Abstract
Background
Environmental contexts associated with drug use can trigger craving in humans and the renewal of drug-seeking behaviours in animals. Here, we tested the hypothesis that context-induced renewal of Pavlovian-conditioned alcohol-seeking is mediated by dopamine.
Methods
Male, Long-Evans rats were trained to discriminate between two, 10-sec, auditory conditioned stimuli. One stimulus (CS+) was consistently paired with 15% ethanol (v/v, 0.2 mL per CS+) and the second stimulus (CS−) was not. Each CS occurred 16 times per session, and entries into a fluid port where ethanol was delivered were measured. Pavlovian discrimination training (PDT) occurred in a distinctive context, referred to as Context A. Subsequently, behaviour was extinguished by presenting both cues without ethanol in a different context (Context B). At test, rats were injected with a dopamine D1-like receptor antagonist (R)-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (SCH 23390; 0, 3.33, 10 µg/kg; 1 mL/kg; s.c.) and presented with the CS+ and CS− without ethanol in the prior PDT context (Context A).
Results
Across training rats developed higher response levels to the alcohol-predictive CS+, compared with the CS−. Port-entries during the CS+ decreased across extinction. At test, placement into the alcohol-associated context triggered a selective increase in CS+ responses after saline, which was significantly reduced by SCH 23390 pre-treatment. In separate studies, SCH 23390 did not affect lever-pressing for sucrose under reinforced or extinction conditions, but decreased port-entries relative to saline in both cases.
Conclusions
These data indicate that dopamine is required for context-induced renewal of Pavlovian-conditioned alcohol-seeking, and may also be necessary for preparatory conditioned-approach behaviours.
Keywords: relapse, reinstatement, ethanol, SCH 23390, alcoholism
Introduction
Relapse is a significant problem faced by people with alcohol abuse disorders (McLellan et al., 1994; Monahan and Finney, 1996; Miller et al., 2001). Relapse can be facilitated by craving for alcohol (Evren et al., 2010), which may be elicited by alcohol-predictive environmental cues (Ludwig, & Wikler, 1974; Fox et al., 2007). These cues can include the sensory properties of alcohol, such as the sight, smell or taste of a preferred alcoholic beverage (Litt and Cooney, 1999). Environmental contexts associated with drug use also precipitate craving (Conklin et al., 2008), suggesting that contexts linked with alcohol consumption may provoke relapse.
The capacity of drug contexts to influence drug-seeking has been modeled using the self-administration paradigm in which rats are trained to perform an operant response that results in drug delivery. Subsequently, behavior is extinguished in a different environmental context by withholding the drug. At test, drug-seeking in the absence of drug delivery is assessed following placement into the prior drug-context, which provokes a reinstatement or ‘renewal’ of the extinguished operant response (Crombag and Shaham, 2002; Zironi et al., 2006; Fuchs et al., 2005; Hamlin et al., 2006; Chaudhri et al., 2009). Using this model researchers have found that dopamine D1-like and D2-like receptors are required for context-induced renewal of operant drug-seeking (Crombag et al., 2002; Bossert et al., 2007; Hamlin et al., 2007).
Alcohol-associated contexts can also stimulate the renewal of Pavlovian-conditioned alcohol-seeking. In this procedure rats are trained to behaviorally discriminate between two auditory stimuli, one that is paired with alcohol (CS+) and another that is not (CS−). Pavlovian-conditioned approach, assessed as entries into a fluid port where alcohol is delivered, is recorded during both cues. Once reliable discrimination is achieved, extinction is conducted in a different context where both cues are presented without alcohol. At test, presentations of the CS+ and CS− without alcohol are administered in the prior training context. This manipulation consistently triggers a selective increase in responding to the alcohol-predictive CS+, indicative of context-induced renewal of Pavlovian-conditioned alcohol-seeking (Chaudhri et al., 2010; Chaudhri et al., 2008b). Using this task, we tested the hypothesis that context-induced renewal of Pavlovian-conditioned alcohol-seeking is mediated by dopamine. Specifically, we predicted that blocking dopamine D1-like receptors (referred to hereafter as D1-receptors) with the an antagonist, SCH 23390, would attenuate renewal. Control studies examined the effects of SCH 23390 on responding to the CS+ when it was paired with alcohol, and on lever-pressing for sucrose.
Materials and Methods
Subjects
Subjects were 33 male, Long-Evans rats (Charles River, St-Constant, QC, Canada; 220–240 g on arrival). Twenty-two experimentally naïve rats were used in Experiment 1, while 11 non-naïve rats were used in Experiment 2. Rats were single-housed in plastic cages in a vivarium (21° C) maintained on a 12-hr light/dark cycle (ON at 7:00 am; procedures conducted in light phase). Access to food and water was unrestricted throughout, except as specified below. Rats were weighed and handled for 7 days after arrival. All procedures were approved by the Animal Research Ethics Committee at Concordia University, and are in accordance with recommendations from the Canadian Council on Animal Care.
Apparatus
Behaviour testing was conducted using equipment and software from Med. Associates Inc, (St. Albans, VT, USA). Twelve operant conditioning chambers (ENV-009A) in ventilated, sound attenuating cubicles were used. Chambers comprised of Plexiglas rear walls, ceiling and front wall, and stainless steel left and right walls. Floors comprised metal bars extending from rear to front. The right wall featured a central port (ENV-200R3AM) into which fluid was delivered via a 20-mL syringe located on a pump (PHM-100, 3.33 RPM) outside the sound attenuating cubicle. Entries into the port were measured by interruptions of a photo beam across its entrance. A white light (75W, 100 mA, ENV-215M) was located centrally near the ceiling on the left wall, which also featured a white noise generator (ENV-225SM, 80–85 dB) and clicker stimulus (ENV-135M, 75–80 dB). Ethanol delivery and auditory stimulus presentations were controlled by a PC computer using Med PC IV Software, which also recorded port-entries.
For Experiment 1, each chamber was assigned a context, created by the addition of visual, olfactory and tactile stimuli. For Pavlovian discrimination training sessions and renewal tests, six chambers were designated as Context 1, and six chambers were designated as Context 2. Context 1 consisted of black walls, a smooth Plexiglas floor, and a lemon odor. Context 2 consisted of clear Plexiglas walls, a wire mesh floor, and almond odor. For extinction, chambers configured as Context 1 were re-configured as Context 2, and vice versa.
For Experiment 2, chambers featured a retractable lever (ENV-112BM) on either side of the fluid port. The context in this study consisted of clear walls and a wire mesh floor which remained consistent throughout the experiment.
Drugs and solutions
95% ethanol (EtOH) was mixed in tap water to achieve a 15% ethanol solution (v/v). R(+)-SCH-23390 hydrochloride (Sigma, Oakville, ON, Canada; D054) was dissolved in sterile saline (10 or 3.33 µg/mL). Sucrose was dissolved in tap water to obtain a 10% sucrose solution (w/v). Odors were prepared by adding tap water to lemon oil (SAFC Supply Solutions, St. Louis, USA) or benzaldehyde (almond odor; OMEGA Chemical Company Inc., Levis, Canada) for final concentrations of 10% (v/v), and were applied to waste pans beneath the chamber floors.
