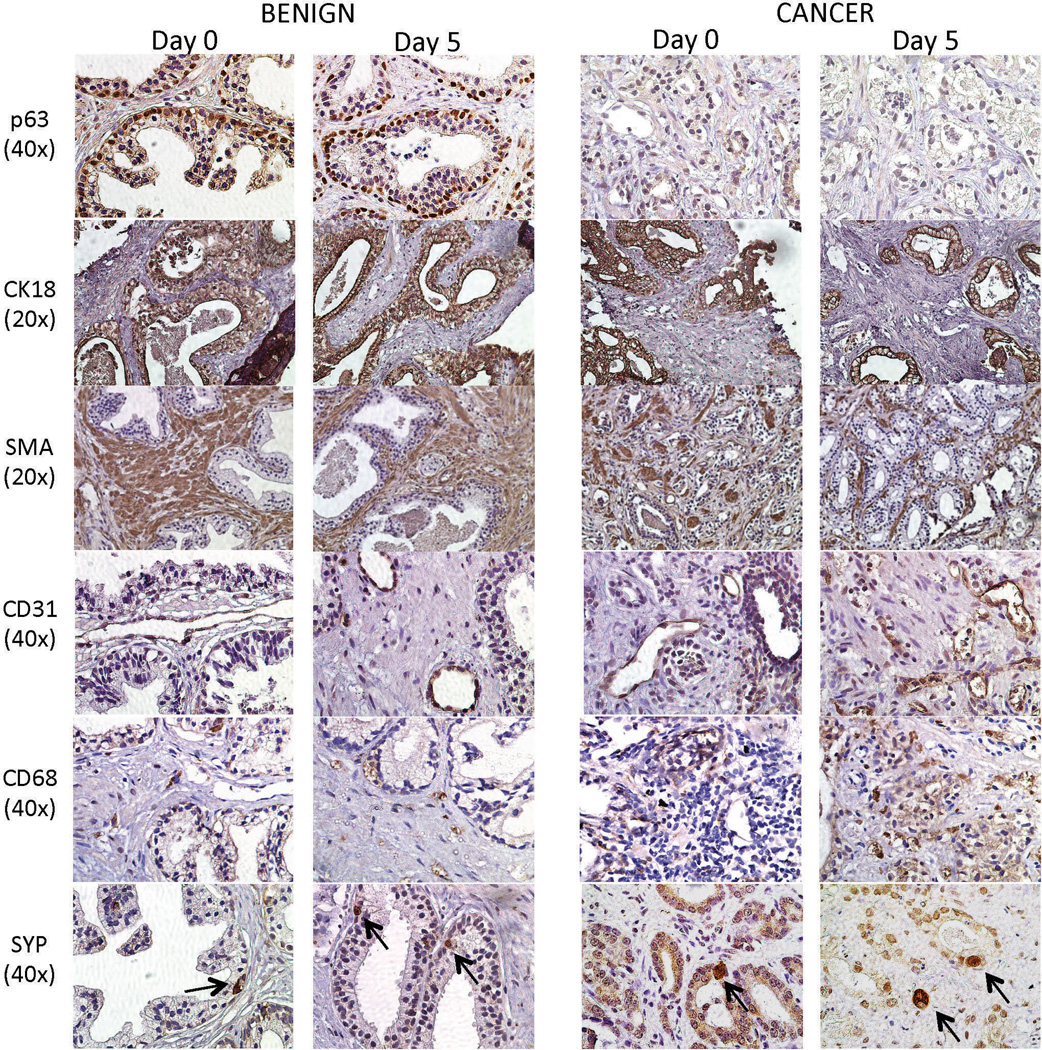
Cellular compositions of benign and PCa TSCs. Cellular markers of basal epithelial cells (p63), luminal epithelial cells and cancer cells (CK18), stromal cells (SMA), endothelial cells (CD31), inflammatory cells (CD68), and neuroendocrine cells (SYP, arrows) were appropriately expressed in benign and PCa TSCs after 5 days in culture. The expression patterns recapitulated those in the native “Day 0” tissues. Representative images were from patient specimens #16, 18, and 27.
Benign and PCa TSCs Retain Biomarker Expression Patterns of Native Tissue
After confirming that prostate TSCs maintained the cellular composition of the native tissue, we next investigated maintenance of expression of biomarkers that are differentially expressed in PCa by IHC in tissue slices cultured for 5 days (Figure 5). AMACR/p63/CK5 triple staining, used to confirm the presence of PCa in clinical specimens, distinguished regions of benign and malignant tissue in TSCs. The TMPRSS2-ERG gene fusion, occuring in ~40% of prostate tumors, leads to androgen-driven upregulation of the ERG transcription factor.43 Using ERG expression as a surrogate marker for the TMPRSS2-ERG fusion,44 we identified PCa specimens that expressed ERG but observed that ERG expression was reduced in the TSCs after 5 days in culture. While it is possible that androgen signaling is less robust in TSCs, TMPRSS2 expression was well-maintained at day 5. Androgen receptor (AR) expression was detected in both benign and PCa native tissues and in TSCs after 5 days in culture. PTEN was identified in both benign and PCa specimens but to a somewhat lesser extent in PCa, which may be due to haploinsufficiency.45, 46 Both benign and PCa TSCs maintained the PTEN expression of the native tissues after 5 days in culture. Prostate-specific membrane antigen (PSMA) was detected in benign and malignant glands as expected in both the native tissue and in TSCs after 5 days.47 In both the native tissue and the Day 5 TSCs, E-cadherin was expressed at the cell membrane of benign epithelial cells but exhibited more diffuse cytoplasmic staining in the PCa cells as expected.48 Overall, the fidelity of protein expression patterns in TSCs to the native tissues together with their cellular and structural fidelities show that the TSC model uniquely recapitulates prostate biology on structural, cellular, and molecular levels.
