Abstract
Background
Prenatal alcohol exposure can disrupt central nervous system development, manifesting as behavioral deficits that include motor, emotional, and cognitive dysfunction. Both clinical and animal studies have reported binge drinking during development to be highly correlated with an increased risk of fetal alcohol spectrum disorders. We hypothesized that binge drinking may be especially damaging because it is associated with episodes of alcohol withdrawal. Specifically, we have been investigating the possibility that NMDA receptor-mediated excitotoxicity occurs during alcohol withdrawal and contributes to developmental alcohol-related neuropathology. Consistent with this hypothesis, administration of the NMDA receptor antagonists MK-801 or eliprodil during withdrawal attenuates behavioral alterations associated with early alcohol exposure. In the present study, we investigated the effects of memantine, a clinically used NMDA receptor antagonist, on minimizing ethanol-induced overactivity and spatial learning deficits.
Methods
Sprague-Dawley pups were exposed to 6.0 g/kg ethanol via intubation on postnatal day (PD) 6, a period of brain development that models late gestation in humans. Controls were intubated with a calorically matched maltose solution. During withdrawal, 24 and 36 hours after ethanol exposure, subjects were injected with a total of either 0, 20, or 30 mg/kg memantine. The subjects’ locomotor levels were recorded in open field activity monitors on PD 18–21 and on a serial spatial discrimination reversal learning task on PD 40–43.
Results
Alcohol exposure induced overactivity and impaired performance in spatial learning. Memantine administration significantly attenuated the ethanol-associated behavioral alterations in a dose-dependent manner. Thus, memantine may be neuroprotective when administered during ethanol withdrawal.
Conclusion
These data have important implications for the treatment of ethanol’s neurotoxic effects and provide further support that ethanol withdrawal significantly contributes to fetal alcohol spectrum disorders.
Keywords: fetal alcohol, treatment, NMDA, excitotoxicity, binge ethanol
Introduction
Gestational ethanol exposure can disrupt the development of the fetus, leading to a range of effects referred to as fetal alcohol spectrum disorders (FASD). Central nervous system (CNS) dysfunction is among the most serious adverse effects of prenatal alcohol exposure, and both human and animal studies have illustrated that developmental alcohol exposure induces significant damage in CNS areas including the cortex, hippocampus, basal ganglia, and cerebellum (O’Leary-Moore et al., 2011, Riley and McGee, 2005). Disruption of CNS development can lead to behavioral alterations that may include hyperactivity, motor dysfunction, and cognitive impairments including deficits in learning and memory (Riley and McGee, 2005, Schneider et al., 2011).
It has been observed that women who drink ethanol in a binge-like manner place their fetus at a higher risk for alcohol-related brain dysfunction (Rasmussen et al., 2009). Binge paradigms in animal models have similarly demonstrated increased risk of FASD (West et al., 1990). This increased risk is attributed to the higher blood ethanol concentrations achieved, but may also be associated with episodes of ethanol withdrawal following the binge exposure.
Ethanol acts at many CNS sites including several neurotransmitter receptors to potentially contribute to symptoms associated with ethanol use and withdrawal. For example, ethanol’s initial sedative and intoxicating effects are thought to be, in part, by-products of ethanol inhibiting the NMDA receptor (Costa et al., 2000, Tsai and Coyle, 1998). Upregulation of the NMDA receptor, along with an increase in the amount of glutamate released from presynaptic neurons are a neurocompensatory response to the initial NMDA receptor inhibition (Honse et al., 2003, Melendez et al., 2005). However, once ethanol leaves the body during periods of withdrawal, the compensatory response can result in an excess of calcium entering the cell, causing excitotoxic cell death (Hoffman and Tabakoff, 1994, Tsai and Coyle, 1998).
It is hypothesized that NMDA receptor-mediated excitotoxicity occurs during these withdrawal periods contributing to the ethanol-related neuropathology and behavioral deficits observed in FASD (e.g. Costa et al., 2000, Nixon et al., 2004). We specifically have demonstrated that blocking NMDA receptors with a noncompetitive antagonist such as MK-801 (Thomas et al., 1997, Thomas et al., 2002b) can attenuate some of ethanol’s adverse effects in the developing rat including deficits in reversal learning and overactivity in the open field (Thomas et al., 2002a, Thomas et al., 2001, Thomas et al., 2002b, Thomas et al., 2004). More importantly, the beneficial effects of the NMDA receptor antagonists are time-dependent (Thomas et al., 2001); MK-801 is only effective when administered during the withdrawal phase (24 and 36 hours following ethanol exposure) – during the period when NMDA receptor-mediated excitotoxicity would be occurring. When administered at the same time as the ethanol binge (0 hours) or when alcohol has not yet been metabolized (9 hours), mortality rates increased and/or behavioral deficits were exacerbated (Thomas et al., 2001). In addition, MK-801 is a particularly potent antagonist that acts at the phencyclidine (PCP) site within the NMDA-receptor-gated channel. Because of this action, a range of side effects may be produced including memory impairment in adult subjects (Sanger, 1992, Svensson, 2000, Wedzony et al., 2000) and inappropriate neurotoxic cell death, the latter having been observed following MK-801 exposure in the rat neonate (Ikonomidou et al., 1999).
Given that MK-801 can induce adverse side effects in vivo, the clinical potential for MK–801 is limited. Eliprodil and agmatine, other NMDA receptor antagonists that bind to the polyamine site of the NMDA receptor and modulate its action, are more clinically relevant drugs that show promise (Lewis et al., 2007, Thomas et al., 2004). We have recently been investigating memantine (3,5-dimethyladamantan-1-amine hydrochloride), a more moderate affinity, uncompetitive NMDA receptor antagonist. Its ability to rapidly leave the NMDA receptor channel (Parsons et al., 1993) make it less likely to interfere with normal synaptic communication and therefore a more tolerable pharmacological agent in pathological conditions. To date, memantine has shown promise in clinical trials for Alzheimer’s disease, attenuating some cognitive deficits associated with that disease, although it may increase the risk for other problems such as weight gain (Shah et al., 2008, Yang et al., 2013). Related to the present study, memantine can suppress signs of ethanol withdrawal in adult rats, preventing development of cognitive deficits associated with chronic alcohol exposure (Lukoyanov and Paula-Barbosa, 2001). In adult mice, the number of alcohol withdrawal-related seizures is significantly reduced following memantine treatment during the withdrawal phase. In addition, memantine is neuroprotective, blocking ethanol withdrawal neurotoxicity in fetal hippocampal cultures (Stepanyan et al., 2008). We have already demonstrated the mitigation of long-lasting alcohol-induced motor coordination deficits, although hippocampal-based effects were not observed when 10, 15, or 20 mg/kg single memantine doses were administered 24 hours after an ethanol binge (Idrus et al., 2011b). This may be due, in part, to memantine concentrations peaking 20–30 minutes after injection and its relatively short half life (4–12 hours) (Beconi et al., 2011, Parsons et al., 2007, Wesemann et al., 1982). It is not known how large the window of excitotoxicity might be, so the present study utilized two administrations of memantine at both 24 and 36 hours post-ethanol.