Home-cage EtOH consumption
Before behavioural testing, rats were acclimated to the taste and pharmacological effects of EtOH in the home-cage across 12 sessions (Wise, 1973, Simms et al., 2008). On Mondays, Wednesdays and Fridays rats were weighed and then given water and EtOH in separate pre-weighed bottles. Twenty-four hours later each bottle was weighed and only the water bottle was placed back on the cage. Thus, on Saturdays, Sundays, Tuesdays and Thursdays rats received water only. Positions of water and EtOH bottles were switched after each session to mitigate side preferences. Spillage was accounted for by subtracting the amount of EtOH lost from bottles placed on empty cages from average EtOH intake during corresponding sessions. EtOH intake escalated across sessions (see Supplementary Fig. 1).
Experiment 1a: Effect of SCH 23390 on context-induced renewal of Pavlovian-conditioned alcohol-seeking
Pavlovian Discrimination Training
Rats were first habituated to the behaviour testing room (15 min/day, 2 days), and then exposed to Context 1 and Context 2 in single 20-min sessions with the chamber light illuminated. Subsequently, Pavlovian discrimination training (PDT) was conducted across 20 daily (Mon-Fri) 54-min sessions. Session onset was indicated by illumination of the chamber light 5-min after placement into the chamber. In each session, 16 random presentations each of a 10-sec continuous white noise and a 10-sec clicker stimulus (2 Hz) were delivered according to a variable-time 67-sec schedule. One of the two stimuli was designated the CS+ and 0.2 mL of EtOH was delivered into the fluid receptacle 4-sec after the onset of each CS+ presentation. The second stimulus (CS−) was not paired with EtOH. A total of 3.2 mL of EtOH was delivered per session, resulting in EtOH intake of 0.74 ± 0.02 g/kg averaged across the last 2 PDT sessions. Ports were checked at the end of each session to ensure that EtOH was consumed.
Before starting PDT rats were counterbalanced into Contexts 1 and 2 based on home-cage EtOH consumption (average of last 2 days). The context used for training and renewal tests, referred to as Context A, was kept consistent for each rat. The use of white noise or clicker as the CS+ was counterbalanced across context. During PDT subcutaneous (s.c.) injections of saline were administered in the colony room after PDT session 17, and then in the behavioural testing room before PDT session 19.
Extinction and renewal tests
Following PDT, extinction was conducted in the context not used during PDT (referred to as Context B). During each extinction session the CS+ and CS− were presented as during PDT, but EtOH was withheld. A saline injection (s.c.) was administered before extinction session 3.
The impact of SCH 23390 (0, 3.33 & 10 µg/kg; 1 mL/kg; s.c.) on renewal was assessed using a counterbalanced, within-subject design. The first renewal test was conducted 24-hrs after extinction session 9. Subsequently, 3 PDT re-training sessions and 6 re-extinction sessions were conducted before renewal tests 2 & 3. To test renewal rats were placed into the prior PDT context (Context A) and presented with the CS+ and CS− as during PDT, but without EtOH. Saline or SCH 23390 injections occured 15–20-min before test.
To achieve a balanced within-subject design each dose of SCH 23390 (0, 3.33 & 10 µg/kg) was also administered before extinction tests that occurred 48-hrs before each renewal test. Thus, extinction tests were separated from renewal tests by a single extinction session. Each rat received 3 extinction tests and 3 renewal tests, with the same treatment administered before pairs of tests.
Experiment 1b: Effect of SCH 23390 on Pavlovian-conditioned alcohol-seeking during PDT
Following the third renewal test, 5 sessions of PDT re-training were conducted in Context A. Injections of saline or 10 µg/kg of SCH 23390 (1 mL/kg, s.c.) were administered before sessions 3 & 5, according to a counterbalanced, within-subject design.
Experiment 2a: Effect of SCH 23390 on lever-pressing for sucrose under extinction conditions
Lever-press training
Rats (n=11, used previously in a Pavlovian conditioning experiment with sucrose) were water deprived for 24-hrs and given a single, 12-hr lever-press training session which began with the onset of the chamber light and the insertion of a response lever located to the right of the fluid receptacle. Lever-presses were reinforced with 10% sucrose (0.1 mL over 3-sec) on a fixed-ratio 1 (FR1) schedule. Upon earning 200 reinforcers the chamber light turned off and the lever retracted to indicate the end of the session.
Sucrose self-administration
Twenty-four hours after lever-press training, instrumental conditioning sessions commenced (Mon-Fri; 30-min/session). Five minutes after initiating the session the chamber light was illuminated and two response levers were inserted into the chamber. Presses on the right (active) lever resulted in sucrose (0.1 mL over 3-sec; FR1), whereas presses on the left (inactive) lever were recorded but had no programmed consequences. Saline injections were administered after session 5 and before session 6 of sucrose self-administration.
Test
To investigate the impact of SCH 23390 on sucrose-seeking under extinction conditions, rats received an injection of saline (1 mL/kg; n=6) or SCH 23390 (10 µg/kg; 1 mL/kg; s.c.; n=5) before session 7, in which active lever-pressing no longer resulted in sucrose. A second test was conducted on session 11, utilizing a within-subject, counterbalanced design in which each rats was tested under each treatment. Tests were separated by 3 sucrose self-administration sessions.
Experiment 2b: Effect of SCH 23390 on lever-pressing for sucrose
Subjects from Experiment 2a were used to test the impact of SCH 23390 on sucrose self-administration when active lever-pressing resulted in sucrose delivery. Rats were allowed to self-administer sucrose as described above for four sessions. Before the last session they received an injection (1mL/kg; s.c.) of saline (n=6) or 10 µg/kg SCH 23390 (n=5).
Statistical Analyses
Dependent measures from Experiment 1 included (i) normalized port-entries during each CS (port-entries during CS minus port-entries during corresponding 10-sec, pre-CS); (ii) total number of port-entries per session; (iii) number of port-entries during each CS+ and CS− trial at test; (iv) non-CS+ responding (total port-entries minus port-entries during CS+).
Data from PDT and extinction were analysed separately using ANOVA with Session (PDT sessions 1–20 or extinction sessions 21–29) and Normalized CS (CS+, CS−) as within-subject variables. Total port-entries were analyzed across Session. The impact of SCH 23390 on renewal was analyzed using ANOVA with Phase (Extinction, Renewal), Dose (0, 3.33, 10 µg/kg) and Normalized CS (CS+, CS−) as within-subject, repeated measures. Total port-entries and non-CS+ responding were analyzed across Phase and Dose. The number of port-entries made during each CS+ and CS− trial during renewal tests were analyzed across the within-subject, repeated-measures of Dose, CS, and Trial (1–16).
Dependent variables from Experiment 2 were (i) number of active lever-presses; (ii) number of inactive lever-presses; (iii) total number of port-entries per session. In addition, patterns of responding were assessed by averaging active lever-presses and port-entries across 1-min time bins during each test.
Training data were analyzed using repeated measures ANOVA with Session (training day 1–6) and Lever (active, inactive) as within-subject, repeated measures. Test data were analysed using ANOVA with Lever as a within subject variable and Treatment (saline, SCH 23390) as a within-subject (Exp 2a) or between-subject (Exp 2b) variable. Similar analyses were conducted on port-entry data. Time-course analyses utilized Time (bins 1–30) as a within-subject repeated measure and Treatment as a within-subject (Exp 2a) or between-subject (Exp 2b) variable
The Huynh Feltd correction was used for significant violations of homogeneity as determined by the Mauchly sphericity test. Significant main effects and interactions were pursued using targeted ANOVA, with post-hoc t-tests for paired or independent samples. Analyses were conducted using SPSS (version 11.0) with a significance level of α=0.05.