Figure 5.
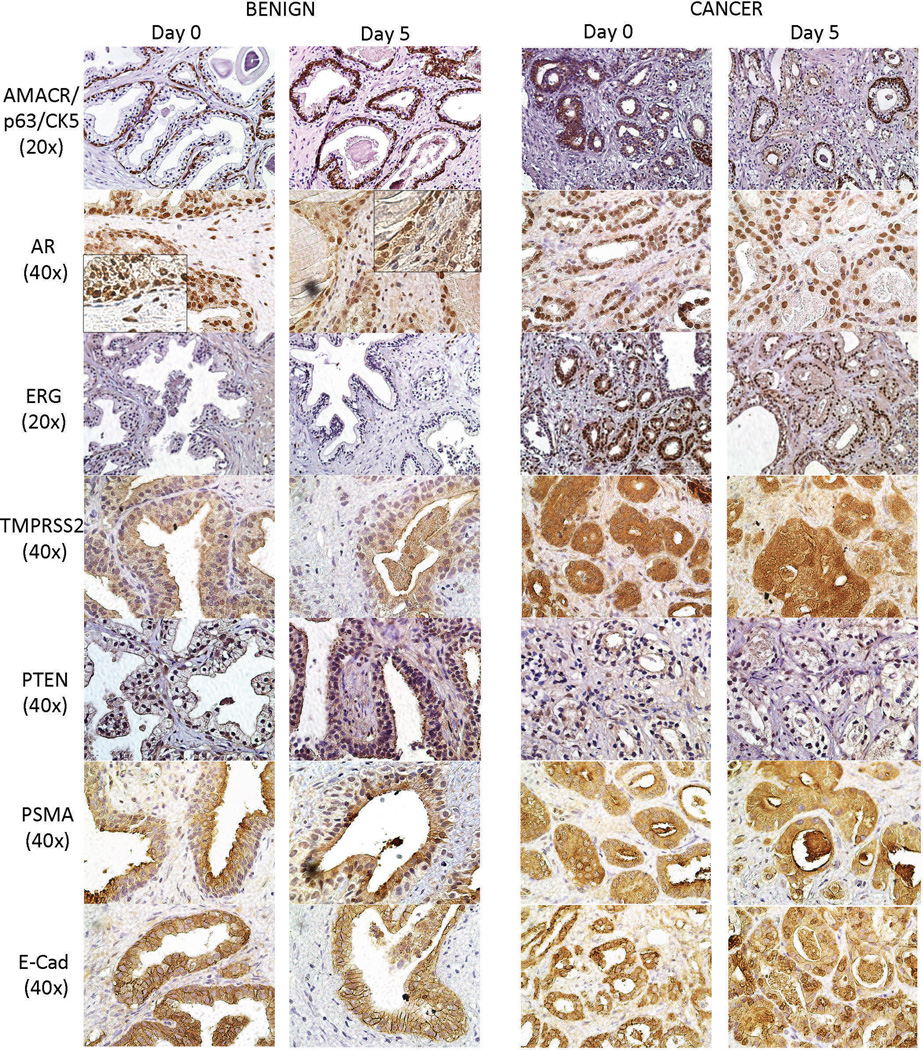
Biomarker expression in benign and PCa TSCs. IHC expression patterns of known biomarkers in benign and PCa TSCs cultured for 5 days mirrored those in the corresponding native “Day 0” tissues. 60×-magnification inserts show the absence of AR staining in basal cells of benign TSCs. Representative images were from patient specimens #18, 27, and 28.
