Abstract
Though anthropogenic impacts are often considered harmful to species, human modifications to the landscape can actually create novel niches to which other species can adapt. These “domestication” processes are especially important in the context of arthropod disease vectors, where ecological overlap of vector and human populations may lead to epidemics. Here, we present results of a global genetic study of one such species, the dengue and yellow fever mosquito, Aedes aegypti, whose evolutionary history and current distribution have been profoundly shaped by humans. We used DNA sequences of four nuclear genes and 1504 SNP markers developed with RAD-tag sequencing to test the hypothesis that Ae. aegypti originated in Africa, where a domestic form arose and spread throughout the tropical and subtropical world with human trade and movement. Results confirmed African ancestry of the species, and supported a single subspeciation event leading to the pantropical domestic form. Additionally, genetic data strongly supported the hypothesis that human trade routes first moved domestic Ae. aegypti out of Africa into the New World, followed by a later invasion from the New World into Southeast Asia and the Pacific. These patterns of domestication and invasion are relevant to many species worldwide, as anthropogenic forces increasingly impact evolutionary processes.
Keywords: Aedes aegypti, population genetics, human association, RAD, nuclear markers
INTRODUCTION
Over the past several thousand years, Homo sapiens has modified the global landscape profoundly. Though many anthropogenic impacts are known to destroy natural habitats and harm species (Chapin et al. 2000; Tilman and Lehman 2001; Pimm et al. 2006), they can also create novel niches for species living in close proximity to humans. Over time, certain species, often hardy generalists (Yeh and Price 2004; Kark et al. 2007), can evolve to live in these disturbed environments and may even adapt to become human-habitat specialists (Schofield et al. 1999; Das et al. 2004; Partecke et al. 2006; Keller 2007; Evans et al. 2009). This “domestication” process, or human commensalism, has impacted the evolutionary paths of species since the beginnings of human civilization, and only continues to grow in importance over time as human-dominated landscapes represent an increasing proportion of available niches worldwide.
Some of the closest ecological relationships between humans and other species are with arthropod vectors of disease, which often depend on human blood and artificial breeding sites for their survival (Schofield et al. 1999; Harrington et al. 2001; Ayala and Coluzzi 2005; Lyimo and Ferguson 2009). These human-adapted vector species can be spread by human movement and trade, with major public health consequences (Lounibos 2002). Humans have been particularly effective at spreading anthropophilic mosquitoes over intercontinental distances, as evidenced by the invasion of the malaria vector Anopheles arabiensis into Brazil from West Africa in the 1930s (Parmakelis et al. 2008), and the cosmopolitan spread of the disease vectors Aedes albopictus and Culex quinquefasciatus, among many other examples (Lounibos 2002). Our study focused on Aedes aegypti, the dengue and yellow fever mosquito, whose evolutionary history has been profoundly impacted by its relationship with humans (Tabachnick 1991). Though an effective vaccine exists for yellow fever, there is no vaccine currently approved for use against dengue viruses. Therefore, understanding and controlling this mosquito vector is essential to preventing disease spread; dengue affects an estimated 50 million people per year, with a full 40% of the world’s population currently at risk for infection (WHO 2009).
Aedes aegypti has long been assumed to have originated in Africa, where the ancestral form was likely a generalist, zoophilic treehole breeder (Mattingly 1957; Tabachnick 1991). Ecologically similar populations of Ae. aegypti still exist today on the African continent as the subspecies Aedes aegypti formosus. The better-known specialized domestic form, Aedes aegypti aegypti, is found in close association with human habitats throughout the tropical and subtropical world outside of Africa. Pure Aedes ae. aegypti mosquitoes are not found on the African continent except for unique, isolated populations in coastal East Africa, which are described below. The two subspecies are genetically distinct with discrete geographic distributions and mean differences in scaling pattern and ecological preferences (Mattingly 1957; McClelland 1974; Tabachnick and Powell 1979; Powell et al. 1980; Wallis et al. 1983; Brown et al. 2011).
It has been hypothesized that the domestic form, Ae. ae. aegypti, originated from a small population of forest-dwelling Ae. aegypti that became isolated in North Africa when a period of severe drying began in the Sahara approximately 4,000 years ago (Petersen 1977; Tabachnick 1991). The harsh landscape could have selected for mosquitoes exhibiting domestic behaviors such as a preference for breeding in artificial water storage containers (Petersen 1977; Tabachnick 1991). As global trade increased over the centuries, highly human-adapted Ae. ae. aegypti were spread across much of the tropical and subtropical world. The species was likely introduced to the New World by slave trade ships between the fifteenth and eighteenth centuries, possibly on multiple occasions (Tabachnick 1991). The spread to Asia may have occurred during the late nineteenth century based on the history of urban dengue emergence in the region (Smith 1956; Tabachnick 1991).
Today, urban domestic populations of traditionally “wild” Ae. ae. formosus are common throughout Africa, which can make subspecies identification of these populations more difficult (Miller et al. 1989; Nasidi et al. 1989; Vazeille-Falcoz et al. 1999; Huber et al. 2008; Paupy et al. 2008; Sylla et al. 2009; Paupy et al. 2010; Brown et al. 2011). Some of these populations may have arisen from hybridization with domestic Ae. ae. aegypti mosquitoes (Miller et al. 1989; Tabachnick 1991; Brown et al. 2011), but others are likely pure Ae. ae. formosus mosquitoes that have opportunistically moved into human-altered habitats across the African landscape. There are also isolated populations of the subspecies Ae. ae. aegypti that exist sympatrically with Ae. ae. formosus in certain locations on the East African coast (e.g. Rabai, Kenya) with little or no hybridization between forms (Tabachnick et al. 1979; Brown et al. 2011). These mosquitoes may represent a unique genetic and ecological variant of the subspecies that breeds exclusively indoors, as compared to typically outdoor-breeding pantropical Ae. ae. aegypti populations.
Due to its role as an invasive species and globally important disease vector, it is critical to understand the patterns by which Ae. aegypti populations have associated with humans, and to assess how humans have moved populations of the species around the world through time. To gain a historical perspective of Ae. aegypti evolution, we sequenced four nuclear markers in mosquitoes from 21 localities worldwide and used phylogenetic methods to explore the hypothesis of African ancestry in the species. We expect that the hypothesized isolation event and accompanying selection leading to the domestic subspecies, Ae. ae. aegypti would have resulted in a population bottleneck, detectable with our genetic markers. Additionally, a panel of 1504 SNP markers was developed using a RAD (restriction site associated DNA) sequencing approach (Baird et al. 2008) to assess fine-scale structure among diverse, global Ae. aegypti populations to better understand the role of humans as a disperser of the species on a more recent time scale.
MATERIALS AND METHODS
Collections
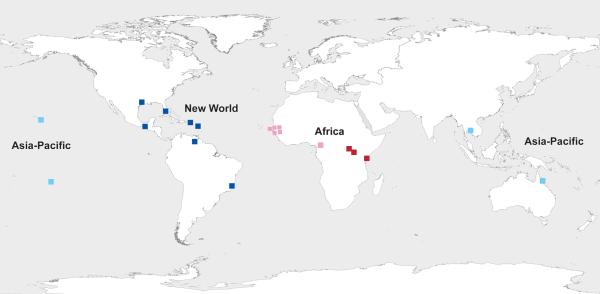
Mosquitoes for this study were collected from 21 localities representing 13 countries worldwide (Fig. 1, Table 1). All collections were performed according to previously published methodology (Brown et al. 2011). Whole genomic DNA was extracted individually from Ae. aegypti mosquitoes (Table 1) and outgroup species, Ae. mascarensis and Ae. bromeliae.