In this study, rats were exposed to a heavy binge of ethanol (peak blood ethanol levels = 397–412 mg/dL) on postnatal day (PD) 6, a period of brain development that is equivalent to a portion of the mid-third trimester in the human fetus (Dobbing and Sands, 1979). This period of development is also marked by high sensitivity to both ethanol-induced teratogenicity (Thomas et al., 2001) and NMDA receptor-mediated excitotoxicity (Ikonomidou et al., 1989). During the phase that follows the ethanol binge, the neonatal rats were injected with memantine at two timepoints within the withdrawal period. Subjects were then raised and tested for locomotor activity and spatial discrimination reversal learning, as both tasks have been previously used to demonstrate the efficacy of other NMDA receptor antagonists in attenuating ethanol-induced behavioral deficits (Thomas et al., 2002b, Thomas et al., 1997). Body growth, blood alcohol level, performance on a motor task, and cerebellar Purkinje cell numbers from these same subjects are provided in a separate report (Idrus et al., 2011a).
Materials and Methods
Subjects
Subjects were Sprague-Dawley rats’ offspring from the breeding colony at the Center for Behavioral Teratology, San Diego State University. Briefly, a Sprague-Dawley male and female were housed together overnight. The presence of a seminal plug on the following morning indicated mating and was designated as gestational day (GD) 0. Pregnant dams were then singly housed in a temperature- and humidity-controlled room with food and water ad libitum. On the morning following birth, litters were pseudorandomly culled to 8 pups, with 4 males and 4 females when possible. To control for potential litter effects, no more than one pup of each sex from a single litter was assigned to a particular treatment group (n = 8–11/sex/treatment).
Treatment
To investigate the effects of memantine in reducing ethanol withdrawal-induced excitotoxicity, pups were randomly assigned to one of six treatment groups (ethanol-treated (EtOH) vs. maltose control (MC); 0, 20, or 30 mg/kg memantine doses). On postnatal day (PD) 6, EtOH subjects received 6 g/kg (13.6% v/v) ethanol in a binge-like manner via two intragastric intubations (27.5 mL/kg). Specifically, subjects received 3 g/kg ethanol in each of the two intubations, separated by two hours. The MC subjects received an equal caloric maltose solution during each intubation (Livy et al., 2003, Thomas et al., 1997). To control for known reductions in nursing that occur in ethanol-treated subjects (Goodlett and Johnson, 1997), EtOH subjects received two additional intubations of a nutritionally balanced milk formula every two hours following the ethanol intubations. The MC subjects were sham intubated during these two additional feedings. Maternal separation was minimized as all subjects were returned to the dam between intubations.
Twenty-four hours after the initial EtOH intubation, all subjects received 0, 10, or 15 mg/kg memantine via intraperitoneal injection. Each subject received another memantine injection (of the same dose) twelve hours thereafter; that is, 36 hours after the initial EtOH intubation. Therefore, the total dose administered was 0, 20, or 30 mg/kg memantine, doses that have shown protective effects following brain damage including those associated with developmental alcohol exposure and other insults (Idrus et al., 2011a, Rao et al., 2001).
Locomotor Activity Testing
On PD 18–21, basic activity level was monitored in a Plexiglas open field (40 × 40 × 30.5 cm) contained within an automated optical beam activity monitor (Hamilton-Kinder, San Diego, CA). The open field was housed in enclosed, ventilated chambers. White noise was present during acclimation (≃30 min) and testing to mask any outside noises. Prior to testing each subject, the chambers were carefully cleaned to eliminate any odor cues. Each subject was then placed in the center of a chamber and infrared beam interruptions were recorded every 5 min during the 60-min test by the Hamilton-Kinder monitor. The total distance traveled and the number of times the rat reared served as performance measures. Subjects were tested for four consecutive days during the dark cycle between 18:00–22:00.
Serial Spatial Discrimination Reversal Learning
On PD 40–43, subjects were tested on a serial spatial discrimination reversal task (Idrus et al., 2011b, Thomas et al., 1997). This task required the subject to escape from a Plexiglas T-maze (19-cm-wide passages, 71-cm-long stem and a 91-cm arm span with 10-cm-wide cul-de-sac at each end) that was partially submerged in a 121-cm diameter tank of water. Each cul-de-sac contained a rod from which a wire escape ladder could be hung. Both cul-de-sacs were constructed of black Plexiglas so that the ladder could not be seen from the start (bottom of the stem) or the choice points in the T-maze. The water in the maze was kept at 26° C (± 1°C) and made opaque with the addition of powdered milk. Between trials, subjects were kept in a heated environment (≃31°C) to prevent hypothermia.
On the first day (PD 40), subjects underwent a 10-trial pre-training session. During each pre-training trial, one arm of the T-maze was blocked, forcing the subject to swim to the other arm to escape. The subject was initially placed at the bottom of the stem facing the experimenter and was required to swim to the location of the escape ladder. If the subject failed to escape within 60 sec, the subject was guided to the goal ladder by the experimenter. Subjects were forced to escape from each arm five times in a pseudorandom order during this pre-training session.
Before the first trial on PD 41, a trial was employed to determine the individual subject’s side preference. Each subject was placed at the bottom of the T-maze and both goal arms were open without an escape ladder. Once the subject had entered into an escape arm, the ladder was placed in the opposite arm. Thus, each subject started training to their non-preferred side. During training trials, both arms were open and the escape ladder placed in only one goal arm. During the position discrimination, a self-correction procedure was used, in which multiple entries could be made into any of the arms until the subject escaped, exiting the water from the correct arm. Entries into the incorrect arm, reentries into the starting stem, and backtracking through the correct arm without escaping were considered errors.
Once the subject reached a criterion of six consecutive successful escapes without errors, the contingencies were reversed; the subject was afforded escape only in the previously non- reinforced arm. Training to this arm continued until the criterion of six consecutive successful trials with no errors was reached once again. Thus, each time the subject reached criterion, the escape ladder’s location was switched. Subjects were trained for three consecutive days, 30 trials a day, with 1–2 min inter-trial intervals. If the subject had four consecutive successful escapes during the last four trials of the day, 2 additional trials were given so that the subject could potentially complete 6 consecutive escapes. The number of successful reversals on the first day, the number of trials to the first successful criterion on the first day, the total number of successful discriminations achieved, and the number of errors over the course of the three testing days served as performance measures, as did the percent of subjects able to reverse the discrimination twice on the first testing day.
Data Analyses
Data were analyzed using SPSS software (Chicago, USA). All data were analyzed with ANOVAs with a 2 (ethanol, maltose) × 3 (0, 20, 30 mg/kg memantine) × 2 (male, female) between-subjects design. Day served as a repeated within-subject variable for serial spatial discrimination reversal learning performance. Day (4) and 5-min bin (12) served as repeated within-subject variables in activity performance. Post hoc comparisons were conducted with Least Significant Difference (LSD) analyses (p’s <0.05). The percent of subjects able to reverse the discrimination twice on the first day was analyzed nonparametrically with Fisher’s exact probability comparisons.