Benign and PCa TSCs Exhibit Androgen-dependence
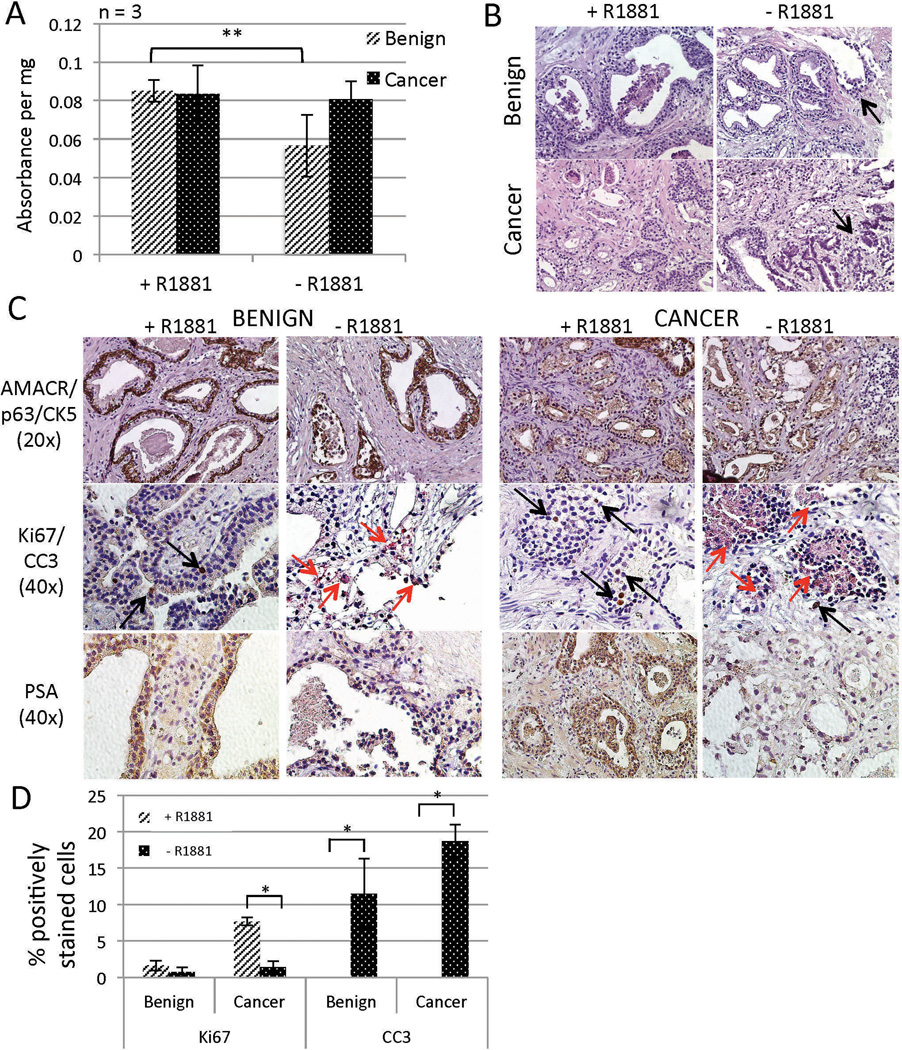
Benign human prostate and PCa are both highly dependent on androgen signaling, and we showed in Figure 5 that both benign and PCa TSCs express AR. In order to further evaluate the authenticity of the TSC model, the response of TSCs to androgen ablation was assessed. Benign and PCa TSCs were cultured in Complete PFMR-4A medium in the presence (50 nM R1881) or absence (0.05% ethanol control) of androgen. Upon histological evaluation after 5 days in culture, there was evidence of ductal degeneration in regions of both benign and cancer TSCs in the absence of androgen (Figure 6B, arrows, Supplementary Tables 1 and 2). An MTS assay showed a statistically significant reduction of viability in benign TSCs but not cancer after 5 days in the absence of androgen (Figure 6A). However, there is likely a heterogeneous response to androgen ablation among the cell types in both benign and cancer TSCs, as the histology suggested, which may not be revealed by the MTS assay.
Figure 6.
Responses of TSCs to androgen ablation. Culture of benign and PCa TSCs in the absence of androgen for 5 days resulted in heterogeneous reduction of cellular viability and glandular integrity according to MTS assays (A) and H&E staining (B, arrows point to degenerating glands), respectively. Luminal degeneration was also evident upon IHC evaluation of benign and PCa TSCs cultured for 5 days in the absence (ethanol control) of R1881 (C). Benign and PCa TSCs were verified by AMACR/p63/CK5 staining (top row). Androgen ablation reduced the number of actively proliferating cells (Ki67-positive, black arrows) and increased the number of cells undergoing apoptosis (CC3-positive, red arrows) in both benign and PCa TSCs. PSA expression was reduced in both benign and PCa TSCs upon 5 days of androgen ablation (bottom row). (D) The percentages of CC3- and Ki67-positive nuclei were quantified from six random 40× fields from each of three tissue slices per condition (average of 90 nuclei per field). ** = p < 0.01; * = p < 0.05. Experiments and representative images were from patient specimens #18, 20, and 21.
We used IHC to further investigate the effects of androgen ablation on TSCs. After 5 days, evidence of proliferating cells (Ki67-positive, black arrows) decreased and evidence of apoptotic cells (CC3-positive, red arrows) increased in both benign and cancer TSCs in the absence of androgen (Figure 6C). Quantification of the percent positively-stained nuclei from six random 40× fields (average of 90 nuclei per field) from each of three tissue slices per condition confirmed statistically-significant increases in apoptotic cells in benign and PCa TSCs in the absence of androgen (Figure 6D). There was a statistically significant reduction in Ki67-positive cells in PCa but not benign TSCs in the absence of androgen (Figure 6D). Furthermore, intensity of PSA, an androgen-regulated gene, diminished in both benign and cancer TSCs as expected (Figure 6C). Together, these results show the androgen-dependency of both benign prostate and primary PCa TSCs, further supporting the biological relevance of the TSC model to the human prostate.