Results
Experiment 1: Effect of SCH 23390 on context-induced renewal of Pavlovian-conditioned alcohol-seeking
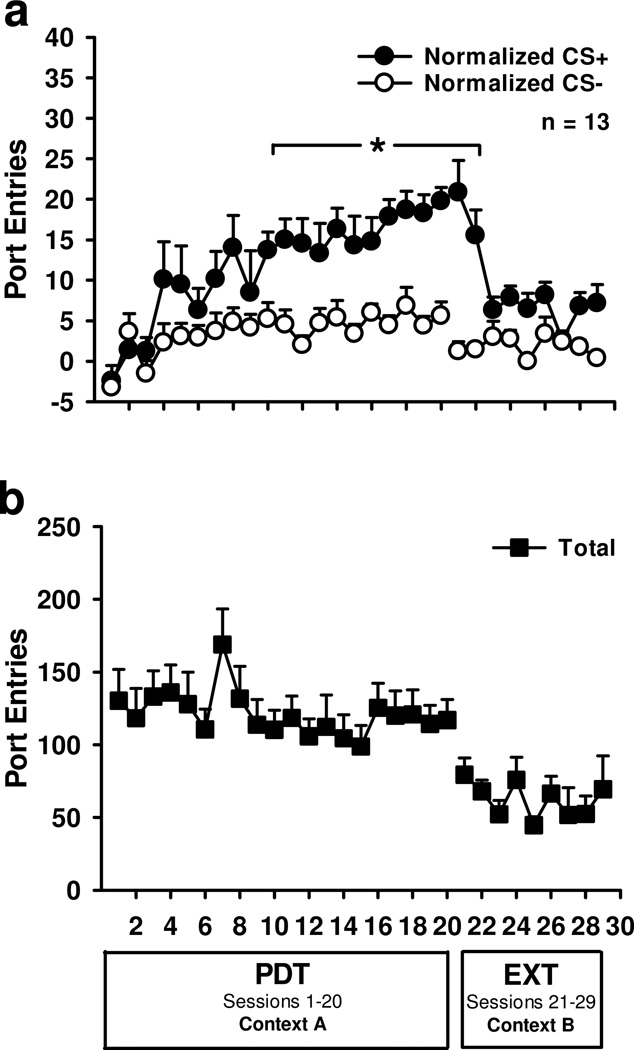
Rats learned to discriminate between the alcohol-predictive CS+ and the CS− across PDT sessions in Context A (Fig. 1a). CS responding increased across session [Session, F19,228=5.59, p<0.001], with CS+ responding stabilizing at a higher level than CS− responding [CS, F1,12=63.94, p<0.001; Session × CS, F19,228=2.57, p<0.01]. Conditioned responding, particularly to the CS+, decreased across extinction sessions in Context B (Fig. 1a) where alcohol was withheld [Session, F8,96=5.09, p<0.001; CS, F1,12=30.29, p<0.001; Session × CS, F8,96=6.48, p<0.001]. The total number of port-entries made per session (Fig. 1b) remained stable across PDT [Session, F19,228=1.42, p>0.05 and extinction [Session, F8,96=1.30, p>0.05].
Figure 1.
Acquisition and extinction of Pavlovian discrimination training. A Mean (± SEM) normalized port-entries during the CS+ (filled circles) and CS− (open circles). B Mean (± SEM) total port-entries. During Pavlovian discrimination training (PDT) in Context A each CS+ trial was paired with alcohol, whereas the CS− was presented without alcohol. During extinction (EXT) in Context B the CS+ and CS− were presented without alcohol. * p < 0.05, normalized CS+ > normalized CS−.
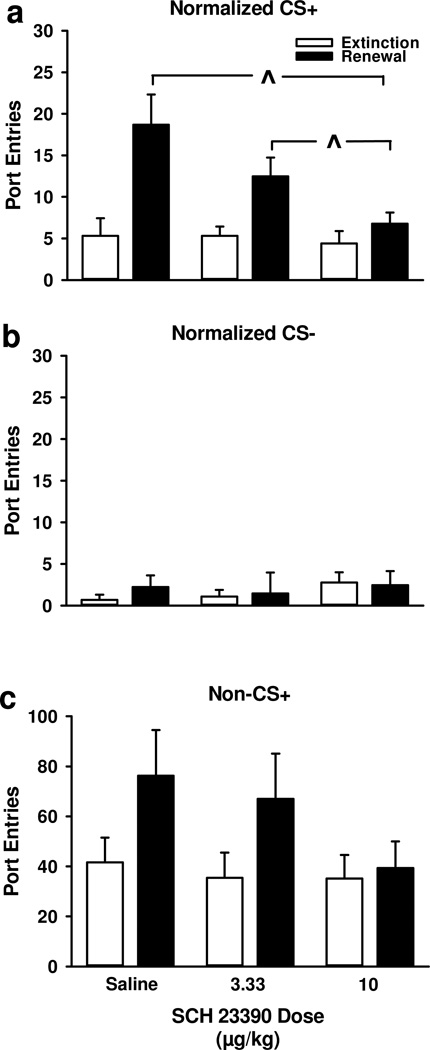
Presentations of the CS+ and CS− without alcohol in Context A following extinction in Context B caused a significant renewal of CS+ responding, with no effect on CS− responding (Fig. 2a and 2b). Furthermore, blocking dopamine D1-receptors significantly attenatued the renewal of CS+ responding (Fig. 2a), with no effect on CS− responding (Fig. 2b). Because the omnibus ANOVA revealed a statistically significant Dose × CS interaction [F2, 24=5.13, p<0.05], separate analyses were conducted on normalized CS+ and normalized CS− responding. ANOVA verified that SCH 23390 selectively attenuated the renewal of CS+ responding [Phase, F1, 12 =33.83, p<0.001; Dose, F2, 24=4.38, p<0.05; Phase × Dose, F2, 24=3.45, p<0.05], with no effect on CS− responding [Phase, F1, 12=0.10, p>0.05; Dose, F2, 24=0.63, p>0.05; Phase × Dose, F2, 24=0.54 p>0.05]. Paired-samples t-tests on CS+ responding found that compared to extinction, significant renewal was observed after injections of saline [t12=−3.91, p<0.01] and 3.33 µg/kg SCH 23390 [t12=−2.85, p<0.05], but not after the 10 µg/kg dose [t12=−1.09, p>0.05]. The 10 µg/kg dose of SCH 23390 significanly reduced CS+ responding compared to saline [t12=3.00, p<0.05] and 3.33 µg/kg SCH 23390 [t12=2.32, p<0.05]. There was no difference between saline and 3.33 µg/kg SCH 23390 [t12=1.36, p>0.05].
Figure 2.
Blocking dopamine D1-receptors reduced context-induced renewal of Pavlovian-conditioned alcohol-seeking. Data represent entries into the fluid port during extinction tests in Context B (open bars) and renewal tests in Context A (filled bars) following injections of saline or SCH 23390 (3.33, 10 µg/kg; 1 mL/kg, s.c.). Rats received the same treatment before pairs of extinction and renewal tests. A Mean (± SEM) normalized port-entries during the CS+. B Mean (± SEM) normalized port-entries during the CS−. C Mean (± SEM) responding during non-CS+ intervals. ^ p < 0.05, significantly different from 10 µg/kg SCH 23390.