Figure 1. Map of worldwide collection sites of Ae. aegypti populations used in this study.
Sites are color-coded by region: Asia-Pacific sites are shown in light blue, New World sites in dark blue, West/Central African sites in pink, and East African sites in red.
Table 1.
Population information, including: collection site, global region, number of individual mosquitoes (N) analyzed with sequenced nuclear loci (nuDNA) and SNPs, number of generations in the lab, and year collected. % Missing data across all SNP loci for each population is shown in the final column.
| Collection Site | Region | N (nuDNA/ SNPs) |
Gen. in Lab |
Year collected |
% Missing |
|---|---|---|---|---|---|
| Kichwamba, Uganda* | E. Africa | 10/8 | 0 | 2009 | 25.9% |
| Bundibugyo, Uganda* | E. Africa | 10/8 | 0 | 2009 | 23.2% |
| Rabai, Kenya (formosus)* | E. Africa | 10/8 | 0 | 2009 | 26.4% |
| Yaounde, Cameroon* | C. Africa | 10/8 | 0 | 2009 | 23.2% |
| Dakar, Senegal* | W. Africa | 10/- | 0 | 2005 | - |
| Goudiry, Senegal* | W. Africa | 10/- | 0 | 2007 | - |
| Ngoye, Senegal* | W. Africa | 10/- | 0 | 2007 | - |
| PK-10, Senegal* | W. Africa | 10/- | 0 | 2006 | - |
| Bijagos, Guinea-Bissau* | W. Africa | 10/8 | 0 | 2009 | 26.5% |
| Bolivar, Venezuela* | New World | 10/- | 2 | 2004 | - |
| Cachoeiro de Itapemirim, Brazil |
New World | 10/8 | 1 | 2010 | 9.3% |
| Dominica* | New World | 10/6 | 0 | 2009 | 8.7% |
| Patillas, Puerto Rico** | New World | 10/8 | 0 | 2010 | 27.7% |
| Vaca Key, FL, USA* | New World | 10/8 | 0 | 2009 | 10.4% |
| Houston, TX, USA* | New World | 10/8 | 0 | 2009 | 12.1% |
| Pijijiapan, Mexico* | New World | 10/8 | 1 | 2008 | 11.5% |
| Hawaii, USA | Asia/Pacific | 10/8 | 0 | 2010 | 12.9% |
| Tahiti, French Polynesia* | Asia/Pacific | 10/8 | 1 | 2010 | 10.8% |
| Cairns, Australia* | Asia/Pacific | 10/6 | 0 | 2009 | 15.4% |
| Rayong, Thailand* | Asia/Pacific | 10/6 | 2 | 2009 | 16.4% |
| Rabai, Kenya (aegypti)* | E. Africa | 10/8 | 0 | 2009 | 13.5% |
| All Sites | - | 210/129 | - | - | - |
|
| |||||
| Outgroup Species | Country |
N (nuDNA/
SNPs) |
Gen. in
Lab |
Year
collected |
%
Missing |
| Ae. mascarensis | Mauritius | 4/7 | 0 | 2011 | 59.7% |
| Ae. bromeliae | Kenya | 4/- | 0 | 2009 | - |
Among those analyzed with microsatellite markers in Brown et al. 2011
Analyzed with microsatellites in Somers et al. 2011
Sequenced nuclear loci
Primers were developed to amplify and sequence variable sections of four nuclear genes: apoLp-2 paralogue (Apolipophorin 2), SDR (Short-chain dehydrogenase/reductase), CYP9J2 (Cytochrome P450), and DVRF1 (dengue virus restriction factor) (Table S1, see Online Supporting Information). In total, 210 Ae. aegypti mosquitoes, representing 10 individuals from each of 21 Ae. aegypti populations (Fig. 1, Table 1) were amplified and sequenced at these four loci. Alleles were phased with TA-cloning and resequencing. The same methods were used with minor modifications (see Online Supporting Information) on four individuals each from the outgroup species Ae. mascarensis and Ae. bromeliae. The locus DVRF1 failed to amplify in outgroup individuals with the exception of two Ae. mascarensis specimens where sequences matched identically to Ae. ae. aegypti sequences, likely a result of interspecific introgression.
Restriction-site associated DNA (RAD)
Additionally, DNA from eight mosquitoes each from a subset of 16 Ae. aegypti populations worldwide was submitted to Floragenex (Eugene, OR, USA) for sequenced restriction site associated DNA (RAD) library preparation, along with 8 individuals of Ae. mascarensis (Table 1). Individually-barcoded RAD libraries were created following published methods (Baird et al. 2008; Emerson et al. 2010; Hohenlohe et al. 2010; Chutimanitsakun et al. 2011). Illumina sequencing adaptors and individual barcodes were ligated to Sbf1-digested total genomic DNA. The libraries were sequenced on the Illumina Genome Analyzer IIx (1 lane, 40 individuals) or HiSeq 2000 (2 lanes, 48 individuals each) platform with 1 × 75 bp chemistry at the Yale Center for Genome Analysis, New Haven, CT, USA. The sequencing produced 65.4 million and 218 million short reads from one lane GAIIx and 2 lanes HiSeq 2000, respectively.
Selection of SNPs from RAD data
Sequence data were filtered and demultiplexed using the process_radtags utility included in Stacks v 0.994 (Catchen et al. 2011), which also discarded low quality reads and rescued reads where the barcode or cut site were one bp different from what was expected. Sequences for each individual were aligned to the Ae. aegypti reference genome (aaegypti.SUPERCONTIGS-Liverpool.AaegL1) on Vectorbase using bwa 0.5.9 (Li and Durbin 2009), allowing for no more than 5 differences across the whole ~70 bp read and no more than 3 differences in 20 base windows of each other while being aligned against the reference (options aln -n 5 -k 3 -l 20). These individual-level alignments were then pooled into population level alignments for use as pseudo-parents for input into Stacks. The reference-aligned version of the pipeline was run using the ref_map.pl script with the expectation of up to 5 differences between individuals and reference. Stacks then compared reads grouped into stacks across populations and assigned SNP calls to stacks of reads that differed across individuals (Catchen et al. 2011). This information was deposited into a database for downstream curation and filtering.
The stacks pipeline identified 184,178 unique tags, 142,448 of which were identical to the reference, i.e. were not polymorphic. Of the 41,730 polymorphic tags, 10,503 had exactly 1 SNP called. From those, we filtered for tags that were found in at least half of the individuals in 16 or 17 populations and only allowed for biallelic SNPs. Seven individuals with poor sequencing success were removed from the dataset, as reflected in Table 1. The final working dataset consisted of 1504 tags, from which we used the 1504 associated SNPs in all further analyses of the 129 included individuals (Table 1). Across the 1504 SNPs, total levels of missing data were quantified for each mosquito population (Excoffier and Lischer 2010). Levels of missing data per population varied from 8.7% to 27.7% within Ae. aegypti (Table 1). A full 59.7% of SNPs had no calls across individuals in the Ae. mascarensis population.