TSCs Validate a Cancer-specific Response to Piperlongumine
Investigations to assess the feasibility of using TSC as a predictive preclinical model for human therapeutic response are ongoing across a variety of organ types.7, 49, 50 Of particular value is the ability to compare responses of cancer versus benign cells in TSCs, as efforts to identify cancer-specific drugs with reduced side effects intensify. The naturally-derived alkaloid piperlongumine (PL) was recently found to selectively kill cancer cells across a range of cell lines and in a transgenic mouse model for breast cancer.51 Golovine et al. subsequently tested the effects of PL on PCa cell lines and reported that after only 3–6 hours, PL depleted AR and reduced cell proliferation.28 There remains the question of whether the effects of PL on PCa cell lines will be recapitulated in the native prostatic microenvironment and whether PL will affect benign prostate cells as well as the cancer. After confirming the androgen-dependence of benign and PCa TSCs, we evaluated the effects of PL in this more biologically-relevant system in which we can compare benign and cancer responses.
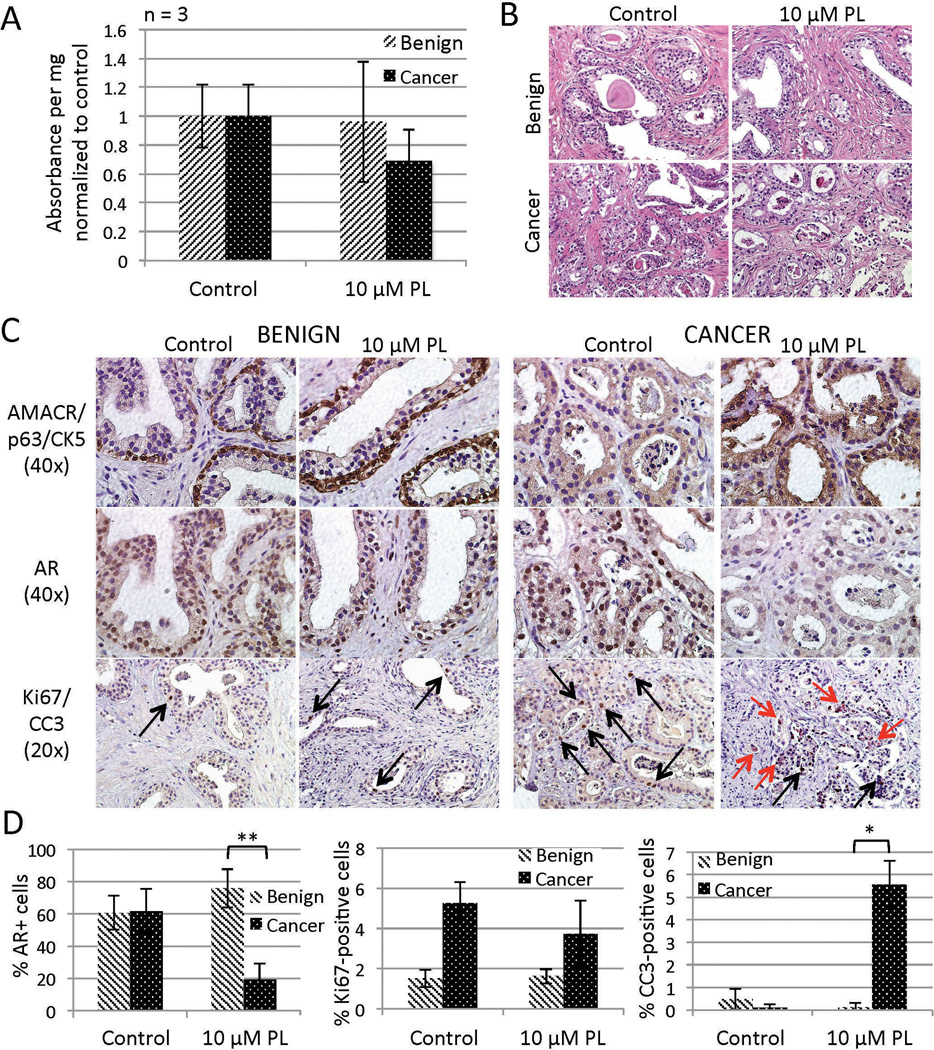
Six hour treatment with low-dose PL (10 µm) appeared to reduce viability and cause epithelial degeneration to a somewhat greater extent in cancer TSCs than in benign as evidenced by MTS assays and H&E staining, respectively (Figure 7A and B). We next used IHC to localize cell-specific effects of PL in the benign and cancer TSCs. AR expression persisted in benign TSCs after 6 hours of PL treatment, while AR was significantly reduced in PL-treated PCa TSCs (Figure 7C and D). Furthermore, while there was a slight decrease in actively proliferating (Ki67-positive) cells in the PL-treated PCa TSCs, there was a significant increase in PCa cells undergoing apoptosis (CC3-positive) compared to benign cells (Figure 7C and D). These results are consistent with and validate the findings by Golovine et al. (although they did not test for apoptosis) while additionally revealing a cancer-specific effect of PL in the prostate that is consistent with findings in other tissue types.51 These findings lend support for further investigation into the use of PL in the clinic, and they exemplify the utility of the TSC model for validating preclinical studies previously executed only in cell lines or animal models.
Figure 7.