Results
Locomotor Activity Testing
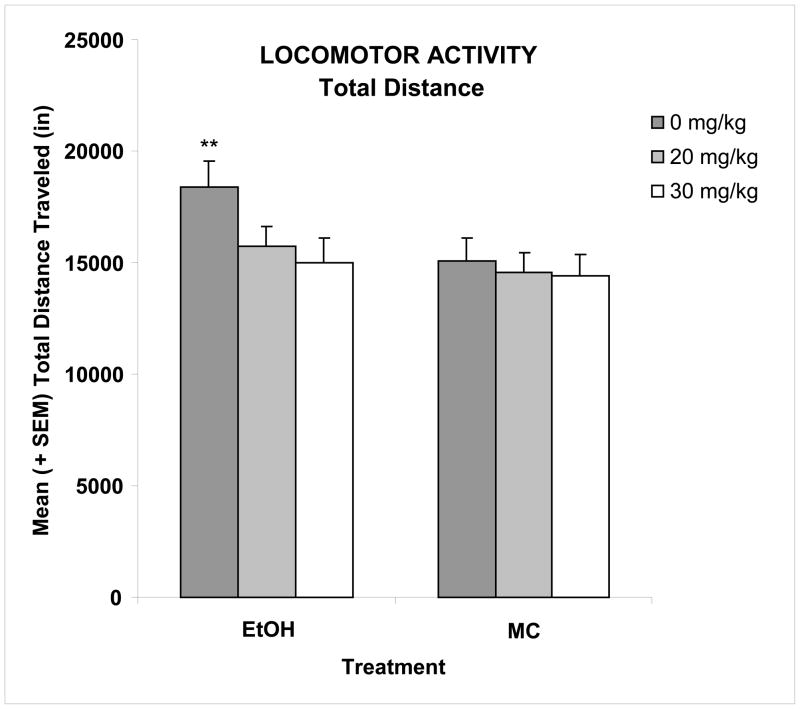
Ethanol exposure on PD 6 led to significant increases in activity level in the open field chamber. This overactivity was reduced with memantine treatment given during the withdrawal period (24 and 36 hrs). Figure 1 shows the total distance traveled collapsed across day and bin. During the 4-day activity testing period, the total distance traveled decreased gradually between sessions (main day effect [F(3,315)=25.6, p<0.01]) (Table 1) and within sessions (main bin effect [F(11,1155)=394.2, p<0.001]) (data not shown). Ethanol-exposed subjects not treated with memantine were significantly more active during the first day of testing and during the first two-thirds of each session, producing significant interactions of ethanol x day [F(3,315)=3.9, p<0.01] and ethanol x bin [F(11,1155)=5.8, p<0.01]. When collapsed across day and bin (Figure 1), there was a main effect of ethanol [F(1,105)=7.6, p<0.01], as well as a significant interaction of ethanol by memantine [F(2,105)=3.2, p< 0.05]. Post hoc analyses showed the ethanol-exposed subjects not treated with memantine traveled significantly further distances compared to sham controls, and that the 30 mg/kg dose of memantine (EtOH + 30) significantly decreased this ethanol-induced overactivity (LSD, p<0.05) to control levels. Activity of the EtOH + 20 group was intermediate, not differing significantly from any other group, including controls. Memantine did not significantly affect activity levels among controls. Finally, there were no significant main or interactive effects of sex on locomotor activity.
Figure 1. Locomotor Activity.
Binge-like ethanol treatment on postnatal day 6 significantly increased activity level, as ethanol-exposed (EtOH) subjects travelled significantly further distances compared to maltose controls (MC). The total distance traveled decreased gradually between sessions. With administration of memantine during the withdrawal phase, this ethanol-induced overactivity was significantly reduced in a dose-dependent manner. ** significantly different from all other groups except EtOH + 20.
EtOH + 0, ethanol-exposed, 0 mg/kg memantine; EtOH + 20, ethanol-exposed, 20 mg/kg memantine; EtOH + 30, ethanol-exposed, 30 mg/kg memantine; MC + 0, maltose control, 0 mg/kg memantine; MC + 20, maltose control, 20 mg/kg memantine; MC + 30, maltose control, 30 mg/kg memantine.
Table 1. Locomotor Activity (mean ± SEM).
A binge ethanol treatment on postnatal day 6 significantly increased activity levels, with ethanol-exposed (EtOH) subjects travelling significantly further distances compared to maltose controls (MC), particularly on the first day (postnatal day 18) (* p<0.05). With administration of memantine during the withdrawal phase, this ethanol-induced overactivity was significantly reduced in a dose-dependent manner.
A binge ethanol treatment on postnatal day 6 also significantly increased the number of times rats reared, with EtOH subjects rearing significantly more compared to MC subjects (* p<0.05). Memantine administration did not significantly affect this measure.
Serial Spatial Discrimination Reversal Learning (mean ± SEM)
Ethanol-treated subjects made significantly more total errors than their control counterparts (* p<0.05), particularly on the first day (postnatal day 41). No effect of memantine was detected in the total number of errors made.
| OPEN FIELD ACTIVITY | ||||
|---|---|---|---|---|
| Total Distance (in) | ||||
| GROUP | Postnatal Day | Memantine 0 mg/kg | Memantine 20 mg/kg | Memantine 30 mg/kg |
| EtOH | 18* | 5832 ± 347 | 4978 ± 334 | 4518 ± 335 |
| 19 | 4379 ± 344 | 3555 ± 257 | 3739 ± 289 | |
| 20 | 4240 ± 268 | 3972 ± 327 | 3434 ± 210 | |
| 21 | 4469 ± 347 | 4348 ± 297 | 3658 ± 322 | |
| MC | 18 | 4065 ± 335 | 4057 ± 314 | 4284 ± 309 |
| 19 | 3411 ± 252 | 3641 ± 208 | 3759 ± 261 | |
| 20 | 3623 ± 298 | 3596 ± 172 | 3646 ± 262 | |
| 21 | 3386 ± 307 | 3715 ± 188 | 3744 ± 229 | |
| Number of Rearings | ||||
| EtOH* | 18–21 | 8386 ± 66 | 7247 ± 67 | 687 ± 76 |
| MC | 18–21 | 575 ± 63 | 623 ± 39 | 640 ± 49 |
| SERIAL SPATIAL DISCRIMINATION REVERSAL LEARNING | ||||
| Number of Errors | ||||
| GROUP | Postnatal Day | Memantine 0 mg/kg | Memantine 20 mg/kg | Memantine 30 mg/kg |
| EtOH | 41* | 19.4 ± 1.3 | 19.2 ± 1.1 | 17.1 ± 0.8 |
| 42 | 15.2 ± 1.0 | 15.2 ± 1.3 | 14.1 ± 0.7 | |
| 43 | 14.3 ± 0.9 | 13.4 ± 1.2 | 13.9 ± 0.7 | |
| MC | 41 | 14.7 ± 0.9 | 13.3 ± 0.4 | 16.3 ± 1.6 |
| 42 | 14.5 ± 0.9 | 14.1 ± 0.8 | 12.5 ± 0.8 | |
| 43 | 12.9 ± 0.9 | 12.6 ± 0.6 | 12.6 ± 0.9 | |
Ethanol exposure on PD 6 also significantly increased the number of rearings, producing a main effect of ethanol [F(1,105)=7.9, p<0.01] (Table 1). Although memantine administration appeared to reduce ethanol-related increases in rearing, no significant interaction of ethanol and memantine was found. As with the total distance, the total number of rearings decreased gradually between sessions (main day effect [F(3,315)=24.2, p<0.01]) and within sessions (main bin effect [F(11,1155)=203.1, p<0.01]). Ethanol-exposed subjects reared more during the first bins of testing, producing a significant interaction of ethanol x bin [F(11,1155)=4.5, p<0.01] (data not shown). As with locomotor activity, no significant sex effects were observed on rearing.