Population structure and evolutionary history
In order to make rooted historical inferences regarding the origins of global Ae. aegypti populations, individual gene trees were created for apoLp-2 paralogue, CYP9J2, SDR, and DVRF1. Sequences were aligned using a slow DNA alignment with default parameters in ClustalW2 (Larkin et al. 2007; Goujon et al. 2010), and were checked by eye in MacClade 4 (Maddison and Maddison 2005). For gene trees of each locus, identical sequences were first collapsed to unique haplotypes using the program ALTER (Glez-Pena et al. 2010). These haplotype sequences were archived to GenBank with accession numbers KF360534-KF360809. The best model of nucleotide evolution was chosen using the Akaike information criterion (AIC) as implemented in the program jModelTest 0.1.1 (Posada 2008). The models of nucleotide evolution selected for the four sequenced loci were TVM+I+G (apoLp2 paralogue), HKY+I+G (DVRF1 and SDR), and SYM+I+G (CYP9J2). Phylogenetic trees were created using the maximum-likelihood approach implemented in the program Garli 2.0 (http://garli.googlecode.com). For each locus, the best tree was selected from 10 search replicates, and node support was assessed using 1000 bootstrap replicates.
The program *BEAST v. 1.5.3 (Heled and Drummond 2010) was used to create a species tree for the 21 Ae. aegypti populations using phased sequence data from each of three loci (apoLp-2 paralogue, CYP9J2, and SDR). MCMC analyses were run for a total of 200 million generations (sampling once every 1000 steps and excluding the first 20% as burn-in), and the analysis was repeated three times. Aedes mascarensis individuals were ultimately not included in the species tree due to problems with the long branch leading to the outgroup. However, the root was inferred from the individual gene trees. DVRF1 sequences were not included in the species tree primarily because there were no outgroup sequences for this locus, and we did not feel comfortable placing a root with DVRF1 included. Additionally, since DVRF1 is a gene known to affect dengue virus replication in Ae. aegypti (Souza-Neto et al. 2009, see Online Supporting Information), the locus may be subject to selection pressures that we have not yet explored in depth, and which could bias the species tree. For each of the four sequenced nuclear loci, we used the program DnaSP v5 (Librado and Rozas 2009) to calculate nucleotide diversity (π) and haplotype diversity (Hd) of Ae. aegypti populations by geographical region.
Detailed relationships between 16 Ae. aegypti populations and one population of Ae. mascarensis were also assessed using the 1504 RAD-associated SNPs. Values of FST were calculated between each pair of populations in GENEPOP 4.1 (Rousset 2008). Cavalli-Sforza and Edwards’s chord distance was calculated between populations in the GENDIST module of PHYLIP 3.69 and used to create a population-level neighbor-joining network in MEGA5 (Tamura et al. 2011). Node confidence was inferred with 1000 bootstrap replicates in PHYLIP 3.69 (modules SEQBOOT, GENDIST, NEIGHBOR, and CONSENSE). Aedes mascarensis individuals were not included in this bootstrapped neighbor-joining network due to the high levels of missing data in the species, which would have prevented a large portion of the SNPs from being included in the analysis in PHYLIP 3.69. Individual level clustering was assessed using a neighbor-joining tree and a principal components analysis (PCA) using R v12.13.1 (R Development Core Team 2008) and the adegenet package v1.3-1 (Jombart 2008; Jombart and Ahmed 2011). All missing data in the individual level analyses were replaced by population means.
We also evaluated patterns of population structure using the Bayesian clustering method implemented in the software program STRUCTURE v2.3.3 (Pritchard et al. 2000; Falush et al. 2003). STRUCTURE was run on all Ae. aegypti and Ae. mascarensis individuals, and no a priori information regarding sampling locations was used. The most likely number of clusters was determined following the guidelines of Pritchard et al. (Pritchard et al. 2000) and by calculating ΔK, which is based on the second order rate of change of the likelihood distribution between values of K (Evanno et al. 2005). STRUCTURE results were visualized using the program DISTRUCT (Rosenberg 2004). To determine the most likely number of clusters (K), we conducted five independent runs for each K = 1-18. For all runs, we assumed an admixture model and correlated allele frequencies and used a burn-in value of 20,000 iterations followed by 50,000 replications.
For several of our analyses, comparisons were made between major genetic groups detected here, and in previous microsatellite analyses of the species (Brown et al. 2011). The two major genetic groups, African and pantropical, correspond to the subspecies Ae. ae. formosus and Ae. ae. aegypti, respectively, and follow the same geographic boundaries. As such, in this manuscript, the pantropical form refers to all Ae. aegypti populations outside of Africa, as well as isolated populations on the East Coast of Africa (i.e. certain mosquitoes from Rabai, Kenya). All other Ae. aegypti populations from Africa included in this study represent the African genetic form.
RESULTS
Genetic diversity
Measures of genetic diversity for all sequenced nuclear loci were higher across African Ae. ae. formosus populations than across pantropical domestic mosquitoes (Table 2). East African populations were the most diverse group by both measures for three of the four loci, with West/Central African populations harboring an intermediate level of diversity, on average (Table 2). Within pantropical Ae. ae. aegypti populations, 15 of 16 values of genetic diversity were lower for Asia-Pacific populations than New World populations (Table 2).
Table 2.
Average values of nucleotide diversity (π) and haplotype diversity (Hd) across populations in selected regions for each of four nuclear loci.
| Group/ Region |
apoLp-2 paralogue |
CYP9J2 | SDR | DVRF1 | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Hd | π | Hd | π | Hd | π | Hd | π | |
| Africa | 0.7152 | 0.0086 | 0.7908 | 0.0224 | 0.7638 | 0.0060 | 0.8871 | 0.0193 |
| East | 0.9493 | 0.0129 | 0.9213 | 0.0306 | 0.7263 | 0.0050 | 0.9613 | 0.0220 |
| West/Central | 0.5982 | 0.0065 | 0.7255 | 0.0183 | 0.7825 | 0.0065 | 0.8500 | 0.0179 |
|
| ||||||||
| Pantropical | 0.5785 | 0.0031 | 0.4938 | 0.0178 | 0.5180 | 0.0032 | 0.4685 | 0.0134 |
| New World | 0.6406 | 0.0038 | 0.5150 | 0.0190 | 0.5700 | 0.0039 | 0.4949 | 0.0123 |
| Asia-Pacific | 0.4843 | 0.0021 | 0.4158 | 0.0130 | 0.4343 | 0.0019 | 0.3750 | 0.0127 |
Population genetic structure
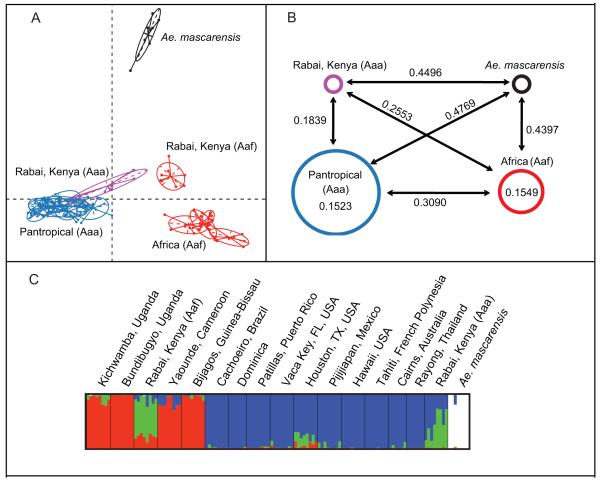
Both the *BEAST species tree based on nuclear genes and multiple cluster analyses of 1504 SNPs clearly separated the two subspecies of Ae. aegypti: Ae. ae. formosus and Ae. ae. aegypti (Fig. 2, Fig. 3). One genetic cluster (African) corresponds to Ae. ae. formosus and is only found in Africa. The other corresponds to Ae. ae. aegypti (pantropical) and represents all populations from outside Africa, as well as an isolated group of mosquitoes from Rabai, Kenya. As seen in microsatellite analyses (Brown et al. 2011), the Ae. ae. aegypti population from Rabai, Kenya (coastal East Africa) formed a somewhat distinct genetic cluster, as did the Ae. ae. formosus population from the same location (Fig. 2a). Additionally, the broad-scale individual-based PCA distinguished Ae. mascarensis outgroup individuals from all Ae. aegypti individuals (Fig. 2a).