Responses of TSCs to piperlongumine (PL). PCa but not benign TSCs treated with PL for 6 hr exhibited a non-significant reduction of viability (MTS assay, A) that was mirrored by sporadic regions of luminal degeneration (H&E, B). Further IHC evaluation of benign and PCa TSCs revealed a cancer-specific decrease of AR expression (middle row C, D) and increase of apoptotic cells (red arrows, bottom row C, D). PL treatment did not significantly affect proliferation among benign or PCa cells (black arrows, bottom row C, D). Total nuclei (average of 114 per field) and positively-stained nuclei were blindly counted from three random 40× fields from each of three tissue slices per treatment (D). ** = p < 0.001; * = p < 0.01. Experiments and representative images were from patient specimen #26.
DISCUSSION
Optimization of culture conditions resulted in maintenance of benign and malignant prostate TSCs for 5 days, a significant improvement over our previous ability to maintain TSCs for only ~2 days without signs of degeneration. Although others have reportedly maintained prostate TSCs for up to a week, maintenance of structure and function was not comprehensively evaluated in those studies, and we could not reproduce those findings. We extended the longevity of TSCs by using a serum-free medium previously developed for primary culture of human prostatic epithelial cells, Complete PFMR-4A, and increasing the androgen supplement, R1881, from 10 to 50 nM. Although 50 nM is a high dose of this synthetic androgen that is often used in cell culture because of its stability compared to the natural androgen, DHT, little is known about hormone dynamics in this experimental context. Changing the medium daily also contributed to increased survival of TSCs. While perfusion culture might prove superior,37 frequent refreshment of medium is a feasible alternative when using standard culture apparati.
Why prostate tissues are so challenging to maintain in culture for long periods is unclear. TSCs from other species or organs can be maintained for weeks or months.7, 25, 27, 52 Organotypic culture models of the human prostate and PCa have taken several forms in the past, but their uses have been hampered by a short lifespan, the need for technical optimization, and lack of thorough characterization to confirm fidelity and reproducibility.
Over the 5-day period of culture in optimized medium, the TSC model maintained the authentic structure, function, and biological characteristics of both the benign prostate and PCa. Basal cell hyperplasia and luminal cell degeneration were minimized and the characteristic single layer of basal cells topped by a layer of secretory luminal cells was preserved in benign epithelial glands. Classic cell-type expression of proteins was retained, as evidenced by the localization of p63 and CK5 to the basal cells and CK18, AR and PSA to the luminal cells. Cancer-associated markers that are specifically or differentially expressed in PCa, including AMACR, TMPRSS2, PTEN, PSMA, and E-cadherin, were appropriately expressed in PCa TSCs. Neuroendocrine differentiation in both benign prostate and PCa TSCs was typical of that observed in native tissue. SMA expression confirmed the survival of smooth muscle cells and myofibroblasts in the stroma of benign tissue and PCa, respectively. Comparable rates of proliferation and apoptosis were observed in TSCs and native tissues, as measured by labeling for Ki67 and CC3.
Recapitulation of the cellular and molecular phenotype of the native tissue is lacking in other in vitro models. Cellular and molecular heterogeneity, in addition to the presence of an intact microenvironment, are ultimately necessary for accurate preclinical evaluations. Our comprehensive characterization also showed for the first time that endothelial cells, macrophages and monocytes survive in prostate TSCs. The presence of these cells may enable preclinical studies of therapeutics targeting the vascular or the immune system.
A notable outcome of optimizing culture conditions was our ability to maintain PCa of different Gleason grades in TSCs. There is a pressing need for model systems that authentically represent low risk (Gleason grade 3) and high risk (Gleason grades 4/5) primary human PCa, since the latter is associated with a high risk of recurrence and current neoadjuvant therapies have not significantly extended overall survival.53 TSCs can fulfill this need, since all Gleason grades were maintained during the 5-day culture period, and can be used to screen novel therapies. Furthermore, PCa-derived TSCs often contain admixed benign glands, uniquely providing an internal control for evaluating cancer-specificity and mechanisms of experimental therapeutics. Taking advantage of this feature, we tested the activity of the natural agent piperlongumine that reportedly reduces AR expression in a cancer-specific manner.28 We validated this finding in the TSC model, supporting further investigation into the efficacy of piperlongumine in the clinical setting.
The androgen-dependence that we demonstrated in TSCs is an essential characteristic of an authentic model of the benign and malignant prostate, especially considering that targeting the AR signaling pathway is a major goal in PCa research and therapy. However, further investigation into the mechanism of androgen signaling in TSCs will be necessary. While expression of the androgen-regulated genes TMPRSS2 and PSA persisted in TSCs, ERG expression (presumably driven by the androgen-responsive promoter of the TMPRSS2-ERG gene fusion) waned. AR signaling dynamics have not yet been investigated in this model system, and persistence of target gene expression may be due to longer half-lives instead of active AR signaling. Regardless, the presence of androgen proved to be critical for maintenance of benign and PCa TSCs.