Serial Spatial Discrimination Reversal Learning
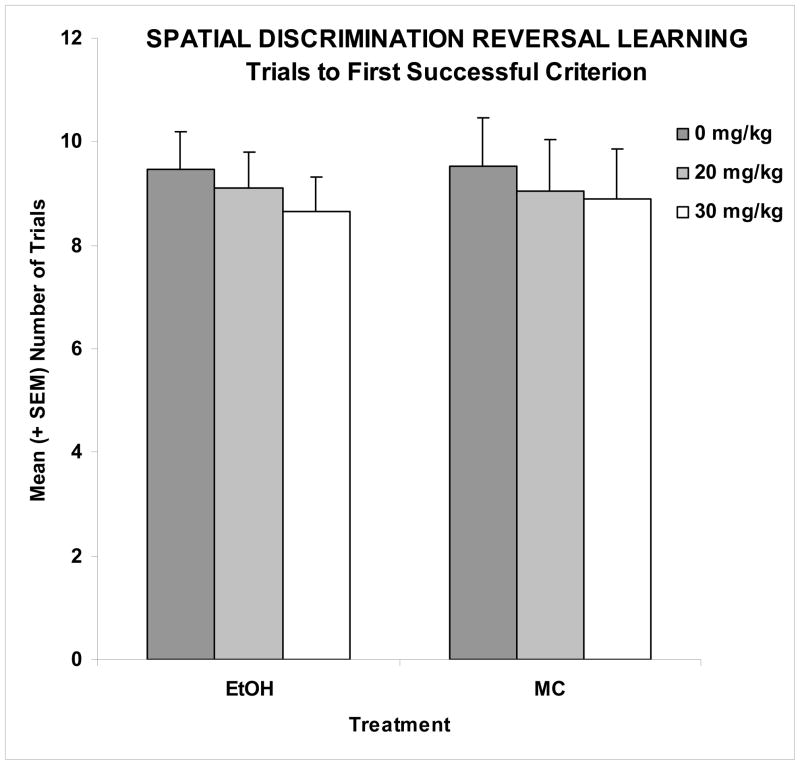
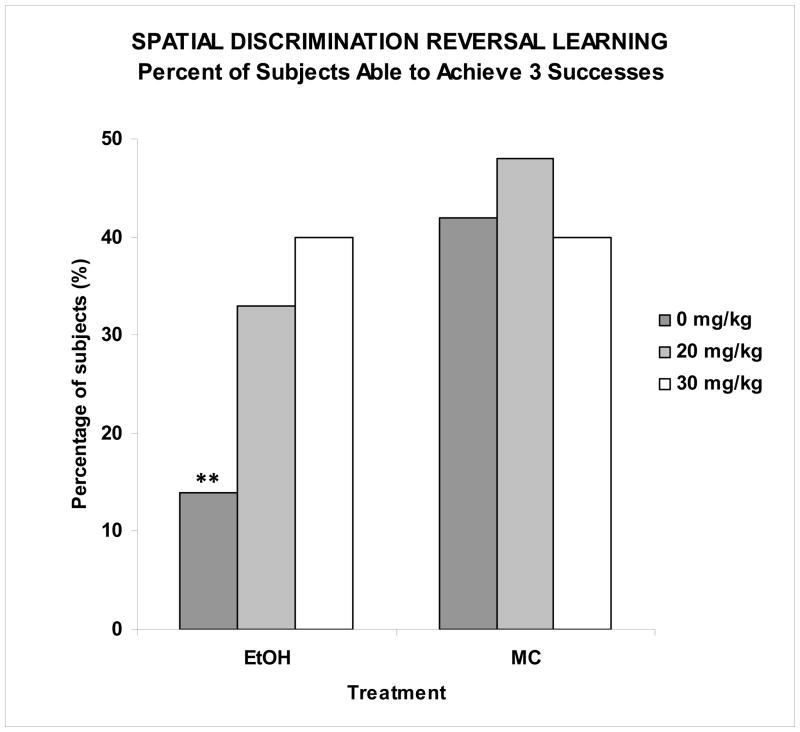
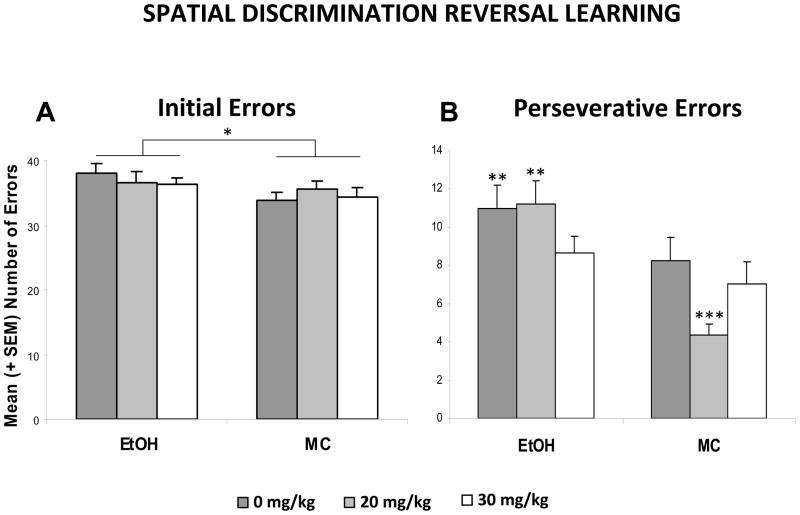
There were no significant effects of either ethanol or memantine on the number of trials to reach criterion to the first discrimination (Figure 2), suggesting that the subjects’ ability to discriminate spatial location was unaffected by ethanol or memantine. However, adverse effects of ethanol were observed in the number of successful discriminations achieved on the first testing day, as shown in Figure 3. On the first testing day, only 14% of the EtOH + 0 memantine subjects were able to reverse the discrimination twice (3 successful criteria attained), a significantly lower percentage than the MC subjects. In fact, fewer subjects in the EtOH + 0 memantine group were able to achieve 3 discriminations compared to all other groups including the EtOH + 30 memantine group, but not the EtOH + 20 memantine group. The EtOH + 20 memantine group did not differ from any other group, including controls. Importantly, the performance of the EtOH + 30 memantine subjects was not significantly different from that of the maltose control subjects (Fisher’s exact probability tests, p’s<0.05). Ethanol exposure on PD 6 also increased the total number of errors committed, producing a significant effect of ethanol [F(1,104)=13.7, p<0.001] (Table 1). No effect of memantine was detected, however females committed more errors than males, producing a significant sex effect [F(1,104)=6.4, p<0.05]. A day effect [F(2,104)=23.4, p<0.001] and a day by ethanol effect [F(2,104)=3.4, p<0.05] were also found, as ethanol-exposed subjects committed significantly more errors than controls on the first day of testing (Table 1). As this behavioral task utilizes a method of self-correction, the subjects could commit an initial error by entering the incorrect arm, as well as perseverative-type repeated errors (returning to the incorrect arm or to the starting stem after committing an initial error). Thus, the total number of errors committed was a combination of the number of trials with errors and the number of perseverative-type errors. As was seen for the total number of errors committed, EtOH-treated subjects likewise committed a significantly greater average number of initial and perseverative errors over the course of the three testing days (Figure 4), producing a main effect of ethanol ([F(1,104)=4.0, p<0.05] and [F(1,104)=17.9, p<0.001], respectively). For perseverative errors, this ethanol effect was ameliorated with administration of 30 mg/kg memantine during the withdrawal period, producing an ethanol by memantine interaction [F(2,104)=2.9, p<0.05]. Follow-up analyses confirmed the number of preservative errors committed by the EtOH + 20 group did not significantly differ from the EtOH + 0 group, but that EtOH + 30 subjects did commit significantly fewer errors compared to both the EtOH + 0 and EtOH + 20 groups, performing comparably to the controls. A memantine effect was found in the control groups, as the MC + 20 group committed significantly fewer perseverative errors than the MC + 0 or MC + 30 groups. Finally, there was a significant interaction of day x ethanol [F(2,104)=3.5, p<0.05], as ethanol-exposed subjects committed significantly more perseverative errors on the first day of testing.