Figure 2. Broad-scale analyses of SNP data for Ae. aegypti and Ae. mascarensis populations.
A) Individual-based PCA with major genetic groups denoted. African (i.e. Aaf) populations appear in red, while pantropical (i.e. Aaa) mosquitoes appear in blue. The Rabai Aaa population is shown in purple. Ae. mascarensis individuals are shown in black. B) Average pairwise FST values between and within major genetic groups of Ae. aegypti and Ae. mascarensis. Size of circles is proportional to number of populations sampled. C) STRUCTURE plot, K=4. Each vertical bar represents the probability of assignment of a single individual to each genetic cluster. Aaa=Ae. ae. aegypti, Aaf = Ae. ae. formosus. Of the four genetic groups identified by STRUCTURE, red corresponds roughly to African Aaf, blue to pantropical Aaa (including Rabai), and green appears to be a unique Rabai, Kenya geographic signal. The white cluster corresponds to outgroup Ae. mascarensis individuals.
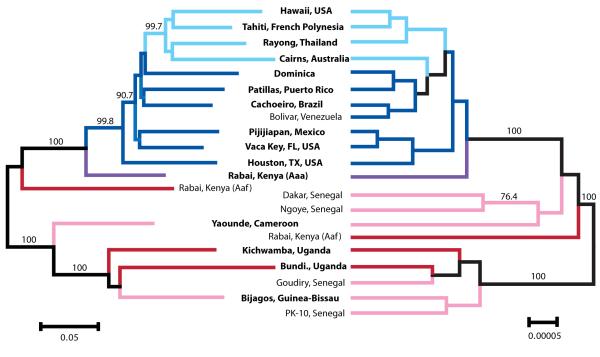
Figure 3. Evolutionary history of Ae. aegypti from SNPs and sequenced nuclear genes.
Bootstrapped neighbor-joining network based on population pairwise chord-distances from 1504 SNPs (left). Species (i.e. population) tree based on phased apoLp-2 paralogue, CYP9J2, and SDR sequences (right). Node support over 75% is shown on relevant branches. East African populations are shaded in red, West and Central African populations in pink, the Rabai (Ae. ae. aegypti) population in purple, New World populations in dark blue, and Asia-Pacific populations are shown in light blue. Rooting was inferred from nuclear gene trees (Fig. 4). Population labels in bold font refer to branches on both sides (i.e. to both the neighbor-joining network and the species tree), while labels in regular font refer only to the nearest branch.
Average FST values between groups reflected similar patterns to the PCA results (Fig. 2b). Pairwise FST values between Ae. aegypti populations were high overall, ranging from 0.0873 to 0.3599. The average pairwise FST between pantropical Ae. ae. aegypti populations was 0.1523, and that among African Ae. ae. formosus populations was a similar value of 0.1549 (Fig. 2b). However, the average population-pairwise FST between the two groups (subspecies) was much higher at 0.3090. The outgroup species, Ae. mascarensis, was clearly divergent from Ae. aegypti populations, falling slightly closer to the African group than to pantropical populations (Fig. 2b). The Bayesian clustering algorithms implemented in STRUCTURE (Pritchard et al. 2000; Falush et al. 2003) revealed similar broad patterns as detected using PCA and FST values (Fig. 2c).
Phylogeography and Phylogenetics
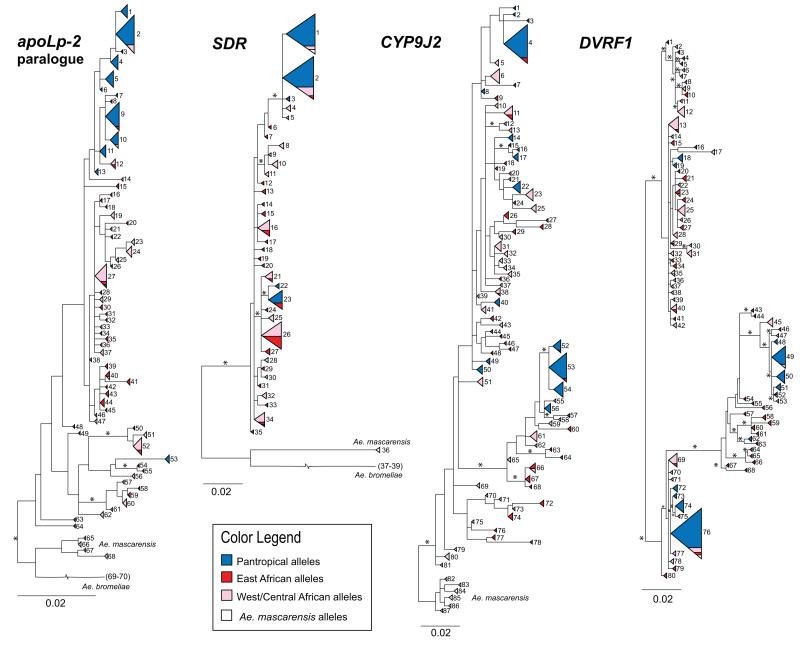
All three rooted nuclear gene trees (apoLp2 paralogue, CYP9J2, SDR) supported African ancestry in Ae. aegypti (Fig. 4, Tables S2-S4). Although structure was observed within Africa in all four loci, there was no clear geographic pattern of alleles within Africa in any of the gene trees (Fig. 4). Nearly all of the pantropical alleles formed a monophyletic group in the apoLp-2 paralogue and SDR gene trees, (Fig.4). In both the apoLp2 paralogue and SDR trees, common pantropical haplotypes were found in some African populations, mainly Ngoye and Dakar, Senegal, with a handful of alleles from Yaounde, Cameroon, and Rabai, Kenya (Fig. 4, Tables S2, S3).
Figure 4.