To determine if D1-receptors are involved in alcohol-seeking that is not elicited by the CS+, port-entries made during non-CS+ intervals (Fig 2c) were analyzed. Compared to extinction, there was a significant increase in non-CS+ responding during renewal tests [Phase, F1, 12=5.71, p<0.05], which was not affected by SCH 23390 [Dose, F2, 24=2.32, p>0.05; Phase × Dose, F2,24=1.99, p>0.05].
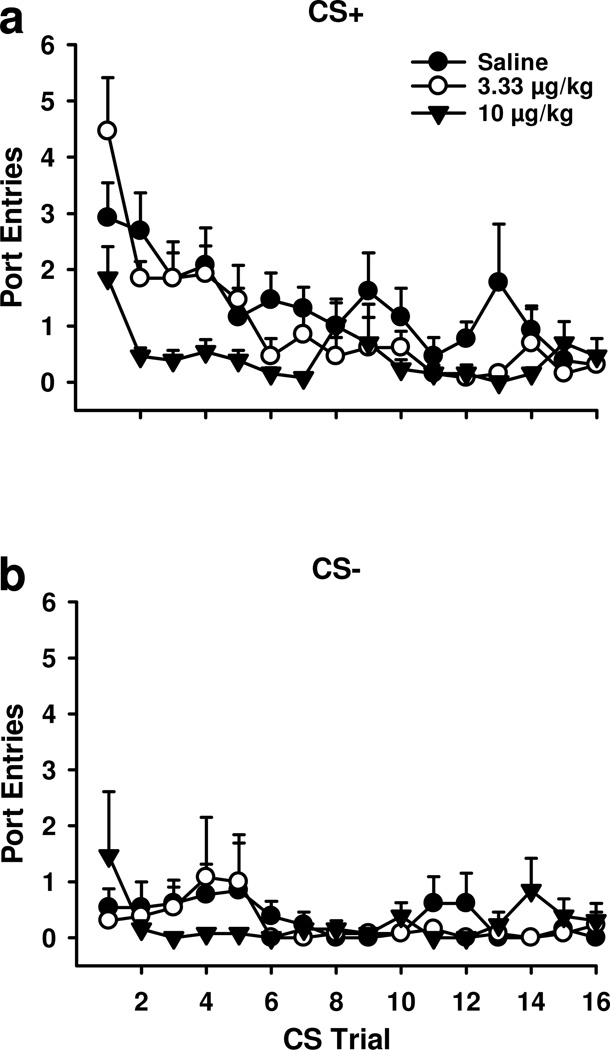
To determine how SCH 23390 affected alcohol-seeking across renewal the number of port-entries made during each CS+ and CS− trial at test (Figs 3a and 3b) were analyzed. Overall, rats responded more to the CS+ than the CS− [CS, F1, 12=47.35, p<0.001] and more at the start of the session than the end [Trial, F15, 180=6.58, p<0.001]. Responding to the CS+ but not the CS− decreased as a function of trial [Trial × CS, F15, 180=3.11, p<0.001]. Blocking dopamine D1-receptors reduced responding [Dose, F2, 24=7.51, p<0.01], specifically of port-entries triggered by the CS+ [Dose × CS, F2, 24=6.20, p<0.01]. Trial × Dose [F30, 360=1.36, p>0.05] and Trial × Dose × CS [F30, 360=1.30, p>0.05] interactions were not significant.
Figure 3.
Blocking dopamine D1-receptors reduced port-entries across CS+ trials during renewal tests. Port-entries during each CS trial following saline (filled circles), 3.33 µg/kg SCH 23390 (open circles) and 10 µg/kg SCH23390 (triangles) are depicted. A Mean (± SEM) port-entries during each CS+ trial. Compared to saline and 3.33 µg/kg SCH 23390, 10 µg/kg of SCH 23390 significantly reduced CS+ responding across trials. B Mean (± SEM) port-entries during each CS− trial. There was no effect of SCH 23390 on CS− responding.
Follow-up ANOVA verified that SCH 23390 selectively affected CS+ responding [Trial, F15, 180=8.49, p<0.001; Dose, F2, 24=8.18, p<0.01; Trial × Dose, F30, 360=1.60, p<0.05] and not CS− responding [Trial, F(15, 180)=0.94, p>0.05; Dose, F2, 24=0.37, p>0.05; Trial × Dose, F30, 360=0.93, p>0.05]. Compared to saline, there was no impact of 3.33 µg/kg SCH 23390 on CS+ responding [Dose, F1, 12=2.15, p>0.05; Trial, F15, 180=7.02, p<0.001; Dose × Trial, F15, 180=1.12, p>0.05]. However, 10 µg/kg SCH 23390 significantly reduced CS+ responding compared to saline [Dose, F1, 12=12.77, p<0.01; Trial, F15, 180=3.53, p<0.001; Dose × Trial, F15, 180=1.37, p>0.05] and compared to 3.33 µg/kg SCH 23390 [Dose, F1, 12=10.81, p<0.01; Trial, F15, 180=7.67, p<0.001; Dose × Trial, F15, 180=2.63, p<0.01].
Because CS+ responding was maximal on the first trial of each renewal test, we analyzed this measure and found a significant effect of Dose [F2, 24=3.53, p<0.05]. Subsequent t-tests for paired samples revealed no difference between saline and the low [t12=−1.73, p>0.05] or high doses of SCH 23390 [t12=1.14, p>0.05]. However, port-entries during the first CS+ trial were reduced after 10 µg/kg SCH 23390, compared to the low dose [t12=2.35, p<0.05].
Latency to the first port-entry made during renewal tests was analyzed to determine if SCH 23390 influenced motor behaviour. There was no effect of SCH 23390 on this (Mean ± SEM: Saline = 62.84 ± 17.83; 3.33 µg/kg = 38.52 ± 6.39; 10 µg/kg = 46.33 ± 14.60) [Dose, F2, 24=0.73, p>0.05].
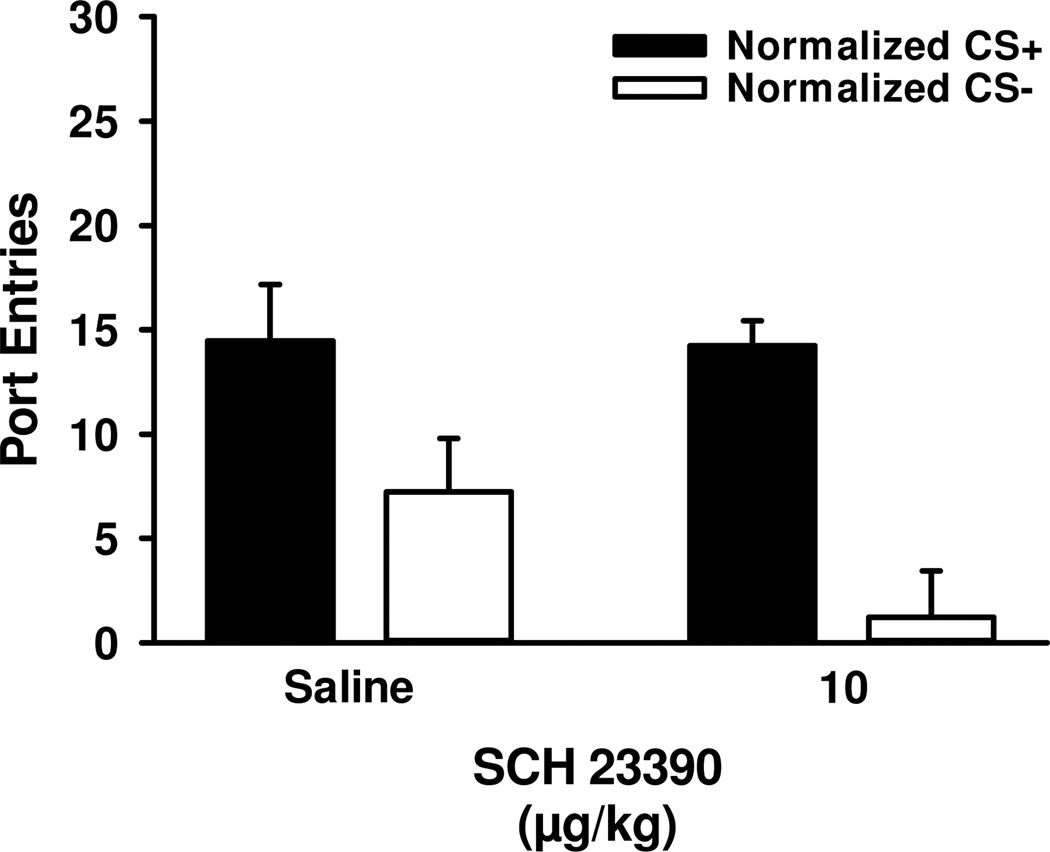
SCH 23390 had no effect on Pavlovian-conditioned alcohol-seeking when the CS+ was paired with alcohol during PDT sessions [Fig. 4; Dose, F1, 12=2.04, p>0.05; CS, F1,12=12.47, p<0.01; Dose × CS, F1,12=1.66, p>0.05].