Figure 2. Serial Spatial Discrimination Reversal Learning.
No significant effects of either ethanol or memantine on the number of trials to reach the first successful discrimination were observed, a measure of a subject’s ability to discriminate spatial location.
Figure 3. Serial Spatial Discrimination Reversal Learning.
Ethanol exposure significantly reduced discrimination reversals on the first day of testing (ability to achieve 3 successful criterions). This effect was significantly attenuated by administration of the 30 mg/kg memantine dose during the withdrawal period. Performance of the EtOH + 20 group did not significantly from any other group. ** significantly different from every other group except EtOH + 20.
Figure 4. Serial Spatial Discrimination Reversal Learning.
Mean (+ SEM) total number of initial and perseverative errors committed over the course of the 3 testing days by the subjects in the spatial discrimination reversal learning task. Ethanol-treated subjects committed a greater number of initial and perseverative-type errors (* p<0.05). For perseverative errors, the ethanol effect was mitigated by 30 mg/kg memantine given during the withdrawal period. ** significantly different from all MC groups and EtOH + 30; *** significantly different from all groups
Discussion
The present study extends our previous findings with MK-801 and eliprodil, illustrating that administration of NMDA receptor antagonists during the alcohol withdrawal period in the neonatal rat reduces the severity of ethanol-related behavioral alterations (Thomas et al., 2001, Thomas et al., 2002b, Thomas et al., 1997). The current study similarly demonstrates that treatment with the low affinity binding NMDA receptor antagonist, memantine, during the withdrawal period, can likewise mitigate ethanol-induced overactivity and deficits in spatial discrimination reversal learning. Importantly, memantine treatment by itself did not adversely affect any of the behavioral measures tested here, indicating that the low affinity and rapid off-rate kinetics of memantine make it a more clinically feasible treatment for fetal alcohol effects (Rogawski et al., 2000).
A single binge ethanol exposure on PD 6 in the rat significantly altered behavioral development, leading to significant elevation of activity levels. This reflects numerous reports of overactivity induced by ethanol exposure during the brain growth spurt, not only in rodents and other animals such as sheep and non-human primates, but also in alcohol-exposed children who otherwise exhibit normal IQ levels (Riley and McGee, 2005, Spear-Smith et al., 2000), illustrating that even a brief exposure to this teratogen can induce lasting neurobehavioral changes. With administration of memantine during the withdrawal period, at both 24 and 36 hours after the ethanol binge, amelioration of ethanol-induced locomotor overactivity was observed in a dose-dependent manner. Although treatment with 20 mg/kg memantine tended to decrease ethanol-induced overactivity, administration of 30 mg/kg memantine significantly mitigated ethanol-related overactivity. In fact, this treatment was so effective that no significant differences in the distance travelled was detected between the EtOH + 30 group and the maltose control subjects.
The amelioration of ethanol-related behavioral deficits after memantine treatment was also observed in the spatial discrimination task. First, ethanol exposure on PD 6 significantly impaired some, not all, performance measures on this task. For example, no significant effect of ethanol was observed on the number of trials to the first successful criterion. Such results are consistent with previous studies (Idrus et al., 2011b). This measure of spatial learning is thought to be less sensitive to developmental alcohol exposure in comparison to the more robust measure of the number of errors committed (e.g. Thomas et al., 2001). Although ethanol-exposed subjects may be able to discriminate spatial location, they have difficulties when they are required to reverse a discrimination, exhibiting a lack of behavioral flexibility. Ethanol-exposed subjects perseverate, as reflected in the number of perseverative-type errors and the impairments in discrimination reversal on the first day of testing. Memantine treatment attenuated these ethanol-induced impairments in the reversal learning task. For example, the ethanol-treated subjects given the highest dose of memantine (30 mg/kg) committed fewer perseverative-type errors and were also more successful at reversing the discrimination on the first day of testing (achieving at least 3 successes) compared to the EtOH group that did not receive memantine.
Currently, the optimal parameters of memantine administration that mitigate ethanol’s effects on cognitive ability are unknown, although we have shown in a separate study that a single administration of memantine (20 mg/kg) 24 hours after ethanol was not sufficient to reduce ethanol-induced overactivity or spatial learning deficits (Idrus et al., 2011b). Instead, memantine had more robust effects on motor behavior, mitigating motor deficits as assessed on a parallel bar task. The subjects that were used in this study also showed mitigation of ethanol- related motor incoordination with the 30 mg/kg memantine dose as well as reduced cerebellar Purkinje cell loss in a dose-dependent manner (Idrus et al., 2011a). When compared to other single dose studies from our laboratory (e.g. Thomas et al., 2001, Thomas et al., 2002b, Thomas et al., 2004, Thomas et al., 1997), MK-801 or eliprodil, when administered in a single dose during the withdrawal period, was sufficient to attenuate ethanol-induced increases in perseverative-type errors and activity levels. The present study however suggests that multiple injections of memantine during the withdrawal period may be required to mitigate these behavioral deficits, perhaps due, in part, to the short half life of memantine (Parsons et al., 2007). For example, other studies using a neonatal rat model of hypoxia-ischemia also report that memantine is neuroprotective when additional doses are given at 12 hour intervals after an initial loading dose (Manning et al., 2008).