Gene trees for apoLp-2 paralogue, SDR, CYP9J2, and DVRF1 (from left to right). Each triangle represents a single, unique haplotype, and the area of the triangle is proportional to the number of alleles with that sequence. Alleles from East Africa are colored red, West/Central African alleles are shown in pink, and pantropical alleles are colored blue. Alleles from Ae. mascarensis individuals are shown in white. Numbers next to each triangle/haplotype represent the haplotype number as denoted on the keys (Tables S2-S5). Nodes with bootstrap support over 60% are indicated with an asterisk. The broken line leading to Ae. bromeliae samples indicates long branch lengths. The distance to Ae. bromeliae is approximately 0.11 average substitutions per site to the tips for the apoLp-2 paralogue and 0.12 substitutions per site for SDR. Aedes bromeliae haplotypes were pruned from the CYP9J2 gene tree due to long branch lengths and unclear placement. The DVRF1 gene tree is not rooted by outgroup sequences.
Despite relatively high levels of variation in CYP9J2 across Ae. aegypti populations, the subspecies relationships remained poorly resolved in this gene tree (Fig. 4). However, interestingly, all CYP9J2 alleles from Ae. ae. aegypti individuals from Rabai, Kenya represented two unique haplotypes, numbers 49 and 52 (Table S4), that were not found in any other Ae. aegypti populations, with an exception of a single allele matching the Ae. ae. formosus population in the same location (Rabai, Kenya). Though the subspecies split was also largely unsupported using DVRF1, it is of note that at least 1 major clade in the tree was essentially missing pantropical alleles (Fig. 4, Table S5). Overall, many nodes within all gene trees remained poorly resolved.
Though Ae. mascarensis and Ae. bromeliae served as useful outgroups for apoLp-2 paralogue, CYP9J2, and SDR, a few SDR and DVRF1 alleles from Ae. mascarensis samples matched identically to alleles found in pantropical Ae. aegypti individuals (Fig. 4, Tables S3, S5), indicating some level of interspecific hybridization between Ae. mascarensis and Ae. ae. aegypti. DVRF1 could not be amplified from most outgroup samples. Each of the four gene trees clearly depicted the large amount of variation across Ae. ae. formosus in Africa, as compared to the smaller number of haplotypes found in pantropical (Ae. ae. aegypti) populations worldwide (Fig. 4).
As in the gene trees, no clear evolutionary genetic patterns were detectable among African populations in the species tree that was created with information from three nuclear genes: apoLp2 paralogue, CYP9J2, and SDR (Fig. 3). However, patterns and structure were consistently detected among pantropical Ae. ae. aegypti populations. The species tree and a neighbor-joining analysis of the RAD SNPs detected distinct New World and Asia-Pacific clusters, with the well-supported Asia-Pacific group appearing the most distant (derived) from ancestral African populations (Fig. 3). Furthermore, an individual-based neighbor-joining network provided a near complete discrimination of pantropical populations from one another, with 96.3% of pantropical individuals clustering uniquely with other individuals from their population of origin (Figure S1).
DISCUSSION
Subspeciation in Aedes aegypti
Our results support the hypothesis that Ae. aegypti originated in Africa as a generalist mosquito similar to modern Ae. ae. formosus, from which a specialized domestic form (Ae. ae. aegypti) evolved through a single subspeciation event. All three rooted nuclear gene trees (Fig. 4, Tables S2-S4) indicated African ancestry in Ae. aegypti, and a genetic bottleneck was apparent in the domestic pantropical form, Ae. ae. aegypti (Table 2, Fig. 4). Broad-scale cluster analyses cleanly separated the subspecies of Ae. aegypti (i.e. the African and pantropical genetic forms), and support the position of Ae. mascarensis as a close but distinct outgroup (Fig. 2). Together, these data are consistent with the historical hypothesis that the subspecies Ae. ae. aegypti originated from a population of ancestral Ae. aegypti that became isolated in North Africa during the start of a drying period in the region (Petersen 1977; Tabachnick 1991). However, the data are equally congruent with numerous other scenarios within Africa, and the exact origin of Ae. ae. aegypti on the continent remains unresolved.
An interesting finding here (Fig. 2, 3) and in previous studies (Brown et al. 2011) is that genetically pure populations of Ae. ae. aegypti are largely absent from Africa despite the well-supported origin of the subspecies on that continent. One possible part of the explanation lies in populations of indoor-dwelling (i.e. true domestic) Ae. ae. aegypti mosquitoes that used to be common throughout the arid regions of North Africa, the Middle East, and the Mediterranean until the mid-20th century (Holstein 1967). Today, it is nearly impossible to find any such populations of Ae. ae. aegypti due to the advent of modern plumbing and sanitation, and the resulting lack of indoor water storage (Holstein 1967). These indoor populations may have been very ecologically similar to the Ae. ae. aegypti ancestors in North Africa (or elsewhere) described in the scenario above. Though these populations have gone extinct throughout most of their native, albeit artificial, habitat, the Ae. ae. aegypti in Rabai, Kenya may represent a rare, relic population.
Uniquely, the Rabai Ae. ae. aegypti mosquitoes were found only indoors, despite ample breeding sites outside of homes (Brown et al. 2011). In contrast, Ae. ae. aegypti populations throughout the rest of the world typically exhibit peridomestic behavior. Though genetically pantropical, as designated by previous microsatellite analyses (Brown et al. 2011), the Ae. ae. aegypti population from Rabai, Kenya (coastal East Africa) remained noticeably different from other groups (Fig. 2). This population also harbored private haplotypes in the CYP9J2 nuclear gene (Fig. 4, Table S4), indicating a unique evolutionary history. Because the vast majority of sequenced haplotypes from Rabai, Kenya were identical to those from other pantropical populations (Fig. 4, Tables S2-S3, S5), we hypothesize that Ae. ae. aegypti were introduced to coastal East Africa, including Rabai, after the subspeciation event (Tabachnick 1991). However, without other arid, indoor-only populations to compare to, we can only make informed speculation.
Due to the exact match of Rabai, Kenya Ae. ae. aegypti haplotypes to those from other pantropical populations for apoLp2, SDR, and DVRF1, it should be noted that the apparent basal position of the Ae. ae. aegypti population from Rabai, Kenya to the rest of Ae. ae. aegypti (Fig. 3) may be attributable to low levels of introgression over time from the sympatric Ae. ae. formosus population (Fig. 4, Tables S2-S5), rather than to true evolutionary history. In addition, the broad-scale analyses of the SNPs (Figure 2) show that the Ae. ae. formosus population in Rabai also clusters distinctly from, though close to, other African populations. It is likely that, as noted above, low levels of introgression in the region may have contributed to the unique SNP-based clustering patterns of both populations.
Another part of the explanation for a lack of pure Ae. ae. aegypti through most of Africa may be related to the ease with which the subspecies seem to interbreed in West and Central Africa. Though the two subspecies appear distinct based on multiple analyses of many types of genetic markers, nuclear sequences revealed recent introgression between Ae. ae. formosus and Ae. ae. aegypti in a few (mostly urban) West and Central African locations (Fig. 4, Tables S2-S5). Evidence for mixing of the subspecies has also been shown using microsatellite data (Brown et al. 2011), and hybridization between subspecies is further supported by morphological and behavioral evidence in the region (Miller et al. 1989; Tabachnick 1991). Since Ae. ae. aegypti populations harbor less genetic diversity than Ae. ae. formosus, this phenomenon also provides an explanation for lower values of genetic diversity within West and Central African as compared to East Africa (Table 2). This hybridization of forms, combined with the existence of rare and/or extinct indoor populations, may help explain the apparent paradox of Ae. ae. aegypti moving to the New World from a region with few, if any, remaining populations of pure Ae. ae. aegypti mosquitoes. Historical Aedes ae. aegypti populations in West and Central Africa may have been absorbed into Ae. ae. formosus populations and or may have gone extinct.