Figure 4.
Blocking dopamine D1-receptors had no impact on Pavlovian-conditioned alcohol-seeking when each CS+ trial was paired with alcohol. Data represent mean (± SEM) normalized port-entries during the CS+ (filled bars) and CS− (open bars), following saline or 10 µg/kg SCH 23390.
Experiment 2a: Effect of SCH 23390 on lever-pressing for sucrose under extinction conditions
Rats acquired lever-pressing for sucrose during self-administration sessions (see Table 1). There was no impact of SCH 23390 on lever-pressing when active lever responding was not reinforced with sucrose (Fig. 5a). Rats discriminated between the levers [Lever, F1, 10=12.63, p<0.01], with no differences as a function of Treatment [F1, 10=0.75, p>0.05; Treatment × Lever, F1, 10=1.18, p>0.05]. An analysis of the pattern of active lever responding (Fig. 5b) indicated that responding decreased across the session [Time, F24, 240=25.36, p<0.001], with no differences as a function of Treatment [F1, 10=1.42, p>0.05; Time × Treatment, F24, 240=0.89, p>0.05].
Table 1.
Mean ± SEM responses on the active and inactive levers. Data are averaged across the last 2 sessions of sucrose self-administration.
| Active Lever | Inactive Lever | |
|---|---|---|
| Experiment 2a | ||
| (n=11) | 180.05 ± 7.96 * | 5.95 ± 0.90 |
| Experiment 2b | ||
| Saline (n=6) | 198.58 ± 7.69 * | 3.75 ± 1.07 |
| SCH 23390 (n=5) | 193.30 ± 25.24 * | 1.9 ± 0.38 |
p < 0.05, Active > Inactive
Figure 5.
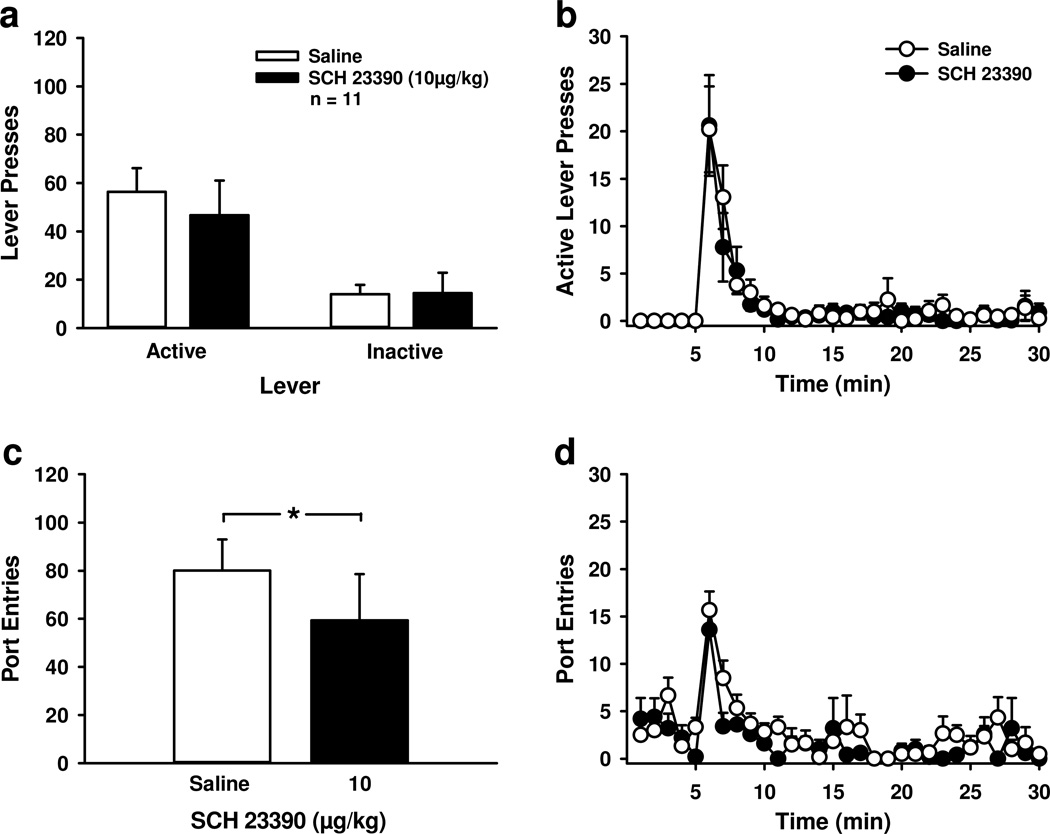
Blocking dopamine D1-receptors had no impact on lever-pressing under extinction conditions, but reduced total port-entries. Rats were trained to self-administer sucrose; however, at test active lever-presses had no programmed consequences. A Mean (± SEM) active and inactive lever-presses following saline (open bars) or 10 µg/kg SCH 23390 (filled bars) B Mean (± SEM) active lever-presses per minute following saline (open circles) or SCH 23390 (filled circles). Levers were inserted into the chambers at minute 5. C Mean (± SEM) total port-entries following saline (open bar) or SCH 23390 (filled bar). D Mean (± SEM) port-entries per minute following saline (open circles) or SCH 23390 (filled circles). * p < 0.05, saline > SCH 23390.
In contrast to lever-pressing, SCH 23390 reduced the total number of port-entries at test [Fig. 5c; t10=2.41, p<0.05]. The distribution of port-entries across session is depicted in Figure 5d. Separate analyses were conducted on data from the first 5-min when levers were retracted, and the remaining 25-min when levers were extended. While there was a trend for port-entries to decrease across the first 5-min [Time, F4, 40=2.51, p=0.057], there was no effect of SCH 2339 on port-entries at this time [Treatment, F1, 10=2.62, p>0.05; Time × Treatment, F4, 40=1.76, p>0.05]. When levers were available, port-entries did not differ as a function of Treatment [F1, 10=1.38, p>0.05]. However, there was a significant reduction in port-entries across session [Time, F24, 240=13.58, p<0.001] and a near significant Time × Treatment interaction [F24, 240=1.56, p=0.05]. Paired-samples t-tests indicated less responding following SCH 23390 compared to saline at minutes 7 and 24 (p<0.05 for each comparison).