The faithful retention of tissue structure and function in the TSC model presents an ideal opportunity to evaluate multifaceted effects of therapeutic compounds on an organ.15 TSC may be used to identify potential biomarkers of drug response, determine drug concentrations that affect organ function or morphology, carry out gene or protein expression profiling, identify species-specific or cell-line-specific effects, and elucidate mechanisms relevant to human safety.49 A recent study evaluated a new HSP90 inhibitor in PCa cell lines and organotypic PCa tissue cultures and reported that the tissue cultures provided information on the drug response that was not previously observed in cell lines or animal models.50 Further studies testing the boundaries of preclinical assessment with TSCs are warranted.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part with funding from NIH 2T32DK007217-37, DAMD W81XWH-10-1-0336, and DAMD W81XWH-13-1-0268.
ABBREVIATIONS
- AMACR
α methylacyl coenzyme A racemase
- AR
androgen receptor
- CC3
cleaved caspase 3
- CK5
cytokeratin 5
- CK18
cytokeratin 18
- DHT
dihydrotestosterone
- FFPE
formalin-fixed, parrafin-embedded
- H&E
hematoxylin and eosin
- IHC
immunohistochemistry
- LDH
lactate dehydrogenase
- PCa
prostate cancer
- PL
piperlongumine
- PSA
prostate-specific antigen
- PSMA
prostate-specific membrane antigen
- ROS
reactive oxygen species
- SMA
smooth muscle α-actin
- TSC
tissue slice culture
- TSG
tissue slice graft
Footnotes
Supplementary information is available at Laboratory Investigation’s website.
DISCLOSURE/CONFLICT OF INTEREST
The authors report no disclosures or conflicts of interest.
REFERENCES
- 1.Pienta KJ, Abate-Shen C, Agus DB, et al. The current state of preclinical prostate cancer animal models. Prostate. 2008;68(6):629–639. doi: 10.1002/pros.20726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toivanen R, Taylor RA, Pook DW, et al. Breaking through a roadblock in prostate cancer research: an update on human model systems. J Steroid Biochem Mol Biol. 2012;131(3–5):122–131. doi: 10.1016/j.jsbmb.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Taylor RA, Risbridger GP. Prostatic tumor stroma: a key player in cancer progression. Current cancer drug targets. 2008;8(6):490–497. doi: 10.2174/156800908785699351. [DOI] [PubMed] [Google Scholar]
- 4.Shappell SB, Thomas GV, Roberts RL, et al. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 2004;64(6):2270–2305. doi: 10.1158/0008-5472.can-03-0946. [DOI] [PubMed] [Google Scholar]
- 5.Peehl DM. Primary cell cultures as models of prostate cancer development. Endocr Relat Cancer. 2005;12(1):19–47. doi: 10.1677/erc.1.00795. [DOI] [PubMed] [Google Scholar]
- 6.Parrish AR, Gandolfi AJ, Brendel K. Precision-cut tissue slices: applications in pharmacology and toxicology. Life sciences. 1995;57(21):1887–1901. doi: 10.1016/0024-3205(95)02176-j. [DOI] [PubMed] [Google Scholar]
- 7.Holliday DL, Moss MA, Pollock S, et al. The practicalities of using tissue slices as preclinical organotypic breast cancer models. J Clin Pathol. 2013;66(3):253–255. doi: 10.1136/jclinpath-2012-201147. [DOI] [PubMed] [Google Scholar]
- 8.Fisher RL, Vickers AE. Preparation and culture of precision-cut organ slices from human and animal. Xenobiotica; the fate of foreign compounds in biological systems. 2013;43(1):8–14. doi: 10.3109/00498254.2012.728013. [DOI] [PubMed] [Google Scholar]
- 9.Enk CD, Jacob-Hirsch J, Gal H, et al. The UVB-induced gene expression profile of human epidermis in vivo is different from that of cultured keratinocytes. Oncogene. 2006;25(18):2601–2614. doi: 10.1038/sj.onc.1209292. [DOI] [PubMed] [Google Scholar]
- 10.Schmid JO, Dong M, Haubeiss S, et al. Cancer cells cue the p53 response of cancer-associated fibroblasts to cisplatin. Cancer Res. 2012;72(22):5824–5832. doi: 10.1158/0008-5472.CAN-12-1201. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs ER, Bodiga S, Ali I, et al. Tissue protection and endothelial cell signaling by 20-HETE analogs in intact ex vivo lung slices. Exp Cell Res. 2012;318(16):2143–2152. doi: 10.1016/j.yexcr.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brauer HA, Makowski L, Hoadley KA, et al. Impact of tumor microenvironment and epithelial phenotypes on metabolism in breast cancer. Clin Cancer Res. 2013;19(3):571–585. doi: 10.1158/1078-0432.CCR-12-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graaf IA, Groothuis GM, Olinga P. Precision-cut tissue slices as a tool to predict metabolism of novel drugs. Expert opinion on drug metabolism & toxicology. 2007;3(6):879–898. doi: 10.1517/17425255.3.6.879. [DOI] [PubMed] [Google Scholar]