The clear evidence of hybridization between subspecies in West Africa is very different from the situation in certain East African coastal locations like Rabai where the subspecies remain distinct, sympatric forms with low levels of introgression (Brown et al. 2011, Fig. 2, Fig. 4, Tables S2-5). Since the two subspecies, including the sympatric forms from Rabai, are fully capable of interbreeding in the laboratory (Moore 1979), the underlying reasons for this disparity remain unknown. Future molecular and ecological studies of mating behavior and niche competition in and between subspecies in East and West/Central Africa (and outside Africa) will be critical to teasing apart this complex evolutionary situation. Additional genetic and behavioral studies may also help address the evolution of the incredibly complicated range and breadth of domestication-related traits seen in populations of both subspecies of Ae. aegypti.
Pantropical invasions and the spread of Aedes aegypti aegypti
Strong among-population relationships (Fig. 3) support the historical hypothesis that humans first moved Ae. aegypti out of Africa into the New World with the slave trade. Within pantropical Ae. ae. aegypti, our results clearly support a secondary invasion of Ae. ae. aegypti from the New World to the Asia-Pacific region, perhaps as recently as the 20th century (Smith 1956; Tabachnick 1991). New World populations cluster distinctly from a Southeast Asia-Pacific group, and the Southeast Asia-Pacific cluster is the most distant from ancestral African populations, suggesting a more recent invasion of the region (Smith 1956; Tabachnick 1991). This pattern appears in both the species tree and the SNP-based neighbor-joining network, though is better supported by the latter (Fig. 3). Genetic diversity measures also support a more recent founder event in the Southeast Asia-Pacific group, as nearly all values were lower for Asia-Pacific populations than New World populations (Table 2).
Population discrimination and gene flow
Though some population signal can be seen within Africa in an individual-based neighbor-joining network (Figure S1), there is very little geographic clustering and little historical information within the continent. This pattern has now been detected using SNPs, sequenced nuclear loci, and microsatellites (Brown et al. 2011). However, there is obvious geographical structuring in the rest of the world. In addition to distinct New World and Asia-Pacific clusters, the individual-based neighbor-joining network provided a near complete discrimination of pantropical populations from one another, with 96.3% of pantropical individuals clustering uniquely with other individuals from their population of origin (Figure S1).
All genetic evidence here and in our previous study (Brown et. al 2011) suggests that Ae. ae. formosus individuals in Africa have remained relatively panmictic over the course of the species history. Up until the past few decades, Ae. ae. formosus populations bred primarily in natural environments (e.g. tree holes) (Mattingly 1957; McClelland 1974), and we hypothesize that members of this subspecies would have been forced to move further to find suitable breeding locations during the dry season, as well as seek out hosts since they did not have the luxury of constant access to humans in a domestic habitat. There is evidence that a lack of local, suitable oviposition sites can lead to increased dispersal in Ae. aegypti (Reiter et al. 1995; Honorio et al. 2003; Maciel-de-Freitas and Lourenco-de-Oliveira 2009). This increased movement would have led to higher levels of gene flow between locations, and may explain the low population genetic signal within this subspecies.
In contrast, recent but clear geographic structure can be observed across pantropical Ae. ae. aegypti populations using allele frequency and genotype-based analyses with both SNPs and microsatellites (Brown et al. 2011). This structure may be explained by recent founder events out of Africa and across the tropics and subtropics, as well the discontinuous nature of domestic habitats available to this highly human-adapted subspecies. Pantropical Ae. ae. aegypti mosquitoes tend to stay within approximately 10 to 500 m flight distance during their entire lifetimes (McDonald 1977; Trpis and Hausermann 1986; Reiter et al. 1995; Harrington et al. 2005; Maciel-de-Freitas and Lourenco-de-Oliveira 2009) when stable breeding sites and host blood are readily available, as would be likely for many domestic populations. With little dispersal or gene flow, genetic structure could be formed and maintained even on a small geographic scale.
Conclusions
We used genetic markers to show that anthropogenic forces have profoundly shaped the evolutionary history and distribution of Ae. aegypti across time and space. Human domestic habitats provided the ecological basis for the subspeciation of the highly invasive and epidemiologically important subspecies, Ae. ae. aegypti, from ancestral Ae. ae. formosus. Soon after, human trade and movement facilitated the invasion of Ae. aegypti across the tropical and subtropical world. The history of Ae. aegypti represents just one example of how anthropogenic impacts can shape the evolutionary path of other species, and influence patterns of genetic diversity.
Supplementary Material
SUPPORTING INFORMATION Figure S1. Individual-level neighbor-joining network based on 1504 SNPs.
ACKNOWLEDGMENTS
We would like to thank Greg Lanzaro, Durland Fish, Charles Jeannin, Tuterarii Paoaafaite, Henri Frogier, Barry J. Beaty, Gustavo Ponce García, Adriana E. Flores, Larry Hribar, Rosemary Sang, Barry Miller, and John-Paul Mutebi, Jennifer Simpson, Carolyn S. McBride, Petrina Johnson, Scott Ritchie, Christophe Paupy, Hervé Bossin, Joel Lutomiah, Ildefonso Fernandez-Salas, Alongkot Ponlawat, Anthony J. Cornel, William C. Black, IV, Norma Gorrochotegui-Escalante, Ludmel Urdaneta-Marquez, Massamba Sylla, Michel Slotman, Kristy O. Murray, Christopher Walker, Gerard Somers, Roberto Barrera, Denise Valle, Alexandre Peixoto, and Dina Fonseca for their help in providing mosquito samples, and Beckie Symula for her help with statistical and phylogenetic analyses. Data analysis was supported by the Yale University Biomedical High Performance Computing Center. JEB was supported by NIH Pre-doctoral Training in Genetics (T32-GM07499-33) and the Yale Institute for Biospheric Studies (YIBS), Center for Field Ecology pilot grant. The collection in Brazil was supported by the Programa Nacional de Controle da Dengue (PNCD). The rest of the study was supported by NIH R01 AI046018 (JRP) and NSF Doctoral Dissertation Improvement Grant DEB-1011449 (JEB, JRP).
Footnotes
The authors have no conflicts of interest to declare.
Sequence data have been archived to GenBank with accession numbers KF360534-KF360809.