Experiment 2b: Effect of SCH 23390 on lever-pressing for sucrose
Compared to saline, there was no impact of SCH 23390 on active lever-pressing that was reinforced with sucrose (Fig. 6a). Rats responded more on the active lever than the inactive lever [Lever, F1, 9=78.21, p<0.001], with no main effect of Treatment [F1, 9=0.64, p>0.05] or Treatment × Lever interaction [F1, 9=0.63, p>0.05]. Active lever-pressing decreased across session (Fig. 6b) for both groups [Time, F24, 216=15.71 p<0.001; Time × Treatment, F24, 216=0.59, p>0.05], with no effect of SCH 23390 on the pattern of active lever responses [Treatment, F1, 9=0.63, p>0.05].
Figure 6.
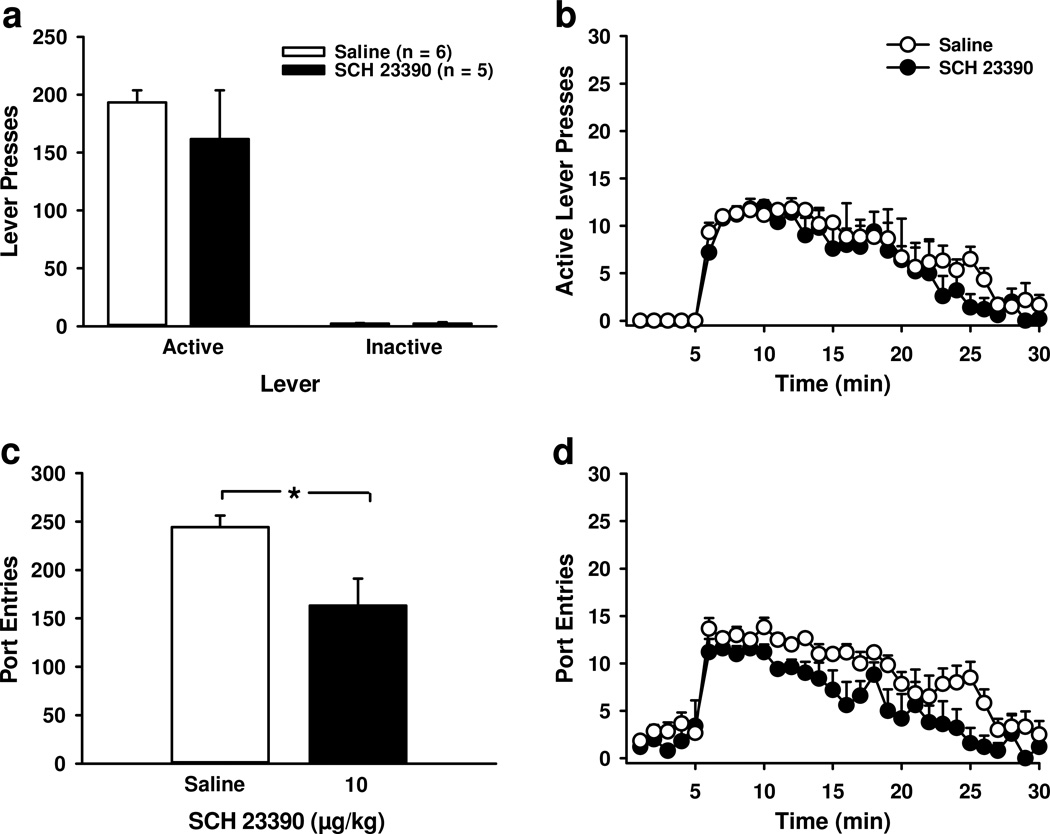
Blocking dopamine D1-receptors had no impact on lever-pressing for sucrose, but reduced total port-entries. Each responses on the active lever resulted in the delivery of 0.1 mL of sucrose into the fluid receptacle. A Mean (± SEM) active and inactive lever-presses following saline (open bars) or SCH 23390 (filled bars) B Mean (± SEM) active lever-presses per minute following saline (open circles) or SCH 23390 (filled circles). Levers were inserted into the chambers at minute 5. C Mean (± SEM) total port-entries following saline (open bar) or SCH 23390 (filled bar). D Mean (± SEM) port-entries per minute following saline (open circles) or SCH 23390 (filled circles). * p < 0.05, saline > SCH 23390.
Consistent with Experiment 2a, SCH 23390 reduced total port-entries at test (Fig. 6c) relative to saline [t9=−2.67, p<0.05]. Responding remained stable across the first 5-min (Fig. 6d) [Time, F4, 36=0.60, p>0.05] and did not differ as a function of Treatment [F1, 9=1.19, p>0.05; Time × Treatment, F4, 36=0.47, p>0.05]. For the remaining 25-min, port-entries decreased as a function of Time [F24, 216=17.27, p<0.001; Time × Treatment, F24, 216=0.77, p>0.05], with SCH 23390 reducing port-entries relative to saline [Treatment, F1, 9=8.28, p<0.05].
Discussion
Placement into an alcohol context following extinction in a different context triggered the renewal of Pavlovian-conditioned alcohol-seeking, which was significantly attenuated by the dopamine D1 receptor antagonist, SCH 23390. In contrast, SCH 23390 had no effect on Pavlovian-conditioned alcohol-seeking in tests where the CS+ was paired with alcohol. In separate studies, instrumental responding was not affected by SCH 23390 under conditions where lever-pressing either did or did not result in sucrose. However, rats made fewer entries into the fluid port following SCH 23390, compared to saline. These results indicate that dopamine D1-receptors are required for context-induced renewal of Pavlovian-conditioned alcohol-seeking. That SCH 23390 had no impact on instrumental responding but consistently reduced port-entries suggests that D1-receptors are particularly important for preparatory conditioned approach behaviours that bring organisms into contact with appetitive (positive) outcomes (Blackburn et al., 1987; Phillips et al., 1993; Blackburn et al., 1989).
The present research studied the impact of systemic SCH 23390 on a number of behaviours in order to generate a comprehensive understanding of the role of dopamine D1-receptors in renewal. Our finding of interest, that context-induced renewal of Pavlovian-conditioned alcohol-seeking was reduced by SCH 23390, is consistent with results obtained using instrumental, alcohol-conditioning procedures (Hamlin et al., 2007; Chaudhri et al., 2009; Liu and Weiss, 2002) and, for the first time, extends a role for dopamine in mediating the impact of context on Pavlovian-conditioned alcohol-seeking. Dopamine in the nucleus accumbens increases upon placement into an alcohol-associated context, suggesting a role for dopamine in context-mediated anticipation of appetitive outcomes (Katner and Weiss, 1999). Blocking D1-receptors might impair the capacity of the context to signal alcohol, resulting in less renewal.