The varying pharmacological profiles of these NMDA receptor antagonists may also account for the differences observed. For example, noncompetitive antagonists such as MK-801 show no selectivity for any NMDA receptor subtype, blocking the receptor by acting within the NMDA-receptor-gated channel at the phencyclidine site. Because of this, side effects have been observed including memory impairment (Sanger, 1992, Svensson, 2000, Wedzony et al., 2000) and inappropriate neurotoxic cell death (Ikonomidou et al., 1999). We have, in fact, demonstrated that when MK-801 is given at the same time as the ethanol binge, mortality rates increase (Thomas et al., 1997, Thomas et al., 2002b). Eliprodil, an NR2B subunit selective antagonist that acts at the polyamine modulatory site of the NMDA receptor, has shown some mitigating effects on ethanol-induced behavioral deficits (Thomas et al., 2004). Its selective capability in blocking the NR2B subunit, receptors that are believed to be more sensitive to alcohol (Allgaier, 2002), make eliprodil a potential treatment for attenuating fetal alcohol effects without the toxic effects associated with MK-801 (Sanger, 1992). However, eliprodil’s promising effects in animal studies have failed to be replicated in clinical trials for the treatment of other disorders of NMDA receptor-mediated excitotoxic cell death e.g. Alzheimer’s, acute ischemic stroke (Ikonomidou and Turski, 2002). Another agent that works at the polyamine site is agmatine. When administered during ethanol withdrawal, ethanol-induced cerebellar deficits, as assessed on the rotarod, are mitigated (Lewis et al., 2007). To date, agmatine has shown limited neuroprotective properties in clinical trials as an antidepressant (Shopsin, 2012) and an analgesic for neuropathic pain (Keynan et al., 2010), although studies have shown neuroprotective effects in cell culture and animal model systems (Zhu et al., 2006, Hong et al., 2007, Arndt et al., 2009).
In comparison, memantine has been approved for the treatment of Alzheimer’s disease, showing attenuation of some memory deficits (Shah et al., 2008). Its effectiveness in a range of other disorders, including alcohol dependence (Muhonen et al., 2008), is also currently being tested in clinical trials and shows promise. Similar to eliprodil, the current study showed no adverse effects of the memantine treatment on behavior in the control subjects. In this way, memantine as a more moderate affinity NMDA receptor antagonist, can inhibit NMDA receptor-mediated excitotoxic cell death during the withdrawal period, while preserving the physiological functions of the NMDA receptor and not displaying the adverse side effects observed with drugs such as MK-801 (Rogawski et al., 2000). However, in addition to action at NMDA receptors, memantine also acts at serotonergic and nicotinic receptor sites (Rammes et al., 2008). Prenatal alcohol exposure reduces the number of 5-HT binding sites, which has been prevented by simultaneous treatment with 5-HT1A agonists (Tajuddin and Druse, 2001). Since memantine can act as a 5-HT antagonist (Rammes et al., 2008), mitigating effects may be less likely to be observed. Interestingly, 5-HT receptors are found in high numbers within the hippocampus, but not the cerebellum (Hoyer et al., 2002). This may well explain why memantine seems to be effective in attenuating alcohol’s effects on cerebellar-related behaviors with exposure parameters that do not effectively attenuate alcohol-related alterations in cognitive behaviors (Idrus et al., 2011a, Idrus et al., 2011b).
To further demonstrate memantine’s mitigating effects on alcohol-related deficits, analysis of related neuroprotection could shed some light. Significant increases in both activity levels and the numbers of perseverative errors suggest that the ethanol-exposed subjects suffer from response inhibition deficits. Ethanol-induced response inhibition deficits may be a function of either hippocampal or prefrontal cortical damage (Bardgett et al., 2006, Riley et al., 1986). In fact, excitotoxic lesions to the hippocampus increases locomotor activity (Godsil et al., 2005) and induces response inhibition deficits (Bardgett et al., 2006); similar behavioral outcomes also occur following excitotoxic (NMDA-induced) damage to the prefrontal cortex (Lacroix et al., 2002). Thus, memantine’s ability to reverse ethanol’s effects on activity and spatial reversal learning in the current study are consistent with the hypothesis that NMDA receptor-mediated excitotoxic cell death occurs during ethanol withdrawal in the hippocampus and/or prefrontal cortex.
It is currently understood that ethanol exposure will result in a permanent deficit of hippocampal neurons (Bonthius and West, 1990). Administration of the NMDA receptor antagonist, MK-801, during the withdrawal period can protect against ethanol-induced hippocampal cell loss (Thomas et al., 2002b), relating well with the amelioration of the ethanol-related deficits in reversal learning and overactivity in the open field (Thomas et al., 2002a, Thomas et al., 2001, Thomas et al., 1997). Although memantine’s effects on alcohol-related cell loss within the hippocampus or frontal cortex have not directly been investigated, cytotoxicity associated with ethanol withdrawal in hippocampal slice cultures is reduced following memantine administration (Stepanyan et al., 2008). Our laboratory has also found significant protection against alcohol-related Purkinje cell loss in the cerebellum following administration of memantine during the withdrawal period (Idrus et al., 2011a), which relates to performance on a motor task.
As memantine administration during the withdrawal period reduced the severity of alcohol-related hyperactivity and spatial learning, the current study lends further support to the hypothesis that binge drinking may be more damaging because of the episodes of ethanol withdrawal and associated NMDA receptor-mediated excitotoxicity. Moreover, unlike other NMDA receptor antagonists like MK-801 that possess adverse side effects, memantine may hold more promise as an effective intervention for some fetal alcohol spectrum disorders.
Acknowledgments
This research was supported by funds from the National Institute on Alcohol Abuse and Alcoholism (AA06902) and from the Kaplan Fellowship from the Department of Psychology, San Diego State University.
All procedures included in this study were approved by the SDSU IACUC and are in accordance with the NIH Guide for Care and Use of Laboratory Animals.