LITERATURE CITED
- Ayala FJ, Coluzzi M. Chromosome speciation: Humans, Drosophila, and mosquitoes. Proc. Natl. Acad. Sci. USA. 2005;102:6535–6542. doi: 10.1073/pnas.0501847102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird NA, Etter PD, Atwood TS, Currey MC, Shiver AL, Lewis ZA, Selker EU, Cresko WA, Johnson EA. Rapid SNP Discovery and Genetic Mapping Using Sequenced RAD Markers. PLoS One. 2008;3:e3376. doi: 10.1371/journal.pone.0003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JE, McBride CS, Johnson P, Ritchie S, Paupy C, Bossin H, Lutomiah J, Fernandez-Salas I, Ponlawat A, Cornel AJ, Black WC, Gorrochotegui-Escalante N, Urdaneta-Marquez L, Sylla M, Slotman M, Murray KO, Walker C, Powell JR. Worldwide patterns of genetic differentiation imply multiple ‘domestications’ of Aedes aegypti, a major vector of human diseases. Proc. R. Soc. Lond. B. 2011;278:2446–2454. doi: 10.1098/rspb.2010.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catchen JM, Amores A, Hohenlohe P, Cresko W, Postlethwait JH. Stacks: Building and Genotyping Loci De Novo From Short-Read Sequences. G3: Genes, Genomes, Genetics. 2011;1:171–182. doi: 10.1534/g3.111.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin FS, Zavaleta ES, Eviner VT, Naylor RL, Vitousek PM, Reynolds HL, Hooper DU, Lavorel S, Sala OE, Hobbie SE, Mack MC, Diaz S. Consequences of changing biodiversity. Nature. 2000;405:234–242. doi: 10.1038/35012241. [DOI] [PubMed] [Google Scholar]
- Christophers RC. The Yellow Fever Mosquito: Its Life History, Bionomics, and Structure. Cambridge Univ. Press; Cambridge, U. K.: 1960. Aedes aegypti. [Google Scholar]
- Chutimanitsakun Y, Nipper RW, Cuesta-Marcos A, Cistue L, Corey A, Filichkina T, Johnson EA, Hayes PM. Construction and application for QTL analysis of a Restriction Site Associated DNA (RAD) linkage map in barley. BMC Genomics. 2011;12:4. doi: 10.1186/1471-2164-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Mohanty S, Stephan W. Inferring the population structure and demography of Drosophila ananassae from multilocus data. Genetics. 2004;168:1975–1985. doi: 10.1534/genetics.104.031567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson KJ, Merz CR, Catchen JM, Hohenlohe PA, Cresko WA, Bradshaw WE, Holzapfel CM. Resolving postglacial phylogeography using high-throughput sequencing. Proc. Natl. Acad. Sci. USA. 2010;107:16196–16200. doi: 10.1073/pnas.1006538107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol. Ecol. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- Evans KL, Gaston KJ, Frantz AC, Simeoni M, Sharp SP, McGowan A, Dawson DA, Walasz K, Partecke J, Burke T, Hatchwell BJ. Independent colonization of multiple urban centres by a formerly forest specialist bird species. Proc. R. Soc. Lond. B. 2009;276:2403–2410. doi: 10.1098/rspb.2008.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excoffier L, Lischer HEL. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resources. 2010;10:564–567. doi: 10.1111/j.1755-0998.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: Linked loci and correlated allele frequencies. Genetics. 2003;164:1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glez-Pena D, Gomez-Blanco D, Reboiro-Jato M, Fdez-Riverola F, Posada D. ALTER: program-oriented conversion of DNA and protein alignments. Nucleic Acids Res. 2010;38:W14–W18. doi: 10.1093/nar/gkq321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goujon M, McWilliam H, Li WZ, Valentin F, Squizzato S, Paern J, Lopez R. A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Res. 2010;38:W695–W699. doi: 10.1093/nar/gkq313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LC, Edman JD, Scott TW. Why do female Aedes aegypti (Diptera : Culicidae) feed preferentially and frequently on human blood? J. Med. Entomol. 2001;38:411–422. doi: 10.1603/0022-2585-38.3.411. [DOI] [PubMed] [Google Scholar]
- Harrington LC, Scott TW, Lerdthusnee K, Coleman RC, Costero A, Clark GG, Jones JJ, Kitthawee S, Kittayapong P, Sithiprasasna R, Edman JD. Dispersal of the dengue vector Aedes aegypti within and between rural communities. Am. J. Trop. Med. Hyg. 2005;72:209–220. [PubMed] [Google Scholar]
- Heled J, Drummond AJ. Bayesian Inference of Species Trees from Multilocus Data. Mol. Biol. Evol. 2010;27:570–580. doi: 10.1093/molbev/msp274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenlohe PA, Bassham S, Etter PD, Stiffler N, Johnson EA, Cresko WA. Population Genomics of Parallel Adaptation in Threespine Stickleback using Sequenced RAD Tags. PLoS Genet. 2010;6:e1000862. doi: 10.1371/journal.pgen.1000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstein M. Dynamics of Aedes aegypti distribution, density and seasonal prevalence in the Mediterranean area. Bull. Wld. Hlth. Org. 1967;36:541–543. [PMC free article] [PubMed] [Google Scholar]
- Honorio NA, Silva WD, Leite PJ, Goncalves JM, Lounibos LP, Lourenco-de-Oliveira R. Dispersal of Aedes aegypti and Aedes albopictus (Diptera : Culicidae) in an urban endemic dengue area in the State of Rio de Janeiro, Brazil. Mem. Inst. Oswaldo Cruz. 2003;98:191–198. doi: 10.1590/s0074-02762003000200005. [DOI] [PubMed] [Google Scholar]
- Huber K, Ba Y, Dia I, Mathiot C, Sall AA, Diallo M. Aedes aegypti in Senegal: Genetic diversity and genetic structure of domestic and sylvatic populations. Am. J. Trop. Med. Hyg. 2008;79:218–229. [PubMed] [Google Scholar]
- Jombart T. adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics. 2008;24:1403–1405. doi: 10.1093/bioinformatics/btn129. [DOI] [PubMed] [Google Scholar]
- Jombart T, Ahmed I. adegenet 1.3-1: new tools for the analysis of genome-wide SNP data. Bioinformatics. 2011;27:3070–3071. doi: 10.1093/bioinformatics/btr521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kark S, Iwaniuk A, Schalimtzek A, Banker E. Living in the city: can anyone become an ‘urban exploiter’? J. Biogeogr. 2007;34:638–651. [Google Scholar]
- Keller A. Drosophila melanogaster’s history as a human commensal. Curr. Biol. 2007;17:R77–R81. doi: 10.1016/j.cub.2006.12.031. [DOI] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- Lounibos LP. Invasions by insect vectors of human disease. Annu. Rev.Entomol. 2002;47:233–266. doi: 10.1146/annurev.ento.47.091201.145206. [DOI] [PubMed] [Google Scholar]
- Lyimo IN, Ferguson HM. Ecological and evolutionary determinants of host species choice in mosquito vectors. Trends Parasitol. 2009;25:189–196. doi: 10.1016/j.pt.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Maciel-de-Freitas R, Lourenco-de-Oliveira R. Presumed unconstrained dispersal of Aedes aegypti in the city of Rio de Janeiro, Brazil. Rev. Saude Publ. 2009;43:8–12. doi: 10.1590/s0034-89102009000100002. [DOI] [PubMed] [Google Scholar]