- 14.Keshari KR, Sriram R, Van Criekinge M, et al. Metabolic reprogramming and validation of hyperpolarized 13C lactate as a prostate cancer biomarker using a human prostate tissue slice culture bioreactor. Prostate. 2013;73(11):1171–1181. doi: 10.1002/pros.22665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaira V, Fedele G, Pyne S, et al. Preclinical model of organotypic culture for pharmacodynamic profiling of human tumors. Proc Natl Acad Sci U S A. 2010;107(18):8352–8356. doi: 10.1073/pnas.0907676107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varani J, Dame MK, Wojno K, et al. Characteristics of nonmalignant and malignant human prostate in organ culture. Lab Invest. 1999;79(6):723–731. [PubMed] [Google Scholar]
- 17.Papini S, Rosellini A, Campani D, et al. Selective growth of epithelial basal cells from human prostate in a three-dimensional organ culture. Prostate. 2004;59(4):383–392. doi: 10.1002/pros.20021. [DOI] [PubMed] [Google Scholar]
- 18.Parrish AR, Sallam K, Nyman DW, et al. Culturing precision-cut human prostate slices as an in vitro model of prostate pathobiology. Cell Biol Toxicol. 2002;18(3):205–219. doi: 10.1023/a:1015567805460. [DOI] [PubMed] [Google Scholar]
- 19.Blauer M, Tammela TL, Ylikomi T. A novel tissue-slice culture model for non-malignant human prostate. Cell and tissue research. 2008;332(3):489–498. doi: 10.1007/s00441-008-0602-z. [DOI] [PubMed] [Google Scholar]
- 20.Ni X, Zhang Y, Ribas J, et al. Prostate-targeted radiosensitization via aptamer-shRNA chimeras in human tumor xenografts. J Clin Invest. 2011;121(6):2383–2390. doi: 10.1172/JCI45109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiviharju-af Hallstrom TM, Jaamaa S, Monkkonen M, et al. Human prostate epithelium lacks Wee1A-mediated DNA damage-induced checkpoint enforcement. Proc Natl Acad Sci U S A. 2007;104(17):7211–7216. doi: 10.1073/pnas.0609299104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaamaa S, Af Hallstrom TM, Sankila A, et al. DNA damage recognition via activated ATM and p53 pathway in nonproliferating human prostate tissue. Cancer Res. 2010;70(21):8630–8641. doi: 10.1158/0008-5472.CAN-10-0937. [DOI] [PubMed] [Google Scholar]
- 23.Zhu L, Jaamaa S, Af Hallstrom TM, et al. PSA forms complexes with alpha(1)-antichymotrypsin in prostate. Prostate. 2013;73(2):219–226. doi: 10.1002/pros.22560. [DOI] [PubMed] [Google Scholar]
- 24.Jaamaa S, Sankila A, Rantanen V, et al. Contrasting DNA damage checkpoint responses in epithelium of the human seminal vesicle and prostate. Prostate. 2012;72(10):1060–1070. doi: 10.1002/pros.21509. [DOI] [PubMed] [Google Scholar]
- 25.Behrsing HP, Vickers AE, Tyson CA. Extended rat liver slice survival and stability monitored using clinical biomarkers. Biochem Biophys Res Commun. 2003;312(1):209–213. doi: 10.1016/j.bbrc.2003.09.216. [DOI] [PubMed] [Google Scholar]
- 26.Amin K, Ip C, Sato B, et al. Characterization of ANIT-induced toxicity using precision-cut rat and dog liver slices cultured in a dynamic organ roller system. Toxicologic pathology. 2006;34(6):776–784. doi: 10.1080/01926230600918892. [DOI] [PubMed] [Google Scholar]
- 27.Ahlgren H, Henjum K, Ottersen OP, et al. Validation of organotypical hippocampal slice cultures as an ex vivo model of brain ischemia: different roles of NMDA receptors in cell death signalling after exposure to NMDA or oxygen and glucose deprivation. Cell and tissue research. 2011;345(3):329–341. doi: 10.1007/s00441-011-1218-2. [DOI] [PubMed] [Google Scholar]
- 28.Golovine KV, Makhov PB, Teper E, et al. Piperlongumine induces rapid depletion of the androgen receptor in human prostate cancer cells. Prostate. 2013;73(1):23–30. doi: 10.1002/pros.22535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krumdieck CL. Development of a live tissue microtome: reflections of an amateur machinist. Xenobiotica; the fate of foreign compounds in biological systems. 2013;43(1):2–7. doi: 10.3109/00498254.2012.724727. [DOI] [PubMed] [Google Scholar]
- 30.Peehl DM. Human prostatic epithelial cells. In: Freshney R, Freshney M, editors. Culture of Epithelial Cells. 2 ed. New York: Wiley-Liss, Inc; 2002. pp. 171–194. [Google Scholar]
- 31.Zhao H, Nolley R, Chen Z, et al. Tissue slice grafts: an in vivo model of human prostate androgen signaling. Am J Pathol. 2010;177(1):229–239. doi: 10.2353/ajpath.2010.090821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun L, Gu L, Wang S, et al. N-acetylcysteine Protects against Apoptosis through Modulation of Group I Metabotropic Glutamate Receptor Activity. PLoS One. 2012;7(3):e32503. doi: 10.1371/journal.pone.0032503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krause MS, McClenaghan NH, Flatt PR, et al. L-arginine is essential for pancreatic beta-cell functional integrity, metabolism and defense from inflammatory challenge. J Endocrinol. 2011;211(1):87–97. doi: 10.1530/JOE-11-0236. [DOI] [PubMed] [Google Scholar]