References
- ALLGAIER C. Ethanol sensitivity of NMDA receptors. Neurochem Int. 2002;41:377–82. doi: 10.1016/s0197-0186(02)00046-3. [DOI] [PubMed] [Google Scholar]
- ARNDT MA, BATTAGLIA V, PARISI E, LORTIE MJ, ISOME M, BASKERVILLE C, PIZZO DP, IENTILE R, COLOMBATTO S, TONINELLO A, SATRIANO J. The arginine metabolite agmatine protects mitochondrial function and confers resistance to cellular apoptosis. Am J Physiol Cell Physiol. 2009;296:C1411–9. doi: 10.1152/ajpcell.00529.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARDGETT ME, GRIFFITH MS, FOLTZ RF, HOPKINS JA, MASSIE CM, O’CONNELL SM. The effects of clozapine on delayed spatial alternation deficits in rats with hippocampal damage. Neurobiol Learn Mem. 2006;85:86–94. doi: 10.1016/j.nlm.2005.08.010. [DOI] [PubMed] [Google Scholar]
- BECONI MG, HOWLAND D, PARK L, LYONS K, GIULIANO J, DOMINGUEZ C, MUNOZ-SANJUAN I, PACIFICI R. Pharmacokinetics of memantine in rats and mice. PLoS Curr. 2011;3:RRN1291. doi: 10.1371/currents.RRN1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONTHIUS DJ, WEST JR. Alcohol-induced neuronal loss in developing rats: increased brain damage with binge exposure. Alcohol Clin Exp Res. 1990;14:107–18. doi: 10.1111/j.1530-0277.1990.tb00455.x. [DOI] [PubMed] [Google Scholar]
- COSTA ET, SAVAGE DD, VALENZUELA CF. A review of the effects of prenatal or early postnatal ethanol exposure on brain ligand-gated ion channels. Alcohol Clin Exp Res. 2000;24:706–15. [PubMed] [Google Scholar]
- DOBBING J, SANDS J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- GODSIL BP, STEFANACCI L, FANSELOW MS. Bright light suppresses hyperactivity induced by excitotoxic dorsal hippocampus lesions in the rat. Behav Neurosci. 2005;119:1339–52. doi: 10.1037/0735-7044.119.5.1339. [DOI] [PubMed] [Google Scholar]
- GOODLETT CR, JOHNSON TB. Neonatal binge ethanol exposure using intubation: timing and dose effects on place learning. Neurotoxicol Teratol. 1997;19:435–46. doi: 10.1016/s0892-0362(97)00062-7. [DOI] [PubMed] [Google Scholar]
- HOFFMAN PL, TABAKOFF B. The role of the NMDA receptor in ethanol withdrawal. EXS. 1994;71:61–70. doi: 10.1007/978-3-0348-7330-7_7. [DOI] [PubMed] [Google Scholar]
- HONG S, LEE JE, KIM CY, SEONG GJ. Agmatine protects retinal ganglion cells from hypoxia-induced apoptosis in transformed rat retinal ganglion cell line. BMC Neurosci. 2007;8:81. doi: 10.1186/1471-2202-8-81. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- HONSE Y, RANDALL PK, LESLIE SW. Prenatal ethanol exposure modifies [3H]MK-801 binding to NMDA receptors: spermidine and ifenprodil. Alcohol Clin Exp Res. 2003;27:1993–2001. doi: 10.1097/01.ALC.0000099029.55026.C6. [DOI] [PubMed] [Google Scholar]
- HOYER D, HANNON JP, MARTIN GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–54. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- IDRUS NM, MCGOUGH NN, RILEY EP, THOMAS JD. Administration of memantine during ethanol withdrawal in neonatal rats: effects on long-term ethanol-induced motor incoordination and cerebellar Purkinje cell loss. Alcohol Clin Exp Res. 2011a;35:355–64. doi: 10.1111/j.1530-0277.2010.01351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IDRUS NM, MCGOUGH NN, SPINETTA MJ, THOMAS JD, RILEY EP. The effects of a single memantine treatment on behavioral alterations associated with binge alcohol exposure in neonatal rats. Neurotoxicol Teratol. 2011b;33:444–50. doi: 10.1016/j.ntt.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IKONOMIDOU C, BOSCH F, MIKSA M, BITTIGAU P, VOCKLER J, DIKRANIAN K, TENKOVA TI, STEFOVSKA V, TURSKI L, OLNEY JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–4. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- IKONOMIDOU C, MOSINGER JL, SALLES KS, LABRUYERE J, OLNEY JW. Sensitivity of the developing rat brain to hypobaric/ischemic damage parallels sensitivity to N-methyl-aspartate neurotoxicity. J Neurosci. 1989;9:2809–18. doi: 10.1523/JNEUROSCI.09-08-02809.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IKONOMIDOU C, TURSKI L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002;1:383–6. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- KEYNAN O, MIROVSKY Y, DEKEL S, GILAD VH, GILAD GM. Safety and Efficacy of Dietary Agmatine Sulfate in Lumbar Disc-associated Radiculopathy. An Open-label, Dose-escalating Study Followed by a Randomized, Double-blind, Placebo-controlled Trial. Pain Med. 2010;11:356–68. doi: 10.1111/j.1526-4637.2010.00808.x. [DOI] [PubMed] [Google Scholar]
- LACROIX L, WHITE I, FELDON J. Effect of excitotoxic lesions of rat medial prefrontal cortex on spatial memory. Behav Brain Res. 2002;133:69–81. doi: 10.1016/s0166-4328(01)00442-9. [DOI] [PubMed] [Google Scholar]
- LEWIS B, WELLMANN KA, BARRON S. Agmatine reduces balance deficits in a rat model of third trimester binge-like ethanol exposure. Pharmacol Biochem Behav. 2007;88:114–21. doi: 10.1016/j.pbb.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIVY DJ, MILLER EK, MAIER SE, WEST JR. Fetal alcohol exposure and temporal vulnerability: effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicol Teratol. 2003;25:447–58. doi: 10.1016/s0892-0362(03)00030-8. [DOI] [PubMed] [Google Scholar]
- LUKOYANOV NV, PAULA-BARBOSA MM. Memantine, but not dizocilpine, ameliorates cognitive deficits in adult rats withdrawn from chronic ingestion of alcohol. Neurosci Lett. 2001;309:45–8. doi: 10.1016/s0304-3940(01)02037-7. [DOI] [PubMed] [Google Scholar]
- MANNING SM, TALOS DM, ZHOU C, SELIP DB, PARK HK, PARK CJ, VOLPE JJ, JENSEN FE. NMDA receptor blockade with memantine attenuates white matter injury in a rat model of periventricular leukomalacia. J Neurosci. 2008;28:6670–8. doi: 10.1523/JNEUROSCI.1702-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MELENDEZ RI, HICKS MP, CAGLE SS, KALIVAS PW. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcohol Clin Exp Res. 2005;29:326–33. doi: 10.1097/01.alc.0000156086.65665.4d. [DOI] [PubMed] [Google Scholar]
- MUHONEN LH, LAHTI J, SINCLAIR D, LONNQVIST J, ALHO H. Treatment of alcohol dependence in patients with co-morbid major depressive disorder--predictors for the outcomes with memantine and escitalopram medication. Subst Abuse Treat Prev Policy. 2008;3:20. doi: 10.1186/1747-597X-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIXON K, HUGHES PD, AMSEL A, LESLIE SW. NMDA receptor subunit expression after combined prenatal and postnatal exposure to ethanol. Alcohol Clin Exp Res. 2004;28:105–12. doi: 10.1097/01.ALC.0000106311.88523.7B. [DOI] [PubMed] [Google Scholar]