- Maddison DR, Maddison WP. MacClade 4: Analysis of phylogeny and character evolution. Version 4.08a. 2005. [DOI] [PubMed]
- Mattingly PF. Genetical aspects of the Aedes aegypti problem I. Taxonomy and bionomics. Ann. Trop. Med. Parasit. 1957;51:392–408. [PubMed] [Google Scholar]
- McClelland GAH. A worldwide survey of variation in scale pattern of the abdominal tergum of Aedes aegypti (L.) (Diptera: Culicidae) Trans. R. Entomol. Soc. Lond. 1974;126:239–259. [Google Scholar]
- McDonald PT. Population characteristics of domestic Aedes aegypti (Diptera: Culicidae) in villages on the Kenya coast. II. Dispersal within and between villages. J. Med. Entomol. 1977;14:49–53. doi: 10.1093/jmedent/14.1.49. [DOI] [PubMed] [Google Scholar]
- Miller BR, Monath TP, Tabachnick WJ, Ezike VI. Epidemic yellow fever caused by an incompetent mosquito vector. Trop. Med. Parasitol. 1989;40:396–399. [PubMed] [Google Scholar]
- Moore DF. Hybridization and mating behavior in Aedes aegypti (Diptera: Culicidae) J. Med. Entomol. 1979;16:223–226. doi: 10.1093/jmedent/16.3.223. [DOI] [PubMed] [Google Scholar]
- Nasidi A, Monath TP, Decock K, Tomori O, Cordellier R, Olaleye OD, Harry TO, Adeniyi JA, Sorungbe AO, Ajosecoker AO, Vanderlaan G, Oyediran ABO. Urban yellow fever epidemic in western Nigeria, 1987. Trans. R. Soc. Trop. Med. Hyg. 1989;83:401–406. doi: 10.1016/0035-9203(89)90518-x. [DOI] [PubMed] [Google Scholar]
- Parmakelis A, Russello MA, Caccone A, Marcondes CB, Costa J, Forattini OP, Sallum MAM, Wilkerson RC, Powell JR. Short report : Historical analysis of a near disaster: Anopheles gambiae in Brazil. Am. J. Trop. Med. Hyg. 2008;78:176–178. [PubMed] [Google Scholar]
- Partecke J, Gwinner E, Bensch S. Is urbanisation of European blackbirds (Turdus merula) associated with genetic differentiation? J. Ornithol. 2006;147:549–552. [Google Scholar]
- Paupy C, Brengues C, Kamgang B, Herve JP, Fontenille D, Simard F. Gene flow between domestic and sylvan populations of Aedes aegypti (Diptera : Culicidae) in North Cameroon. J. Med. Entomol. 2008;45:391–400. doi: 10.1603/0022-2585(2008)45[391:gfbdas]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Paupy C, Brengues C, Ndiath O, Toty C, Herve JP, Simard F. Morphological and genetic variability within Aedes aegypti in Niakhar, Senegal. Infect. Genet. Evol. 2010;10:473–480. doi: 10.1016/j.meegid.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Petersen JL. Ph.D. thesis. University of Notre Dame; Notre Dame, IN, USA: 1977. Behavior differences in two subspecies of Aedes aegypti (L.) (Diptera: Culicidae) in East Africa. [Google Scholar]
- Pimm S, Raven P, Peterson A, Sekercioglu CH, Ehrlich PR. Human impacts on the rates of recent, present, and future bird extinctions. Proc. Natl. Acad. Sci. USA. 2006;103:10941–10946. doi: 10.1073/pnas.0604181103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- Powell JR, Tabachnick WJ, Arnold J. Genetics and the origin of a vector population: Aedes aegypti, a case study. Science. 1980;208:1385–1387. doi: 10.1126/science.7375945. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2008. [Google Scholar]
- Reiter P, Amador MA, Anderson RA, Clark GG. Dispersal of Aedes aegypti in an urban area after blood feeding as demonstrated by rubidium-marked eggs. Am. J. Trop. Med. Hyg. 1995;52:177–179. doi: 10.4269/ajtmh.1995.52.177. [DOI] [PubMed] [Google Scholar]
- Rosenberg NA. DISTRUCT: a program for the graphical display of population structure. Mol. Ecol. Notes. 2004;4:137–138. [Google Scholar]
- Rousset F. GENEPOP ’ 007: a complete re-implementation of the GENEPOP software for Windows and Linux. Mol. Ecol. Resources. 2008;8:103–106. doi: 10.1111/j.1471-8286.2007.01931.x. [DOI] [PubMed] [Google Scholar]
- Schofield CJ, Diotaiuti L, Dujardin JP. The process of domestication in Triatominae. Mem. Inst. Oswaldo Cruz. 1999;94:375–378. doi: 10.1590/s0074-02761999000700073. [DOI] [PubMed] [Google Scholar]
- Smith CEG. The history of dengue in tropical Asia and its probable relationship to the mosquito Aedes aegypti. J. Trop. Med. Hyg. 1956;59:243–251. [PubMed] [Google Scholar]
- Somers G, Brown JE, Barrera R, Powell JR. Genetics and morphology of Aedes aegypti (Diptera: Culicidae) in septic tanks in Puerto Rico. J. Med. Entomol. 2011;48:1095–1102. doi: 10.1603/me11129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza-Neto JA, Sim S, Dimopoulos G. An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proc. Natl. Acad. Sci. USA. 2009;106:17841–17846. doi: 10.1073/pnas.0905006106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylla M, Bosio C, Urdaneta-Marquez L, Ndiaye M, Black WC. Gene Flow, Subspecies Composition, and Dengue Virus-2 Susceptibility among Aedes aegypti Collections in Senegal. PLoS Negl. Trop. Dis. 2009;3:e408. doi: 10.1371/journal.pntd.0000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick WJ. Evolutionary genetics and insect borne disease. The yellow fever mosquito, Aedes aegypti. Am. Entomol. 1991;37:14–24. [Google Scholar]
- Tabachnick WJ, Munstermann LE, Powell JR. Genetic distinctness of sympatric forms of Aedes aegypti in East Africa. Evolution. 1979;33:287–295. doi: 10.1111/j.1558-5646.1979.tb04682.x. [DOI] [PubMed] [Google Scholar]
- Tabachnick WJ, Powell JR. A world-wide survey of genetic variation in the yellow fever mosquito, Aedes aegypti. Genet. Res. 1979;34:215–229. doi: 10.1017/s0016672300019467. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilman D, Lehman C. Human-caused environmental change: Impacts on plant diversity and evolution. PNAS. 2001;98:5433–5440. doi: 10.1073/pnas.091093198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trpis M, Hausermann W. Dispersal and other population parameters of Aedes aegypti in an African village and their possible significance in epidemiology of vector-borne diseases. Am. J. Trop. Med. Hyg. 1986;35:1263–1279. doi: 10.4269/ajtmh.1986.35.1263. [DOI] [PubMed] [Google Scholar]
- Vazeille-Falcoz M, Failloux AB, Mousson L, Elissa N, Rodhain F. Oral receptivity of Aedes aegypti formosus from Franceville (Gabon, Central Africa) for dengue type 2 virus. Bull. Soc. Pathol. Exot. 1999;92:341–342. [PubMed] [Google Scholar]
- Wallis GP, Tabachnick WJ, Powell JR. Macrogeographic genetic variation in a human commensal: Aedes aegypti, the yellow fever mosquito. Genet. Res. 1983;41:241–258. doi: 10.1017/s0016672300021315. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Dengue and dengue hemorrhagic fever. 2009 WHO Fact Sheet No. 117. http://www.who.int/mediacentre/factsheets/fs117/en/
- Yeh PJ, Price TD. Adaptive phenotypic plasticity and the successful colonization of a novel environment. Am. Nat. 2004;164:531–542. doi: 10.1086/423825. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION Figure S1. Individual-level neighbor-joining network based on 1504 SNPs.