An alternate explanation is that blocking dopamine D1-receptors reduced the incentive-motivational properties of the alcohol-predictive CS+ in the alcohol-associated context, resulting in less renewal (See et al., 2001). This possibility is supported by analyses of port-entries made during consecutive CS+ trials at test. As expected, responding decreased as a function of trial in the absence of alcohol delivery. Interestingly, there was no difference between saline and SCH 23390 conditions in the number of port-entries made during the first CS+ trial at test: however, compared to saline and the low dose of SCH 23390, CS+ responding was reduced across trials following 10 µg/kg of SCH 23390. These results suggest that dopamine D1-receptors may be particularly important for the maintenance of alcohol-seeking behaviour in the absence of alcohol delivery. This interpretation is consistent with pharmacological data showing that dopamine D1-receptors are required for the reinstatement of alcohol-seeking in operant models (Liu and Weiss, 2002), and with an established role for dopamine in the acquisition and expression of incentive salience by appetitive Pavlovian cues (Berridge and Robinson, 1998; Flagel et al., 2011; Saunders and Robinson, 2012).
An important consideration in the interpretation of the present data is the extent to which the reduction in renewal might be attributable to SCH 23390 producing a non-specific motor deficit that could have impaired port-entry responding. To address this issue we examined latency to the first port-entry response during renewal tests, and found no differences as a function of treatment. Furthermore, there was no impact of SCH 23390 on the number of port-entries made during the first CS+ trial during renewal tests. An analysis of port-entry responses made outside CS+ intervals indicated that while placement into the alcohol context triggered a renewal of generalized alcohol-seeking behaviour, there was no impact of SCH 23390 on this effect. Furthermore, in a separate control experiment, SCH 23390 did not alter lever-pressing under conditions in which responding either did or did not result in sucrose delivery. The latter findings are in agreement with published studies that find no impact of SCH 23390 (10 µg/kg) on sucrose self administration (Crombag et al., 2002), and together these results suggest that SCH 23900 did not produce a motor deficit that could account for reductions in renewal.
In contrast to the effect of SCH 23390 on renewal, blocking D1-receptors did not affect port-entries triggered by the CS+ when it was paired with alcohol during PDT sessions. While this result suggests that Pavlovian-conditioned alcohol-seeking in the presence of alcohol delivery does not require D1-receptors, it should be interpreted with caution because it is inconsistent with our unpublished data showing a small but statistically significant reduction in CS+ responding during PDT (Sparks & Chaudhri, unpublished data). A reduction in Pavlovian-conditioned alcohol-seeking following SCH 23390 might be expected given that dopamine is necessary for the acquisition and expression of conditioned approach and preparatory behaviours triggered by appetitive cues (Blackburn et al., 1987; Pfaus and Phillips, 1991; Parkinson et al., 2002; Di Ciano et al., 2001). Furthermore, blocking dopamine D1-receptors systemically attenuates alcohol consumption (Dyr et al., 1993; Sabino et al., 2013) and when injected into the nucleus accumbens core or bed nucleus of the stria terminalis, SCH 23390 decreases operant alcohol self-administration (Hodge et al., 1997; Eiler et al., 2003), suggesting a role for these receptors in alcohol-conditioned behaviours. Furthermore, blocking dopamine D1-receptors in the nucleus accumbens core of mice prevents the acquisition but not expression of alcohol-induced conditioned place preference, emphasizing a role for these receptors in learned associations between alcohol and contextual cues (Young et al., 2013). In light of these reports, a more detailed analysis of the role of the dopaminergic system in the acquisition and expression of Pavlovian-conditioned responding to alcohol-predictive cues is warranted.
In concurrence with the literature, we found that SCH 23390 did not affect sucrose self-administration (Crombag et al., 2002). Our results also indicate that there was also no impact of SCH 23390 on lever-pressing under extinction conditions. The latter observation differs from recent evidence that blocking dopamine D1-receptors reduces lever-pressing in extinction following 1-day of forced abstinence from sucrose self-administration (Grimm et al., 2011). This difference may be accounted for by the fact that active lever-pressing in the present study had no programmed consequences at test, whereas in the study by Grimm and colleagues it resulted in presentations of a discrete, compound stimulus that had been paired with sucrose during self-administration. Thus, dopamine may be particularly important for instrumental responding that is reinforced by reward-paired cues, potentially through effects at D1-receptors in the nucleus accumbens core (Floresco et al., 2008).
Interestingly, SCH 23390 produced a small but significant reduction in entries into the fluid port in tests where lever-pressing either did or did not result in sucrose. To the extent that approach to a fluid port can be considered a ‘preparatory response’ that brings rats into contact with the appetitive outcome (sucrose) in the fluid chamber, the present findings are consistent with evidence that the dopaminergic system is involved in this form of anticipatory behaviour (Blackburn et al., 1987). This explanation is particularly compelling given the relation between lever-pressing and port-entries illustrated in Figures 5 and 6. Compared to the first 5-min of the test sessions, port-entries were dramatically elevated in the time-bin immediately following insertion of the levers into the chambers, suggesting that approach and entry into the fluid port is linked with anticipation of receiving sucrose. Furthermore, the effect of SCH 23390 was only apparent on port-entries made following lever insertion. Interestingly, if dopamine D1-receptors are required for preparatory conditioned approach responses, this explanation may also pertain to the reduction in renewal observed in experiment 1.
The present results can be extended in several ways, with important direction provided by renewal studies conducted using the operant drug self-administration procedure. For instance, blocking dopamine D1-receptors in the nucleus accumbens attenuates context-induced reinstatement of instrumental alcohol-seeking (Chaudhri et al., 2008a, Chaudhri et al., 2009) and heroin seeking (Bossert et al., 2007) in rats. It would be of interest to determine if the same is true for the renewal of Pavlovian-conditioned alcohol-seeking, particularly in light of data indicating that pharmacologically inactivating the nucleus accumbens attenuates context-induced renewal of Pavlovian-conditioned alcohol-seeking (Chaudhri et al., 2010). Additionally, systemic injections of the dopamine D2-receptor antagonist raclopride attenuate context-induced reinstatement of instrumental cocaine-seeking (Crombag et al., 2002). Thus, future research should explore the differential effects of localized injections of dopamine D1- and D2-receptor antagonists on context-induced renewal of Pavlovian-conditioned alcohol-seeking.
In conclusion, we have shown that environmental contexts associated with alcohol can elicit the renewal of Pavlovian-conditioned alcohol-seeking in rats, and that this effect requires dopamine transmission at D1-like receptors. Our data support the hypothesis that dopamine may be particularly important for guiding conditioned approach behaviours that bring organisms into contact with primary, appetitive outcomes. Understanding how dopamine is involved in renewal, as well in the acquisition and expression of Pavlovian-conditioned alcohol-seeking, will advance our knowledge of the multiple factors that influence the development and longevity of alcohol abuse in humans.
Supplementary Material
Acknowledgments
This research was supported by ABMRF/The Foundation for Alcohol Research (NC) and the National Institute of Alcohol Abuse and Alcoholism (RO1 AA14925; Patricia H. Janak, Ph. D, University of California at San Francisco). JMS received a graduate fellowship from the Canadian Institutes of Health Research. NC is the recipient of a Chercheur-Boursier award from Fonds de la recherché en santé Quebec, and is a member of the FRQS-funded CSBN/GRNC. We would like to thank Ida Golovkin for assistance in running Experiment 1.