- 34.Ghoumari AM, Dusart I, El-Etr M, et al. Mifepristone (RU486) protects Purkinje cells from cell death in organotypic slice cultures of postnatal rat and mouse cerebellum. Proc Natl Acad Sci U S A. 2003;100(13):7953–7958. doi: 10.1073/pnas.1332667100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tilles AW, Baskaran H, Roy P, et al. Effects of oxygenation and flow on the viability and function of rat hepatocytes cocultured in a microchannel flat-plate bioreactor. Biotechnology and bioengineering. 2001;73(5):379–389. doi: 10.1002/bit.1071. [DOI] [PubMed] [Google Scholar]
- 36.Rambani K, Vukasinovic J, Glezer A, et al. Culturing thick brain slices: an interstitial 3D microperfusion system for enhanced viability. Journal of neuroscience methods. 2009;180(2):243–254. doi: 10.1016/j.jneumeth.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schumacher K, Khong YM, Chang S, et al. Perfusion culture improves the maintenance of cultured liver tissue slices. Tissue engineering. 2007;13(1):197–205. doi: 10.1089/ten.2006.0046. [DOI] [PubMed] [Google Scholar]
- 38.Dabir PD, Ottosen P, Hoyer S, et al. Comparative analysis of three- and two-antibody cocktails to AMACR and basal cell markers for the immunohistochemical diagnosis of prostate carcinoma. Diagnostic pathology. 2012;7:81. doi: 10.1186/1746-1596-7-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cunha GR, Hayward SW, Wang YZ. Role of stroma in carcinogenesis of the prostate. Differentiation. 2002;70(9–10):473–485. doi: 10.1046/j.1432-0436.2002.700902.x. [DOI] [PubMed] [Google Scholar]
- 40.De Nunzio C, Kramer G, Marberger M, et al. The controversial relationship between benign prostatic hyperplasia and prostate cancer: the role of inflammation. European urology. 2011;60(1):106–117. doi: 10.1016/j.eururo.2011.03.055. [DOI] [PubMed] [Google Scholar]
- 41.Bigler SA, Deering RE, Brawer MK. Comparison of microscopic vascularity in benign and malignant prostate tissue. Hum Pathol. 1993;24(2):220–226. doi: 10.1016/0046-8177(93)90304-y. [DOI] [PubMed] [Google Scholar]
- 42.di Sant'Agnese PA. Neuroendocrine differentiation in prostatic carcinoma: an update on recent developments. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2001;12(Suppl 2):S135–S140. [PubMed] [Google Scholar]
- 43.Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310(5748):644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 44.Chaux A, Albadine R, Toubaji A, et al. Immunohistochemistry for ERG expression as a surrogate for TMPRSS2-ERG fusion detection in prostatic adenocarcinomas. The American journal of surgical pathology. 2011;35(7):1014–1020. doi: 10.1097/PAS.0b013e31821e8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kwabi-Addo B, Giri D, Schmidt K, et al. Haploinsufficiency of the Pten tumor suppressor gene promotes prostate cancer progression. Proc Natl Acad Sci U S A. 2001;98(20):11563–11568. doi: 10.1073/pnas.201167798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McMenamin ME, Soung P, Perera S, et al. Loss of PTEN expression in paraffin-embedded primary prostate cancer correlates with high Gleason score and advanced stage. Cancer Res. 1999;59(17):4291–4296. [PubMed] [Google Scholar]
- 47.Wright GL, Jr, Haley C, Beckett ML, et al. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol Oncol. 1995;1(1):18–28. doi: 10.1016/1078-1439(95)00002-y. [DOI] [PubMed] [Google Scholar]
- 48.Jaggi M, Johansson SL, Baker JJ, et al. Aberrant expression of E-cadherin and beta-catenin in human prostate cancer. Urol Oncol. 2005;23(6):402–406. doi: 10.1016/j.urolonc.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 49.Vickers AE, Fisher RL. Evaluation of drug-induced injury and human response in precision-cut tissue slices. Xenobiotica; the fate of foreign compounds in biological systems. 2013;43(1):29–40. doi: 10.3109/00498254.2012.732714. [DOI] [PubMed] [Google Scholar]
- 50.Centenera MM, Gillis JL, Hanson AR, et al. Evidence for efficacy of new Hsp90 inhibitors revealed by ex vivo culture of human prostate tumors. Clin Cancer Res. 2012;18(13):3562–3570. doi: 10.1158/1078-0432.CCR-12-0782. [DOI] [PubMed] [Google Scholar]
- 51.Raj L, Ide T, Gurkar AU, et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 2011;475(7355):231–234. doi: 10.1038/nature10167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Meneghel-Rozzo T, Rozzo A, Poppi L, et al. In vivo and in vitro development of mouse pancreatic beta-cells in organotypic slices. Cell and tissue research. 2004;316(3):295–303. doi: 10.1007/s00441-004-0886-6. [DOI] [PubMed] [Google Scholar]
- 53.Sonpavde G, Palapattu GS. Neoadjuvant therapy preceding prostatectomy for prostate cancer: rationale and current trials. Expert review of anticancer therapy. 2010;10(3):439–450. doi: 10.1586/era.10.17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.