- O’LEARY-MOORE SK, PARNELL SE, LIPINSKI RJ, SULIK KK. Magnetic resonance-based imaging in animal models of fetal alcohol spectrum disorder. Neuropsychol Rev. 2011;21:167–85. doi: 10.1007/s11065-011-9164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARSONS CG, GRUNER R, ROZENTAL J, MILLAR J, LODGE D. Patch clamp studies on the kinetics and selectivity of N-methyl-D-aspartate receptor antagonism by memantine (1-amino-3,5-dimethyladamantan) Neuropharmacology. 1993;32:1337–50. doi: 10.1016/0028-3908(93)90029-3. [DOI] [PubMed] [Google Scholar]
- PARSONS CG, STOFFLER A, DANYSZ W. Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system--too little activation is bad, too much is even worse. Neuropharmacology. 2007;53:699–723. doi: 10.1016/j.neuropharm.2007.07.013. [DOI] [PubMed] [Google Scholar]
- RAMMES G, DANYSZ W, PARSONS CG. Pharmacodynamics of memantine: an update. Curr Neuropharmacol. 2008;6:55–78. doi: 10.2174/157015908783769671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAO VL, DOGAN A, TODD KG, BOWEN KK, DEMPSEY RJ. Neuroprotection by memantine, a non-competitive NMDA receptor antagonist after traumatic brain injury in rats. Brain Res. 2001;911:96–100. doi: 10.1016/s0006-8993(01)02617-8. [DOI] [PubMed] [Google Scholar]
- RASMUSSEN SA, ERICKSON JD, REEF SE, ROSS DS. Teratology: from science to birth defects prevention. Birth Defects Res A Clin Mol Teratol. 2009;85:82–92. doi: 10.1002/bdra.20506. [DOI] [PubMed] [Google Scholar]
- RILEY EP, BARRON S, DRISCOLL CD, HAMLIN RT. The effects of physostigmine on open-field behavior in rats exposed to alcohol prenatally. Alcohol Clin Exp Res. 1986;10:50–3. doi: 10.1111/j.1530-0277.1986.tb05613.x. [DOI] [PubMed] [Google Scholar]
- RILEY EP, MCGEE CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood) 2005;230:357–65. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- ROGAWSKI MA, WASTERLAIN CG, MAZARATI AM. Re: Mazarati et al. “clinically available [antiepileptic drug] with a moderate affinity for the glycine site of the N-methyl-D-aspartate (NMDA) receptor. Epilepsia. 2000;41:918–9. doi: 10.1111/j.1528-1157.2000.tb00265.x. [DOI] [PubMed] [Google Scholar]
- SANGER DJ. NMDA antagonists disrupt timing behaviour in rats. Behav Pharmacol. 1992;3:593–600. [PubMed] [Google Scholar]
- SCHNEIDER ML, MOORE CF, ADKINS MM. The effects of prenatal alcohol exposure on behavior: rodent and primate studies. Neuropsychol Rev. 2011;21:186–203. doi: 10.1007/s11065-011-9168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAH RS, LEE HG, XIONGWEI Z, PERRY G, SMITH MA, CASTELLANI RJ. Current approaches in the treatment of Alzheimer’s disease. Biomed Pharmacother. 2008;62:199–207. doi: 10.1016/j.biopha.2008.02.005. [DOI] [PubMed] [Google Scholar]
- SHOPSIN B. The clinical antidepressant effect of exogenous agmatine is not reversed by parachlorophenylalanine: a pilot study. Acta Neuropsychiatrica. 2012 doi: 10.1111/j.1601-5215.2012.00675.x. [DOI] [PubMed] [Google Scholar]
- SPEAR-SMITH J, BRIEN JF, GRAFE M, ALLRICH R, REYNOLDS JD. Chronic ethanol exposure during late gestation produces behavioral anomalies in neonatal lambs. Neurotoxicol Teratol. 2000;22:205–12. doi: 10.1016/s0892-0362(99)00059-8. [DOI] [PubMed] [Google Scholar]
- STEPANYAN TD, FAROOK JM, KOWALSKI A, KAPLAN E, BARRON S, LITTLETON JM. Alcohol withdrawal-induced hippocampal neurotoxicity in vitro and seizures in vivo are both reduced by memantine. Alcohol Clin Exp Res. 2008;32:2128–35. doi: 10.1111/j.1530-0277.2008.00801.x. [DOI] [PubMed] [Google Scholar]
- SVENSSON TH. Dysfunctional brain dopamine systems induced by psychotomimetic NMDA-receptor antagonists and the effects of antipsychotic drugs. Brain Res Brain Res Rev. 2000;31:320–9. doi: 10.1016/s0165-0173(99)00048-x. [DOI] [PubMed] [Google Scholar]
- TAJUDDIN NF, DRUSE MJ. A persistent deficit of serotonin neurons in the offspring of ethanol-fed dams: protective effects of maternal ipsapirone treatment. Brain Res Dev Brain Res. 2001;129:181–8. doi: 10.1016/s0165-3806(01)00199-7. [DOI] [PubMed] [Google Scholar]
- THOMAS JD, EDWARDS RB, CHEN WJA. MK-801 admnistered during withdrawal attenuates hippocampal CA1 pyramidal cell loss in rats neonatally exposed to alcohol. Alcohol. 2002a;26:134A. [Google Scholar]
- THOMAS JD, FLEMING SL, RILEY EP. MK-801 can exacerbate or attenuate behavioral alterations associated with neonatal alcohol exposure in the rat, depending on the timing of administration. Alcohol Clin Exp Res. 2001;25:764–73. [PubMed] [Google Scholar]
- THOMAS JD, FLEMING SL, RILEY EP. Administration of low doses of MK-801 during ethanol withdrawal in the developing rat pup attenuates alcohol’s teratogenic effects. Alcohol Clin Exp Res. 2002b;26:1307–13. doi: 10.1097/01.ALC.0000025888.60664.D9. [DOI] [PubMed] [Google Scholar]
- THOMAS JD, GARCIA GG, DOMINGUEZ HD, RILEY EP. Administration of eliprodil during ethanol withdrawal in the neonatal rat attenuates ethanol-induced learning deficits. Psychopharmacology (Berl) 2004;175:189–95. doi: 10.1007/s00213-004-1806-x. [DOI] [PubMed] [Google Scholar]
- THOMAS JD, WEINERT SP, SHARIF S, RILEY EP. MK-801 administration during ethanol withdrawal in neonatal rat pups attenuates ethanol-induced behavioral deficits. Alcohol Clin Exp Res. 1997;21:1218–25. [PubMed] [Google Scholar]
- TSAI G, COYLE JT. The role of glutamatergic neurotransmission in the pathophysiology of alcoholism. Annu Rev Med. 1998;49:173–84. doi: 10.1146/annurev.med.49.1.173. [DOI] [PubMed] [Google Scholar]
- WEDZONY K, MACKOWIAK M, ZAJACZKOWSKI W, FIJAL K, CHOCYK A, CZYRAK A. WAY 100135, an antagonist of 5-HT1A serotonin receptors, attenuates psychotomimetic effects of MK-801. Neuropsychopharmacology. 2000;23:547–59. doi: 10.1016/S0893-133X(00)00150-0. [DOI] [PubMed] [Google Scholar]
- WESEMANN W, SCHOLLMEYER JD, STURM G. Distribution of memantine in brain, liver, and blood of the rat. Arzneimittelforschung. 1982;32:1243–5. [PubMed] [Google Scholar]
- WEST JR, GOODLETT CR, BONTHIUS DJ, HAMRE KM, MARCUSSEN BL. Cell population depletion associated with fetal alcohol brain damage: mechanisms of BAC-dependent cell loss. Alcohol Clin Exp Res. 1990;14:813–8. doi: 10.1111/j.1530-0277.1990.tb01820.x. [DOI] [PubMed] [Google Scholar]
- YANG Z, ZHOU X, ZHANG Q. Effectiveness and Safety of Memantine Treatment for Alzheimer’s Disease. J Alzheimers Dis. 2013 doi: 10.3233/JAD-130395. [DOI] [PubMed] [Google Scholar]
- ZHU MY, WANG WP, BISSETTE G. Neuroprotective effects of agmatine against cell damage caused by glucocorticoids in cultured rat hippocampal neurons. Neuroscience. 2006;141:2019–27. doi: 10.1016/j.neuroscience.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]