References
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Blackburn JR, Phillips AG, Fibiger HC. Dopamine and preparatory behavior: I. Effects of pimozide. Behav Neurosci. 1987;101:352–360. doi: 10.1037//0735-7044.101.3.352. [DOI] [PubMed] [Google Scholar]
- Blackburn JR, Phillips AG, Jakubovic A, Fibiger HC. Dopamine and preparatory behavior: II. A neurochemical analysis. Behav Neurosci. 1989;103:15–23. doi: 10.1037//0735-7044.103.1.15. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Cone JJ, Janak PH. Reinstated ethanol-seeking in rats is modulated by environmental context and requires the nucleus accumbens core. Eur J Neurosci. 2008a;28:2288–2298. doi: 10.1111/j.1460-9568.2008.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Janak PH. Context-induced relapse of conditioned behavioral responding to ethanol cues in rats. Biol Psychiatry. 2008b;64:203–210. doi: 10.1016/j.biopsych.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Janak PH. Ethanol seeking triggered by environmental context is attenuated by blocking dopamine D1-receptors in the nucleus accumbens core and shell in rats. Psychopharmacology (Berl) 2009;207:303–314. doi: 10.1007/s00213-009-1657-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Schairer WW, Janak PH. Separable roles of the nucleus accumbens core and shell in context- and cue-induced alcohol-seeking. Neuropsychopharmacology. 2010;35:783–791. doi: 10.1038/npp.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Robin N, Perkins KA, Salkeld RP, McClernon FJ. Proximal versus distal cues to smoke: the effects of environments on smokers' cue-reactivity. Exp Clin Psychopharmacol. 2008;16:207–214. doi: 10.1037/1064-1297.16.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Grimm JW, Shaham Y. Effect of dopamine receptor antagonists on renewal of cocaine seeking by reexposure to drug-associated contextual cues. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2002;27:1006–1015. doi: 10.1016/S0893-133X(02)00356-1. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci. 2002;116:169–173. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Cardinal RN, Cowell RA, Little SJ, Everitt BJ. Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of pavlovian approach behavior. J Neurosci. 2001;21:9471–9477. doi: 10.1523/JNEUROSCI.21-23-09471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyr W, McBride WJ, Lumeng L, Li TK, Murphy JM. Effects of D1 and D2 dopamine receptor agents on ethanol consumption in the high-alcohol-drinking (HAD) line of rats. Alcohol. 1993;10:207–212. doi: 10.1016/0741-8329(93)90037-o. [DOI] [PubMed] [Google Scholar]
- Eiler WJ, 2nd, Seyoum R, Foster KL, Mailey C, June HL. D1 dopamine receptor regulates alcohol-motivated behaviors in the bed nucleus of the stria terminalis in alcohol-preferring (P) rats. Synapse. 2003;48:45–56. doi: 10.1002/syn.10181. [DOI] [PubMed] [Google Scholar]
- Evren C, Cetin R, Durkaya M, Dalbudak E. Clinical factors associated with relapse in male alcohol dependents during six-month follow-up. Bulletin of Clinical Psychopharmacology. 2010;20:14–22. [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, McLaughlin RJ, Haluk DM. Opposing roles for the nucleus accumbens core and shell in cue-induced reinstatement of food-seeking behavior. Neuroscience. 2008;154:877–884. doi: 10.1016/j.neuroscience.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Fox HC, Bergquist KL, Hong KI, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcohol Clin Exp Res. 2007;31:395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Harkness JH, Ratliff C, Barnes J, North K, Collins S. Effects of systemic or nucleus accumbens-directed dopamine D1 receptor antagonism on sucrose seeking in rats. Psychopharmacology (Berl) 2011;216:219–233. doi: 10.1007/s00213-011-2210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Blatchford KE, McNally GP. Renewal of an extinguished instrumental response: neural correlates and the role of D1 dopamine receptors. Neuroscience. 2006;143:25–38. doi: 10.1016/j.neuroscience.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Newby J, McNally GP. The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience. 2007;146:525–536. doi: 10.1016/j.neuroscience.2007.01.063. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Samson HH, Chappelle AM. Alcohol self-administration: further examination of the role of dopamine receptors in the nucleus accumbens. Alcohol Clin Exp Res. 1997;21:1083–1091. doi: 10.1111/j.1530-0277.1997.tb04257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katner SN, Weiss F. Ethanol-associated olfactory stimuli reinstate ethanol-seeking behavior after extinction and modify extracellular dopamine levels in the nucleus accumbens. Alcohol Clin Exp Res. 1999;23:1751–1760. [PubMed] [Google Scholar]
- Litt MD, Cooney NL. Inducing craving for alcohol in the laboratory. Alcohol Res Health. 1999;23:174–178. [PMC free article] [PubMed] [Google Scholar]
- Liu X, Weiss F. Reversal of ethanol-seeking behavior by D1 and D2 antagonists in an animal model of relapse: differences in antagonist potency in previously ethanol-dependent versus nondependent rats. J Pharmacol Exp Ther. 2002;300:882–889. doi: 10.1124/jpet.300.3.882. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Alterman AI, Metzger DS, Grissom GR, Woody GE, Luborsky L, O'Brien CP. Similarity of outcome predictors across opiate, cocaine, and alcohol treatments: role of treatment services. J Consult Clin Psychol. 1994;62:1141–1158. doi: 10.1037//0022-006x.62.6.1141. [DOI] [PubMed] [Google Scholar]
- Miller WR, Walters ST, Bennett ME. How effective is alcoholism treatment in the United States? J Stud Alcohol. 2001;62:211–220. doi: 10.15288/jsa.2001.62.211. [DOI] [PubMed] [Google Scholar]
- Monahan SC, Finney JW. Explaining abstinence rates following treatment for alcohol abuse: a quantitative synthesis of patient, research design and treatment effects. Addiction. 1996;91:787–805. doi: 10.1046/j.1360-0443.1996.9167876.x. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Dalley JW, Cardinal RN, Bamford A, Fehnert B, Lachenal G, Rudarakanchana N, Halkerston KM, Robbins TW, Everitt BJ. Nucleus accumbens dopamine depletion impairs both acquisition and performance of appetitive Pavlovian approach behaviour: implications for mesoaccumbens dopamine function. Behav Brain Res. 2002;137:149–163. doi: 10.1016/s0166-4328(02)00291-7. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Phillips AG. Role of dopamine in anticipatory and consummatory aspects of sexual behavior in the male rat. Behav Neurosci. 1991;105:727–743. doi: 10.1037//0735-7044.105.5.727. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Atkinson LJ, Blackburn JR, Blaha CD. Increased extracellular dopamine in the nucleus accumbens of the rat elicited by a conditional stimulus for food: an electrochemical study. Can J Physiol Pharmacol. 1993;71:387–393. doi: 10.1139/y93-059. [DOI] [PubMed] [Google Scholar]
- Sabino V, Kwak J, Rice KC, Cottone P. Pharmacological characterization of the 20% alcohol intermittent access model in sardinian alcohol-preferring rats: a model of binge-like drinking. Alcohol Clin Exp Res. 2013;37:635–643. doi: 10.1111/acer.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. Eur J Neurosci. 2012;36:2521–2532. doi: 10.1111/j.1460-9568.2012.08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, Kruzich PJ, Grimm JW. Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology (Berl) 2001;154:301–310. doi: 10.1007/s002130000636. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29:203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- Young EA, Dreumont SE, Cunningham CL. Role of nucleus accumbens dopamine receptor subtypes in the learning and expression of alcohol-seeking behavior. Neurobiol Learn Mem. 2013 doi: 10.1016/j.nlm.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zironi I, Burattini C, Aicardi G, Janak PH. Context is a trigger for relapse to alcohol. Behav Brain Res. 2006;167:150–155. doi: 10.1016/j.bbr.2005.09.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.